Synthesis, Characterization and Optimization of Hydrothermally Fabricated Binary Palladium Alloys PdNix for Use as Counter Electrode Catalysts in Dye Sensitized Solar Cells
Abstract
:1. Introduction
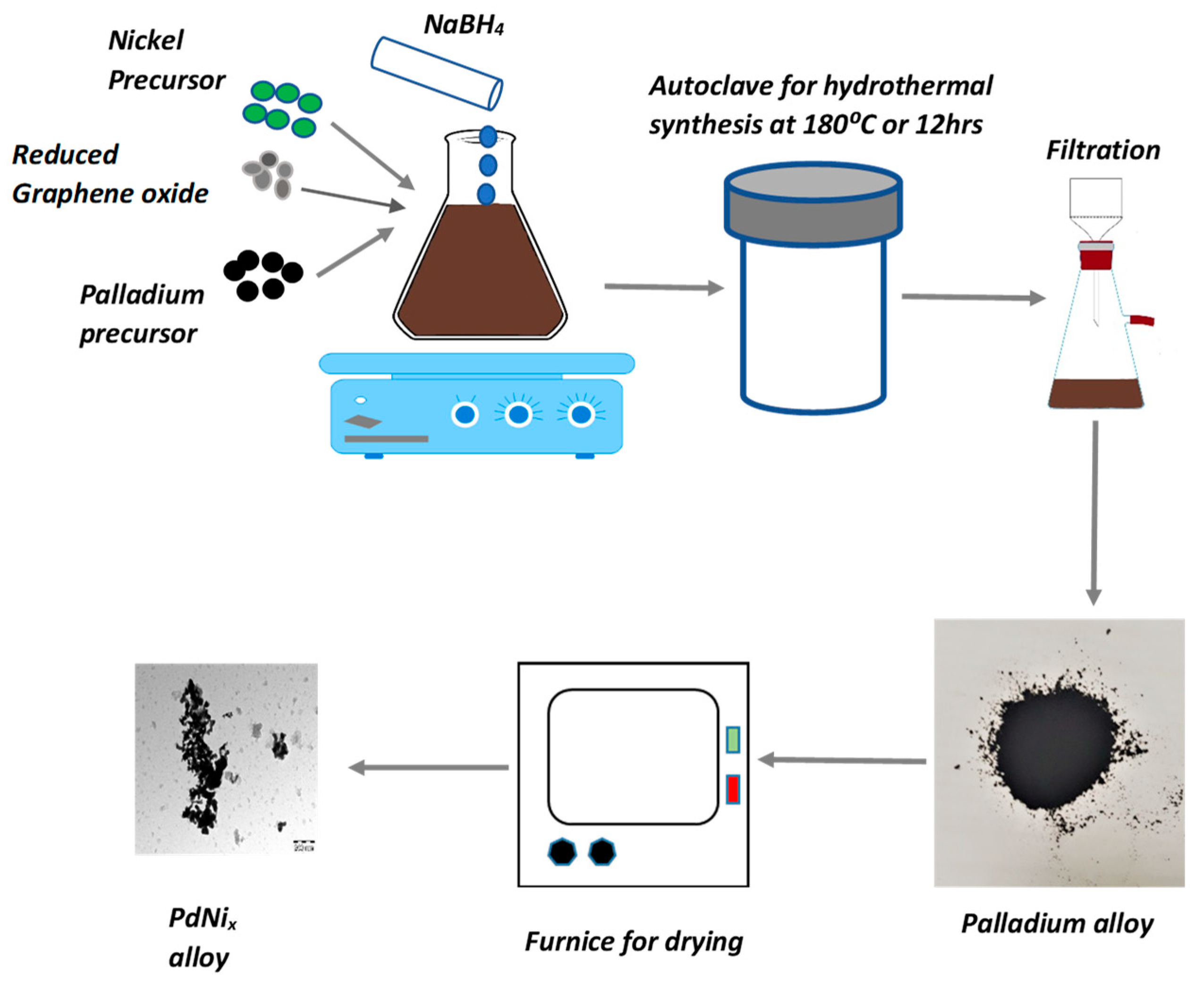
2. Materials and Methods
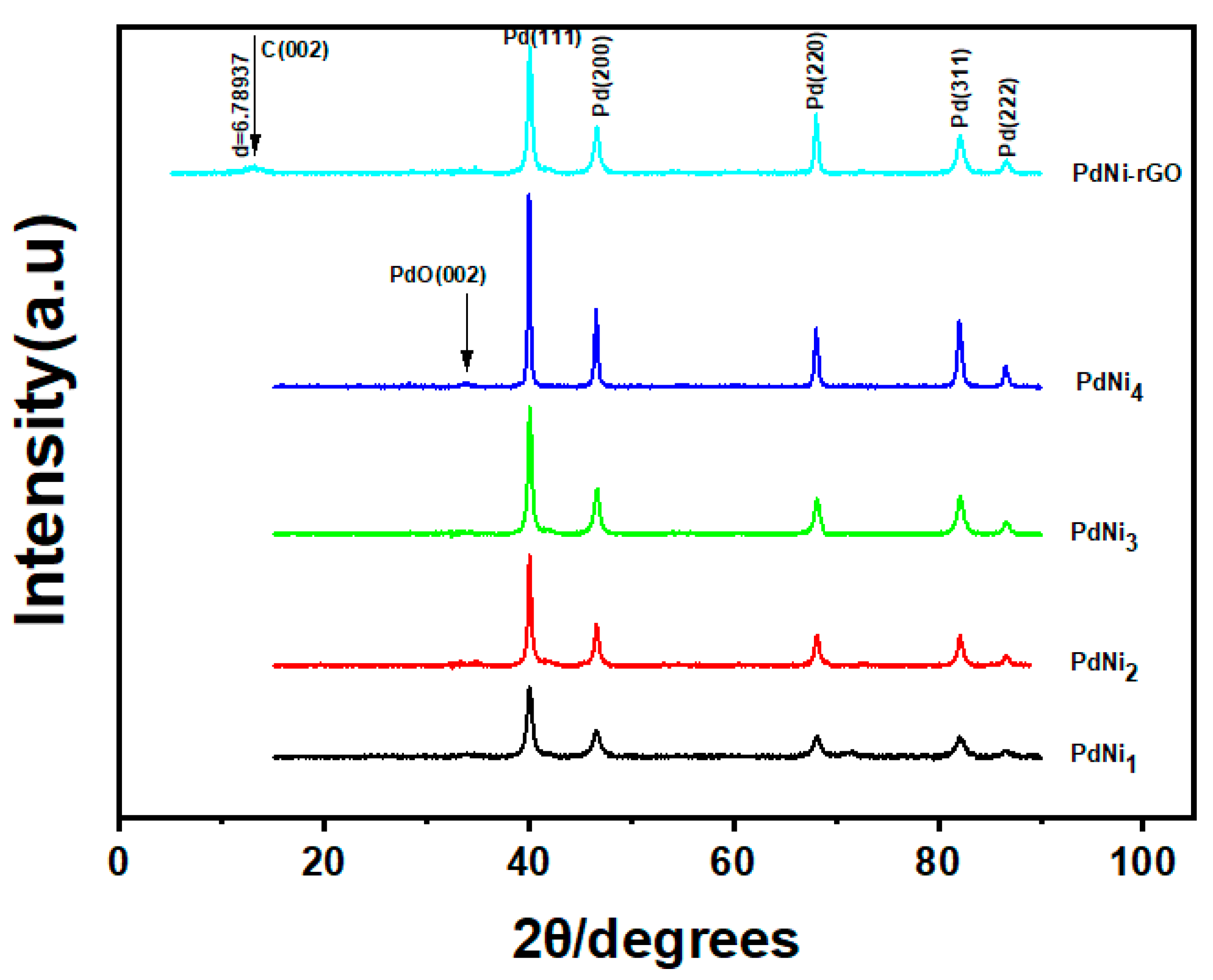
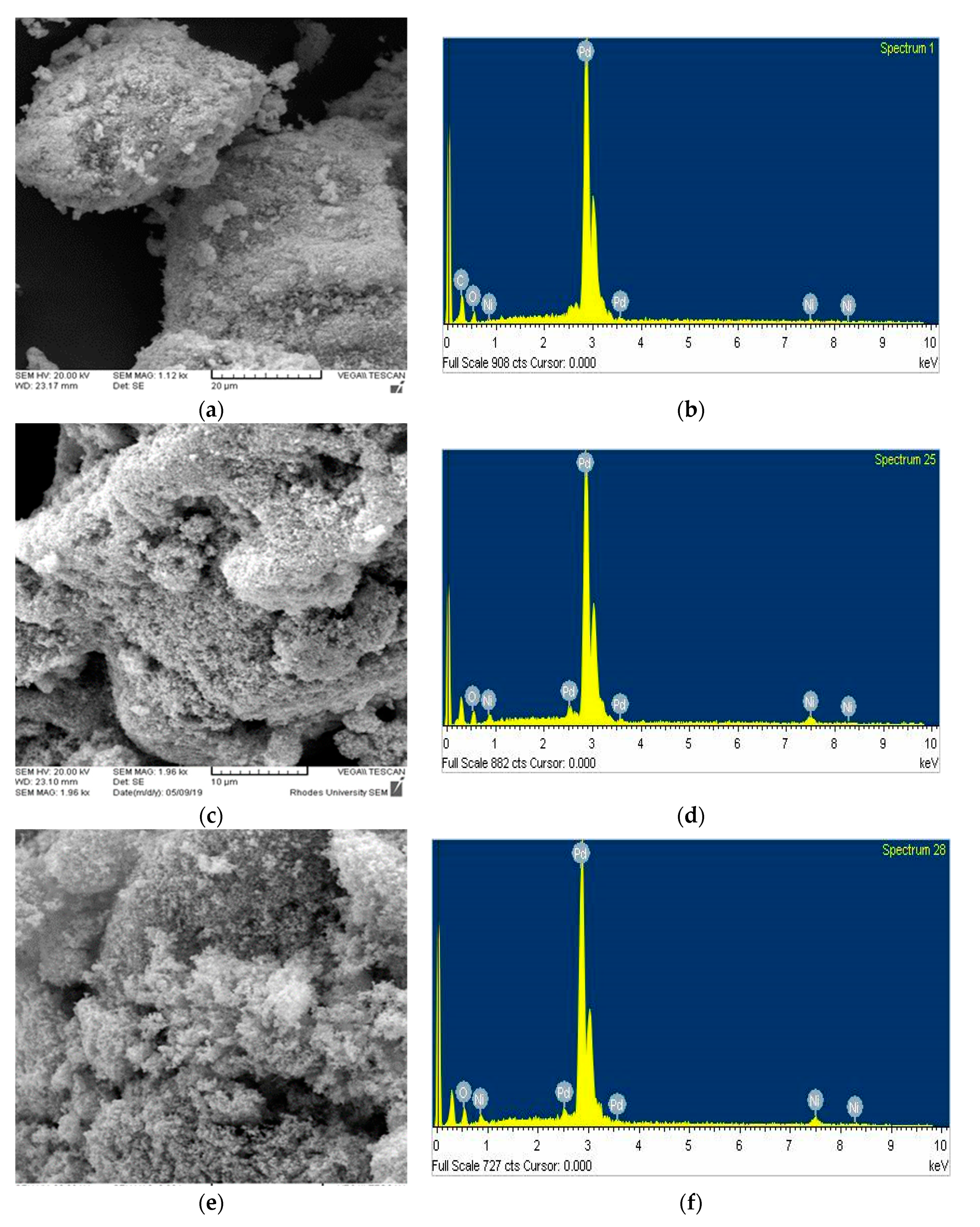
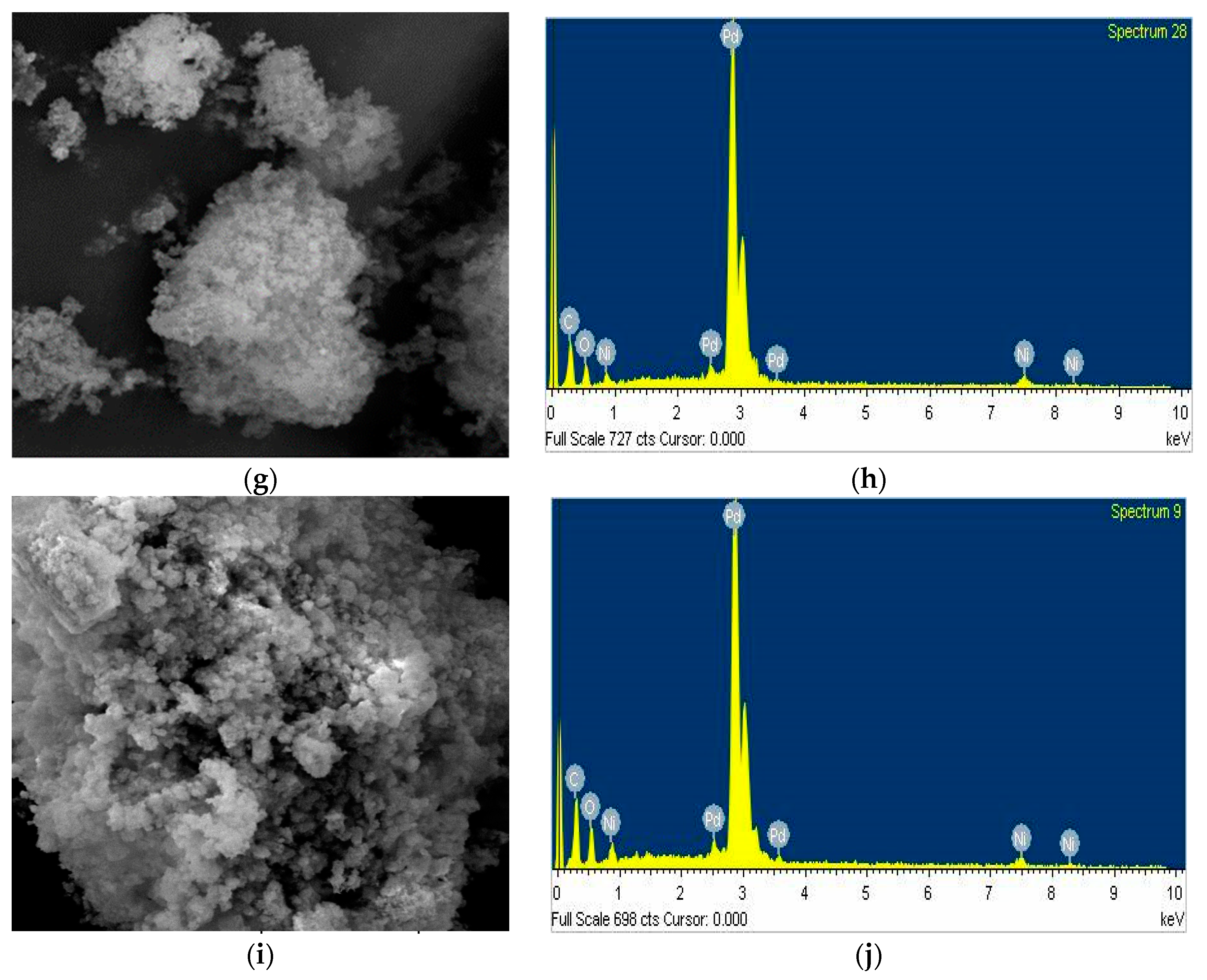
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeager, K.; Dayo, F.; Fisher, B.; Fouquet, R.; Gilau, A.; Rogner, H.A.; Haug, M.; Hosier, R.; Miller, A.; Schnitteger, S.; et al. Energy and Economy. In Global Energy Assessment: Toward a Sustainable Future; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; Chapter 6; pp. 386–422. Available online: http://www.iiasa.ac.at/web/home/research/Flagship-Projects/Global-Energy-Assessment/GEA_Chapter6_economy_lowres.pdf%5Cnhttp://www.iiasa.ac.at/web/home/research/Flagship-Projects/Global-Energy-Assessment/Global_Energy_Assessment_FullReport.pdf (accessed on 15 July 2019).
- Bischof-niemz, T. Statistics of Utility-Scale Solar PV, Wind and CSP in South Africa in 2016; CSIR: Pretoria, South Africa, 2017. [Google Scholar]
- National Renewable Energy Laboratory (NREL). Best Research-Cell Efficiencies Chart; NREL: Lakewood, CO, USA, 2018.
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Taziwa, R.; Mutukwa, D.; Zingwe, N. A Review on the Advancement of Ternary Alloy Counter Electrodes for Use in Dye-Sensitised Solar Cells. Metals 2018, 8, 1080. [Google Scholar] [CrossRef]
- Regan, B.O.; Gratzelt, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Asher, S.; Pershan, P.S. Parabolic Focal Conics and Polygonal Textures in Lipid Liquid Crystals. J. Phys. 1979, 40, 161–173. [Google Scholar] [CrossRef]
- Oh, H.J.; Dao, V.D.; Choi, H.S. Cost-effective CoPd alloy/reduced graphene oxide counter electrodes as a new avenue for high-efficiency liquid junction photovoltaic devices. J. Alloys Compd. 2017, 705, 610–617. [Google Scholar] [CrossRef]
- Huo, J.; Wu, J.; Zheng, M.; Tu, Y.; Lan, Z. Flower-like nickel cobalt sulfide microspheres modified with nickel sulfide as Pt-free counter electrode for dye-sensitized solar cells. J. Power Sources 2016, 304, 266–272. [Google Scholar] [CrossRef]
- Anuratha, K.; Ramaprakash, M.; Panda, S.K.; Mohan, S. Studies on synergetic effect of rGO-NiCo2S4 nanocomposite as an effective counter electrode material for DSSC. Ceram. Int. 2017, 43, 10174–10182. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Q.; He, B.; Yang, P. Counter electrodes from polymorphic platinum-nickel hollow alloys for high-efficiency dye-sensitized solar cells. J. Power Sources 2016, 328, 185–194. [Google Scholar] [CrossRef]
- Bae, K.; Dao, V.; Choi, H. Utility of Pt in PtNi alloy counter electrodes as a new avenue for cost effective and highly efficient liquid junction photovoltaic devices. J. Colloid Interface Sci. 2017, 495, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Tang, Q. A branching NiCuPt alloy counter electrode for high-efficiency dye-sensitized solar cell. Appl. Surf. Sci. 2016, 362, 28–34. [Google Scholar] [CrossRef]
- He, B.; Tang, Q.; Yu, L.; Yang, P. Cost−effective alloy counter electrodes as a new avenue for high−efficiency dye−sensitized solar cells. Electrochim. Acta 2015, 158, 397–402. [Google Scholar] [CrossRef]
- Rajesh, D.; Neel, P.I.; Pandurangan, A.; Mahendiran, C. Pd-NiO decorated multiwalled carbon nanotubes supported on reduced graphene oxide as an efficient electrocatalyst for ethanol oxidation in alkaline medium. Appl. Surf. Sci. 2018, 442, 787–796. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
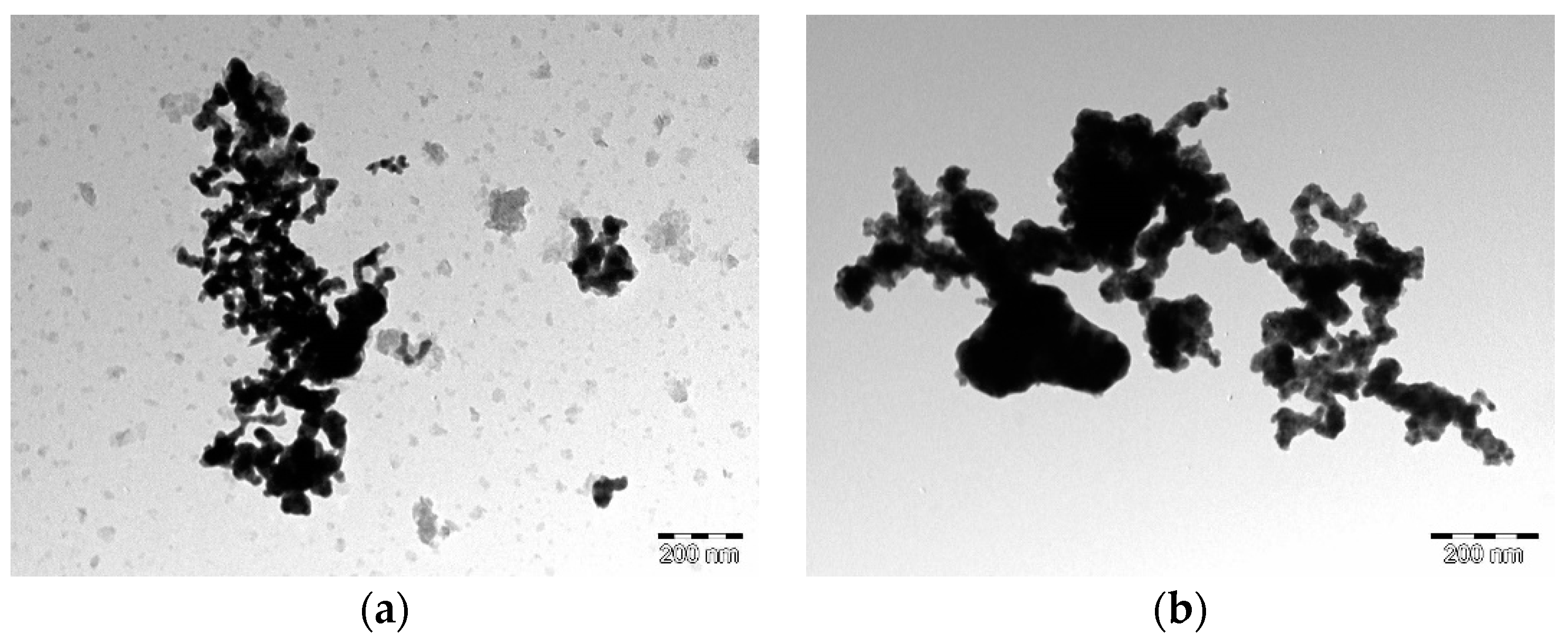
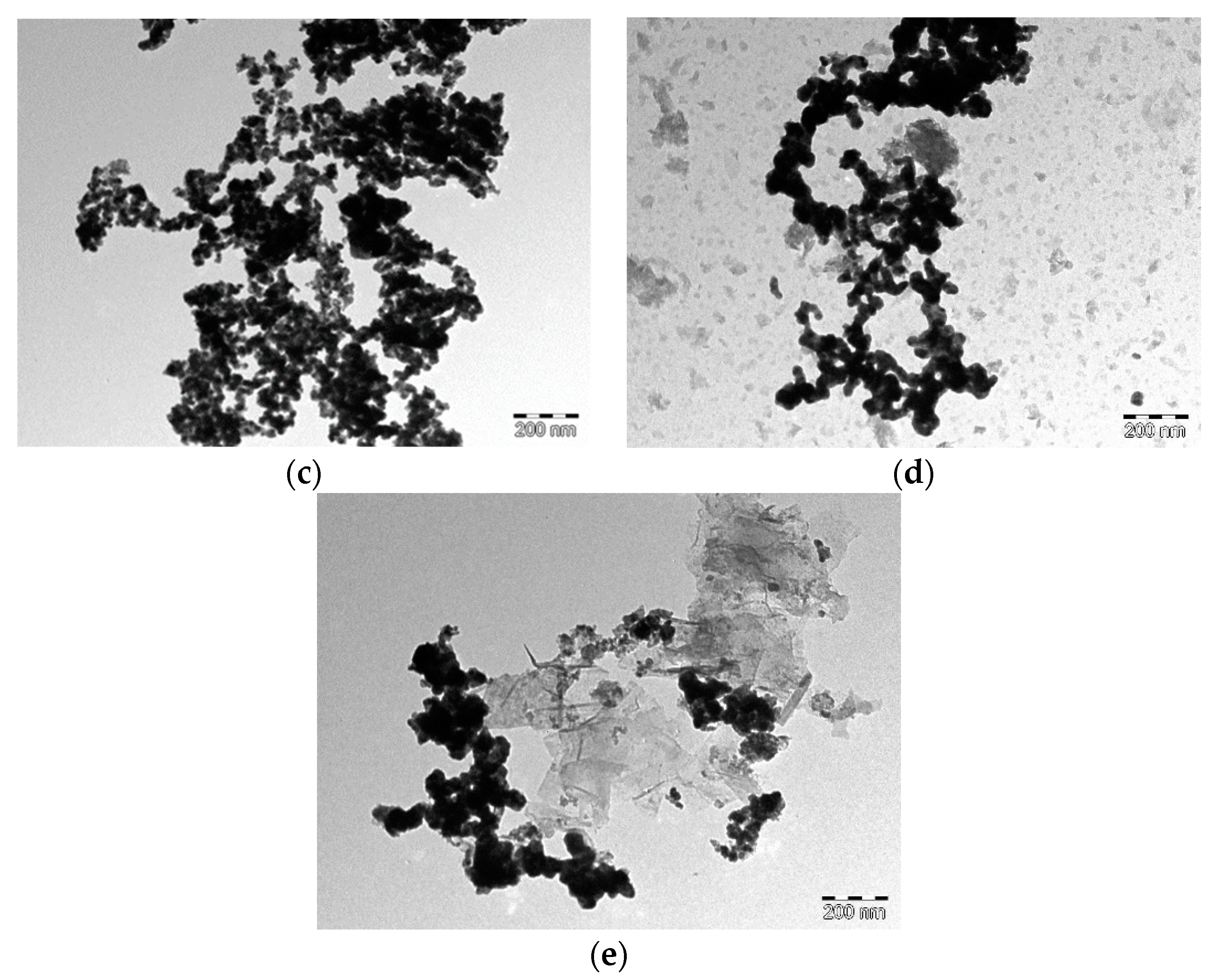
| Alloy | Molar Quantities of Reactants | |
|---|---|---|
| K2PdCl4 | Ni(NO3)2∙6H2O | |
| PdNi1 | 0.0015 | 0.01 |
| PdNi2 | 0.0024 | 0.0086 |
| PdNi3 | 0.003 | 0.0076 |
| PdNi4 | 0.0037 | 0.005 |
| PdNi-rGO | 0.003 | 0.0076 |
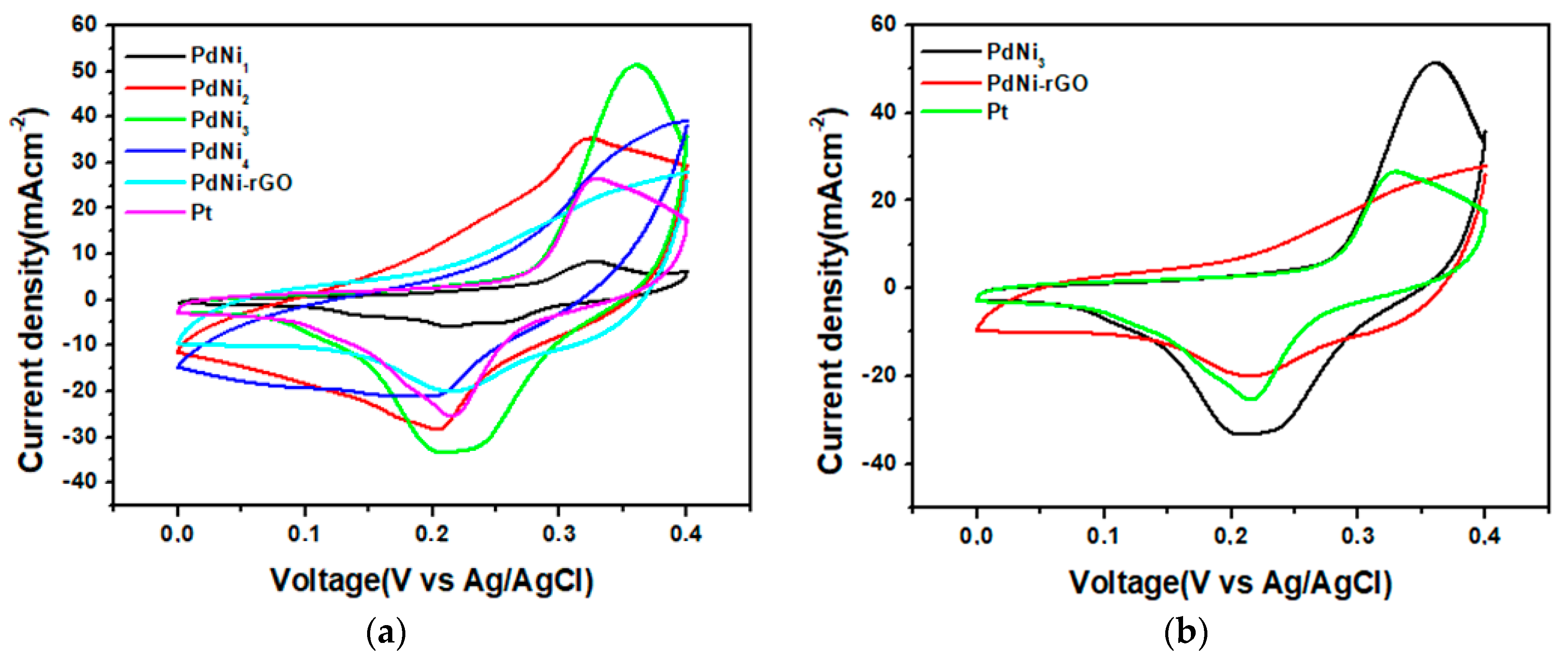
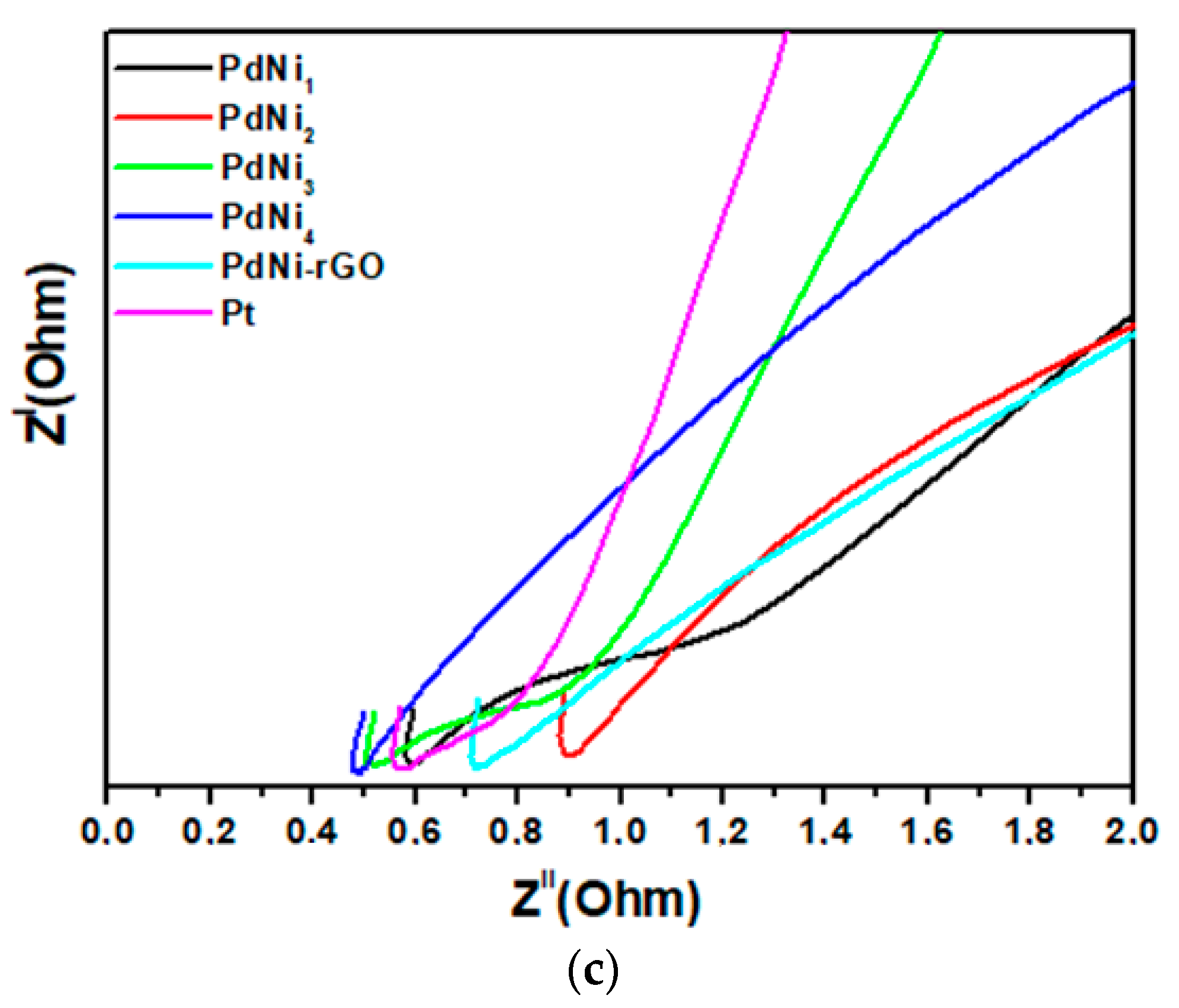
| Counter Electrode Catalyst | Reduction Current Density (JP)/mA cm−2 | Peak to Peak Potential Difference (∆EPP)/mV | Charge Transfer Resistance (RCT)/Ω |
|---|---|---|---|
| PdNi1 | 7 | 0.11 | 0.58 |
| PdNi2 | 30 | 0.11 | 0.9 |
| PdNi3 | 35 | 0.15 | 0.47 |
| PdNi4 | 21 | 0.13 | 0.47 |
| PdNi-rGO | 20 | 0.09 | 0.74 |
| Platinum | 25 | 0.1 | 0.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zingwe, N.; Meyer, E.; Mbese, J. Synthesis, Characterization and Optimization of Hydrothermally Fabricated Binary Palladium Alloys PdNix for Use as Counter Electrode Catalysts in Dye Sensitized Solar Cells. Materials 2019, 12, 3116. https://doi.org/10.3390/ma12193116
Zingwe N, Meyer E, Mbese J. Synthesis, Characterization and Optimization of Hydrothermally Fabricated Binary Palladium Alloys PdNix for Use as Counter Electrode Catalysts in Dye Sensitized Solar Cells. Materials. 2019; 12(19):3116. https://doi.org/10.3390/ma12193116
Chicago/Turabian StyleZingwe, Nyengerai, Edson Meyer, and Johannes Mbese. 2019. "Synthesis, Characterization and Optimization of Hydrothermally Fabricated Binary Palladium Alloys PdNix for Use as Counter Electrode Catalysts in Dye Sensitized Solar Cells" Materials 12, no. 19: 3116. https://doi.org/10.3390/ma12193116
APA StyleZingwe, N., Meyer, E., & Mbese, J. (2019). Synthesis, Characterization and Optimization of Hydrothermally Fabricated Binary Palladium Alloys PdNix for Use as Counter Electrode Catalysts in Dye Sensitized Solar Cells. Materials, 12(19), 3116. https://doi.org/10.3390/ma12193116






