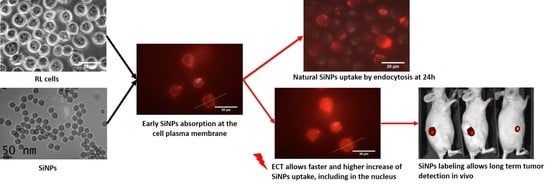
Increasing Uptake of Silica Nanoparticles with Electroporation: From Cellular Characterization to Potential Applications
Abstract
1. Introduction
2. Results
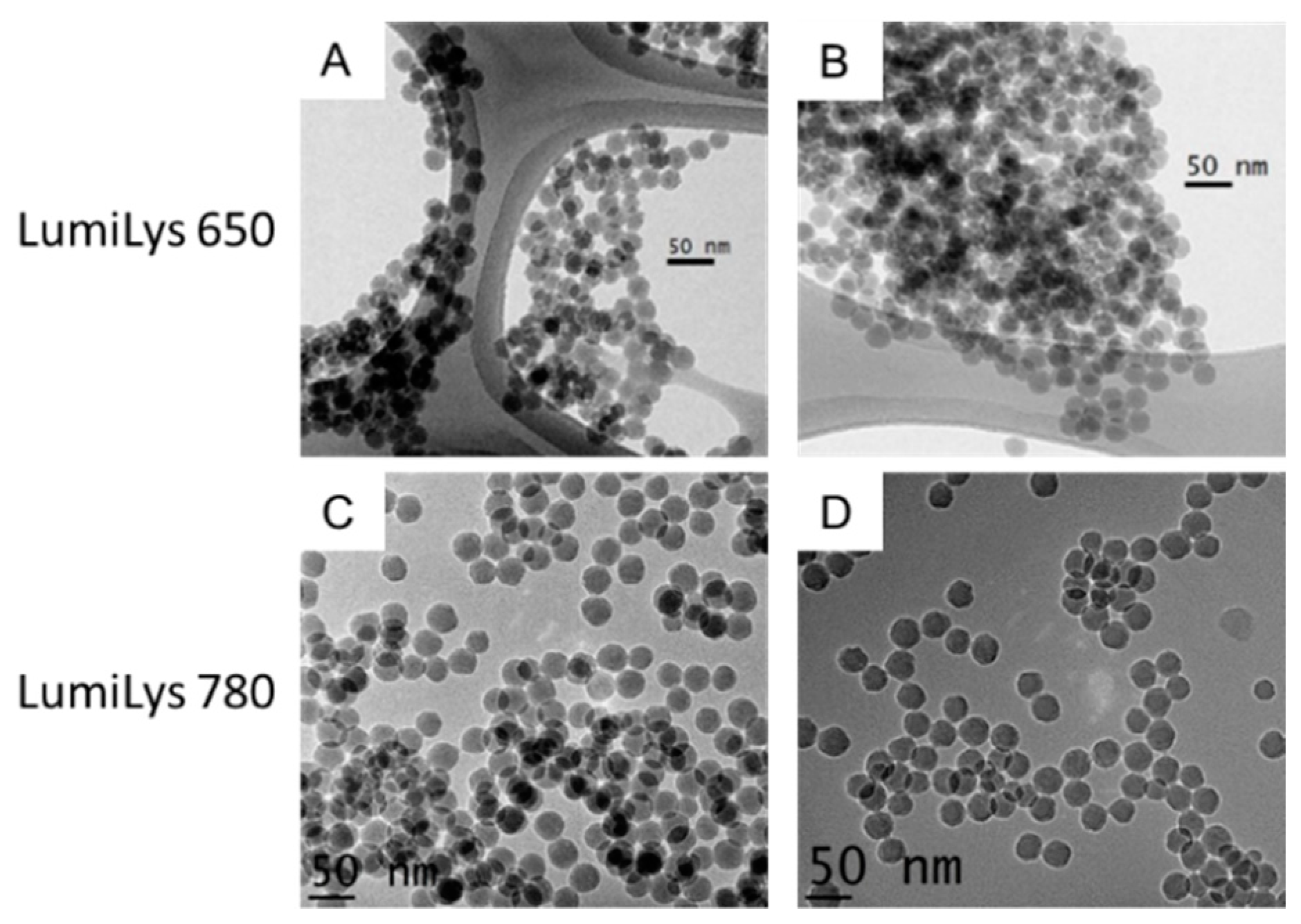
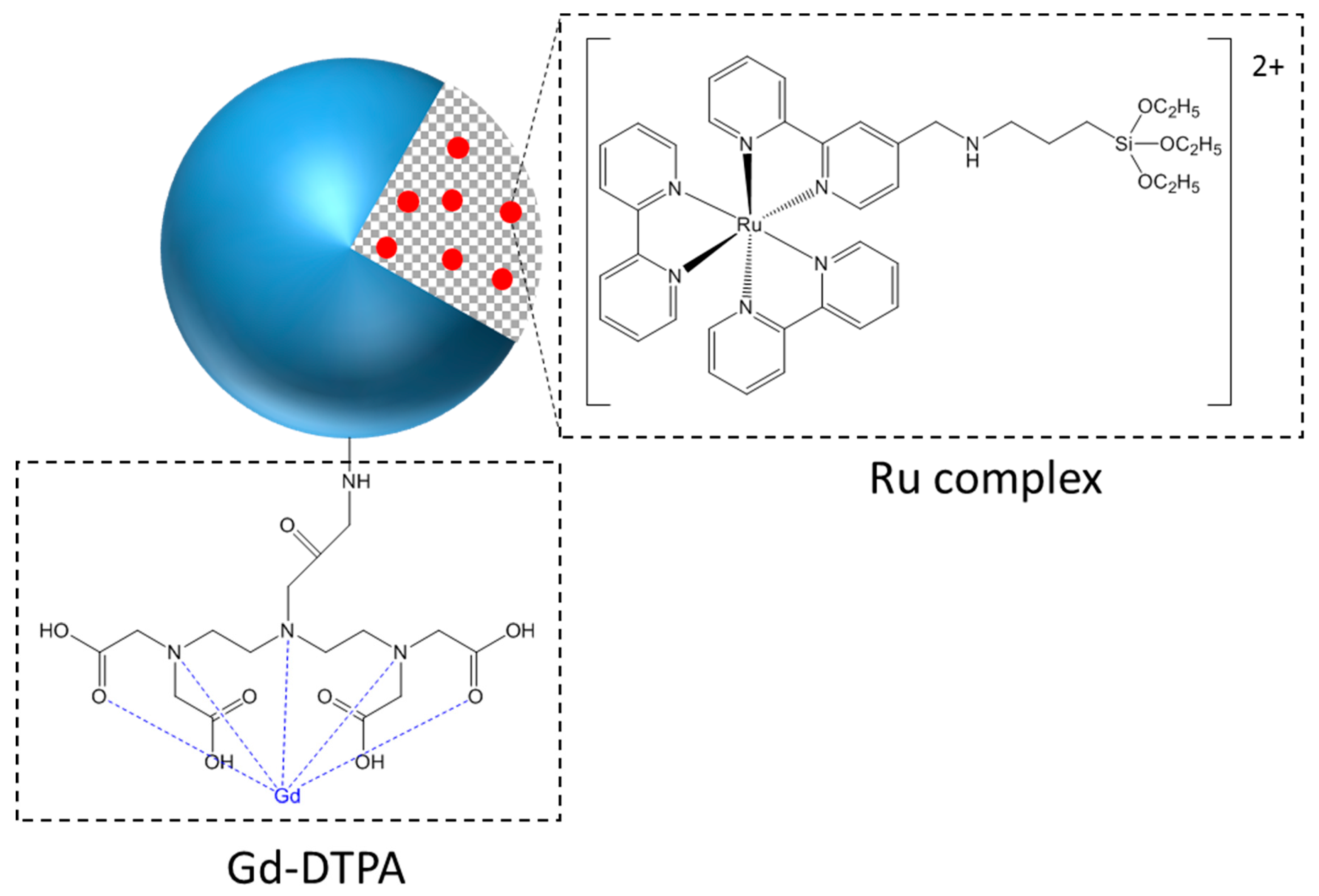
2.1. SiNPs Characterizations
2.2. Passive Incorporation of LumiLys 650 NPs into Cancer Cell Lines
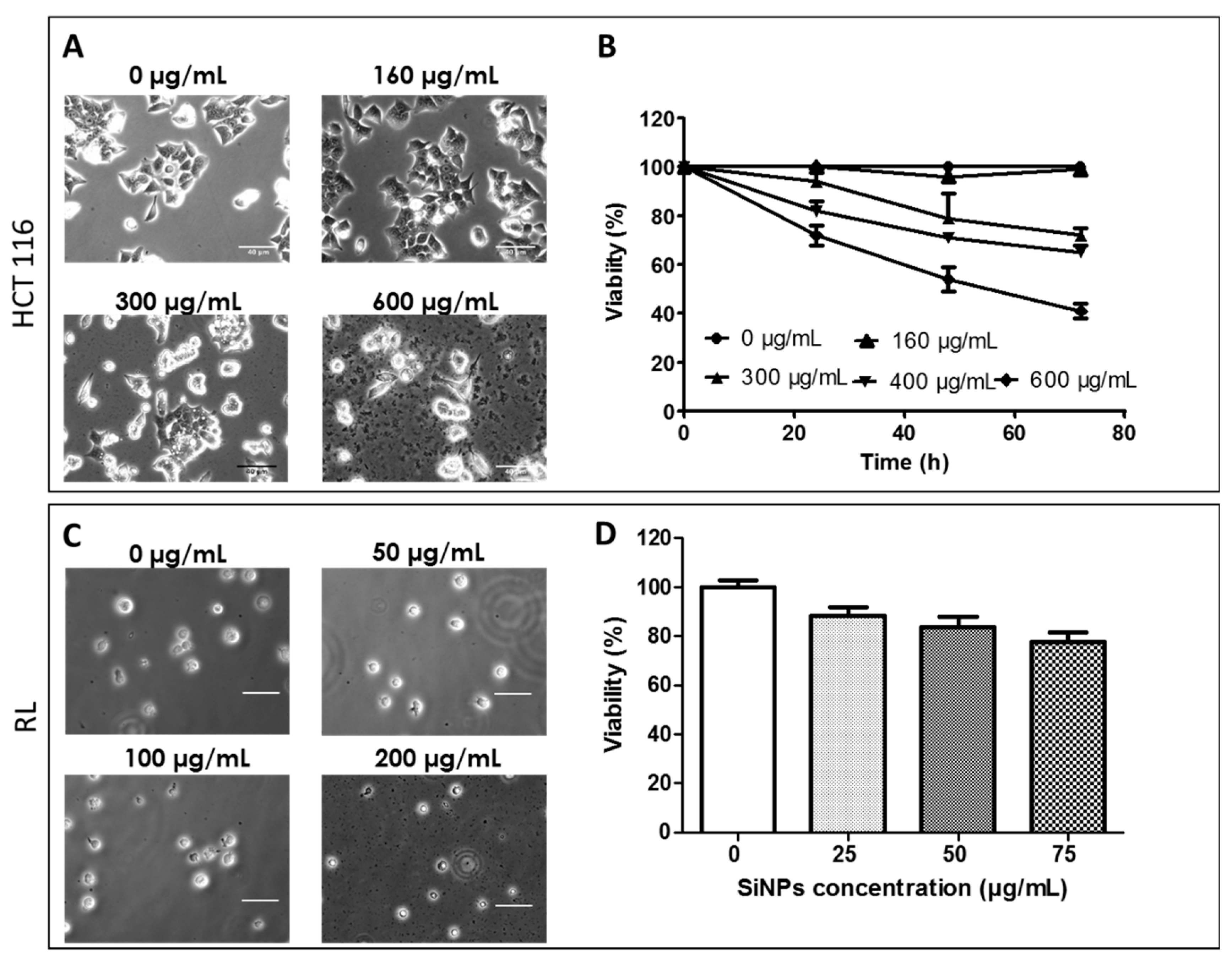
2.2.1. Effect of LumiLys 650 NPs Concentrations on Cancer Cell Viability
2.2.2. LumiLys 650 NPs Incorporation Kinetics into HCT-116 and RL Cells
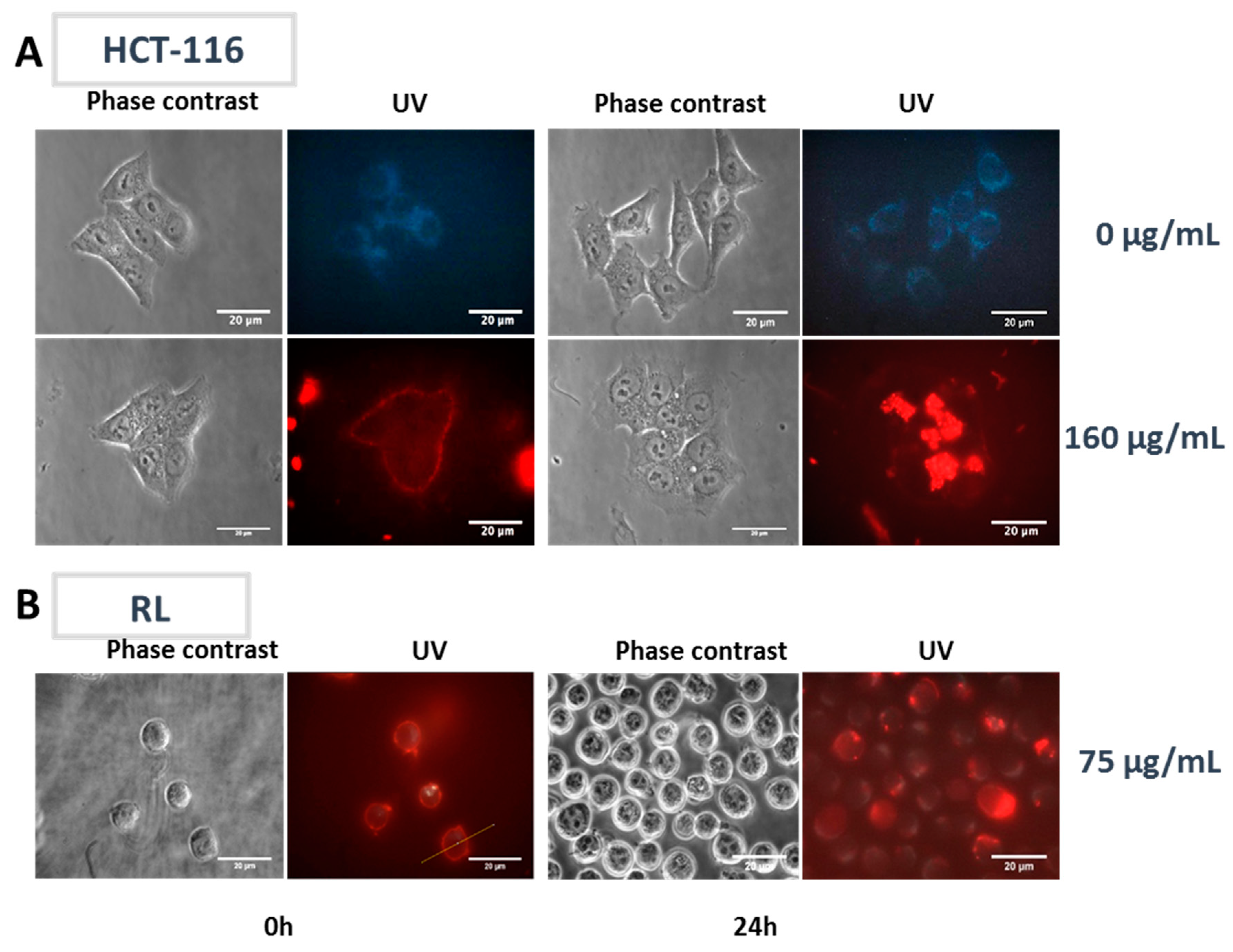
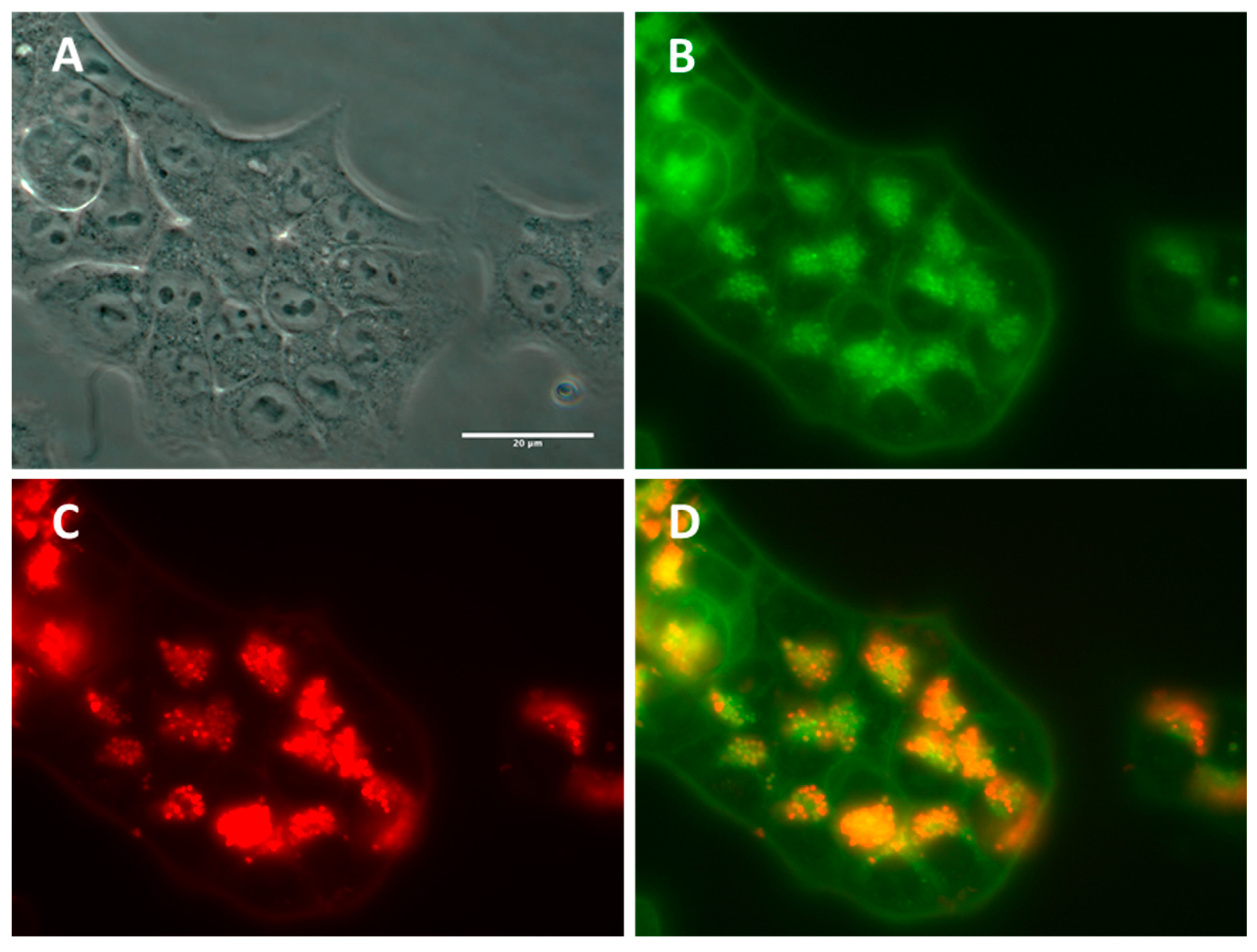
2.2.3. Subcellular NP Localization in Cancer Cell Lines
2.3. Effect of Electric Field Application on SiNPs’ Incorporation into Cancer Cells
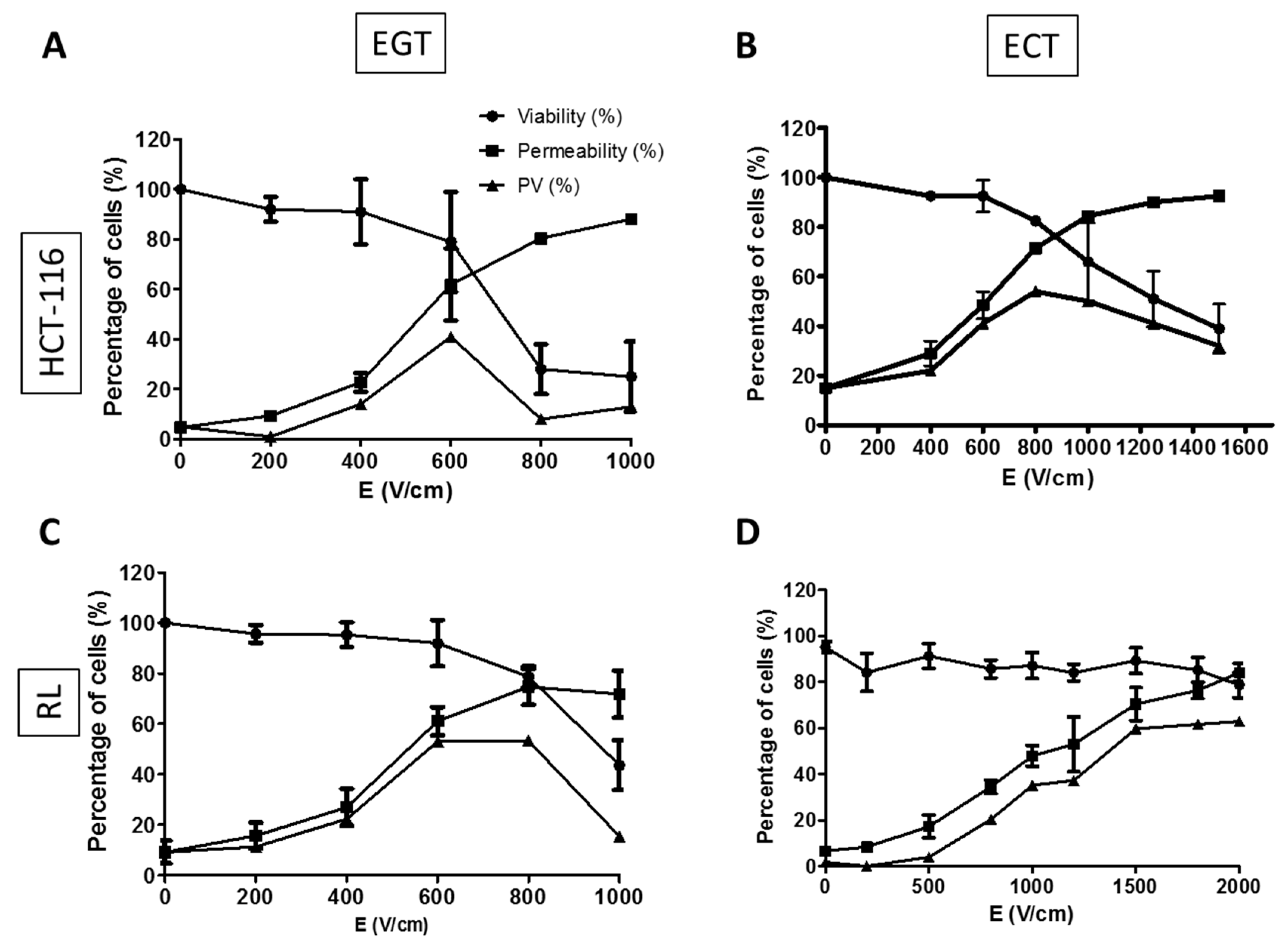
2.3.1. Determination of Electrical Parameters for HCT-116 and RL Cells
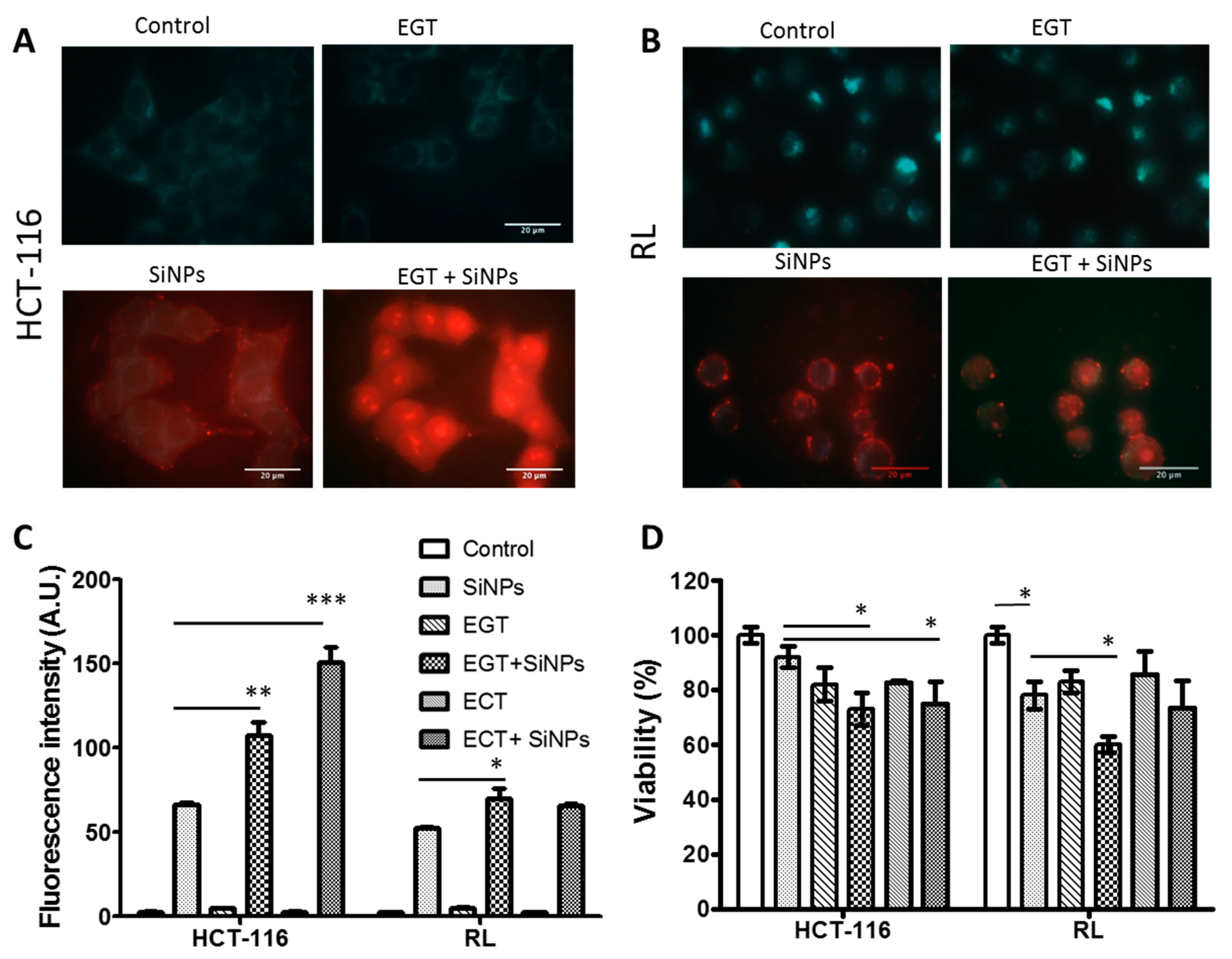
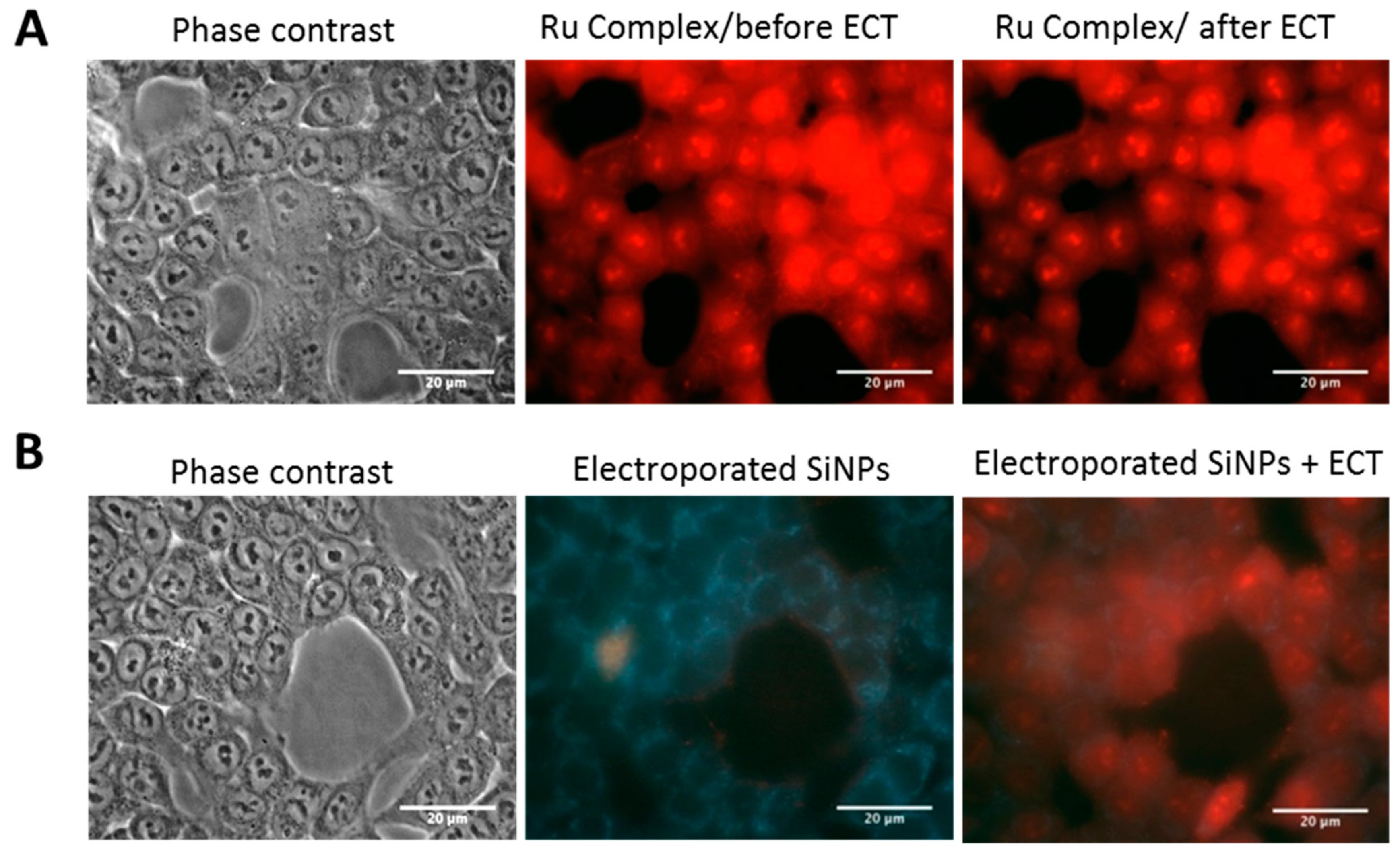
2.3.2. SiNPs Intracellular Localization after Cancer Cell Electropermeabilization
2.3.3. Effect of Electropermeabilization on SiNPs Trafficking into Cells
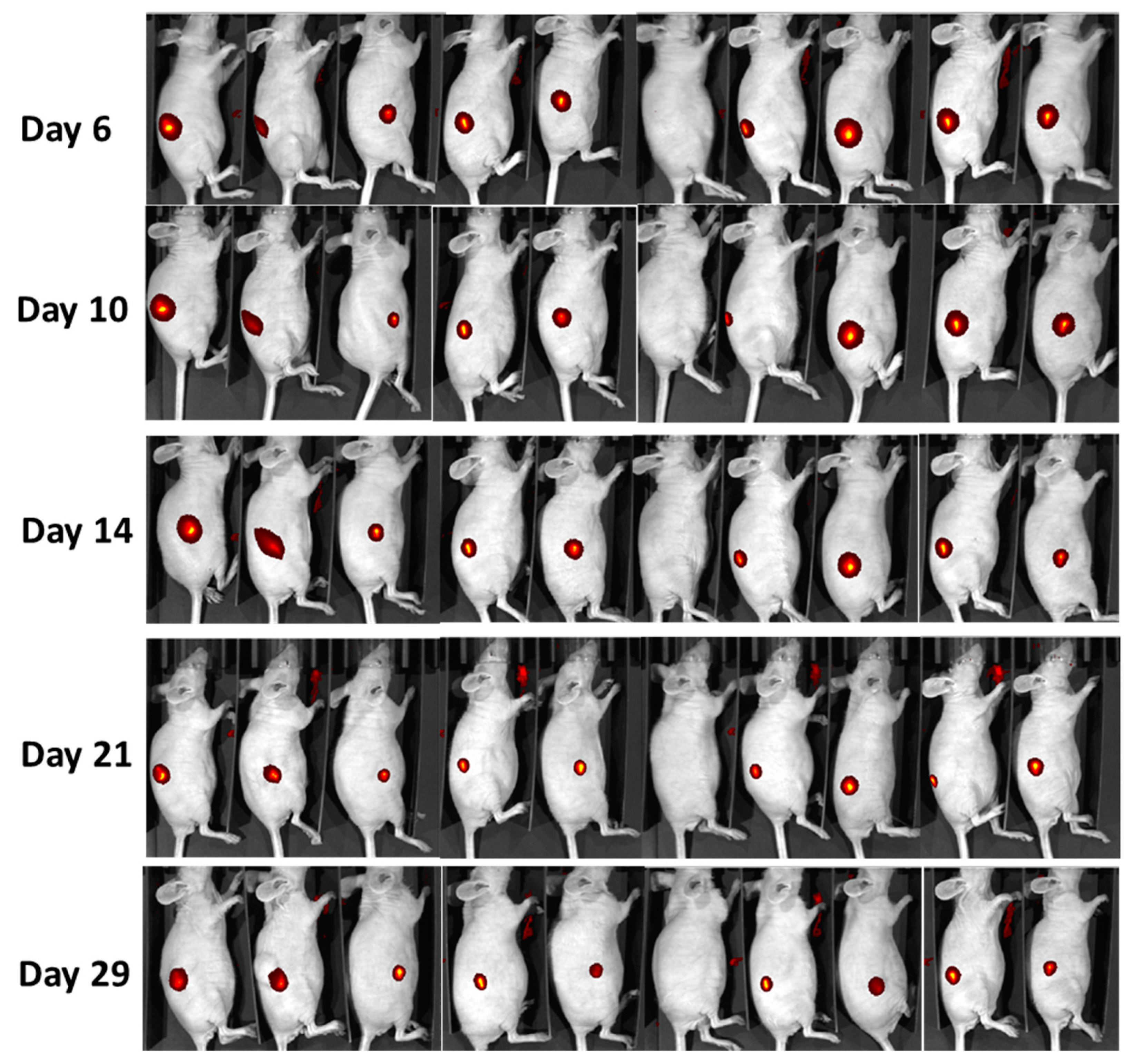
2.4. In Vivo Monitoring of LumiLys 780 NPs Labelled Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Grafting of Gd-DTPA
4.3. Instrumentation
4.4. Cell Culture, Cytotoxicity Tests, and Fluorescence Imaging
4.5. Electroporation of Cells
4.6. Microscopic Acquisition and Data Processing
4.7. In Vivo Experiments
4.8. Statistical Analysis
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Kah Mun, G.L.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 2, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.P.; Ma, B.Y.; Wei, X.W.; Qian, Z.Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, L.; Tanwir, F.; Babadi, V.Y. In vitro toxicity of iron oxide nanoparticle: Oxidative damages on Hep G2 cells. Exp. Toxicol. Pathol. 2015, 67, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Jie, L.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef]
- He, X.; Nie, H.; Wang, K.; Tan, W.; Wu, X.; Zhang, P. In vivo study of biodistribution and urinary excretion of surface-modified silica nanoparticles. Anal. Chem. 2008, 80, 9597–9603. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.A.; Choi, S.; Jeon, S.M.; Yu, J. Silica nanoparticle stability in biological media revisited. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.V.R.; Sameti, M.; Mohapatra, S.S.; Kong, X.; Lockey, R.F.; Bakowsky, U.; Lindenblatt, G.; Schmidt, H.; Lehr, C.M.J. Cationic Silica Nanoparticles as Gene Carriers: Synthesis, Characterization and Transfection Efficiency In vitro and In vivo. Nanosci. Nanotechnol. 2004, 4, 876–881. [Google Scholar] [CrossRef]
- Graf, C.; Dembski, S.; Hofmann, A.; Ruhl, E. A General Method for the Controlled Embedding of Nanoparticles in Silica Colloids. Langmuir 2006, 22, 5604–5610. [Google Scholar] [CrossRef] [PubMed]
- Vestal, C.R.; Zhang, Z.J. Synthesis and Magnetic Characterization of Mn and Co Spinel Ferrite-Silica Nanoparticles with Tunable Magnetic Core. Nano Lett. 2003, 3, 1739–1743. [Google Scholar] [CrossRef]
- Tago, T.; Hatsuta, T.; Miyajima, K.; Kishida, M.; Tashiro, S.; Wakabayashi, K. Novel synthesis of silica-coated ferrite nanoparticles prepared using water-in-oil microemulsion. J. Am. Ceram. Soc. 2004, 85, 2188–2194. [Google Scholar] [CrossRef]
- Grasset, F.; Labhsetwar, N.; Li, D.; Park, D.C.; Saito, N.; Haneda, H.; Cador, O.; Roisnel, T.; Mornet, S.; Duguet, E.; et al. Synthesis and magnetic characterization of zinc ferrite nanoparticles with different environments: powder, colloidal solution, and zinc ferrite−silica core−shell nanoparticles. Langmuir 2002, 18, 8209–8216. [Google Scholar] [CrossRef]
- Barbe, C.; Bartlett, J.; Kong, L.; Finnie, K.; Lin, H.Q.; Larkin, M.; Calleja, S.; Bush, A.; Calleja, G. Silica particles: A novel drug-delivery system. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Nann, T.; Mulvaney, P. Single quantum dots in spherical silica particles. Angew. Chem. Int. Ed. 2004, 43, 5393. [Google Scholar] [CrossRef] [PubMed]
- Ow, H.; Larson, D.R.; Srivastava, M.; Baird, B.A.; Webb, W.W.; Wiesner, U. Bright and stable core-shell fluorescent silica nanoparticles. Nano Lett. 2005, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, M.; Zhao, J.X. Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy. Nanomedicine 2014, 10, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef]
- Rossi, L.M.; Shi, L.; Quina, F.H.; Rosenzweig, Z. Stöber synthesis of monodispersed luminescent silica nanoparticles for bioanalytical assays. Langmuir 2005, 21, 4277–4280. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.J. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, J.; Milosevic, A.; Hauser, D.; Lehner, R.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Biodistribution, Clearance, and Long-Term Fate of Clinically Relevant Nanomaterials. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.A.; Lee, S.G.; Lee, W.G. Seeing the electroporative uptake of cell-membrane impermeable fluorescent molecules and nanoparticles. Nanoscale 2012, 4, 5051–5058. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Lee, W.G. Role of weakly polarized nanoparticles in electroporation. Nanoscale 2011, 3, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M. Therapeutic perspectives of in vivo cell electropermeabilization. Bioelectrochemistry 2001, 53. [Google Scholar] [CrossRef]
- Chabot, S.; Teissié, J.; Golzio, M. Targeted electro-delivery of oligonucleotides for RNA interference: siRNA and antimir. Adv. Drug Deliv. Rev. 2015, 81, 161–168. [Google Scholar] [CrossRef]
- Paganin-Gioanni, A.; Bellard, E.; Escoffre, J.M.; Rols, M.P.; Teissié, J.; Golzio, M. Direct visualization at the single-cell level of siRNA electrotransfer into cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 10443–10447. [Google Scholar] [CrossRef]
- Teissie, J.; Golzio, M.; Rols, M.P. Mechanisms of cell membrane electropermeabilization: A minireview of our present (lack of ?) knowledge. Biochim. Biophys. Acta 2005, 1724, 270–280. [Google Scholar] [CrossRef]
- Di Monta, G.; Caracò, C.; Benedetto, L.; La Padula, S.; Marone, U.; Tornesello, M.L.; Buonaguro, F.M.; Simeone, E.; Ascierto, P.A.; Mozzillo, N. Electrochemotherapy as “new standard of care” treatment for cutaneous Kaposi’s sarcoma. Eur. J. Surg. Oncol. 2014, 40, 61–66. [Google Scholar] [CrossRef]
- Rotunno, R.; Campana, L.G.; Quaglino, P.; De Terlizzi, F.; Kunte, C.; Odili, J.; Gehl, J.; Ribero, S.; Liew, S.H.; Marconato, R.; et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: Analysis of 57 patients from the International Network for Sharing Practices of Electrochemotherapy registry. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef]
- Golzio, M.; Teissie, J.; Rols, M.P. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc. Natl. Acad. Sci. USA 2002, 99, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Teissié, J.; Escoffre, J.M.; Paganin, A.; Chabot, S.; Bellard, E.; Wasungu, L.; Rols, M.P.; Golzio, M. Drug delivery by electropulsation: Recent developments in oncology. Int. J. Pharm. 2012, 423, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Carton, F.; Marengo, A.; Berlier, G.; Stella, B.; Arpicco, S.; Malatesta, M. Fluorescence and electron microscopy to visualize the intracellular fate of nanoparticles for drug delivery. Eur. J. Histochem. 2016, 60, 2640. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A.; Tarannum, M.; Vivero-Escoto, J.L. Cellular Endocytosis and Trafficking of Cholera Toxin B-Modified Mesoporous Silica Nanoparticles. J. Mater. Chem. B 2016, 4, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Rols, M.P.; Teissie, J. Electropermeabilization of mammalian cells to macromolecules: Control by pulse duration. Biophys. J. 1998, 75, 1415–1423. [Google Scholar] [CrossRef]
- Mlakar, V.; Todorovic, V.; Cemazar, M.; Glavac, D.; Sersa, G. Electric pulses used in electrochemotherapy and electrogene therapy do not significantly change the expression profile of genes involved in the development of cancer in malignant melanoma cells. BMC Cancer 2009, 9, 299. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gehl, J. A Review on Differences in Effects on Normal and Malignant Cells and Tissues to Electroporation-Based Therapies: A Focus on Calcium Electroporation. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef]
- Pavliha, D.; Kos, B.; Zupanič, A.; Marčan, M.; Serša, G.; Miklavčič, D. Patient-specific treatment planning of electrochemotherapy: Procedure design and possible pitfalls. Bioelectrochemistry 2012, 87, 265–273. [Google Scholar] [CrossRef]
- Jorritsma, S.H.T.; Gowans, E.J.; Grubor-Bauk, B.; Wijesundara, D.K. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine 2016, 34, 5488–5494. [Google Scholar] [CrossRef] [PubMed]
- Bellard, E.; Teissie, J. Double Pulse Approach of Electropulsation: A Fluorescence Analysis of the Nucleus Perturbation at the Single Cell Level. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1267–1272. [Google Scholar] [CrossRef]
- Lechevallier, S.; Mauricot, R.; Gros-Dagnac, H.; Chevreux, S.; Lemercier, G.; Phonesouk, E.; Golzio, M.; Verelst, M. Silica-Based Nanoparticles as Bifunctional and Bimodal Imaging Contrast Agents. ChemPlusChem 2017, 82, 770–777. [Google Scholar] [CrossRef]
- Gabriel, B.; Teissié, J. Control by electrical parameters of short- and long-term cell death resulting from electropermeabilization of Chinese hamster ovary cells. Biochim. Biophys. Acta 1995, 1266, 171–178. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phonesouk, E.; Lechevallier, S.; Ferrand, A.; Rols, M.-P.; Bezombes, C.; Verelst, M.; Golzio, M. Increasing Uptake of Silica Nanoparticles with Electroporation: From Cellular Characterization to Potential Applications. Materials 2019, 12, 179. https://doi.org/10.3390/ma12010179
Phonesouk E, Lechevallier S, Ferrand A, Rols M-P, Bezombes C, Verelst M, Golzio M. Increasing Uptake of Silica Nanoparticles with Electroporation: From Cellular Characterization to Potential Applications. Materials. 2019; 12(1):179. https://doi.org/10.3390/ma12010179
Chicago/Turabian StylePhonesouk, Erick, Séverine Lechevallier, Audrey Ferrand, Marie-Pierre Rols, Christine Bezombes, Marc Verelst, and Muriel Golzio. 2019. "Increasing Uptake of Silica Nanoparticles with Electroporation: From Cellular Characterization to Potential Applications" Materials 12, no. 1: 179. https://doi.org/10.3390/ma12010179
APA StylePhonesouk, E., Lechevallier, S., Ferrand, A., Rols, M.-P., Bezombes, C., Verelst, M., & Golzio, M. (2019). Increasing Uptake of Silica Nanoparticles with Electroporation: From Cellular Characterization to Potential Applications. Materials, 12(1), 179. https://doi.org/10.3390/ma12010179