Highly Efficient Liquid-Phase Hydrogenation of Naringin Using a Recyclable Pd/C Catalyst
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Preparation of Pd/C Catalyst
2.3. Procedures Used to Carry Out Hydrogenation Reactions
3. Results and Discussion
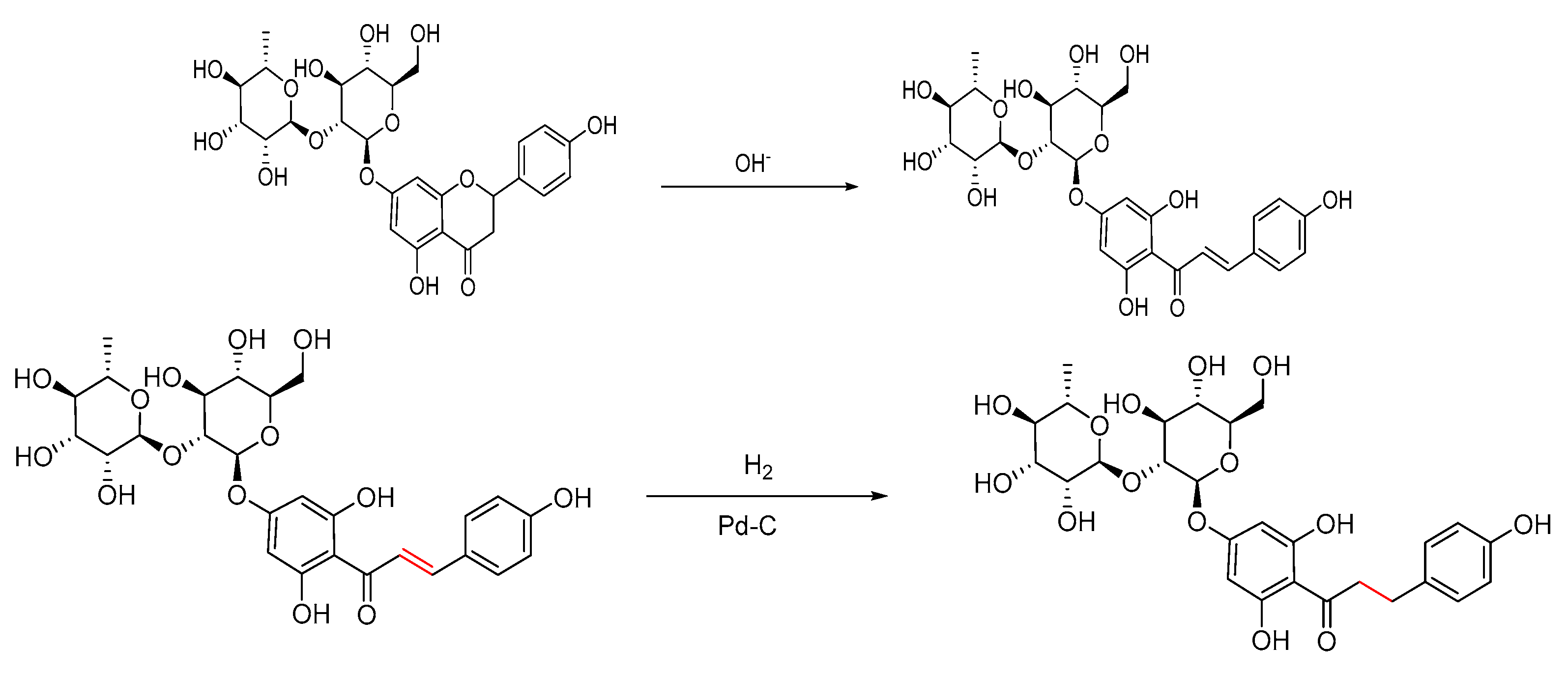
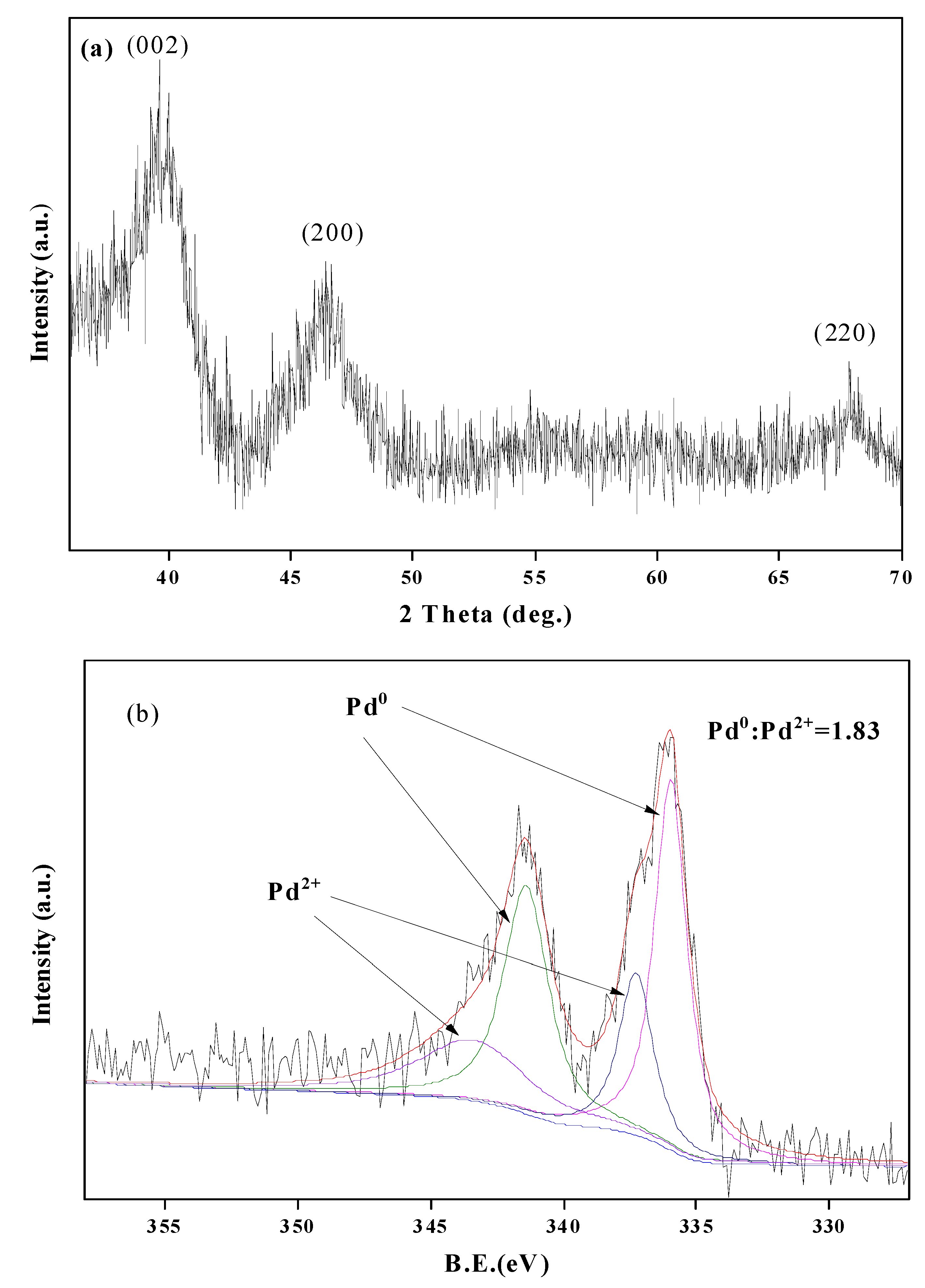
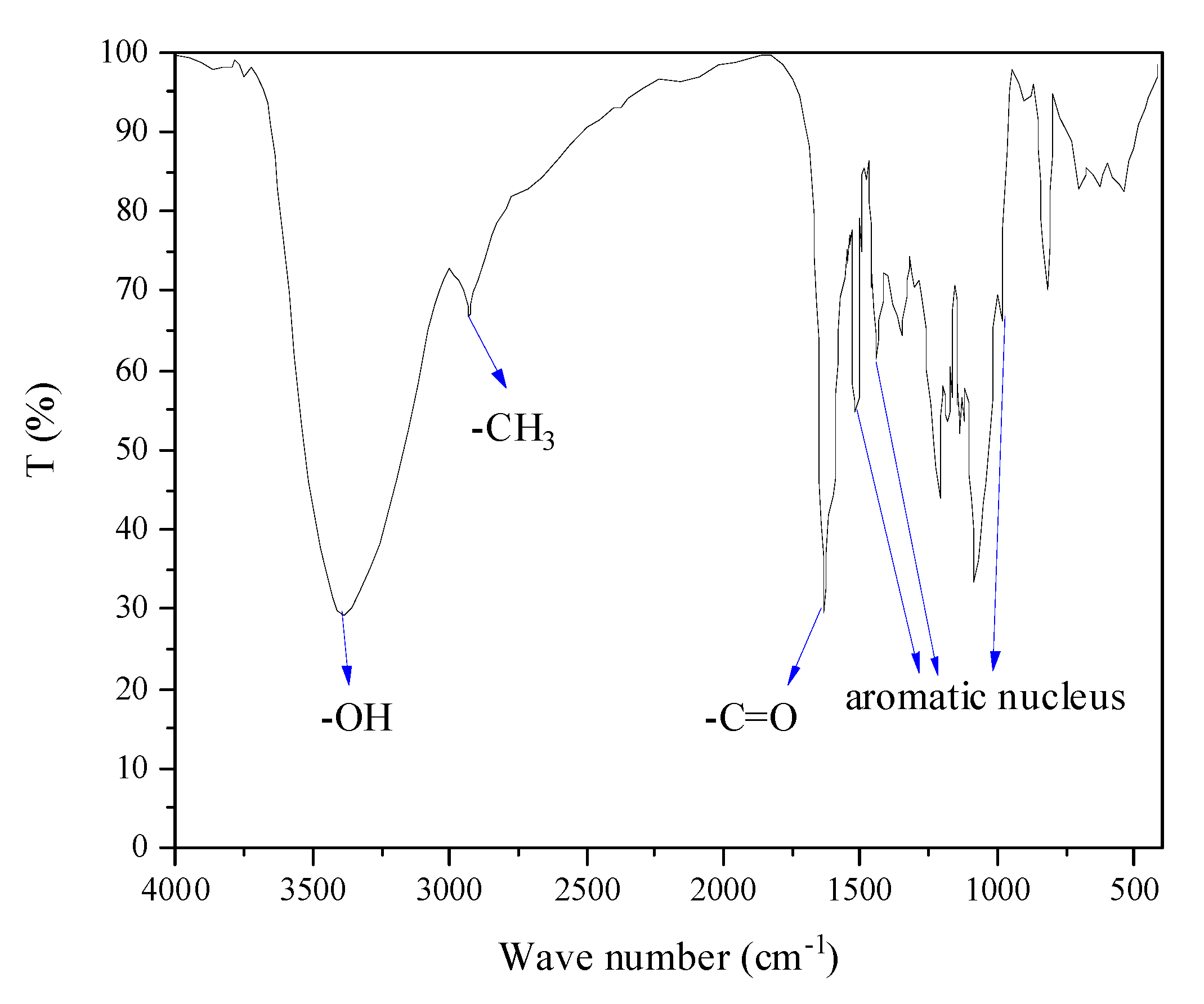
3.1. Characterization of Pd/C and the Product DHC
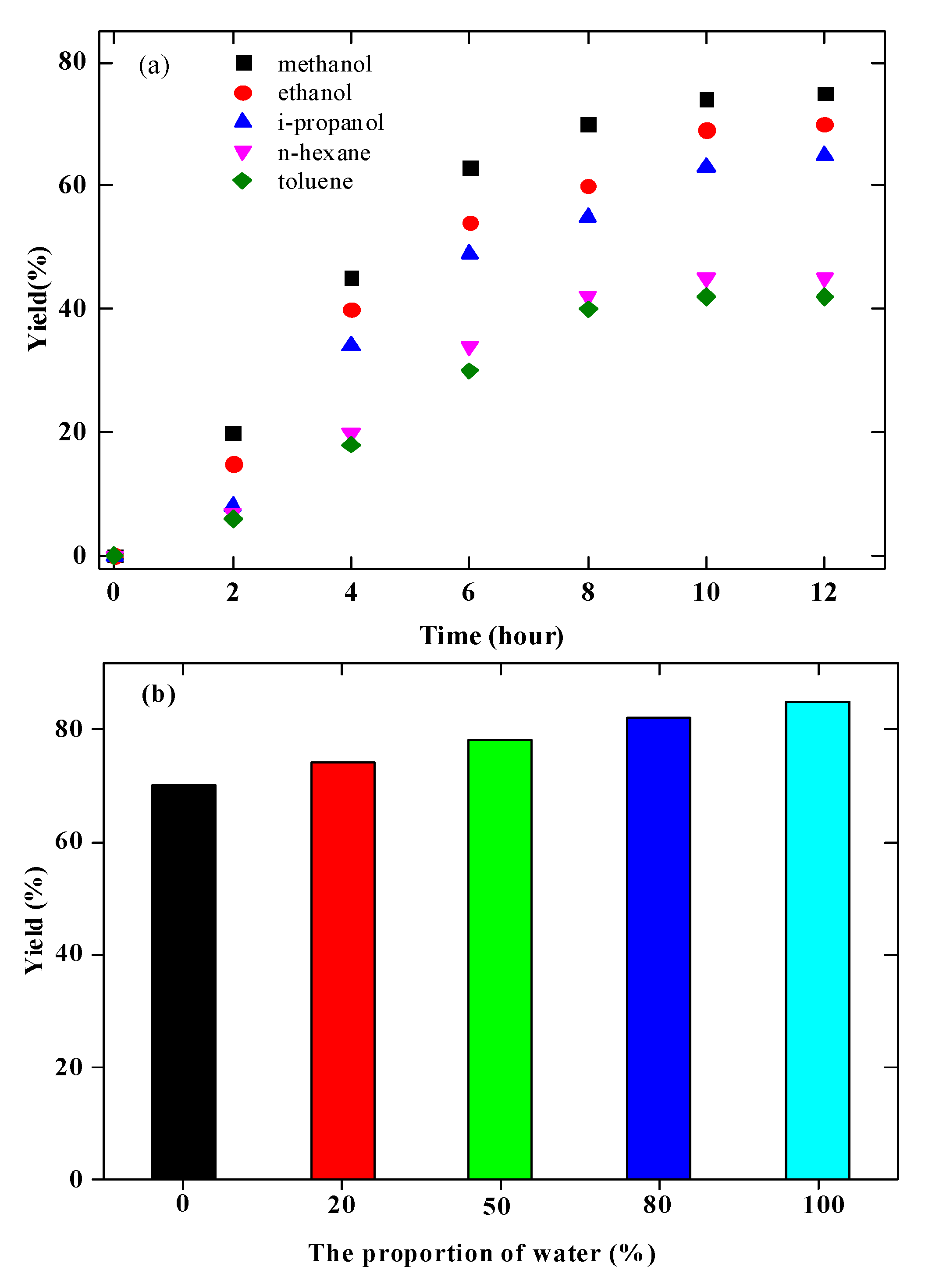
3.2. Solvent Effect on the Hydrogenation Reaction of Naringin over Pd/C Catalyst
3.3. The Influence of Solvent on Pd/C Catalyst Structure
3.4. Optimal Reaction Conditions for Naringin Hydrogenation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Horowitz, R.M.; Gentili, B. Flavonoids of citrus-VI. Tetrahedron 1963, 19, 773–782. [Google Scholar] [CrossRef]
- Eichenberger, M.; Lehka, B.J.; Folly, C.; Fischer, D.; Martens, S.; Simón, E.; Naesby, M. Metabolic engineering of Saccharomyces cerevisiae for de novo production of dihydrochalcones with known antioxidant, antidiabetic, and sweet tasting properties. Metab. Eng. 2017, 39, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, A.; Bungaruang, L.; Weber, H.; Leypold, M.; Breinbauer, R.; Nidetzky, B. Towards the synthesis of glycosylated dihydrochalcone natural products using glycosyltransferase-catalysed cascade reactions. Green Chem. 2014, 16, 4417–4425. [Google Scholar] [CrossRef]
- Chao, E.C. SGLT-2 Inhibitors: A New Mechanism for Glycemic Control. Clin. Diabetes 2014, 32, 4–11. [Google Scholar] [CrossRef]
- Scheen, A.J. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin. Pharmacokinet. 2015, 54, 691–708. [Google Scholar] [CrossRef]
- Snijman, P.W.; Joubert, E.; Ferreira, D.; Li, X.-C.; Ding, Y.; Green, I.R.; Gelderblom, W.C. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and Trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef]
- Janvier, S.; Goscinny, S.; Donne, C.L.; Loco, J.V. Low-calorie sweeteners in food and food supplements on the Italian market. Food Addit. Contam. Part B 2015, 8, 298–308. [Google Scholar] [CrossRef]
- Tang, D.-M.; Zhu, C.-F.; Zhong, S.-A.; Zhou, M.-D. Extraction of naringin from pomelo peels as dihydrochalcone’s precursor. J. Sep. Sci. 2011, 34, 113–117. [Google Scholar] [CrossRef]
- Yuan, W.; Feng, Y.; Xie, A.; Zhang, X.; Huang, F.; Li, S.; Zhang, X.; Shen, Y. Nitrogen-doped nanoporous carbon derived from waste pomelo peel as a metal-free electrocatalyst for the oxygen reduction reaction. Nanoscale 2016, 8, 8704–8711. [Google Scholar] [CrossRef]
- Huang, R.; Cao, M.; Guo, H.; Qi, W.; Su, R.; He, Z. Enhanced ethanol production from pomelo peel waste by integrated hydrothermal treatment, multienzyme formulation, and fed-batch operation. J. Agric. Food Chem. 2014, 62, 4643–4651. [Google Scholar] [CrossRef]
- Wild, J.; Huber, U. Process for Making Neohesperidine Dihydrochalcone. U.S. Patent No. 3,947,405, 30 March 1976. [Google Scholar]
- Wang, Y.; Yao, J.; Li, H.; Su, D.; Antonietti, M. Highly selective hydrogenation of phenol and derivatives over a Pd@ carbon nitride catalyst in aqueous media. J. Am. Chem. Soc. 2011, 133, 2362–2365. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.-A.; Vallat, A.; Süss-Fink, G. Highly selective C C bond hydrogenation in α, β-unsaturated ketones catalyzed by hectorite-supported ruthenium nanoparticles. J. Mol. Catal. A Chem. 2012, 355, 168–173. [Google Scholar] [CrossRef]
- Kantam, L.; Parsharamulu, T.; Manorama, S. Layered double hydroxides supported nano palladium: An efficient catalyst for the chemoselective hydrogenation of olefinic bonds. J. Mol. Catal. A Chem. 2012, 365, 115–119. [Google Scholar]
- Zhao, B.H.; Chen, J.G.; Liu, X.; Liu, Z.W.; Hao, Z.; Xiao, J.; Liu, Z.T. Selective hydrogenation of cinnamaldehyde over Pt and Pd supported on multiwalled carbon nanotubes in a CO2-expanded alcoholic medium. Ind. Eng. Chem. Res. 2012, 51, 11112–11121. [Google Scholar] [CrossRef]
- Morimoto, N.; Yamamoto, S.-I.; Takeuchi, Y.; Nishina, Y. Palladium on graphene: The in situ generation of a catalyst for the chemoselective reduction of α, β-unsaturated carbonyl compounds. RSC Adv. 2013, 3, 15608–15612. [Google Scholar] [CrossRef]
- Delbecq, F.; Sautet, P. Competitive C=C and C=O Adsorption of α-β-Unsaturated Aldehydes on Pt and Pd Surfaces in Relation with the Selectivity of Hydrogenation Reactions: A Theoretical Approach. J. Catal. 1995, 152, 217–236. [Google Scholar] [CrossRef]
- Pallassana, V.; Neurock, M. Theoretical density functional study of the hydrogenation of maleic acid over Pd and Re surfaces. Chem. Eng. Sci. 1999, 54, 3423–3431. [Google Scholar] [CrossRef]
- Manyar, H.G.; Yang, B.; Daly, H.; Moor, H.; McMonagle, S.; Tao, Y.; Yadav, G.D.; Goguet, A.; Hu, P.; Hardacre, C. Selective Hydrogenation of α, β-Unsaturated Aldehydes and Ketones using Novel Manganese Oxide and Platinum Supported on Manganese Oxide Octahedral Molecular Sieves as Catalysts. ChemCatChem 2013, 5, 506–512. [Google Scholar] [CrossRef]
- Mukherjee, S.; Vannice, M.A. Solvent effects in liquid-phase reactions: I. Activity and selectivity during citral hydrogenation on Pt/SiO2 and evaluation of mass transfer effects. J. Catal. 2006, 243, 108–130. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, P.; Xu, X.; Li, Y.; Li, H.; Wang, Y. A novel catalyst Pd@ ompg-C3N4 for highly chemoselective hydrogenation of quinoline under mild conditions. J. Catal. 2013, 297, 272–280. [Google Scholar] [CrossRef]
- Wei, Z.; Gong, Y.; Xiong, T.; Zhang, P.; Li, H.; Wang, Y. Highly efficient and chemoselective hydrogenation of α, β-unsaturated carbonyls over Pd/N-doped hierarchically porous carbon. Catal. Sci. Technol. 2015, 5, 397–404. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Li, X.; Xu, J.; Gu, G.; Xia, C. Water: The most effective solvent for liquid-phase hydrodechlorination of chlorophenols over Raney Ni catalyst. Appl. Catal. B Environ. 2015, 165, 351–359. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, S.; Xia, C. Water-promoted catalytic hydrodechlorination of transformer oil-contained PCBs in liquid system under mild conditions. Appl. Catal. B Environ. 2014, 144, 580–587. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Rajadhyaksha, R.; Karwa, S. Solvent effects in catalytic hydrogenation. Chem. Eng. Sci. 1986, 41, 1765–1770. [Google Scholar] [CrossRef]
- Lide, D.; Haynes, W. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; Lide, D.R., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2009. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Akpa, B.; D’Agostino, C.; Gladden, L.; Hindle, K.; Manyar, H.; McGregor, J.; Li, R.; Neurock, M.; Sinha, N.; Stitt, E.H. Solvent effects in the hydrogenation of 2-butanone. J. Catal. 2012, 289, 30–41. [Google Scholar] [CrossRef]
- Wan, H.; Vitter, A.; Chaudhari, R.V.; Subramaniam, B. Kinetic investigations of unusual solvent effects during Ru/C catalyzed hydrogenation of model oxygenates. J. Catal. 2014, 309, 174–184. [Google Scholar] [CrossRef]




| Peak _ ID | Area | Centre | AT (wt %) | |||
|---|---|---|---|---|---|---|
| Ethanol-Water | Ethanol | Ethanol-Water | Ethanol | Ethanol-Water | Ethanol | |
| O1s | 18,667 | 12,383 | 532.60 | 532.42 | 83.4 | 83.63 |
| Na1s | 2700 | 3323 | 1071.52 | 1071.47 | 4.63 | 11.87 |
| Pd3d | 19,280 | 6107 | 336.39 | 336.11 | 11.96 | 4.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yuan, Y.; Meng, X.; Duan, L.; Zhou, R. Highly Efficient Liquid-Phase Hydrogenation of Naringin Using a Recyclable Pd/C Catalyst. Materials 2019, 12, 46. https://doi.org/10.3390/ma12010046
Zhao J, Yuan Y, Meng X, Duan L, Zhou R. Highly Efficient Liquid-Phase Hydrogenation of Naringin Using a Recyclable Pd/C Catalyst. Materials. 2019; 12(1):46. https://doi.org/10.3390/ma12010046
Chicago/Turabian StyleZhao, Jiamin, Ying Yuan, Xiuhong Meng, Linhai Duan, and Rujin Zhou. 2019. "Highly Efficient Liquid-Phase Hydrogenation of Naringin Using a Recyclable Pd/C Catalyst" Materials 12, no. 1: 46. https://doi.org/10.3390/ma12010046
APA StyleZhao, J., Yuan, Y., Meng, X., Duan, L., & Zhou, R. (2019). Highly Efficient Liquid-Phase Hydrogenation of Naringin Using a Recyclable Pd/C Catalyst. Materials, 12(1), 46. https://doi.org/10.3390/ma12010046





