Effects of Cell Temperature and Reactant Humidification on Anion Exchange Membrane Fuel Cells
Abstract
1. Introduction
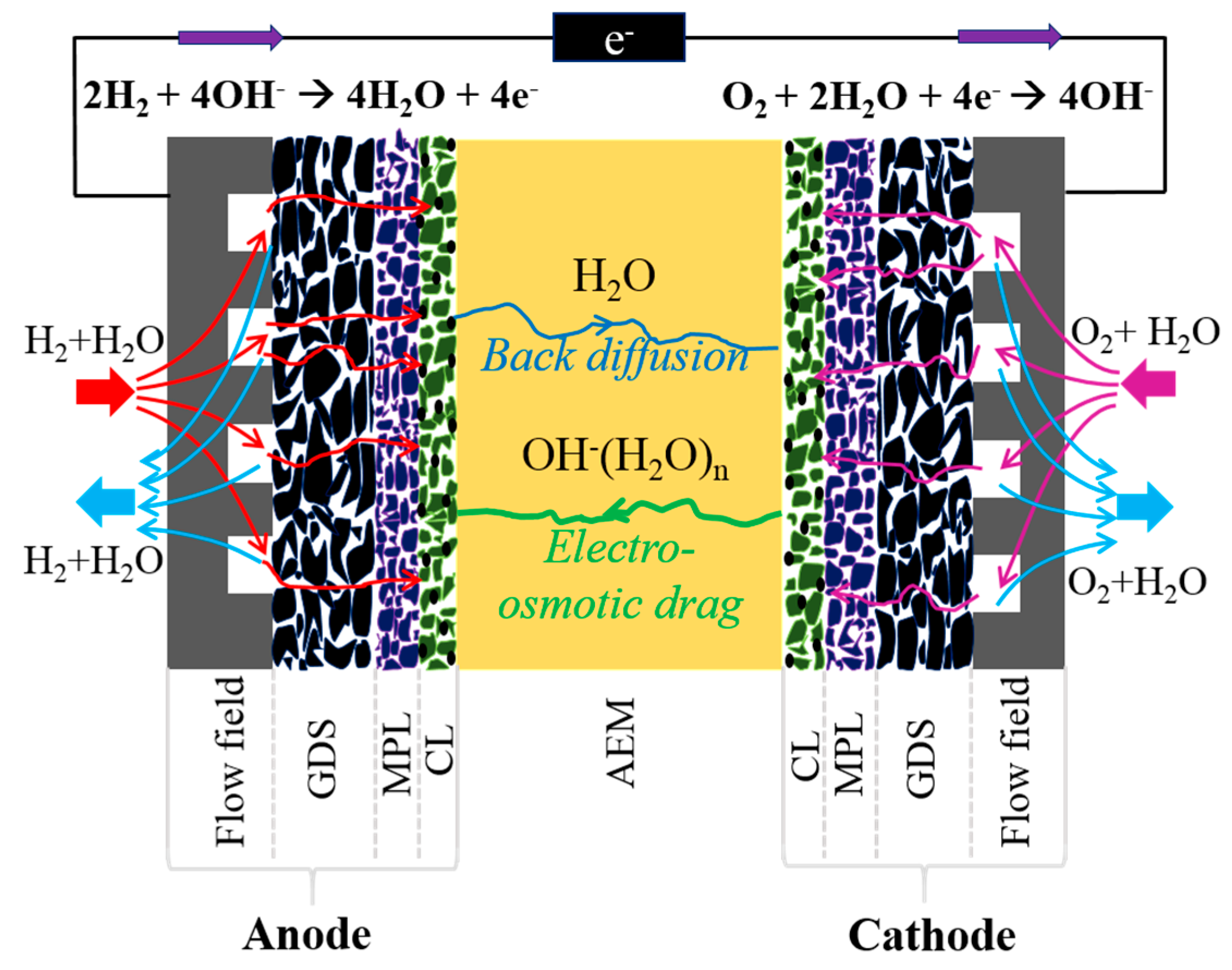
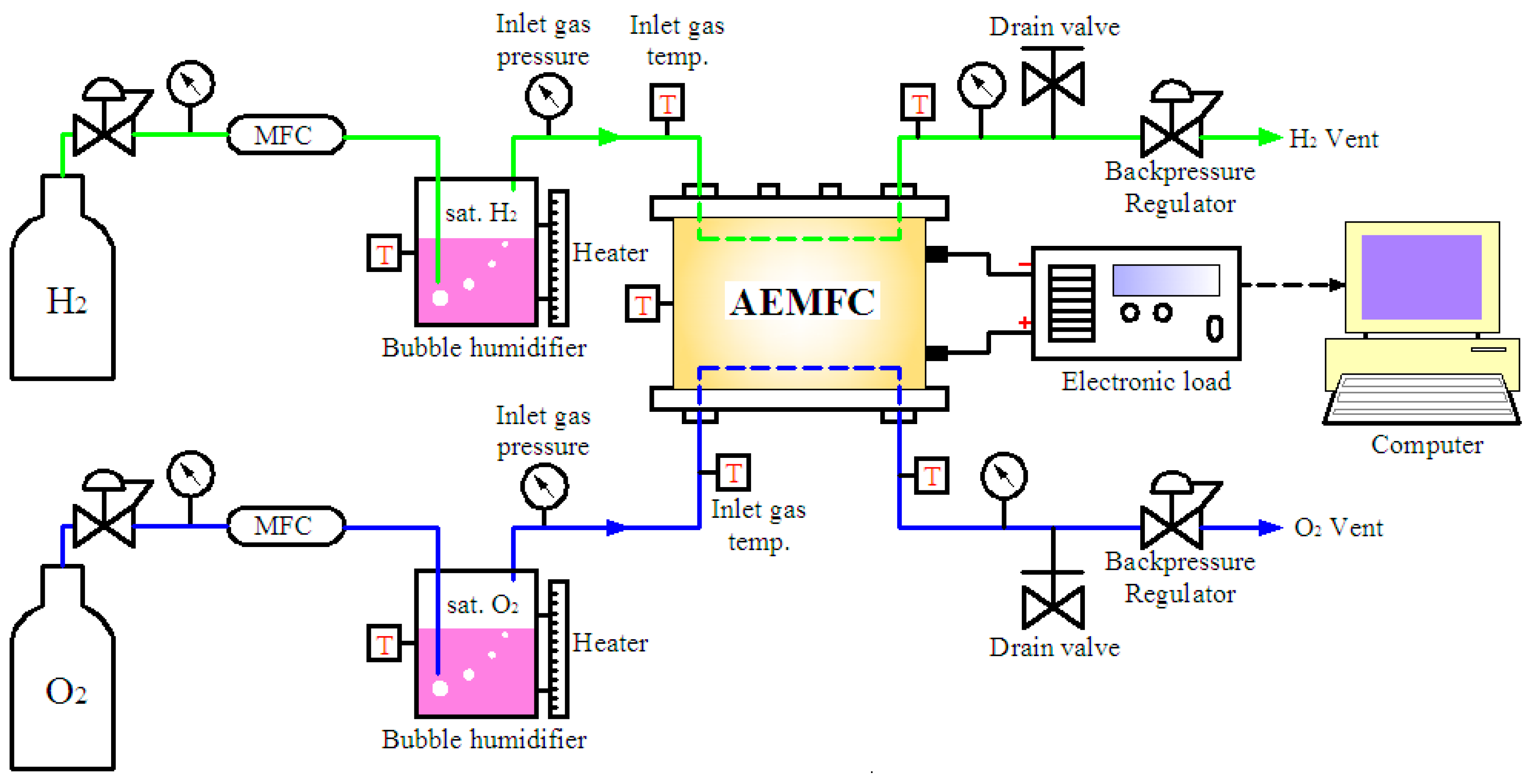
2. Experimental
3. Results and Discussion
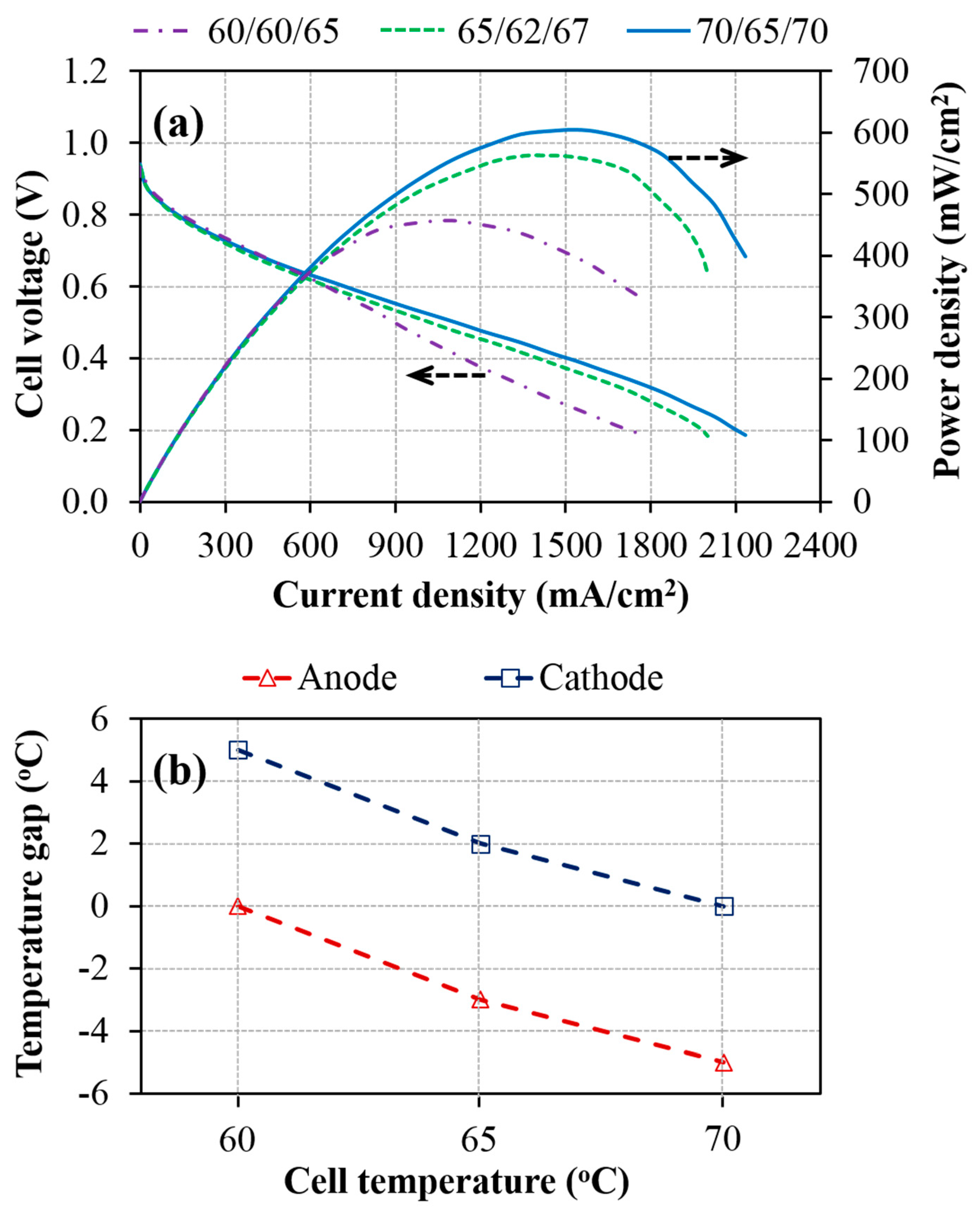
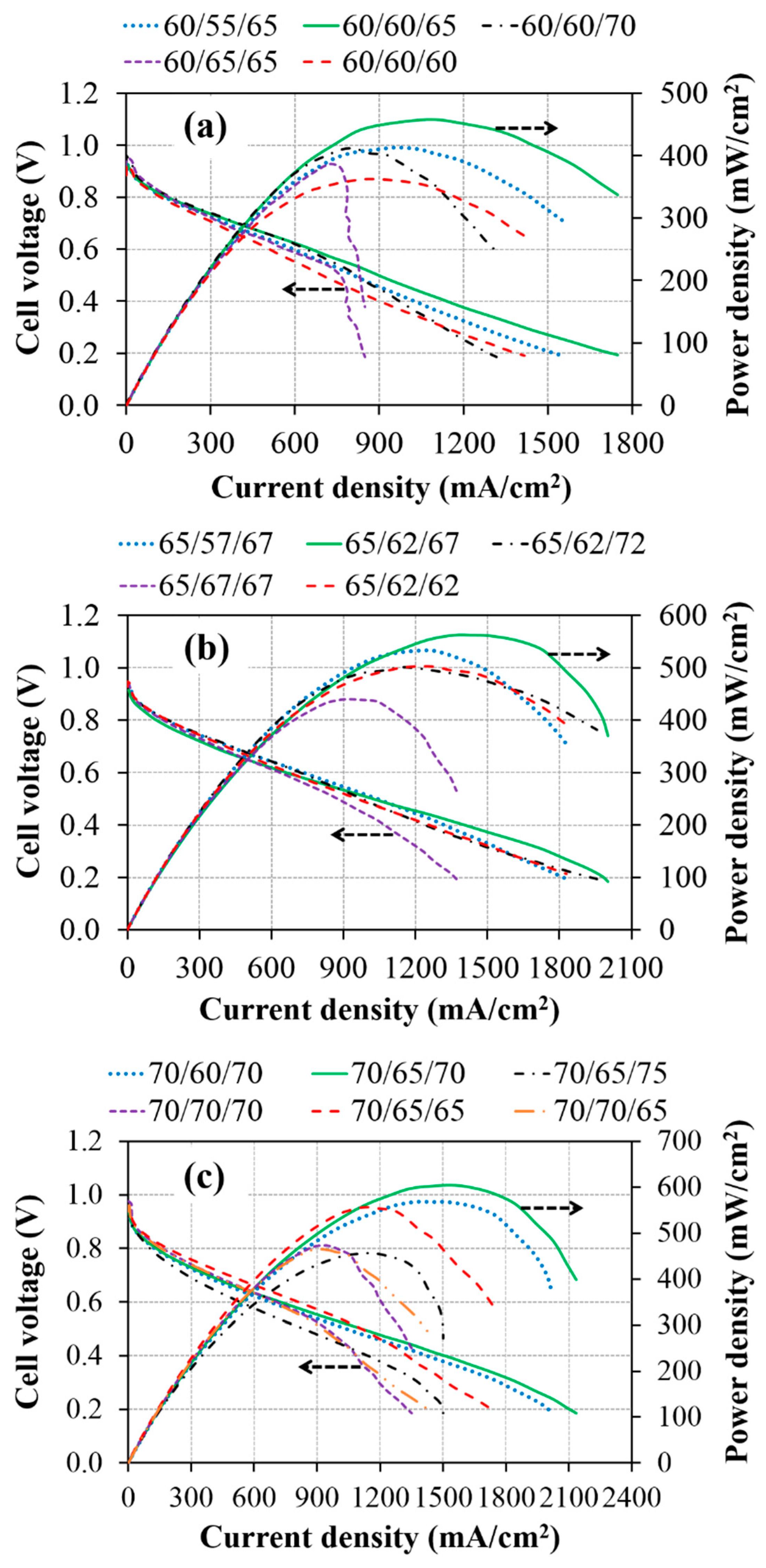
3.1. Effect of Cell Operating Temperature
3.2. Effect of Inlet Gas Humidification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burchardt, T.; Gouérec, P.; Sanchez-Cortezon, E.; Karichev, Z.; Miners, J.H. Alkaline fuel cells: Contemporary advancement and limitations. Fuel 2002, 81, 2151–2155. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. Acs Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Shen, S.Y.; Guo, Y.G.; Wei, G.H.; Luo, L.X.; Li, F.; Zhang, J.L. A perspective on the promoting effect of Ir and Au on Pd toward the ethanol oxidation reaction in alkaline media. Front. Energy 2018, 12, 501–508. [Google Scholar] [CrossRef]
- Yang, W.; Fellinger, T.-P.; Antonietti, M. Efficient Metal-Free Oxygen Reduction in Alkaline Medium on High-Surface-Area Mesoporous Nitrogen-Doped Carbons Made from Ionic Liquids and Nucleobases. J. Am. Chem. Soc. 2011, 133, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Kostowskyj, M.A.; Gilliam, R.J.; Kirk, D.W.; Thorpe, S.J. Silver nanowire catalysts for alkaline fuel cells. Int. J. Hydrog. Energy 2008, 33, 5773–5778. [Google Scholar] [CrossRef]
- Paidar, M.; Fateev, V.; Bouzek, K. Membrane electrolysis—History, current status and perspective. Electrochim. Acta 2016, 209, 737–756. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Goodpaster, J.D.; Kenis, P.J.A.; Wieckowski, A. Mechanism of CO Oxidation on Pt(111) in Alkaline Media. J. Phys. Chem. B 2006, 110, 9545–9555. [Google Scholar] [CrossRef]
- Spendelow, J.S.; Wieckowski, A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media. Phys. Chem. Chem. Phys. 2007, 9, 2654–2675. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukasawa, K.; Nishino, E.; Yamaguchi, S.; Yamada, K.; Tanaka, H.; Bae, B.; Miyatake, K.; Watanabe, M. Anion Conductive Block Poly(arylene ether)s: Synthesis, Properties, and Application in Alkaline Fuel Cells. J. Am. Chem. Soc. 2011, 133, 10646–10654. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Y.; Shen, S. Anionexchange membrane direct ethanol fuel cells: Status and perspective. Front. Energy Power Eng. 2010, 4, 443–458. [Google Scholar] [CrossRef]
- Zhu, L.D.; Zhao, T.S.; Xu, J.B.; Liang, Z.X. Preparation and characterization of carbon-supported sub-monolayer palladium decorated gold nanoparticles for the electro-oxidation of ethanol in alkaline media. J. Power Sources 2009, 187, 80–84. [Google Scholar] [CrossRef]
- Kostowskyj, M.A.; Kirk, D.W.; Thorpe, S.J. Ag and Ag–Mn nanowire catalysts for alkaline fuel cells. Int. J. Hydrog. Energy 2010, 35, 5666–5672. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.T. Prospects for Alkaline Anion-Exchange Membranes in Low Temperature Fuel Cells. Fuel Cells 2005, 5, 187–200. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhao, T.S.; Liang, Z.X. Performance of alkaline electrolyte-membrane-based direct ethanol fuel cells. J. Power Sources 2009, 187, 387–392. [Google Scholar] [CrossRef]
- Varcoe, J.; Beillard, M.; Halepoto, D.M.; Kizewski, J.; Poynton, S.; Slade, R. Membrane and Electrode Materials for Alkaline Membrane Fuel Cells. Ecs Trans. 2008, 16, 1819–1834. [Google Scholar] [CrossRef]
- Yanagi, H.; Fukuta, K. Anion Exchange Membrane and Ionomer for Alkaline Membrane Fuel Cells (AMFCs). Ecs Trans. 2008, 16, 257–262. [Google Scholar] [CrossRef]
- Xu, C.; Shen, P.K.; Liu, Y. Ethanol electrooxidation on Pt/C and Pd/C catalysts promoted with oxide. J. Power Sources 2007, 164, 527–531. [Google Scholar] [CrossRef]
- Roche, I.; Chaînet, E.; Chatenet, M.; Vondrák, J. Carbon-Supported Manganese Oxide Nanoparticles as Electrocatalysts for the Oxygen Reduction Reaction (ORR) in Alkaline Medium: Physical Characterizations and ORR Mechanism. J. Phys. Chem. C 2007, 111, 1434–1443. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Gößling, S.; Klages, M.; Haußmann, J.; Beckhaus, P.; Messerschmidt, M.; Arlt, T.; Kardjilov, N.; Manke, I.; Scholta, J.; Heinzel, A. Analysis of liquid water formation in polymer electrolyte membrane (PEM) fuel cell flow fields with a dry cathode supply. J. Power Sources 2016, 306, 658–665. [Google Scholar] [CrossRef]
- Tötzke, C.; Manke, I.; Hilger, A.; Choinka, G.; Kardjilov, N.; Arlt, T.; Markötter, H.; Schröder, A.; Wippermann, K.; Stolten, D.; et al. Large area high resolution neutron imaging detector for fuel cell research. J. Power Sources 2011, 196, 4631–4637. [Google Scholar] [CrossRef]
- Boillat, P.; Lehmann, E.H.; Trtik, P.; Cochet, M. Neutron imaging of fuel cells—Recent trends and future prospects. Curr. Opin. Electrochem. 2017, 5, 3–10. [Google Scholar] [CrossRef]
- Huo, S.; Deng, H.; Chang, Y.; Jiao, K. Water management in alkaline anion exchange membrane fuel cell anode. Int. J. Hydrog. Energy 2012, 37, 18389–18402. [Google Scholar] [CrossRef]
- Jiao, K.; He, P.; Du, Q.; Yin, Y. Three-dimensional multiphase modeling of alkaline anion exchange membrane fuel cell. Int. J. Hydrog. Energy 2014, 39, 5981–5995. [Google Scholar] [CrossRef]
- Deng, H.; Huo, S.; Chang, Y.; Zhou, Y.; Jiao, K. Transient analysis of alkaline anion exchange membrane fuel cell anode. Int. J. Hydrog. Energy 2013, 38, 6509–6525. [Google Scholar] [CrossRef]
- Dekel, D.R.; Rasin, I.G.; Page, M.; Brandon, S. Steady state and transient simulation of anion exchange membrane fuel cells. J. Power Sources 2018, 375, 191–204. [Google Scholar] [CrossRef]
- Huo, S.; Zhou, J.; Wang, T.; Chen, R.; Jiao, K. Experimental and analytical analysis of polarization and water transport behaviors of hydrogen alkaline membrane fuel cell. J. Power Sources 2018, 382, 1–12. [Google Scholar] [CrossRef]
- Oshiba, Y.; Hiura, J.; Suzuki, Y.; Yamaguchi, T. Improvement in the solid-state alkaline fuel cell performance through efficient water management strategies. J. Power Sources 2017, 345, 221–226. [Google Scholar] [CrossRef]
- Omasta, T.J.; Wang, L.; Peng, X.; Lewis, C.A.; Varcoe, J.R.; Mustain, W.E. Importance of balancing membrane and electrode water in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 205–213. [Google Scholar] [CrossRef]
- Truong, V.M.; Wang, C.-L.; Yang, M.; Yang, H. Effect of tunable hydrophobic level in the gas diffusion substrate and microporous layer on anion exchange membrane fuel cells. J. Power Sources 2018, 402, 301–310. [Google Scholar] [CrossRef]
- Ozen, D.N.; Timurkutluk, B.; Altinisik, K. Effects of operation temperature and reactant gas humidity levels on performance of PEM fuel cells. Renew. Sustain. Energy Rev. 2016, 59, 1298–1306. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Srinivasan, S.; Appleby, A.J.; Martin, C.R. Temperature Dependence of the Electrode Kinetics of Oxygen Reduction at the Platinium/Nafion Interface—A Microelectrode Investigation. J. Electrochem. Soc. 1992, 139, 2530–2537. [Google Scholar] [CrossRef]
- Gopi, H.; Peera, G.; Bhat, S.D.; Parthasarathi, S.; Pitchumani, S. 3-Methyltrimethylammonium poly(2,6-dimethyl-1,4-phenylene oxide) based anion exchange membrane for alkaline polymer electrolyte fuel cells. Bull. Mater. Sci. 2013, 37, 877–881. [Google Scholar] [CrossRef]
- Gopi, K.H.; Peera, S.G.; Bhat, S.D.; Sridhar, P.; Pitchumani, S. Preparation and characterization of quaternary ammonium functionalized poly(2,6-dimethyl-1,4-phenylene oxide) as anion exchange membrane for alkaline polymer electrolyte fuel cells. Int. J. Hydrog. Energy 2014, 39, 2659–2668. [Google Scholar] [CrossRef]
- Park, G.-G.; Sohn, Y.-J.; Yang, T.-H.; Yoon, Y.-G.; Lee, W.-Y.; Kim, C.-S. Effect of PTFE contents in the gas diffusion media on the performance of PEMFC. J. Power Sources 2004, 131, 182–187. [Google Scholar] [CrossRef]
- Benziger, J.; Kimball, E.; Mejia-Ariza, R.; Kevrekidis, I. Oxygen Mass Transport Limitations at the Cathode of Polymer Electrolyte Membrane Fuel Cells. AIChE J. 2011, 57, 2505–2517. [Google Scholar] [CrossRef]
- Owejan, J.P.; Trabold, T.A.; Mench, M.M. Oxygen transport resistance correlated to liquid water saturation in the gas diffusion layer of PEM fuel cells. Int. J. Heat Mass Transf. 2014, 71, 585–592. [Google Scholar] [CrossRef]
- Zhu, L.; Pan, J.; Wang, Y.; Han, J.; Zhuang, L.; Hickner, M.A. Multication Side Chain Anion Exchange Membranes. Macromolecules 2016, 49, 815–824. [Google Scholar] [CrossRef]
- Espiritu, R.; Mamlouk, M.; Scott, K. Study on the effect of the degree of grafting on the performance of polyethylene-based anion exchange membrane for fuel cell application. Int. J. Hydrog. Energy 2016, 41, 1120–1133. [Google Scholar] [CrossRef]
| Thickness (mm) | Polytetrafluoroethylene (PTFE) (wt.%) | Air Permeability (s) | Through Plane Resistance (mΩ cm2) | Mean Pore Diameter (µm) | Porosity (%) | Contact Angle (o) | ||
|---|---|---|---|---|---|---|---|---|
| Microporous Layer (MPL) | Back | MPL | Back | |||||
| 0.31 | 30 | 30 | 99.5 | 11.9 | 36.69 | 64.06 | 146.2 | 147.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, V.M.; Duong, N.B.; Wang, C.-L.; Yang, H. Effects of Cell Temperature and Reactant Humidification on Anion Exchange Membrane Fuel Cells. Materials 2019, 12, 2048. https://doi.org/10.3390/ma12132048
Truong VM, Duong NB, Wang C-L, Yang H. Effects of Cell Temperature and Reactant Humidification on Anion Exchange Membrane Fuel Cells. Materials. 2019; 12(13):2048. https://doi.org/10.3390/ma12132048
Chicago/Turabian StyleTruong, Van Men, Ngoc Bich Duong, Chih-Liang Wang, and Hsiharng Yang. 2019. "Effects of Cell Temperature and Reactant Humidification on Anion Exchange Membrane Fuel Cells" Materials 12, no. 13: 2048. https://doi.org/10.3390/ma12132048
APA StyleTruong, V. M., Duong, N. B., Wang, C.-L., & Yang, H. (2019). Effects of Cell Temperature and Reactant Humidification on Anion Exchange Membrane Fuel Cells. Materials, 12(13), 2048. https://doi.org/10.3390/ma12132048






