Morphology of Aluminum Alloy Foams Produced with Dolomite via Partial Sintering of Precursors
Abstract
1. Introduction
2. Materials and Methods
2.1. Precursor Fabrication
2.2. Partial Sintering Protocol
2.3. Foaming Procedure
2.4. Characterization Techniques
3. Results and Discussion
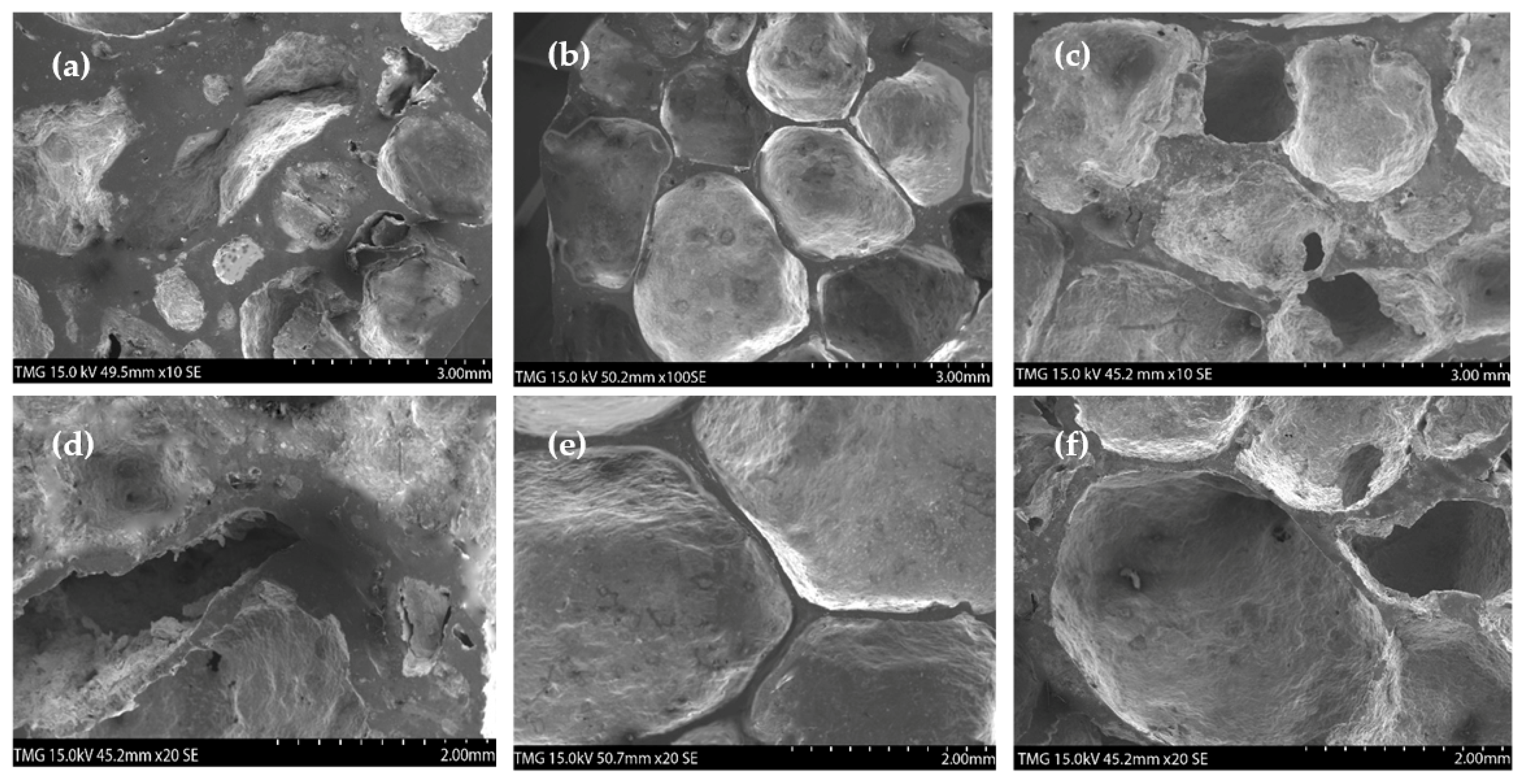
3.1. Influence of Partial Sintering of the Precursors on Foaming Evolution
3.2. Influence of Nickel and Dolomite Additions on Foaming Behavior
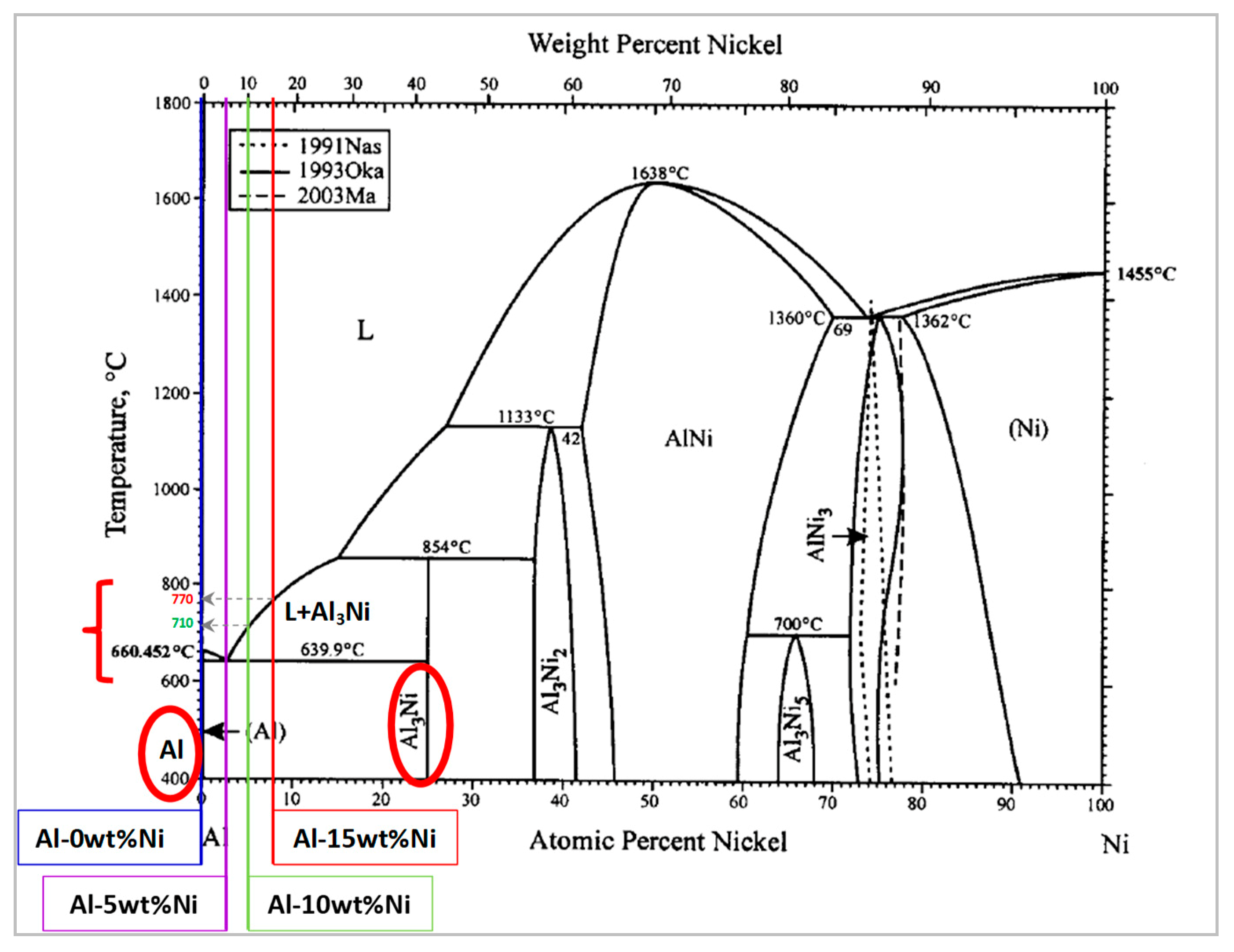
3.2.1. Role of Nickel
3.2.2. Role of Dolomite
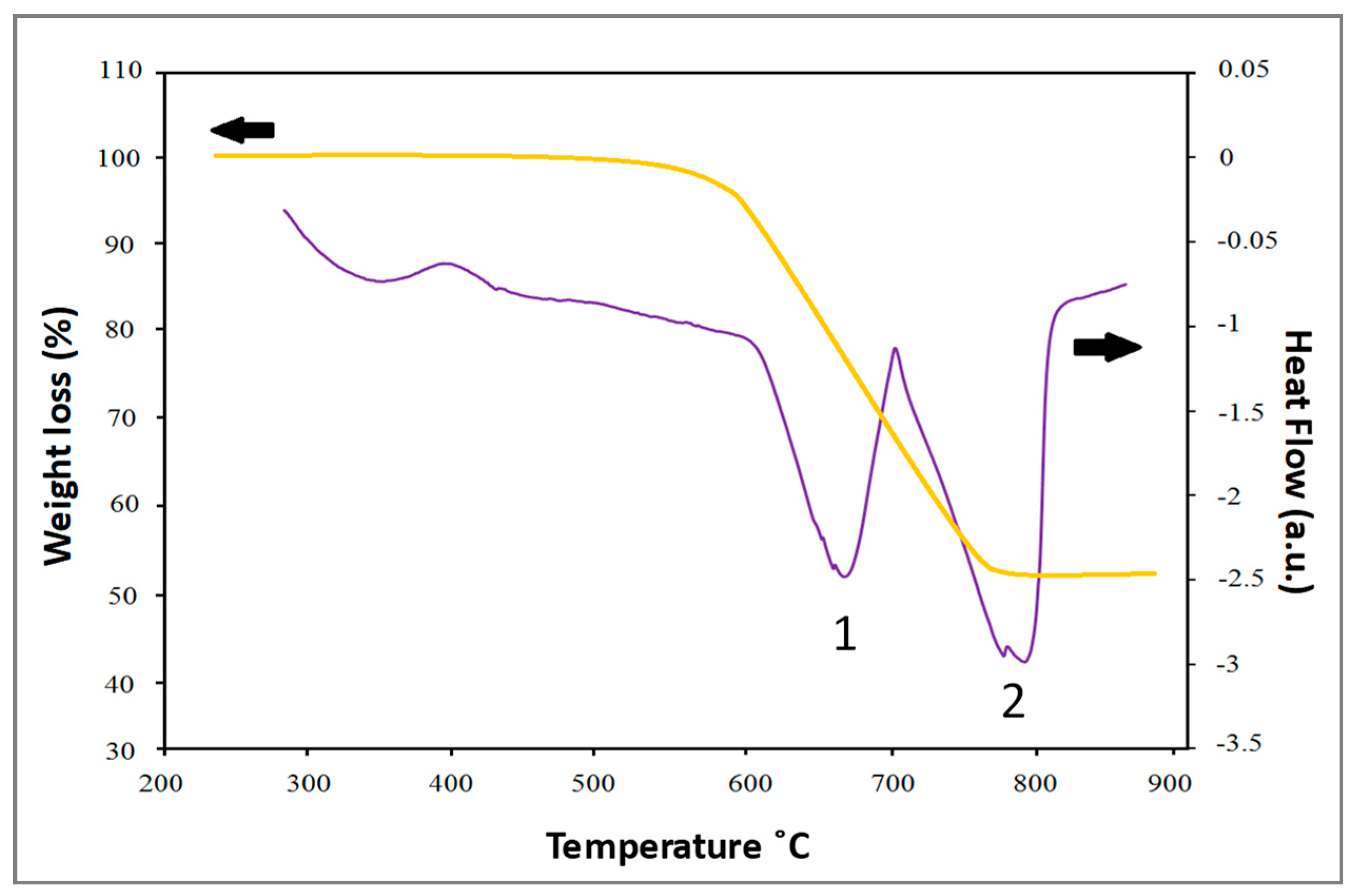
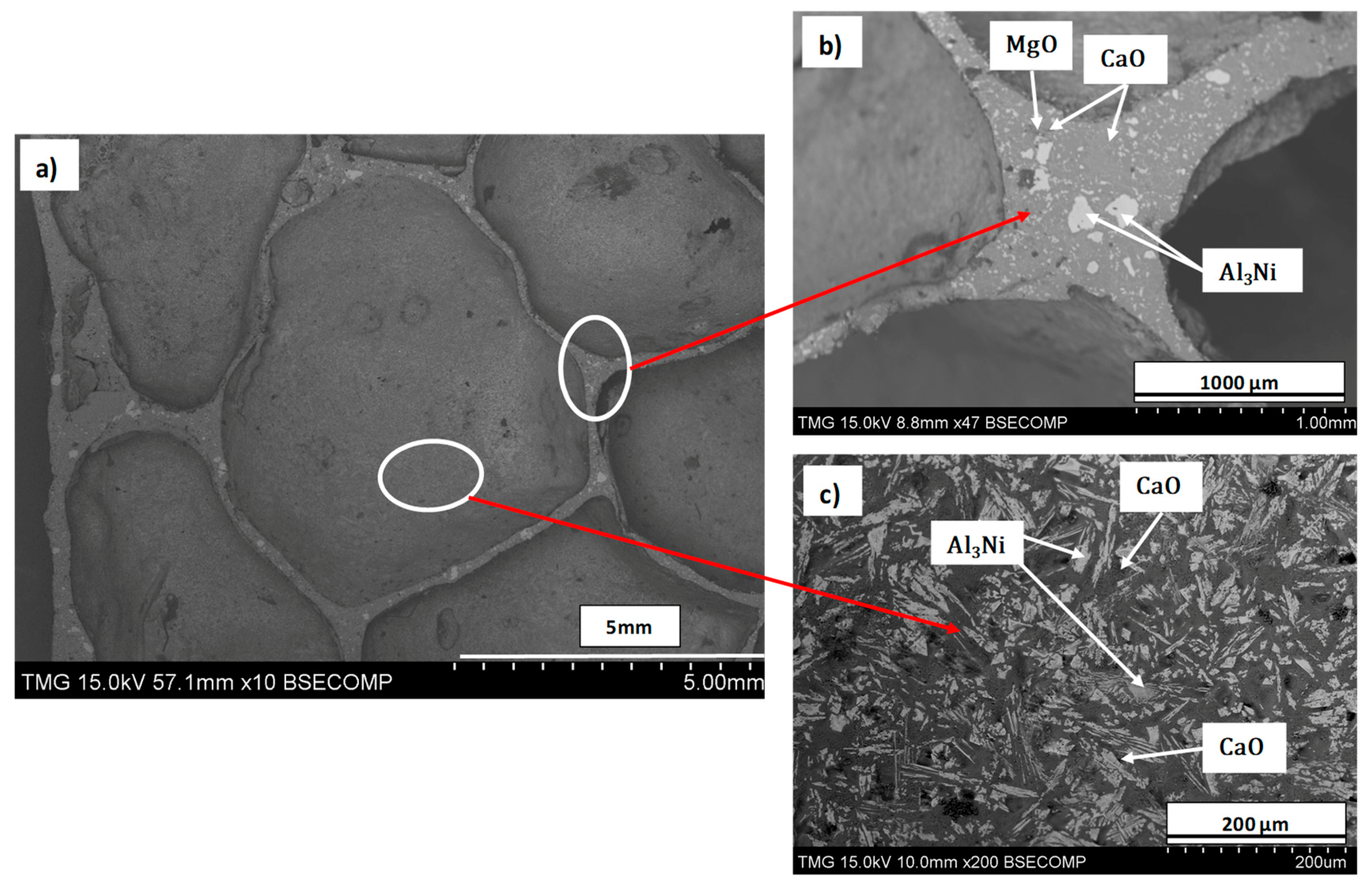
3.3. Microstructure, Phase Analysis, and Stabilization Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lefebvre, L.P.; Banhart, J.; Dunand, D.C. Porous Metals and Metallic Foams: Current Status and Recent Developments. Adv. Eng. Mater. 2008, 10, 775–787. [Google Scholar] [CrossRef]
- Banhart, J.; Baumeister, J.; Weber, M. Powder metallurgical technology for the production of metallic foams. In Proceedings of the European Conference on Advanced PM Materials, Birmingham, UK, 23–25 October 1995. [Google Scholar]
- Deshpande, V.S.; Fleck, N.A. High strain rate compressive behaviour of aluminium alloy foams. Int. J. Impact Eng. 2000, 24, 277–298. [Google Scholar] [CrossRef]
- Markaki, A.E.; Clyne, T.W. The effect of cell wall microstructure on the deformation and fracture of aluminium-based foams. Acta Mater. 2001, 49, 1677–1686. [Google Scholar] [CrossRef]
- Kevorkijan, V.; Skapin, S.D.; Paulin, I.; Sustarsic, B.; Jenko, M. Synthesis and characterization of closed cells aluminum foams containing dolomite powder as foaming agent. Mater. Technol. 2010, 44, 363–371. [Google Scholar]
- Shunmugasamy, V.C.; Mansoor, B. Compressive behavior of a rolled open-cell aluminum foam. Mater. Sci. Eng. A 2018, 715, 281–294. [Google Scholar] [CrossRef]
- Banhart, J. Aluminium foams for lighter vehicles. Int. J. Veh. Des. 2005, 37, 114–125. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. Fatigue performance of selective laser melted Ti6Al4V components: State of the art. Mater. Res. Express 2018, 6, 012002. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Yavari, S.A.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Additively Manufactured Open-Cell Porous Biomaterials Made from Six Different Space-Filling Unit Cells: The Mechanical and Morphological Properties. Materials (Basel) 2015, 8, 1871–1896. [Google Scholar] [CrossRef] [PubMed]
- Banhart, J.; Baumeister, J. Production Methods for Metallic Foams. Mater. Res. Soc. Symp. Proc. 1998, 521, 121. [Google Scholar] [CrossRef]
- Banhart, J. Manufacturing routes for metallic foams. JOM 2000, 52, 22–27. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Griffith, W.M.; Froes, F.H. Surface Oxides in P/M Aluminum Alloys. JOM 1985, 37, 27–33. [Google Scholar] [CrossRef]
- Schaffer, G.B. Powder Processed Aluminium Alloys. In Proceedings of the 9th International Conference on Aluminium Alloys, Brisbane, Australia, 2–5 August 2004; Nie, J.F., Morton, A.J., Muddle, B.C., Eds.; Institute of Materials Engineering Australasia Ltd.: Melbourne, Australia, 2004; Volume 28, p. 65. [Google Scholar]
- Asavavisithchai, S.; Kennedy, A.R. The effect of oxides in various aluminium powders on foamability. Procedia Eng. 2012, 32, 714–721. [Google Scholar] [CrossRef][Green Version]
- Körner, C.; Arnold, M.; Singer, R.F. Metal foam stabilization by oxide network particles. Mater. Sci. Eng. A 2005, 396, 28–40. [Google Scholar] [CrossRef]
- Wübben, T.; Stanzick, H.; Banhart, J.; Odenbach, O. Stability of metallic foams studied under microgravity. J. Phys. Condens. Matter 2003, 15, S427. [Google Scholar] [CrossRef]
- Banhart, J. Metal Foams: The mystery of stabilisation. In Proceedings of the Porous Metal Metal Foaming Technology (CellMat2005), Kyoto, Japan, 21–23 September 2005; Japan Institute of Metals: Sendai, Japan, 2005; pp. 75–787. [Google Scholar]
- Banhart, J. Metal Foams: Production and Stability. Adv. Eng. Mater. 2006, 8, 781–794. [Google Scholar] [CrossRef]
- Prakash, O.; Sang, H.; Embury, J.D. Structure and properties of Al SiC foam. Mater. Sci. Eng. A 1995, 199, 195–203. [Google Scholar] [CrossRef]
- Miyoshi, T.; Itoh, M.; Akiyama, S.; Kitahara, A. ALPORAS Aluminum Foam: Production Process, Properties, and Applications. Adv. Eng. Mater. 2000, 2, 179–183. [Google Scholar] [CrossRef]
- Gergely, V.; Clyne, B. The FORMGRIP Process: Foaming of Reinforced Metals by Gas Release in Precursors. Adv. Eng. Mater. 2000, 2, 175–178. [Google Scholar] [CrossRef]
- Proa-Flores, P.M.; Drew, R.A.L. Production of Aluminum Foams with Ni-coated TiH2 Powder. Adv. Eng. Mater. 2008, 10, 830–834. [Google Scholar] [CrossRef]
- Mandrino, D.; Paulin, I.; Škapin, S.D. Scanning electron microscopy, X-ray diffraction and thermal analysis study of the TiH2 foaming agent. Mater. Charact. 2012, 72, 87–93. [Google Scholar] [CrossRef]
- Orovčík, Ľ.; Nosko, M.; Švec, P.; Nagy, Š.; Čavojský, M.; Simančík, F.; Jerz, J. Effect of the TiH2 pre-treatment on the energy absorption ability of 6061 aluminium alloy foam. Mater. Lett. 2015, 148, 82–85. [Google Scholar] [CrossRef]
- Aguirre-Perales, L.Y.; Drew, R.A.L.; Jung, I.-H. The Effect of In Situ Intermetallic Formation on Al-Sn Foaming Behavior. Metall. Mater. Trans. A 2014, 45, 3714–3727. [Google Scholar] [CrossRef]
- Paulin, I. Synthesis and characterization of al foams produced by powder metallurgy route using dolomite and titanium hydride as a foaming agents. Mater. Technol. 2014, 48, 943–947. [Google Scholar]
- Lehmhus, D.; Vesenjak, M.; Schampheleire, S.; Fiedler, T. From Stochastic Foam to Designed Structure: Balancing Cost and Performance of Cellular Metals. Materials 2017, 10, 922. [Google Scholar] [CrossRef] [PubMed]
- Lehmhus, D.; Baumeister, J.; Haesche, M.; Stöbener, K.; Weise, J.; Lange, D.; Meyer, N. Paths towards cost reduction in precursor material based aluminium foam production processes. In Proceedings of the 11th International Conference on Aluminium Alloys (ICAA 11), Aachen, Germany, 22–26 September 2008; Available online: https://www.researchgate.net/publication/269094024 (accessed on April 2018).
- Papadopoulos, D.P.; Omar, H.; Stergioudi, F.; Tsipas, S.A.; Michailidis, N. The use of dolomite as foaming agent and its effect on the microstructure of aluminium metal foams—Comparison to titanium hydride. Coll. Surf. A 2011, 382, 118–123. [Google Scholar] [CrossRef]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef]
- Haul, R.A.W.; Heystek, H. Differential thermal analysis of the dolomite decomposition. Am. Mineral. 1952, 37, 166–179. [Google Scholar]
- Gergely, V.; Curran, D.C.; Clyne, T.W. The FOAMCARP process: Foaming of aluminium MMCs by the chalk-aluminium reaction in precursors. Compos. Sci. Technol. 2003, 63, 2301–2310. [Google Scholar] [CrossRef]
- Papadopoulos, D.P.; Omar, H.; Stergioudi, F.; Tsipas, S.A.; Lefakis, H.; Michailidis, N. A novel method for producing Al-foams and evaluation of their compression behavior. J. Porous Mater. 2010, 17, 773–777. [Google Scholar] [CrossRef]
- Kevorkijan, V.; Skapin, S.D.; Paulin, I.; Kovacec, U.; Jenko, M. Effect of a foaming agent and its morphology on the foaming behaviour, cell-size distribution and microstructural uniformity of closed-cell aluminium foams. Mater. Technol. 2012, 46, 233–238. [Google Scholar]
- Koizumi, T.; Kido, K.; Kita, K.; Mikado, K.; Gnyloskurenko, S.; Nakamura, T. Effect of Mass Fraction of Dolomite on the Foaming Behavior of AlSiCu Alloy Foam by Powder Metallurgy Route. Metall. Mater. Trans. A 2012, 43, 4377–4382. [Google Scholar] [CrossRef]
- Koizumi, T.; Kido, K.; Kita, K.; Mikado, K.; Gnyloskurenko, S.; Nakamura, T. Method of Preventing Shrinkage of Aluminum Foam Using Carbonates. Metals 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Gnyloskurenko, S.V.; Koizumi, T.; Kita, K.; Nakamura, T. Aluminum metallic foams made by carbonate foaming agents. Resour. Process. 2013, 60, 5–12. [Google Scholar] [CrossRef][Green Version]
- Haesche, M.; Lehmhus, D.; Weise, J.; Wichmann, M.; Mocellin, I.C.M. Carbonates as Foaming Agent in Chip-based Aluminium Foam Precursor. J. Mater. Sci. Technol. 2010, 26, 845–850. [Google Scholar] [CrossRef]
- Mandrino, D.; Paulin, I.; Kržmanc, M.M.; Škapin, S.D. Physical and chemical treatments influence on the thermal decomposition of a dolomite used as a foaming agent. J. Therm. Anal. Calorim. 2018, 131, 1125–1134. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Ni (aluminum-nickel). J. Phase Equilib. Diffus. 2004, 25, 394. [Google Scholar] [CrossRef]
- Kobashi, M.; Kanetake, N. Processing of Intermetallic Foam by Combustion Reaction. Adv. Eng. Mater. 2002, 4, 745–747. [Google Scholar] [CrossRef]
- Baumgärtner, F.; Duarte, I.; Banhart, J. Industrialization of Powder Compact Foaming Process. Adv. Eng. Mater. 2000, 2, 168–174. [Google Scholar] [CrossRef]
- Banhart, J. Light-Metal Foams—History of Innovation and Technological Challenges. Adv. Eng. Mater. 2013, 15, 82–111. [Google Scholar] [CrossRef]
- Baumeister, J.; Schrader, H. Process for Manufacturing Foamable Metal Bodies and Use Thereof. German Patent 4101630, 1991. [Google Scholar]
- Paulin, I.; Sustarsic, B.; Kevorkijan, V.; Skapin, S.D.; Jenko, M. Synthesis of aluminium foams by the powder-metallurgy process: Compacting of precursors. Mater. Technol 2011, 45, 13–19. [Google Scholar]
- German, R.M. Sintering Theory and Practice; Wiley: New York, NY, USA, 1996; ISBN 978-0-471-05786-4. [Google Scholar]
- Lafrance, M. The Reactive Stabilisation of Al-Zn-X Foams via the Formation of a Transient Liquid Phase Using the Powder Metallurgy Approach. Ph.D. Thesis, McGill University Libraries, Montreal, QC, Canada, 2012. [Google Scholar]
- Hartman, M.; Trnka, O.; Veselý, V.; Svoboda, K. Predicting the rate of thermal decomposition of dolomite. Chem. Eng. Sci. 1996, 51, 5229–5232. [Google Scholar] [CrossRef]
- Engler, P.; Santana, M.W.; Mittleman, M.L.; Balazs, D. Non-isothermal, in situ XRD analysis of dolomite decomposition. Thermochim. Acta 1989, 140, 67–76. [Google Scholar] [CrossRef]
- Galai, H.; Pijolat, M.; Nahdi, K.; Trabelsi-Ayadi, M. Mechanism of growth of MgO and CaCO3 during a dolomite partial decomposition. Solid State Ion. 2007, 178, 1039–1047. [Google Scholar] [CrossRef]
- Fazeli, A.R.; Tareen, J.A.K. Thermal decomposition of rhombohedral double carbonates of dolomite type. J. Therm. Anal. Calorim. 1991, 37, 2605–2611. [Google Scholar] [CrossRef]
- Lázaro, J.; Solórzano, E.; Rodríguez-Pérez, M.A.; Rämer, O.; García-Moreno, F.; Banhart, J. Heat Treatment of Aluminium Foam Precursors: Effects on Foam Expansion and Final Cellular Structure. Procedia Mater. Sci. 2014, 4, 287–292. [Google Scholar] [CrossRef]
- Bouche, K.; Barbier, F.; Coulet, A. Phase Formation during Dissolution of Nickel in Liquid Aluminium. Z. Fuer Met. 1997, 88, 446. [Google Scholar]
- Matijasevic, B.; Banhart, J. Improvement of aluminium foam technology by tailoring of blowing agent. Scr. Mater. 2006, 54, 503–508. [Google Scholar] [CrossRef]
- Paulin, I. Stability of close-cell al foams depending on the usage of different foaming agents. Mater. Technol. 2015, 49, 983–988. [Google Scholar] [CrossRef]
- Ke, L.; Huang, C.; Xing, L.; Huang, K. Al–Ni intermetallic composites produced in situ by friction stir processing. J. Alloys Compd. 2010, 503, 494–499. [Google Scholar] [CrossRef]
- Rajan, T.P.D.; Pillai, R.M.; Pai, B.C. Functionally graded Al–Al3Ni in situ intermetallic composites: Fabrication and microstructural characterization. J. Alloys Compd. 2008, 453, L4–L7. [Google Scholar] [CrossRef]
- Adabi, M.; Amadeh, A.A. Formation mechanisms of Ni–Al intermetallics during heat treatment of Ni coating on 6061 Al substrate. Trans. Nonferrous Met. Soc. China 2015, 25, 3959–3966. [Google Scholar] [CrossRef]
- Alimadadi, H.; Kjartansdóttir, C.; Burrows, A.; Kasama, T.; Møller, P. Nickel-aluminum diffusion: A study of evolution of microstructure and phase. Mater. Charact. 2017, 130, 105–112. [Google Scholar] [CrossRef]
- Körner, C.; Hirschmann, M.; Bräutigam, V.; Singer, R.F. Endogenous Particle Stabilization during Magnesium Integral Foam Production. Adv. Eng. Mater. 2004, 6, 385–390. [Google Scholar] [CrossRef]








| Pure Al | Al-5Ni | Al-10Ni | Al-15Ni | |
|---|---|---|---|---|
| As-Compacted Average Vol. Expansion (%) | 4 | 3 | 30 | 20 |
| Partial-Sintered Average Vol. Expansion (%) | 17 | 25 | 244 | 188 |
| Expansion Factor (Partial-Sintered/As-Compacted) | ~4 | ~8 | ~8 | ~9 |
| HEAT TREATMENT CONDITIONS | PRECURSOR PROPERTIES | FOAMS CHARACTERISTICS | |||
|---|---|---|---|---|---|
| Al–10Ni + 7 wt.% Dolomite | Temperature (°C) | Time (min) | TRS (MPa) | Average Vol. Expansion (%) | Average Porosity (%) |
| As-Compacted | n/a | n/a | 58 | 30 | 6 |
| Partial-Sintered I | 450 | 15 | 123 | 244 | 77 |
| Partial-Sintered II | 450 | 20 | 157 | 243 | 70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina Ramirez, A.M.; Vintila, R.R.; Drew, R.A.L. Morphology of Aluminum Alloy Foams Produced with Dolomite via Partial Sintering of Precursors. Materials 2019, 12, 1691. https://doi.org/10.3390/ma12101691
Medina Ramirez AM, Vintila RR, Drew RAL. Morphology of Aluminum Alloy Foams Produced with Dolomite via Partial Sintering of Precursors. Materials. 2019; 12(10):1691. https://doi.org/10.3390/ma12101691
Chicago/Turabian StyleMedina Ramirez, Ana Maria, Ramona Roxana Vintila, and Robin A. L. Drew. 2019. "Morphology of Aluminum Alloy Foams Produced with Dolomite via Partial Sintering of Precursors" Materials 12, no. 10: 1691. https://doi.org/10.3390/ma12101691
APA StyleMedina Ramirez, A. M., Vintila, R. R., & Drew, R. A. L. (2019). Morphology of Aluminum Alloy Foams Produced with Dolomite via Partial Sintering of Precursors. Materials, 12(10), 1691. https://doi.org/10.3390/ma12101691





