Enhancement of Photocatalytic Activity under Visible Light Irradiation via the AgI@TCNQ Core-Shell Structure
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Photocatalysts
2.2. Characterization
2.3. Photocatalytic Activity
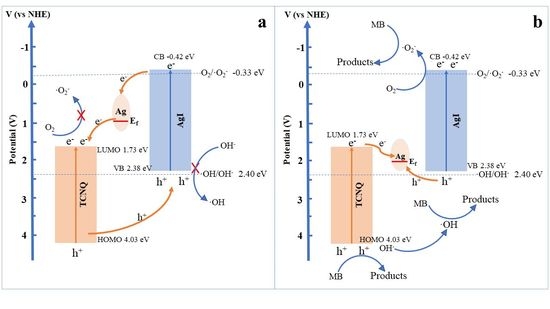
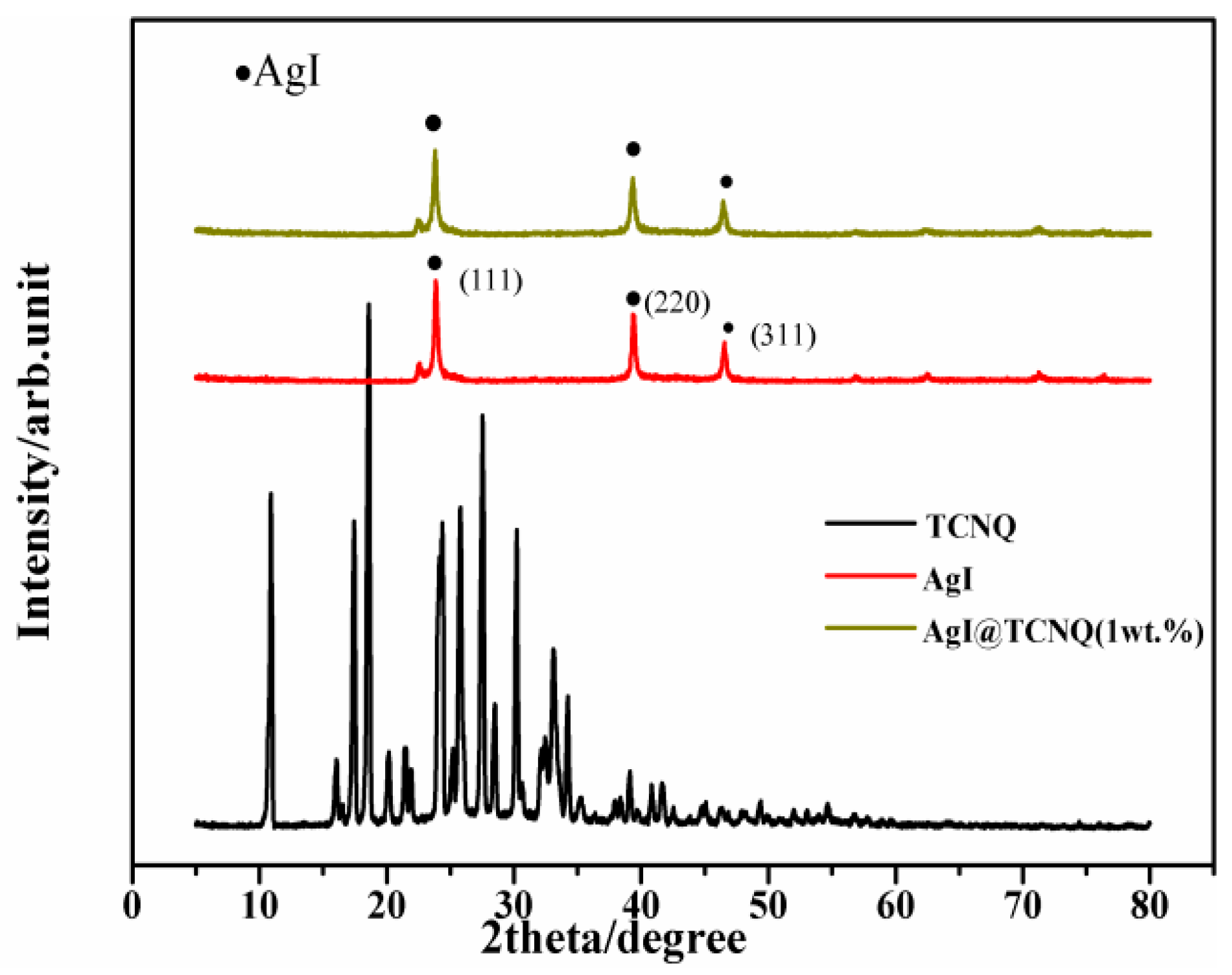
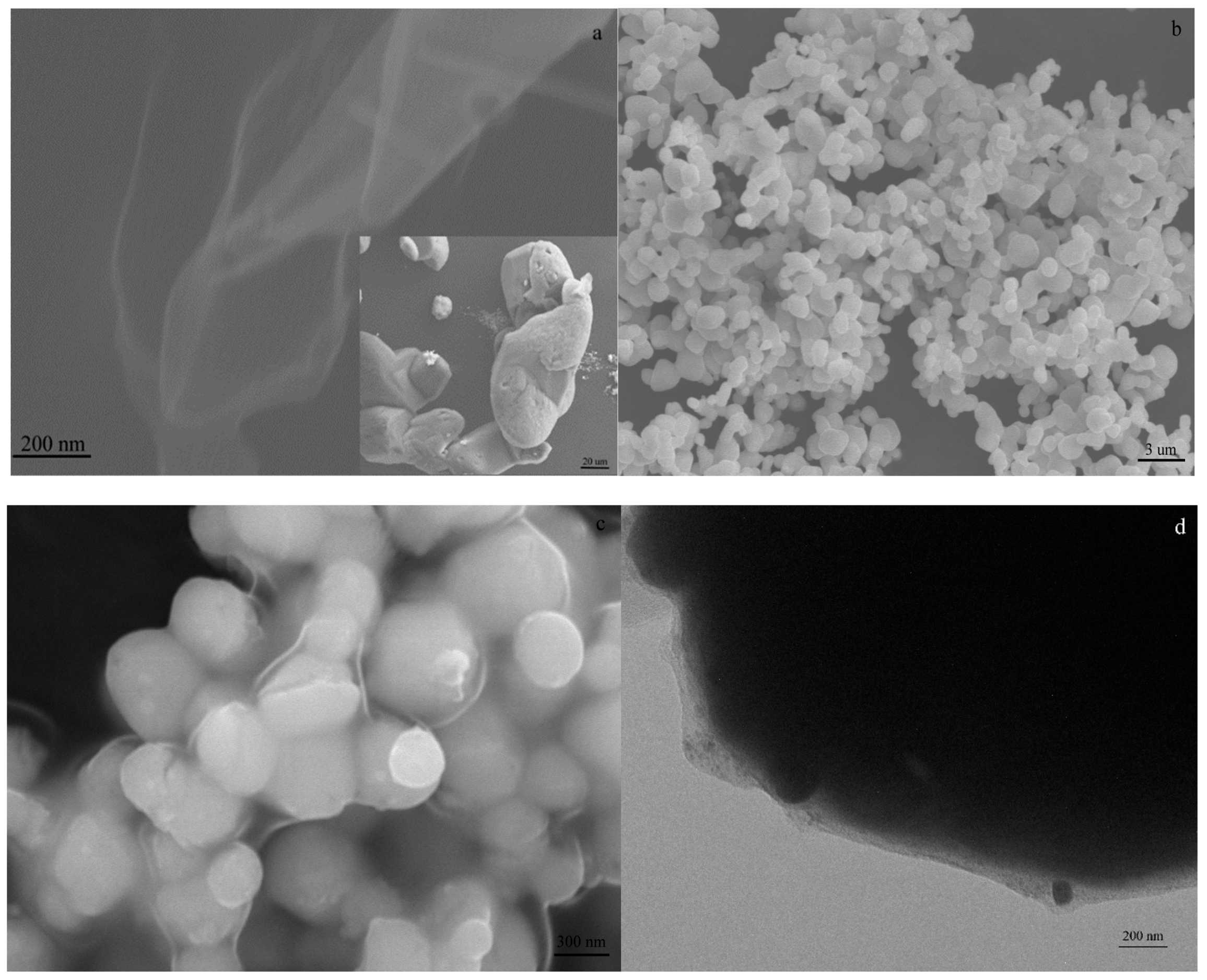
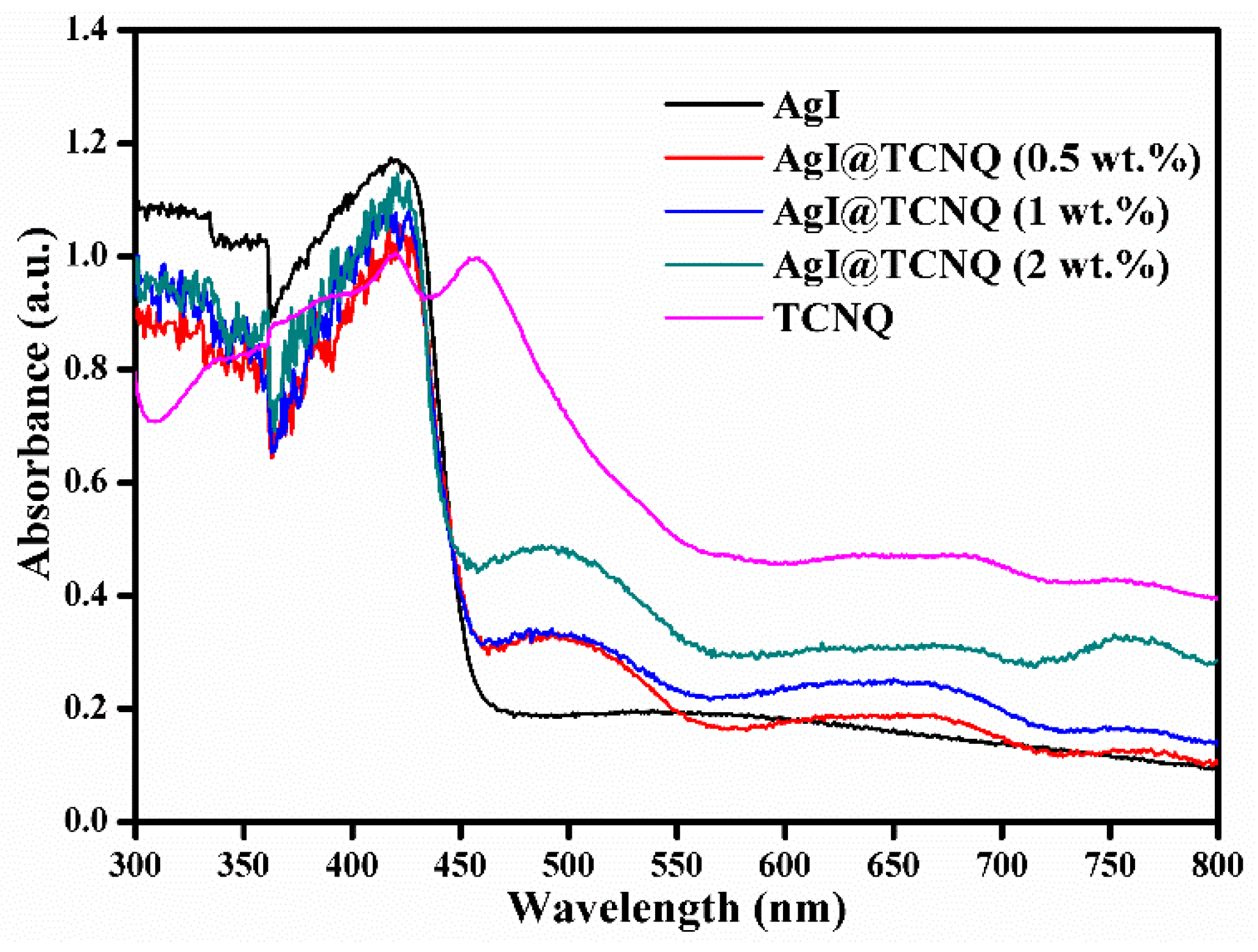
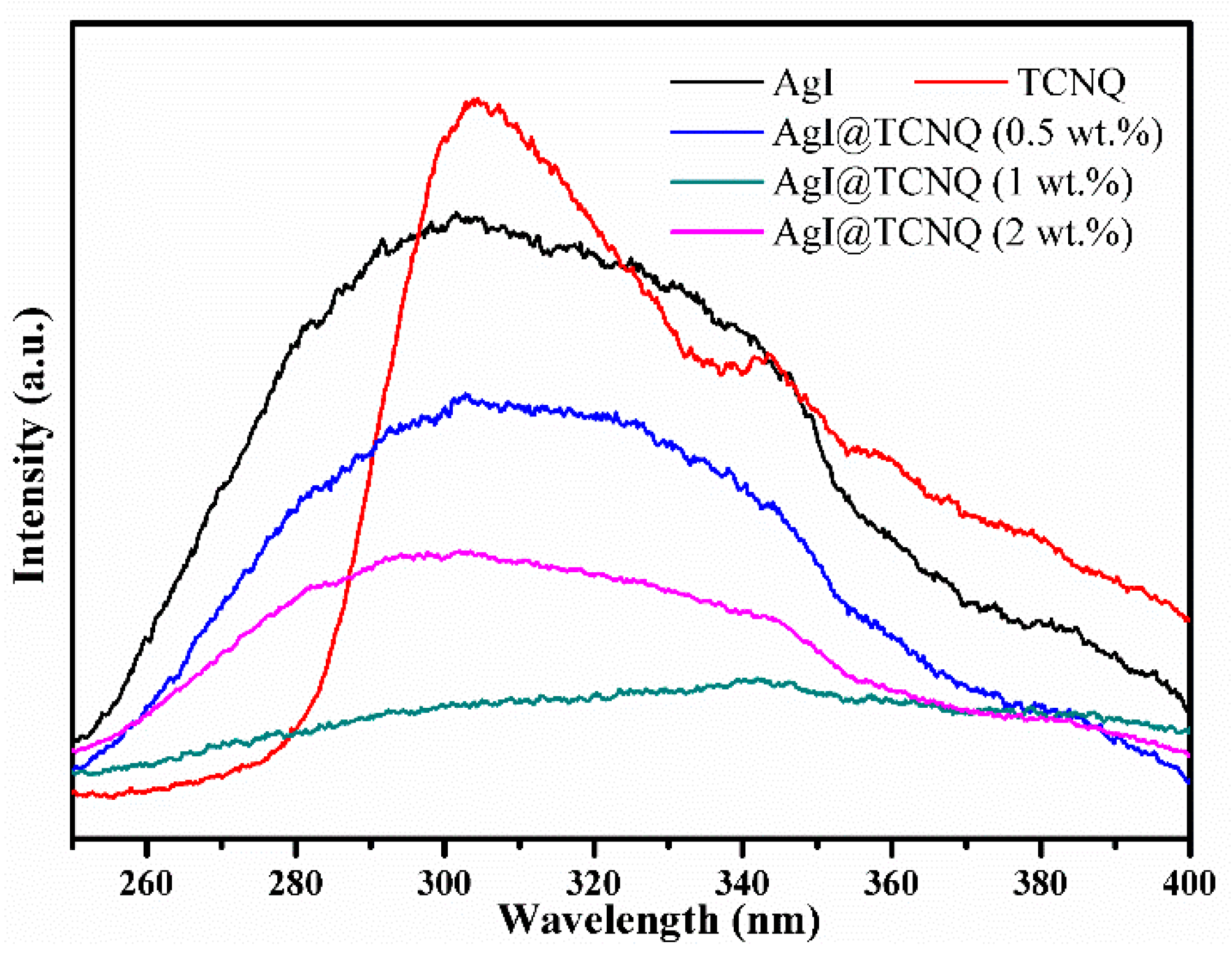
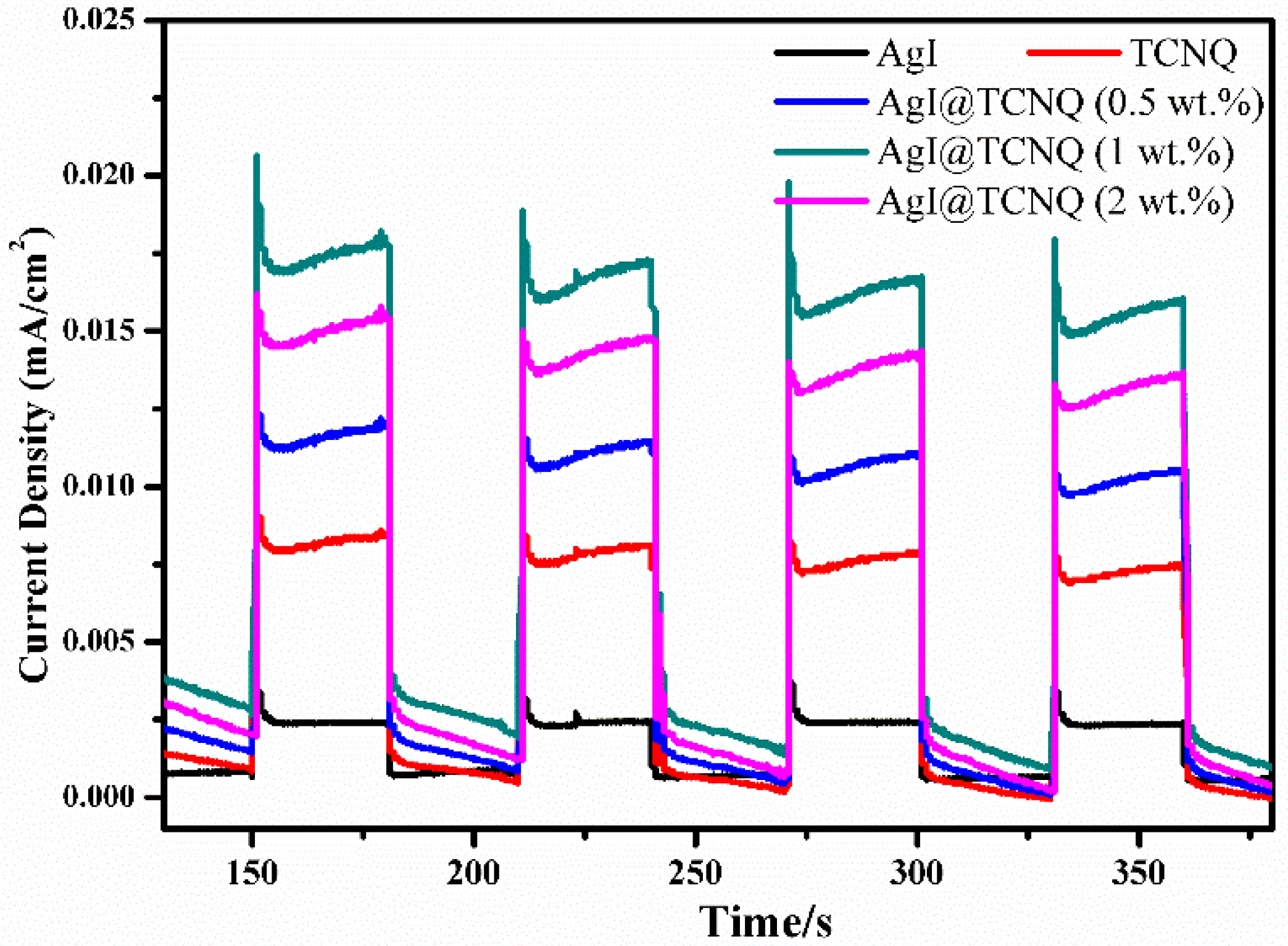
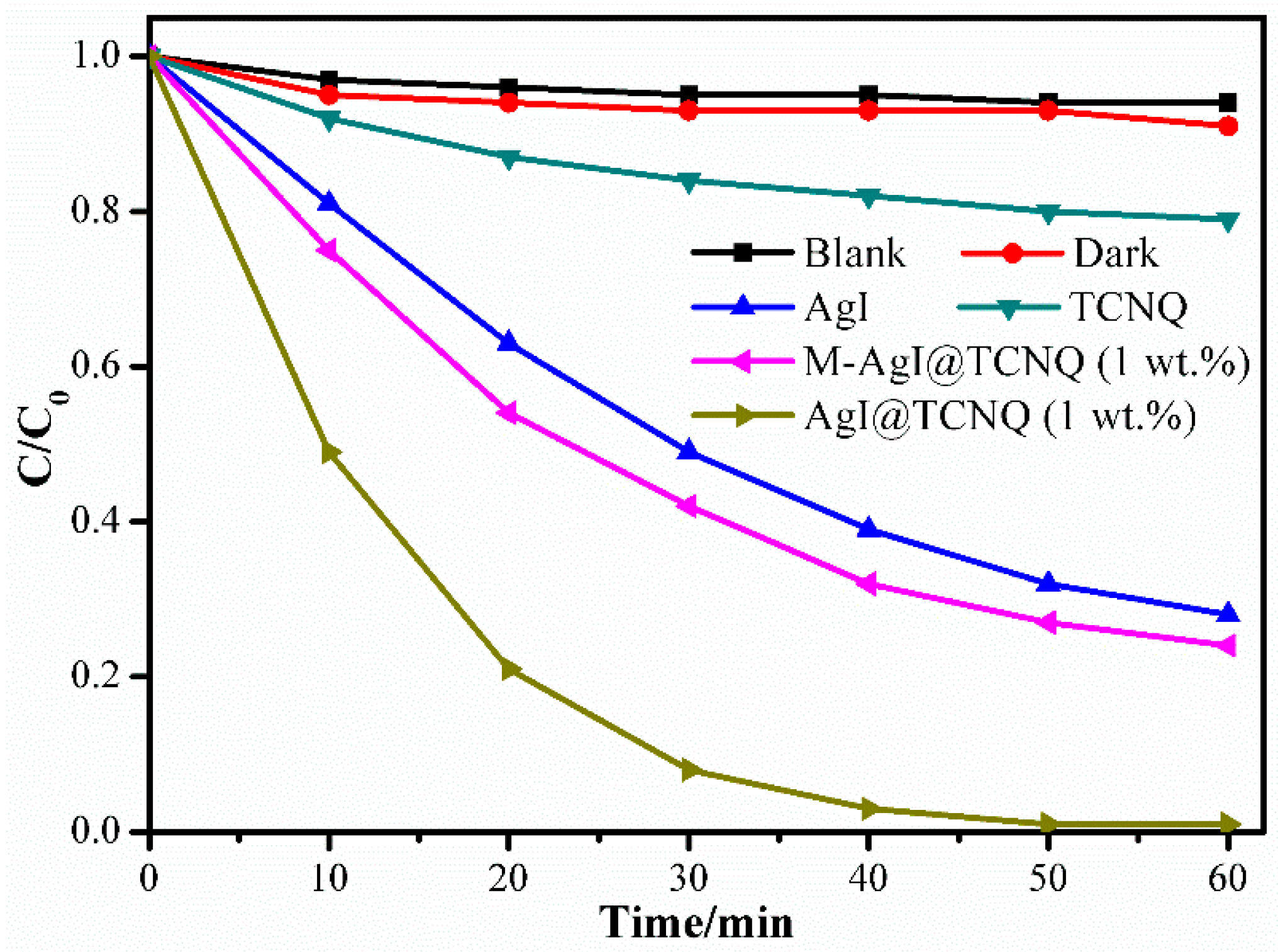
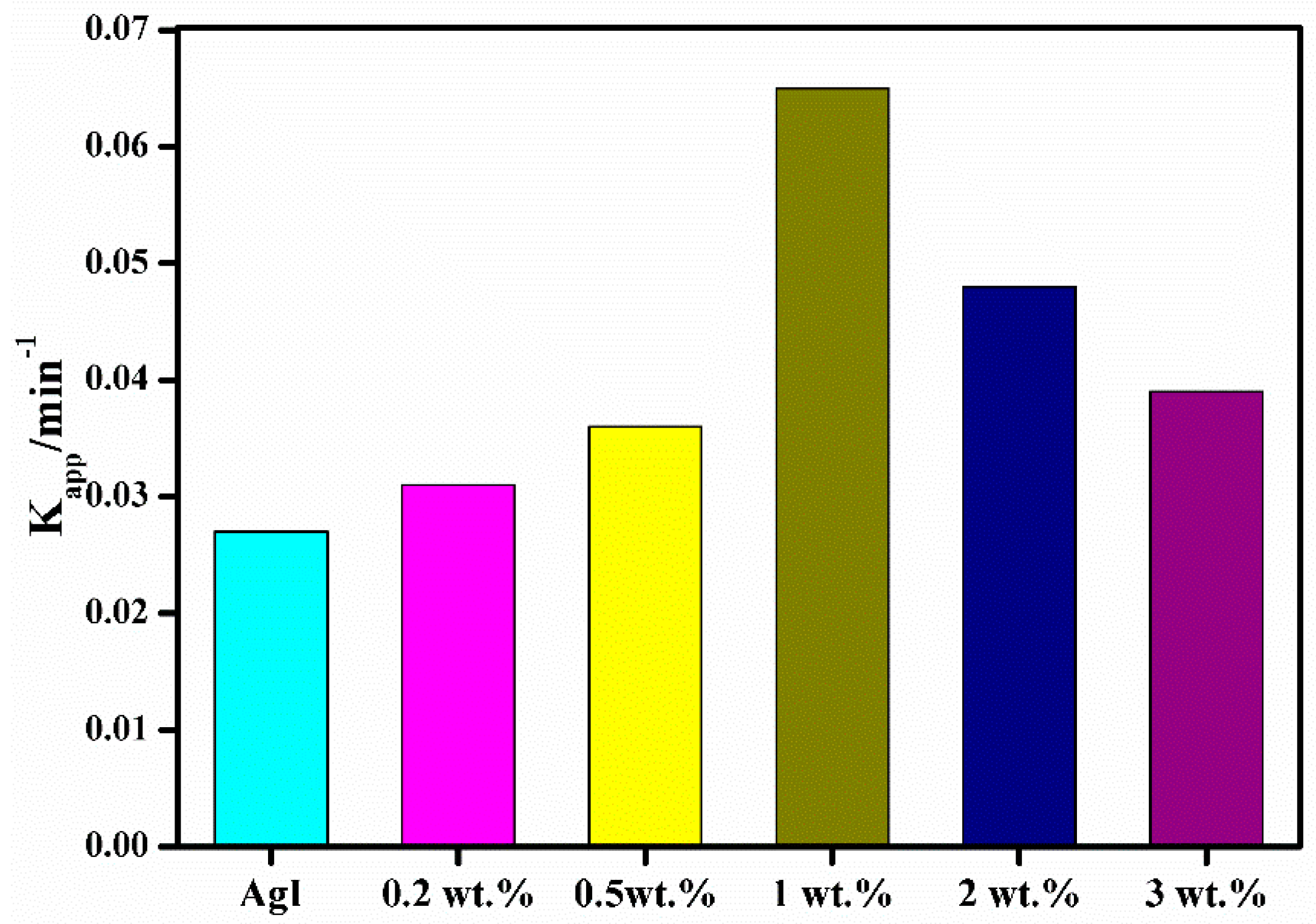
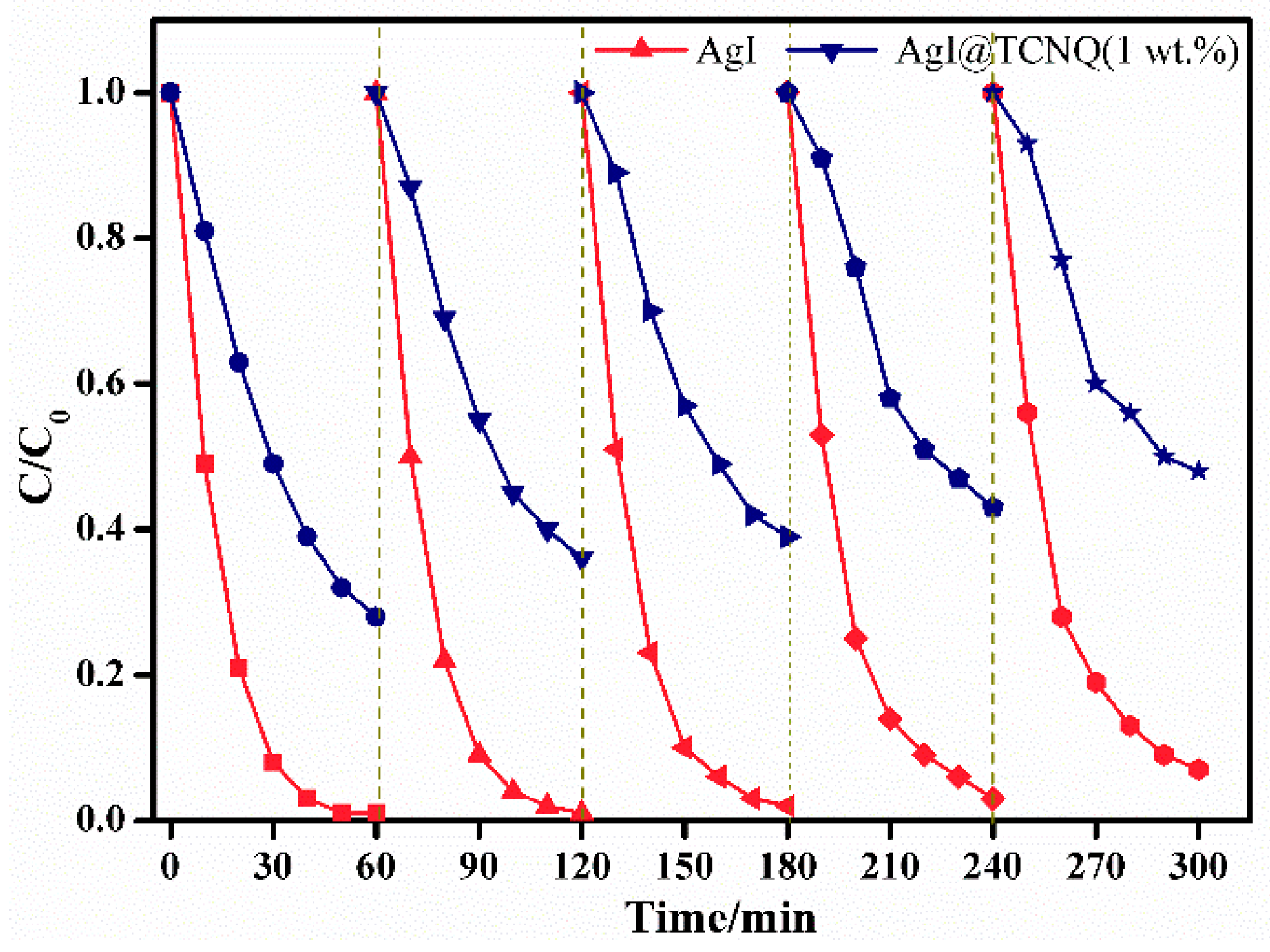
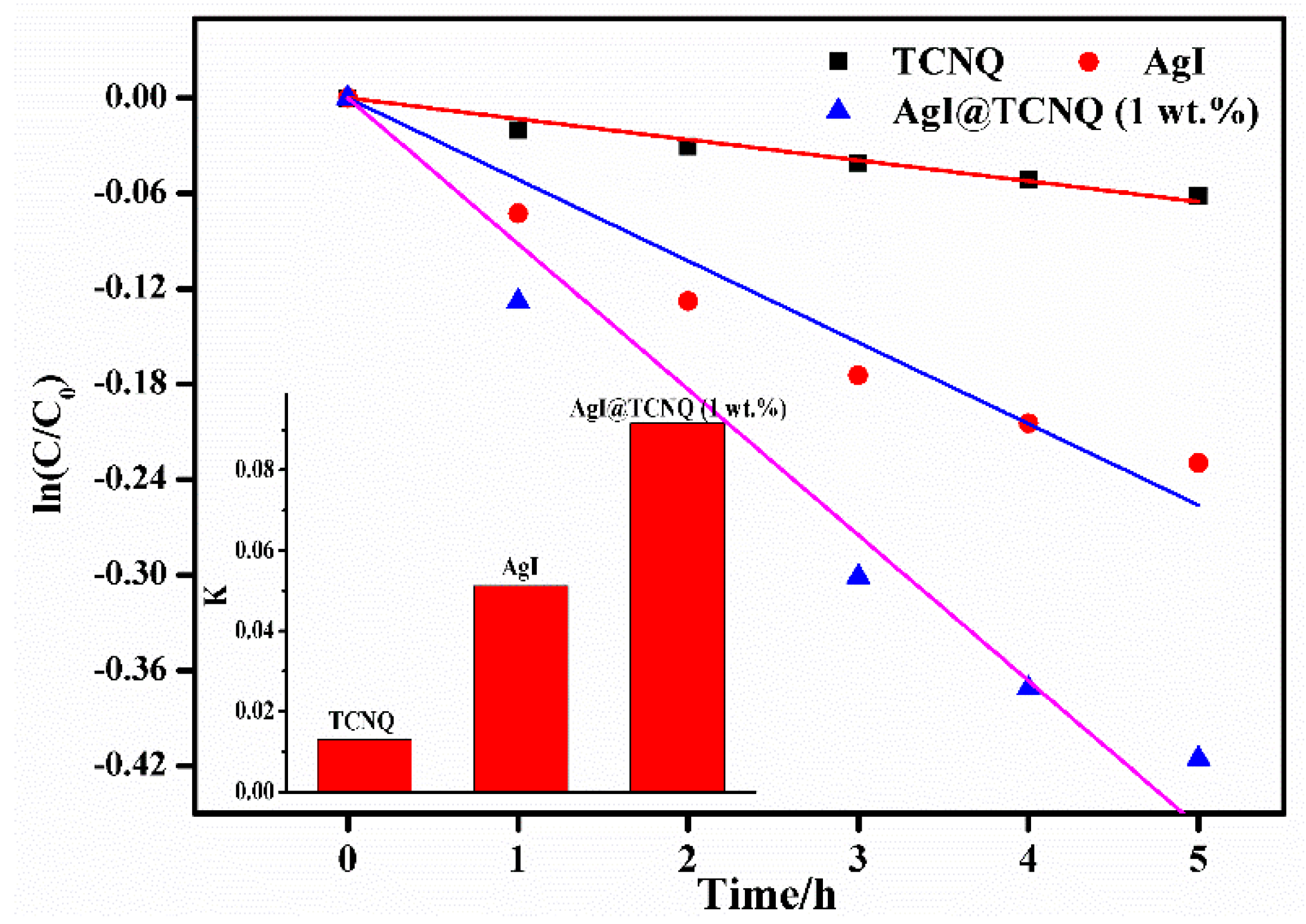
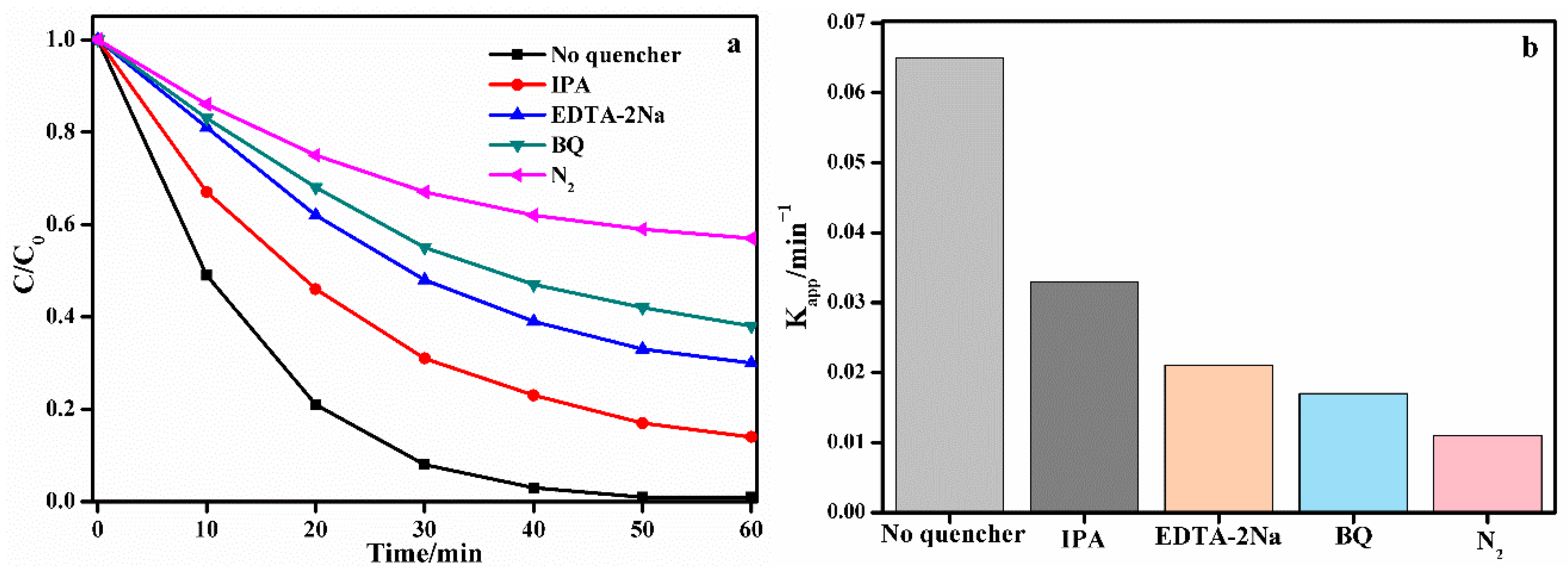
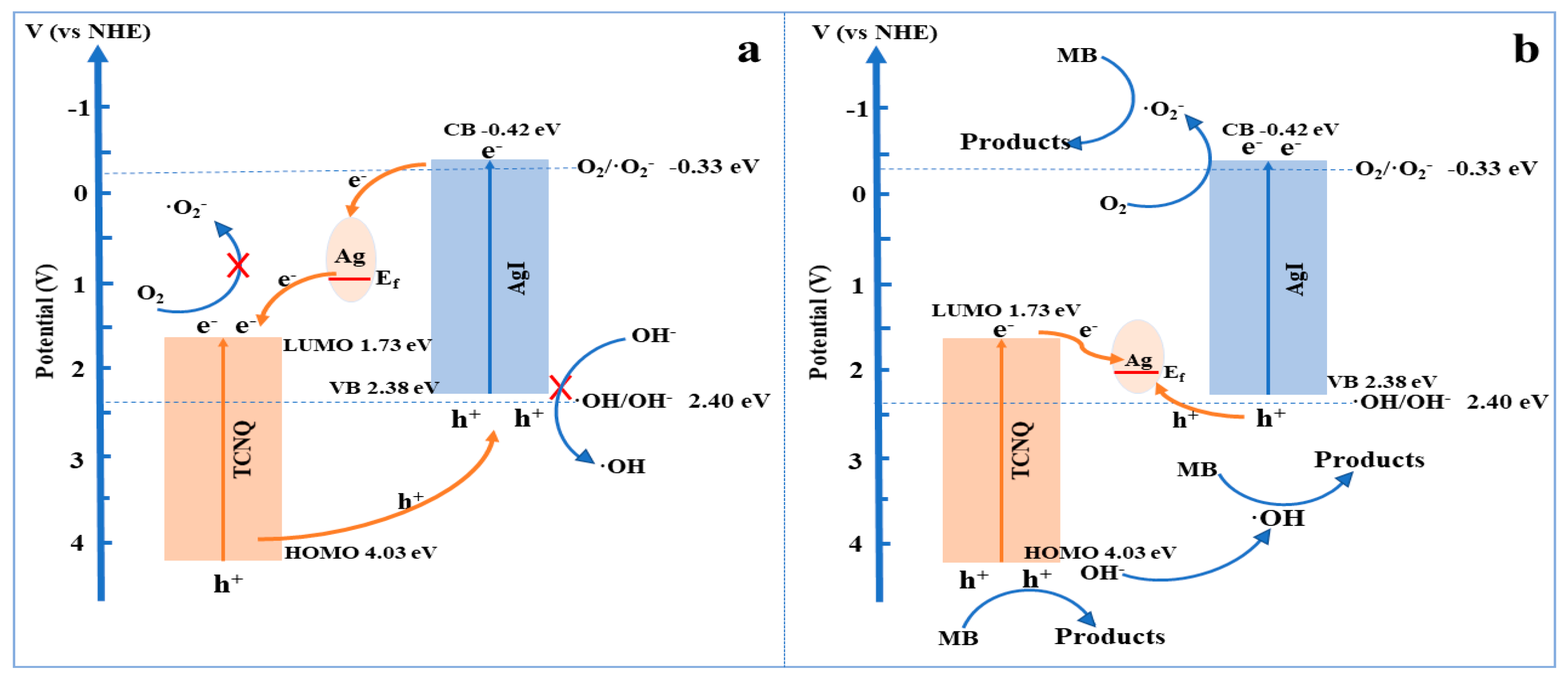
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tian, L.Y.; Rui, Y.L.; Sun, K.L.; Cui, W.Q.; An, W.J. Surface decoration of ZnWO4 nanorods with Cu2O nanoparticles to build heterostructure with enhanced photocatalysis. Nanomaterials 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, M.V. (Ed.) Novel Photoactive Materials; Special Issue; MDPI: Basel, Switzerland, 2018. [Google Scholar]
- Cheng, H.F.; Wang, W.J.; Huang, B.B.; Wang, Z.Y.; Zhan, J.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. Tailoring AgI nanoparticles for the assembly of AgI/BiOI hierarchical hybrids with size-dependent photocatalytic activities. J. Mater. Chem. A 2013, 1, 7131–7136. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Lai, C.; Xu, P.; Zeng, G.M.; Huang, D.L.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Liu, Y.; et al. In situ grown AgI/Bi12O17Cl2 heterojunction photocatalysts for visible light degradation of sulfamethazine: Efficiency, pathway, and mechanism. ACS Sustain. Chem. Eng. 2018, 6, 4174–4184. [Google Scholar] [CrossRef]
- An, C.H.; Wang, J.Z.; Liu, J.X.; Wang, S.T.; Zhang, Q.H. Plasmonic enhancement of photocatalysis over Ag incorporated AgI hollow nanostructures. RSC Adv. 2014, 4, 2409–2413. [Google Scholar] [CrossRef]
- Han, Y.; Bai, C.P.; Zhang, L.X.; Wu, J.B.; Meng, H.; Xu, J.L.; Xu, Y.; Liang, Z.Q.; Zhang, X. A facile strategy for fabricating AgI-MIL-53(Fe) composites: Superior interfacial contact and enhanced visible light photocatalytic performance. New J. Chem. 2018, 42, 3799–3807. [Google Scholar] [CrossRef]
- Pongsaton, A.; Sumetha, S. Comparative study of the photocatalytic decolorization of rhodamine B dye by AgI-Ag3PO4 prepared from co-precipitation and ion-exchange methods. J. Alloys Compd. 2017, 720, 582–588. [Google Scholar]
- Chen, F.; Yang, Q.; Sun, J.; Yao, F.B.; Wang, S.N.; Wang, Y.L.; Wang, X.L.; Li, X.M.; Niu, C.G.; Wang, D.B.; Zeng, G.M. Enhanced photocatalytic degradation of tetracycline by AgI/BiVO4 heterojunction under visible-light irradiation: Mineralization efficiency and mechanism. ACS Appl. Mater. Interfaces 2016, 8, 32887–32900. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.A.; Choi, J.H.; Lee, S.; Ma, R.; Kim, T.K. Green synthesis of AgI nanoparticle functionalized reduced graphene oxide aerogels with enhanced catalytic performance and facile recycling. RSC Adv. 2015, 5, 67394–67404. [Google Scholar] [CrossRef]
- Liu, L.; Ding, L.; Liu, Y.G.; An, W.J.; Lin, S.L.; Liang, Y.H.; Cui, W.Q. A stable Ag3PO4@PANI core@shell hybrid: Enrichment photocatalytic degradation with π-π conjugation. Appl. Catal. B Environ. 2017, 201, 92–104. [Google Scholar] [CrossRef]
- Panchariya, D.K.; Rai, R.K.; Kumar, E.A.; Singh, S.K. Core-shell zeolitic imidazolate frameworks for enhanced hydrogen storage. ACS Omega 2018, 3, 167–175. [Google Scholar] [CrossRef]
- Wang, C.X.; Zhong, H.L.; Wu, W.J.; Pan, C.W.; Wei, X.R.; Zhou, G.L.; Yang, F. Fe3O4@C core-shell carbon hybrid materials as magnetically separable adsorbents for the removal of dibenzothiophene in fuels. ACS Omega 2019, 4, 1652–1661. [Google Scholar] [CrossRef]
- Ho, K.C.; Lin, L.Y. A review of electrode materials based on core-shell nanostructures for electrochemical supercapacitors. J. Mater. Chem. A 2019, 7, 3516–3530. [Google Scholar] [CrossRef]
- Miao, H.; Yang, J.; Wei, Y.X.; Li, W.L.; Zhu, Y.F. Visible-light photocatalysis of PDI nanowires enhanced by plasmonic effect of the gold nanoparticles. Appl. Catal. B Environ. 2018, 239, 61–67. [Google Scholar] [CrossRef]
- Mu, C.F.; Zhang, Y.; Cui, W.Q.; Liang, Y.H.; Zhu, Y.F. Removal of bisphenol A over a separation free 3D Ag3PO4-graphene hydrogel via an adsorption-photocatalysis synergy. Appl. Catal. B Environ. 2017, 212, 41–49. [Google Scholar] [CrossRef]
- Yang, J.; Miao, H.; Wei, Y.X.; Li, W.L.; Zhu, Y.F. π-π Interaction between self-assembled perylene diimide and 3D graphene for excellent visible-light photocatalytic activity. Appl. Catal. B Environ. 2019, 240, 225–233. [Google Scholar] [CrossRef]
- Wang, X.W.; Sun, G.Z.; Routh, P.; Kim, D.H.; Wei, H.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Ge, J.; Wang, L.L.; Gong, M.; Deng, M.S.; Kong, Q.; Song, L.; Jiang, J.; Zhang, Q.; Luo, Y.; et al. A unique semiconductor-metal-graphene stack design to harness charge flow for photocatalysis. Adv. Mater. 2014, 26, 5578. [Google Scholar] [CrossRef]
- Li, M.Z.; Wang, Y.O.; Tang, P.; Xie, N.H.; Zhao, Y.X.; Liu, X.; Hu, G.; Xie, J.L.; Zhao, Y.F.; Tang, J.W.; Zhang, T.R.; Ma, D. Graphene with atomic-level in-plane decoration of h-BN domains for efficient photocatalysis. Chem. Mater. 2017, 29, 2769–2776. [Google Scholar] [CrossRef]
- Zeng, X.K.; Lan, S.Y.; Lo, I.M. Rapid disinfection of, E. coli by a ternary BiVO4/Ag/g-C3N4 composite under visible light: Photocatalytic mechanism and performance investigation in authentic sewage. Environ. Sci. Nano 2019, 6, 610–623. [Google Scholar] [CrossRef]
- Fu, J.W.; Yu, J.G.; Jiang, C.J.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2017, 8, 1701503. [Google Scholar] [CrossRef]
- Pan, Y.T.; Li, D.D.; Jiang, H.L. Sodium-doped C3N4/MOF heterojunction composites with tunable band structures for photocatalysis: Interplay between light harvesting and electron transfer. Chemistry 2018, 24, 18403–18407. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, S.H.; Yang, C.P.; Chen, M.; Li, X. Preparation of size-controlled silver phosphate catalysts and their enhanced photocatalysis performance via synergetic effect with MWCNTs and PANI. Appl. Catal. B Environ. 2019, 245, 71–86. [Google Scholar] [CrossRef]
- Chen, F.Y.; An, W.J.; Li, Y.; Liang, Y.H.; Cui, W.Q. Fabricating 3D porous PANI/TiO2-graphene hydrogel for the enhanced UV-light photocatalytic degradation of BPA. Appl. Surf. Sci. 2018, 427, 123–132. [Google Scholar] [CrossRef]
- Qu, X.H.; Lu, J.Z.; Boas, J.F.; Bond, A.M.; Martin, L.L. Two-step electrochemically directed synthesis of Pr4N (TCNQ)n(n = 1, 2):preparation, structure, and properties of a magnetically isolated dimer and a quasi-one-dimensional chain. Chemistry 2011, 17, 9350–9358. [Google Scholar] [CrossRef]
- Jiang, W.J.; Zhang, M.; Wang, J.; Liu, Y.F.; Zhu, Y.F. Dramatic visible activity in phenol degradation of TCNQ@TiO2 photocatalyst with core-shell structure. Appl. Catal. B Environ. 2014, 160–161, 44–50. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, W.Q.; Lv, Y.H.; Bai, X.J.; Liu, Y.F.; Jiang, W.J.; Zhu, Y.F. Enhancement of mineralization ability of C3N4 via a lower valence position by a tetracyanoquinodimethane organic semiconductor. J. Mater. Chem. A 2014, 2, 11432–11438. [Google Scholar] [CrossRef]
- Hu, P.R.; Liu, L.; An, W.J.; Liang, Y.H.; Cui, W.Q. Use of a core-shell composite Ag3PO4@TCNQ to improve photocatalytic activity and stability. Appl. Surf. Sci. 2017, 425, 329–339. [Google Scholar] [CrossRef]
- Liu, L.; Qi, Y.H.; Yang, J.Y.; Cui, W.Q.; Li, X.A.; Zhang, Z.S. An AgI@g-C3N4 hybrid core@shell structure: Stable and enhanced photocatalytic degradation. Appl. Surf. Sci. 2015, 358, 319–327. [Google Scholar] [CrossRef]
- Takata, T.; Pan, C.S.; Nakabayashi, M.; Shibata, N.; Domen, K. Fabrication of a core-shell-type photocatalyst via photodeposition of group IV and V transition metal oxyhydroxides: An effective surface modification method for overall water splitting. J. Am. Chem. Soc. 2015, 137, 9627–9634. [Google Scholar] [CrossRef]
- Duan, Y.; Deng, L.; Shi, Z.; Zhu, L.; Li, G.C. Assembly of graphene on Ag3PO4/AgI for effective degradation of carbamazepine under visible-light irradiation: Mechanism and degradation pathways. Chem. Eng. J. 2019, 359, 1379–1390. [Google Scholar] [CrossRef]
- Pham, T.T.; Shin, E.W. Influence of g-C3N4 precursors in g-C3N4/NiTiO3 composites on photocatalytic behavior and the interconnection between g-C3N4 and NiTiO3. Langmuir 2018, 34, 13144–13154. [Google Scholar] [CrossRef]
- Cui, W.Q.; An, W.J.; Liu, L.; Hu, J.S.; Liang, Y.H. Novel Cu2O quantum dots coupled flower-like BiOBr for enhancedphotocatalytic degradation of organic contaminant. J. Hazard. Mater. 2014, 280, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.F.; Chen, Y.J.; Duan, M.; Ren, Z.H.; Hu, M. Construction and mechanism of a highly efficient and stable Z-scheme Ag3PO4/reduced graphene oxide/Bi2MoO6 visible-light photocatalyst. Catal. Sci. Technol. 2018, 8, 3818–3832. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Bai, J.; Liang, H.O.; Li, C.P. Synthesis of the novel nanostructured AgI-BiOI/PAN composite photocatalyst with highly enhanced visible-light catalytic performances. J. Photochem. Photobiol. A 2018, 357, 132–139. [Google Scholar] [CrossRef]
- Martín, N.; Segura, J.L.; Seoane, C. Design and synthesis of TCNQ and DCNQI type electron acceptor molecules as precursors for ‘organicmetals’. J. Mater. Chem. 1997, 7, 1661–1676. [Google Scholar]
- Xue, W.J.; Peng, Z.W.; Huang, D.L.; Zeng, G.M.; Wen, X.J.; Deng, R.; Yang, Y.; Yan, X.L. In situ synthesis of visible-light-driven Z-scheme AgI/Bi2WO6 heterojunction photocatalysts with enhanced photocatalytic activity. Ceram Int. 2019, 45, 6340–6349. [Google Scholar] [CrossRef]
- Huang, D.L.; Li, Z.H.; Zeng, G.M.; Zhou, C.Y.; Xue, W.J.; Gong, X.M.; Yan, X.L.; Chen, S.; Wang, W.J.; Cheng, M. Megamerger in photocatalytic field: 2D g-C3N4 nanosheets serve as support of 0D nanomaterials for improving photocatalytic performance. Appl. Catal. B Environ. 2019, 240, 153–173. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Chao, L.Z.; Guo, L.H.; Zeng, G.M. Photocatalytic degradation of ciprofloxacin by a novel Z-scheme CeO2-Ag/AgBr photocatalyst: Influencing factors, possible degradation pathways, and mechanism insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Yu, H.B.; Huang, B.B.; Wang, H.; Yuan, X.Z.; Jiang, L.B.; Wu, Z.B.; Zhang, J.; Zeng, G.M. Facile construction of novel direct solid-state Z-scheme AgI/BiOBr photocatalysts for highly effective removal of ciprofloxacin under visible light exposure: Mineralization efficiency and mechanisms. J. Colloid. Interf. Sci. 2018, 522, 82–94. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Liu, L.; Cui, W.; An, W. Enhancement of Photocatalytic Activity under Visible Light Irradiation via the AgI@TCNQ Core-Shell Structure. Materials 2019, 12, 1679. https://doi.org/10.3390/ma12101679
Xie W, Liu L, Cui W, An W. Enhancement of Photocatalytic Activity under Visible Light Irradiation via the AgI@TCNQ Core-Shell Structure. Materials. 2019; 12(10):1679. https://doi.org/10.3390/ma12101679
Chicago/Turabian StyleXie, Wanli, Li Liu, Wenquan Cui, and Weijia An. 2019. "Enhancement of Photocatalytic Activity under Visible Light Irradiation via the AgI@TCNQ Core-Shell Structure" Materials 12, no. 10: 1679. https://doi.org/10.3390/ma12101679
APA StyleXie, W., Liu, L., Cui, W., & An, W. (2019). Enhancement of Photocatalytic Activity under Visible Light Irradiation via the AgI@TCNQ Core-Shell Structure. Materials, 12(10), 1679. https://doi.org/10.3390/ma12101679