In-Situ Raman Characterization of Initial Corrosion Behavior of Copper in Neutral 3.5% (wt.) NaCl Solution
Abstract
1. Introduction
2. Experimental
2.1. Medium and Electrodes
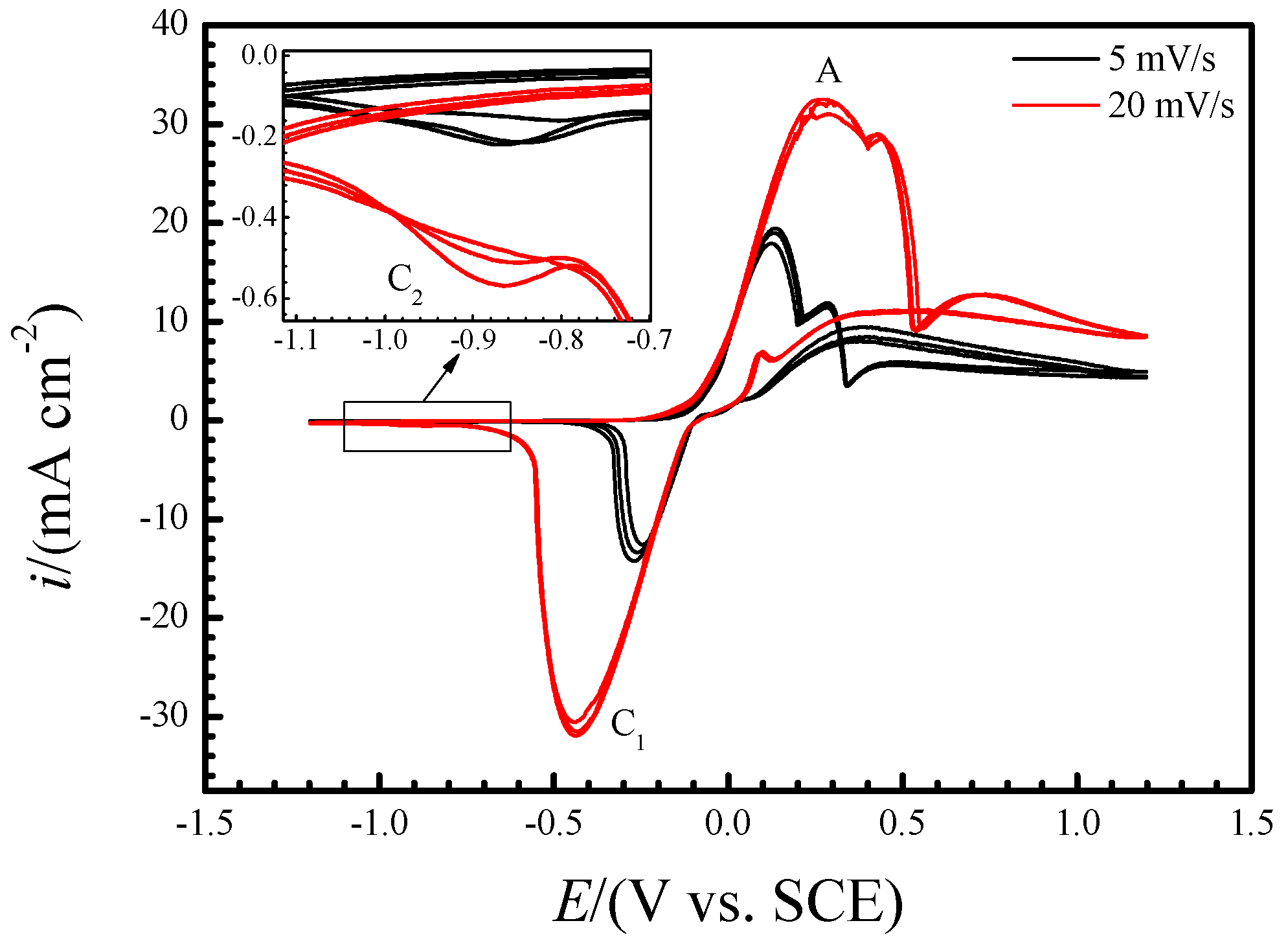
2.2. Electrochemical Tests
2.3. In-Situ Raman Characterization
3. Results and Discussion
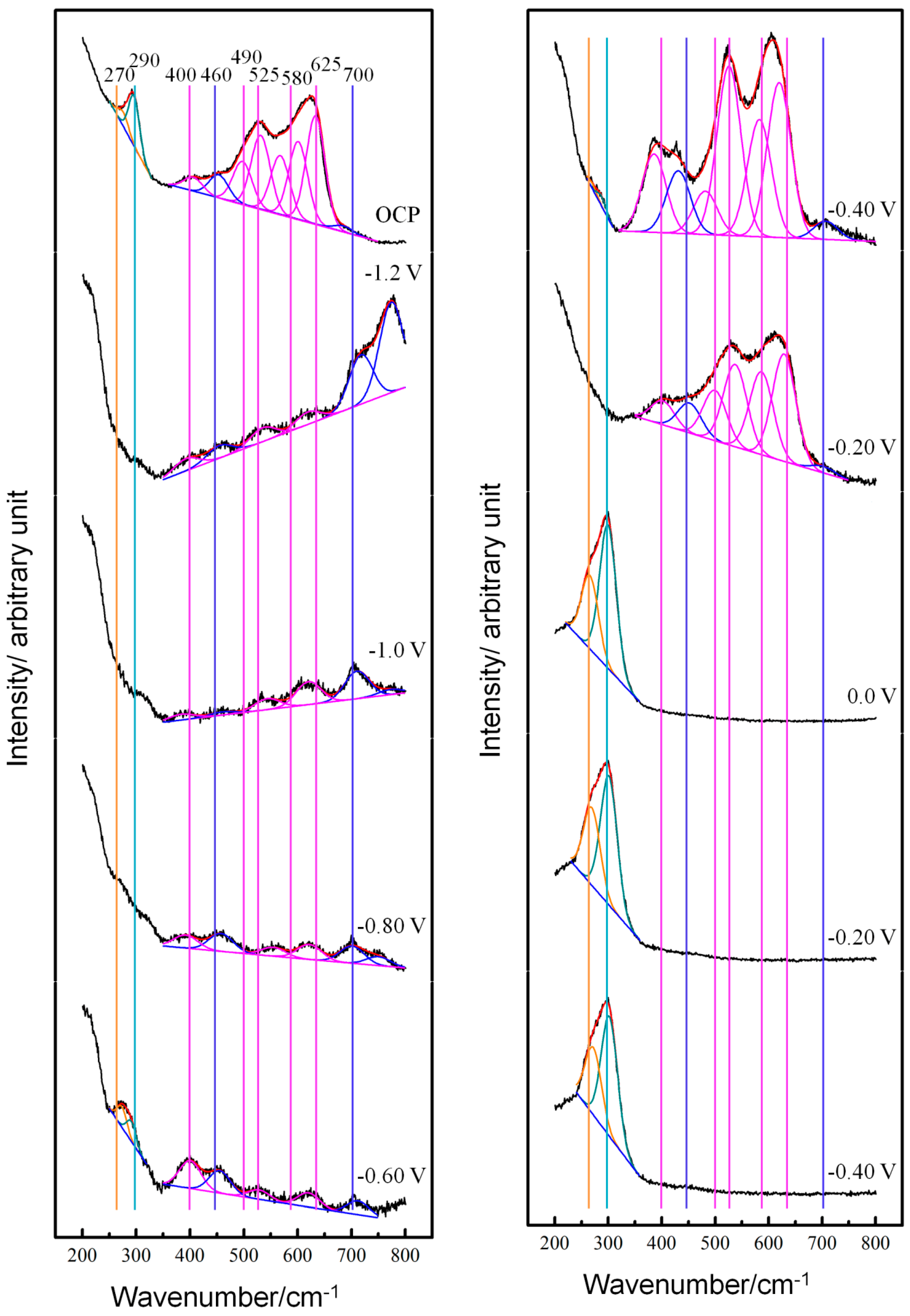
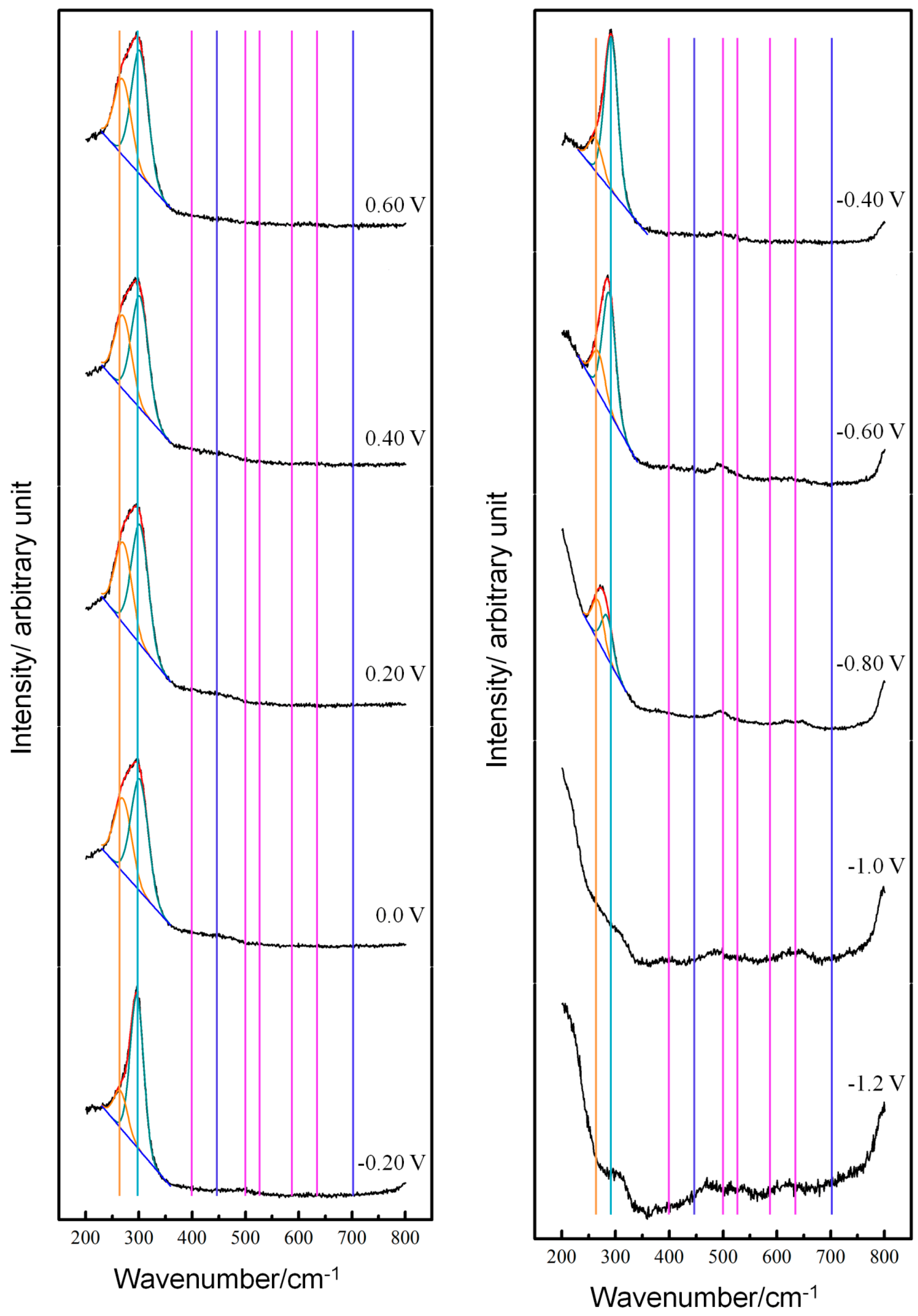
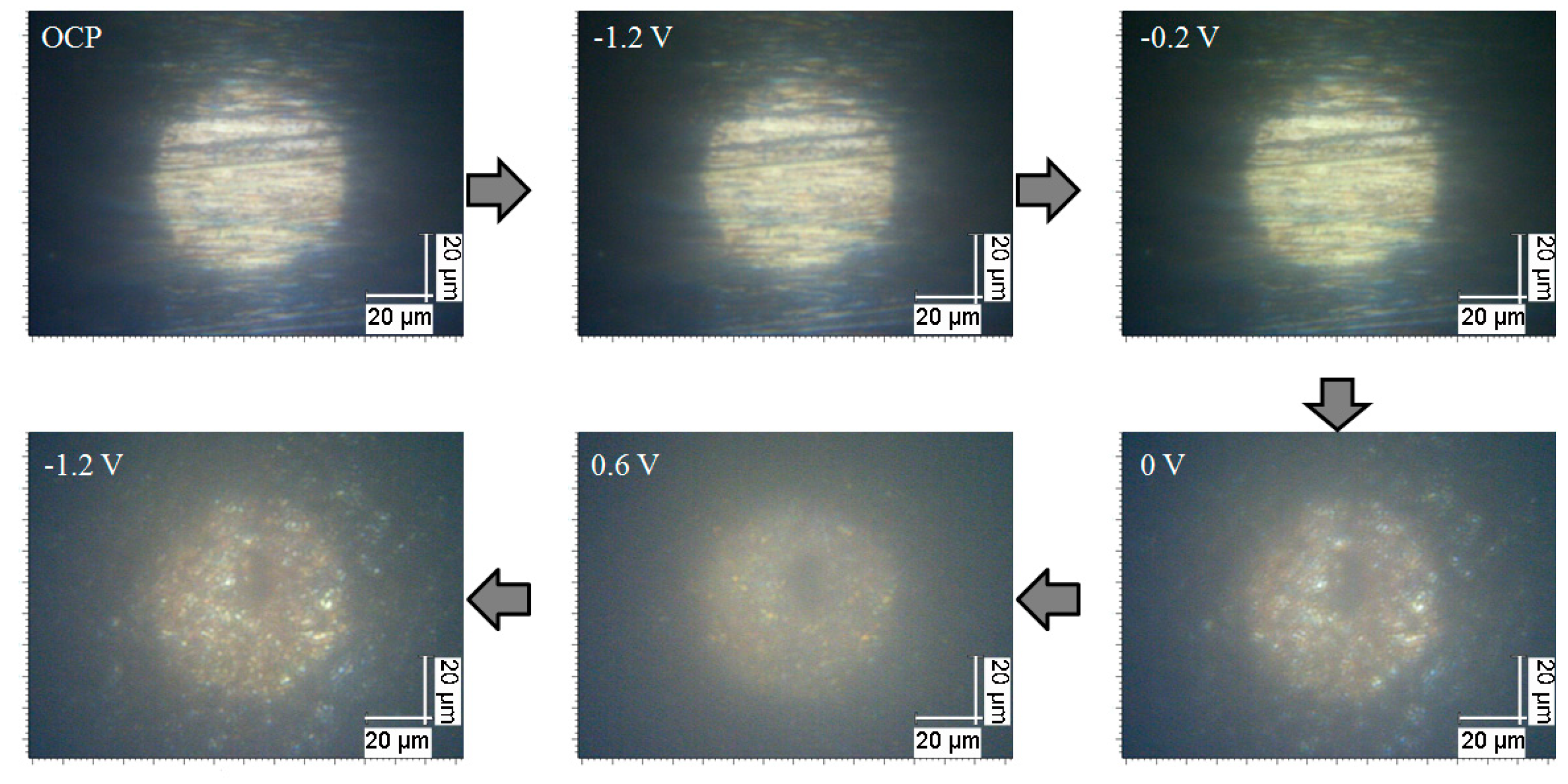
3.1. Potential-Dependent Raman Spectra
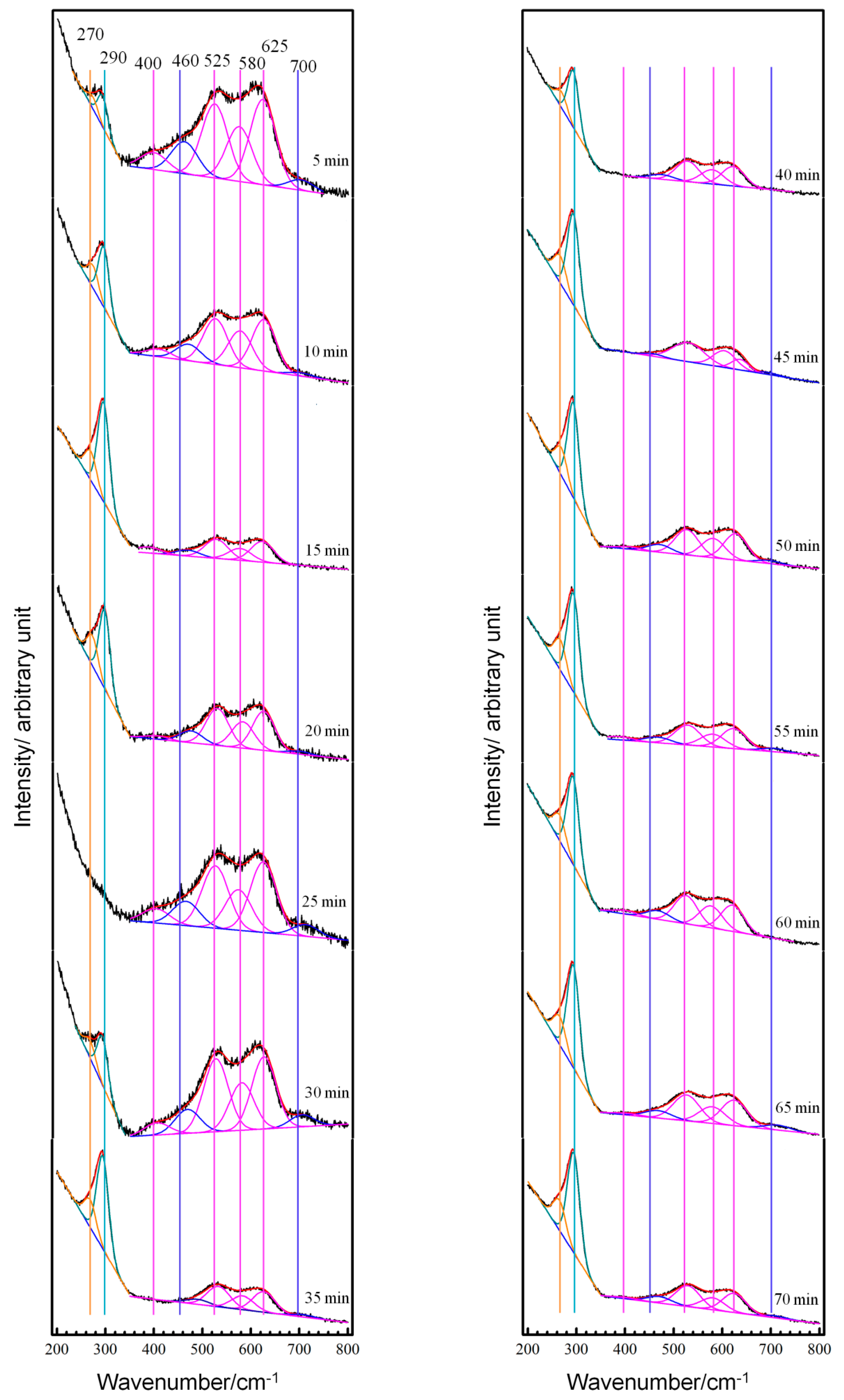
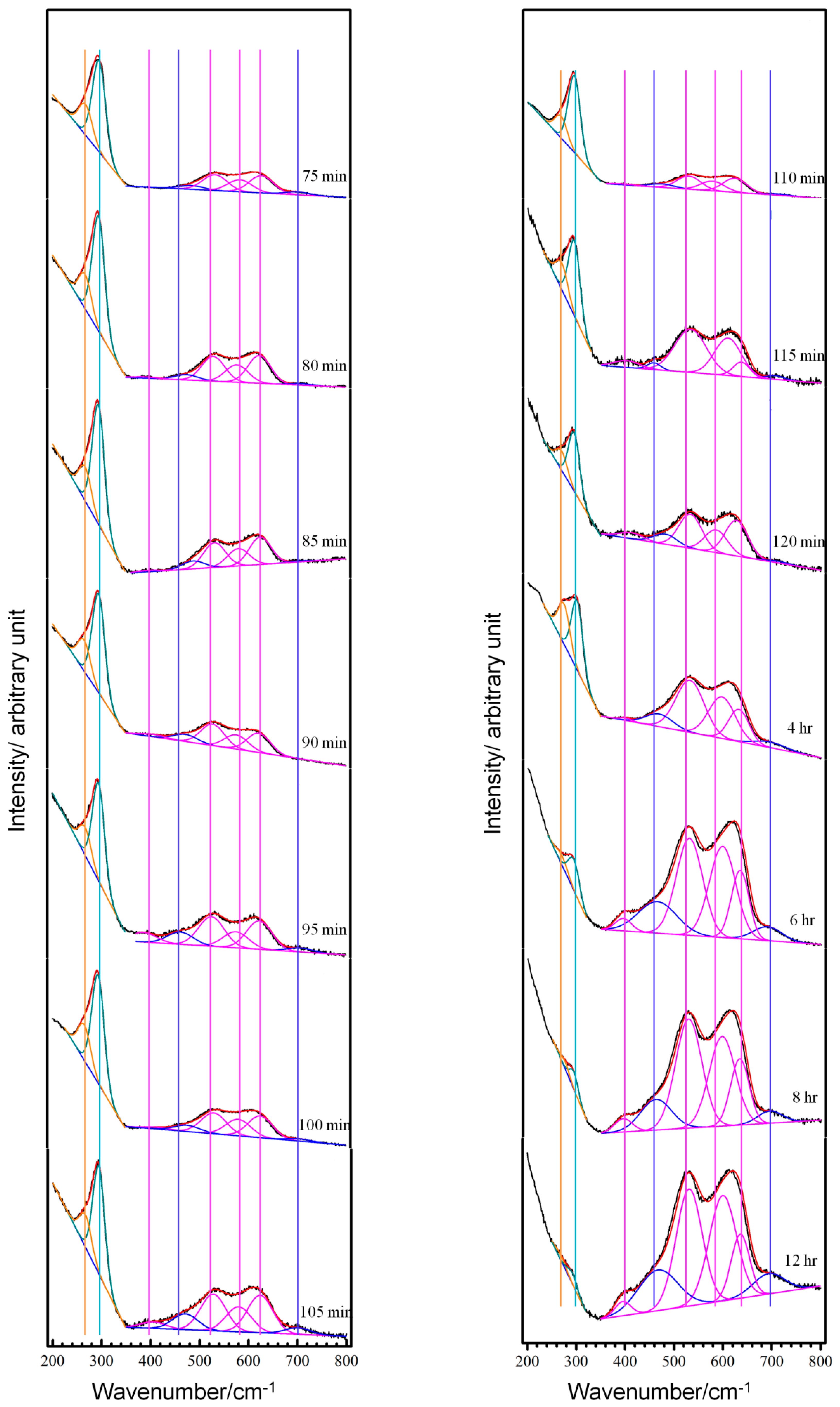
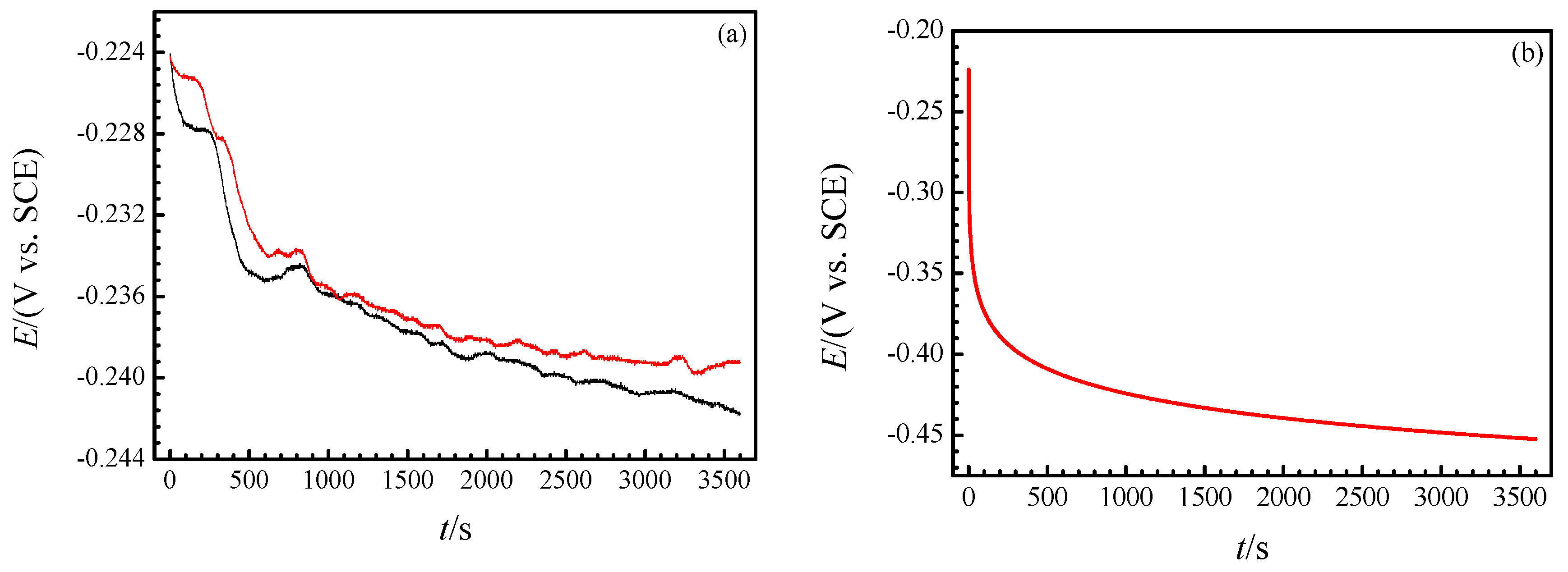
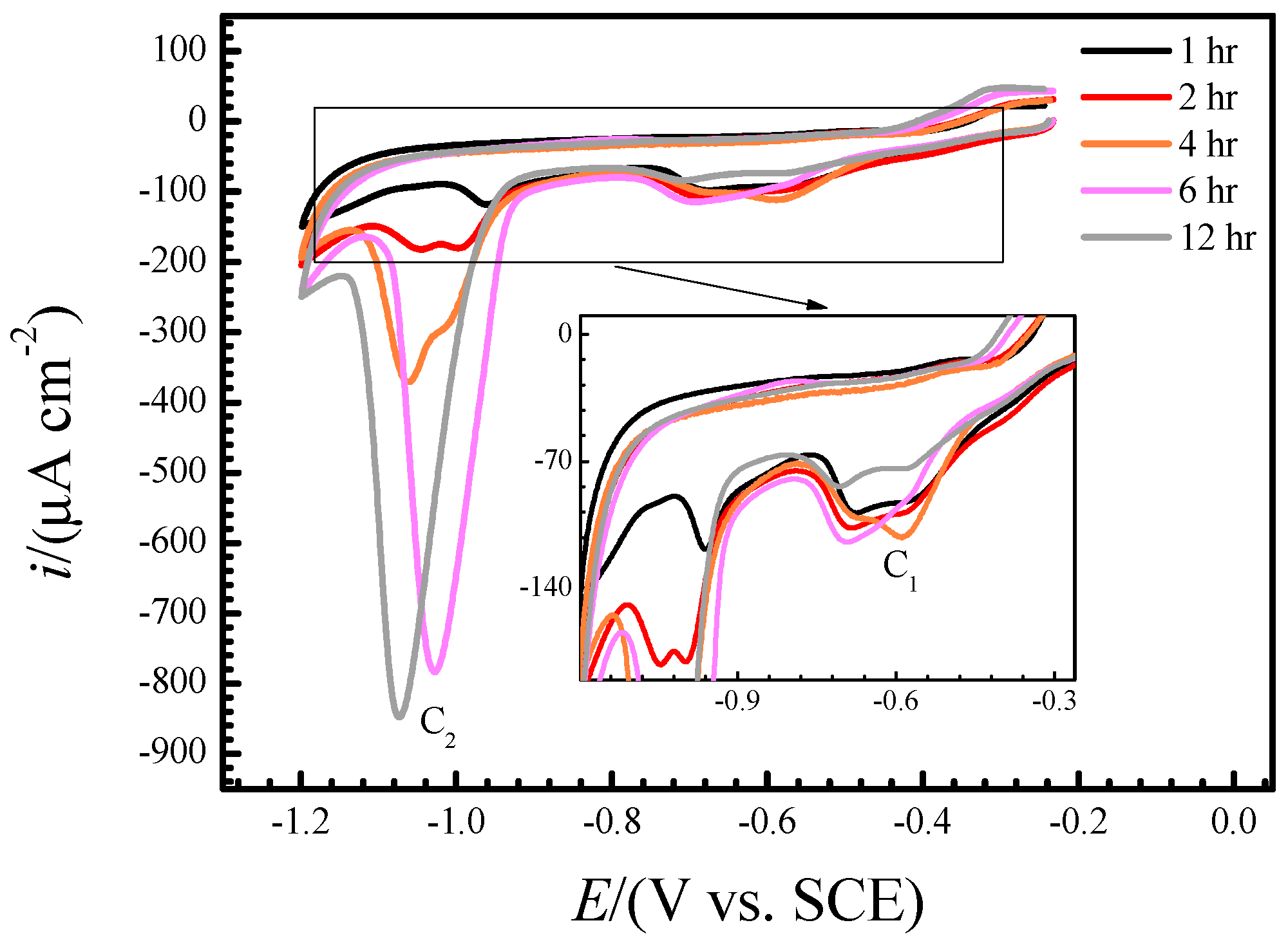
3.2. Real-Time Raman Spectra at OCP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosborg, B.; Werme, L. The Swedish nuclear waste program and the long-term corrosion behaviour of copper. J. Nucl. Mater. 2008, 379, 142–153. [Google Scholar] [CrossRef]
- Qin, Z.; Daljeet, R.; Ai, M.; Farhangi, N.; Noël, J.; Ramamurthy, S.; Shoesmith, D.; King, F.; Keech, P. The active/passive conditions for copper corrosion under nuclear waste repository environment. Corros. Eng. Sci. Technol. 2017, 52, 45–49. [Google Scholar] [CrossRef]
- Kong, D.; Xu, A.; Dong, C.; Mao, F.; Xiao, K.; Li, X.; Macdonald, D.D. Electrochemical investigation and ab initio computation of passive film properties on copper in anaerobic sulphide solutions. Corros. Sci. 2017, 116, 34–43. [Google Scholar] [CrossRef]
- Dong, C.; Mao, F.; Gao, S.; Sharifi-Asl, S.; Lu, P.; Macdonald, D.D. Passivity Breakdown on Copper: Influence of Temperature. J. Electrochem. Soc. 2016, 163, 707–717. [Google Scholar] [CrossRef]
- Xiao, K.; Gao, X.; Yan, L.; Yi, P.; Zhang, D.; Dong, C.; Wu, J.; Li, X. Atmospheric corrosion factors of printed circuit boards in a dry-heat desert environment: Salty dust and diurnal temperature difference. Chem. Eng. J. 2018, 336, 92–101. [Google Scholar] [CrossRef]
- Conseil-Gudla, H.; Jellesen, M.S.; Ambat, R. Printed circuit board surface finish and effects of chloride contamination, electric field, and humidity on corrosion reliability. J. Electron. Mater. 2017, 46, 817–825. [Google Scholar] [CrossRef]
- Xiao, K.; Yi, P.; Dong, C.; Zou, S.; Li, X. Role of mold in electrochemical migration of copper-clad laminate and electroless nickel/immersion gold printed circuit boards. Mater. Lett. 2018, 210, 283–286. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Liao, X.; Cao, F.; Zheng, L.; Liu, W.; Chen, A.; Zhang, J.; Cao, C. Corrosion behaviour of copper under chloride−containing thin electrolyte layer. Corros. Sci. 2011, 53, 3289–3298. [Google Scholar] [CrossRef]
- Nagiub, A.M. Comparative electrochemical noise study of the corrosion of different alloys exposed to chloride Media. Engineering 2014, 6, 1007. [Google Scholar] [CrossRef]
- Martinez-Lombardia, E.; Gonzalez-Garcia, Y.; Lapeire, L.; De Graeve, I.; Verbeken, K.; Kestens, L.; Mol, J.M.C.; Terryn, H. Scanning electrochemical microscopy to study the effect of crystallographic orientation on the electrochemical activity of pure copper. Electrochim. Acta 2014, 116, 89–96. [Google Scholar] [CrossRef]
- Narayanan, B.; Deshmukh, S.; Ramanathan, S.; Sankaranarayanan, S.K. Atomistic Insights into the Interaction of Copper Oxide Surfaces with Chloride Ions in Aqueous Media. J. Am. Coll. Cardiol. 2015, 63, 1069. [Google Scholar]
- Lee, H.P.; Nobe, K. Kinetics and mechanisms of Cu electrodissolution in chloride media. J. Electrochem. Soc. 1986, 133, 2035–2043. [Google Scholar] [CrossRef]
- Suggs, D.W.; Bard, A.J. Scanning tunneling microscopic study with atomic resolution of the dissolution of Cu(100) electrodes in aqueous chloride media. J. Phys. Chem. 1995, 99, 8349–8355. [Google Scholar] [CrossRef]
- Suggs, D.W.; Bard, A.J. Scanning tunneling microscopic study with atomic resolution of the dissolution of Cu(111) in aqueous chloride solutions. J. Am. Chem. Soc. 1994, 116, 10725–10733. [Google Scholar] [CrossRef]
- Kunze, J.; Maurice, V.; Klein, L.H.; Strehblow, H.H.; Marcus, P. In situ STM study of the effect of chlorides on the initial stages of anodic oxidation of Cu(111) in alkaline solutions. Electrochim. Acta 2003, 48, 1157–1167. [Google Scholar] [CrossRef]
- Bianchi, G.; Longhi, P. Copper in sea-water, potential-pH diagrams. Corros. Sci. 1973, 13, 853–864. [Google Scholar] [CrossRef]
- Persson, I.; Sandstroem, M.; Steel, A.T.; Zapatero, M.J.; Aakesson, R. A large-angle X-ray scattering, XAFS, and vibrational spectroscopic study of copper(I) halide complexes in dimethyl sulfoxide, acetonitrile, pyridine, and aqueous solutions. Inorg. Chem. 1991, 30, 4075–4081. [Google Scholar] [CrossRef]
- Frost, R.L.; Williams, P.A.; Kloprogge, J.T.; Martens, W. Raman spectroscopy of the copper chloride minerals nantokite, eriochalcite and claringbullite—Implications for copper corrosion. Neues JB. Miner. Monat. 2003, 2003, 433–445. [Google Scholar] [CrossRef]
- Brown, G.M.; Hope, G.A. A SERS study of /Cl− ion adsorption at a copper electrode in-situ. J. Electroanal. Chem. 1996, 405, 211–216. [Google Scholar] [CrossRef]
- Chan, H.Y.H.; Takoudis, C.G.; Weaver, M.J. Oxide film formation and oxygen adsorption on copper in aqueous media as probed by surface-enhanced Raman spectroscopy. J. Phys. Chem. B 1999, 103, 357–365. [Google Scholar] [CrossRef]
- Hamilton, J.C.; Farmer, J.C.; Anderson, R.J. In situ Raman spectroscopy of anodic films formed on copper and silver in sodium hydroxide solution. J. Electrochem. Soc. 1986, 133, 739–745. [Google Scholar] [CrossRef]
- Gong, Y.S.; Lee, C.; Yang, C.K. Atomic force microscopy and Raman spectroscopy studies on the oxidation of Cu thin films. J. Appl. Phys. 1995, 77, 5422–5425. [Google Scholar] [CrossRef]
- Bouchard, M.; Smith, D.C. Catalogue of 45 reference Raman spectra of minerals concerning research in art history or archaeology, especially on corroded metals and coloured glass. Spectrochim. Acta Part A 2003, 59, 2247–2266. [Google Scholar] [CrossRef]
- Melendres, C.A.; Xu, S.; Tani, B. A laser Raman spectroscopic study of anodic corrosion films on silver and copper. J. Electroanal. Chem. Interfacial Electrochem. 1984, 162, 343–349. [Google Scholar] [CrossRef]
- Frost, R.L. Raman spectroscopy of selected copper minerals of significance in corrosion. Spectrochim. Acta Part A 2003, 59, 1195–1204. [Google Scholar] [CrossRef]
- Niaura, G. Surface-enhanced Raman spectroscopic observation of two kinds of adsorbed OH− ions at copper electrode. Electrochim. Acta 2000, 45, 3507–3519. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: Identification of CuIII oxides as catalytically active species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Singhal, A.; Pai, M.R.; Rao, R.; Pillai, K.T.; Lieberwirth, I.; Tyagi, A.K. Copper (I) oxide nanocrystals—One step synthesis, characterization, formation mechanism, and photocatalytic properties. Eur. J. Inorg. Chem. 2013, 2013, 2640–2651. [Google Scholar] [CrossRef]
- Chrzanowski, J.; Irwin, J.C. Raman scattering from cupric oxide. Solid State Commun. 1989, 70, 11–14. [Google Scholar] [CrossRef]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Kruger, P. Vibrational properties of CuO and Cu4O3 from first-principles calculations, and Raman and infrared spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, C.; Li, L.; Chen, S. Real time observation of the anodic dissolution of copper in NaCl solution with the digital holography. Electrochem. Commun. 2009, 11, 1373–1376. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, C.; Li, L.; Chen, S. Investigation of the effects of the magnetic field on the anodic dissolution of copper in NaCl solutions with holography. Corros. Sci. 2012, 58, 69–78. [Google Scholar] [CrossRef]
- Bianchi, G.; Fiori, G.; Longhi, P.; Mazza, F. “Horse Shoe” Corrosion of Copper Alloys in Flowing Sea Water: Mechanism, and Possibility of Cathodic Protection of Condenser Tubes in Power Stations. Corrosion 1978, 34, 396–406. [Google Scholar] [CrossRef]
- Crousier, J.; Pardessus, L.; Crousier, J.P. Voltammetry study of copper in chloride solution. Electrochim. Acta 1988, 33, 1039–1042. [Google Scholar] [CrossRef]
- Gennero De Chialvo, M.R.; Marchiano, S.L.; Arvía, A.J. The mechanism of oxidation of copper in alkaline solutions. J. Appl. Electrochem. 1984, 14, 165–175. [Google Scholar] [CrossRef]
- Modestov, A.D.; Zhou, G.D.; Ge, H.H.; Loo, B.H. A study by voltammetry and the photocurrent response method of copper electrode behavior in acidic and alkaline solutions containing chloride ions. J. Electroanal. Chem. 1995, 380, 63–68. [Google Scholar] [CrossRef]
- Shim, J.J.; Kim, J.G. Copper corrosion in potable water distribution systems: Influence of copper products on the corrosion behavior. Mater. Lett. 2004, 58, 2002–2006. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part, I. Long-term immersion, 3D-profilometry, and electrochemistry. Corros. Sci. 2013, 72, 82–89. [Google Scholar] [CrossRef]

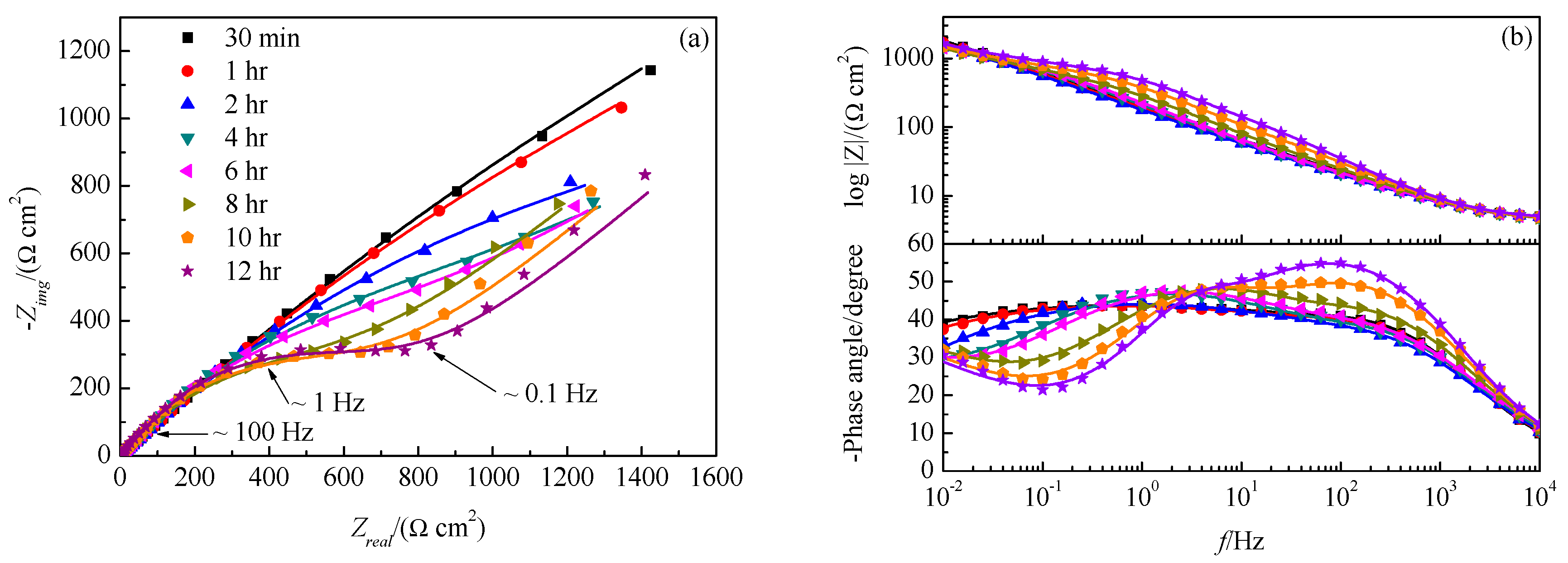
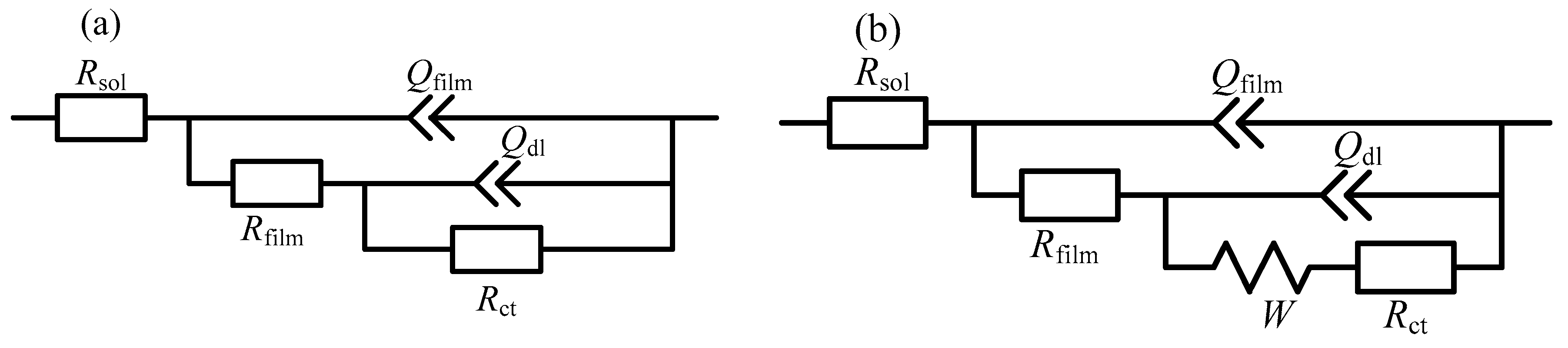
| Immersion Time | Rsol/ | Qfilm/ | nfilm | Rfilm/ | Qdl/ | ndl | Rct/ | W/ |
|---|---|---|---|---|---|---|---|---|
| 30 min | 4.5 | 3.4 × 10−4 | 0.72 | 27.4 | 1.7 × 10−3 | 0.48 | 1.46 × 104 | |
| 1 h | 4.4 | 4.0 × 10−4 | 0.70 | 29.4 | 1.8 × 10−3 | 0.48 | 1.03 × 104 | |
| 2 h | 4.3 | 4.6 × 10−4 | 0.69 | 23.8 | 1.7 × 10−3 | 0.49 | 4577 | |
| 4 h | 4.2 | 5.1 × 10−4 | 0.67 | 35.7 | 1.0 × 10−3 | 0.57 | 1622 | 4.8 × 10−3 |
| 6 h | 4.0 | 6.5 × 10−4 | 0.65 | 53.8 | 6.9 × 10−4 | 0.61 | 1209 | 4.4 × 10−3 |
| 8 h | 4.2 | 4.5 × 10−4 | 0.69 | 65.8 | 4.2 × 10−4 | 0.68 | 667.4 | 3.9 × 10−3 |
| 10 h | 4.3 | 3.4 × 10−4 | 0.71 | 179.1 | 2.0 × 10−4 | 0.84 | 529 | 3.9 × 10−3 |
| 12 h | 4.4 | 2.6 × 10−4 | 0.73 | 373.3 | 1.2 × 10−4 | 0.99 | 338.4 | 3.7 × 10−3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Li, J. In-Situ Raman Characterization of Initial Corrosion Behavior of Copper in Neutral 3.5% (wt.) NaCl Solution. Materials 2019, 12, 2164. https://doi.org/10.3390/ma12132164
Liu M, Li J. In-Situ Raman Characterization of Initial Corrosion Behavior of Copper in Neutral 3.5% (wt.) NaCl Solution. Materials. 2019; 12(13):2164. https://doi.org/10.3390/ma12132164
Chicago/Turabian StyleLiu, Ming, and Jun Li. 2019. "In-Situ Raman Characterization of Initial Corrosion Behavior of Copper in Neutral 3.5% (wt.) NaCl Solution" Materials 12, no. 13: 2164. https://doi.org/10.3390/ma12132164
APA StyleLiu, M., & Li, J. (2019). In-Situ Raman Characterization of Initial Corrosion Behavior of Copper in Neutral 3.5% (wt.) NaCl Solution. Materials, 12(13), 2164. https://doi.org/10.3390/ma12132164






