Design of SnO2 Aggregate/Nanosheet Composite Structures Based on Function-Matching Strategy for Enhanced Dye-Sensitized Solar Cell Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of SnO2 Aggregates
2.3. Preparation of SnO2 Nanosheets
2.4. Preparation of SnO2 Hybrid Photoanode and Cell Assembly
2.5. Characterizations
3. Results and Discussion
3.1. XRD Patterns and BET Surface Area Analysis
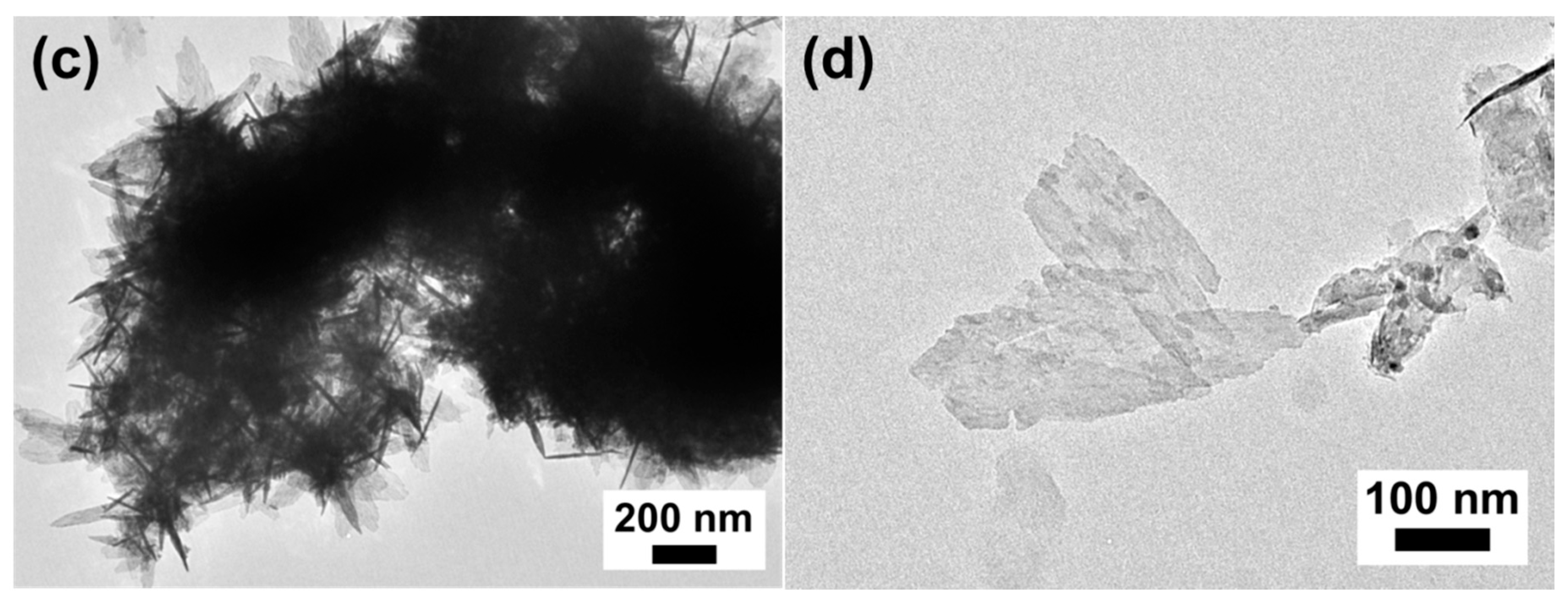
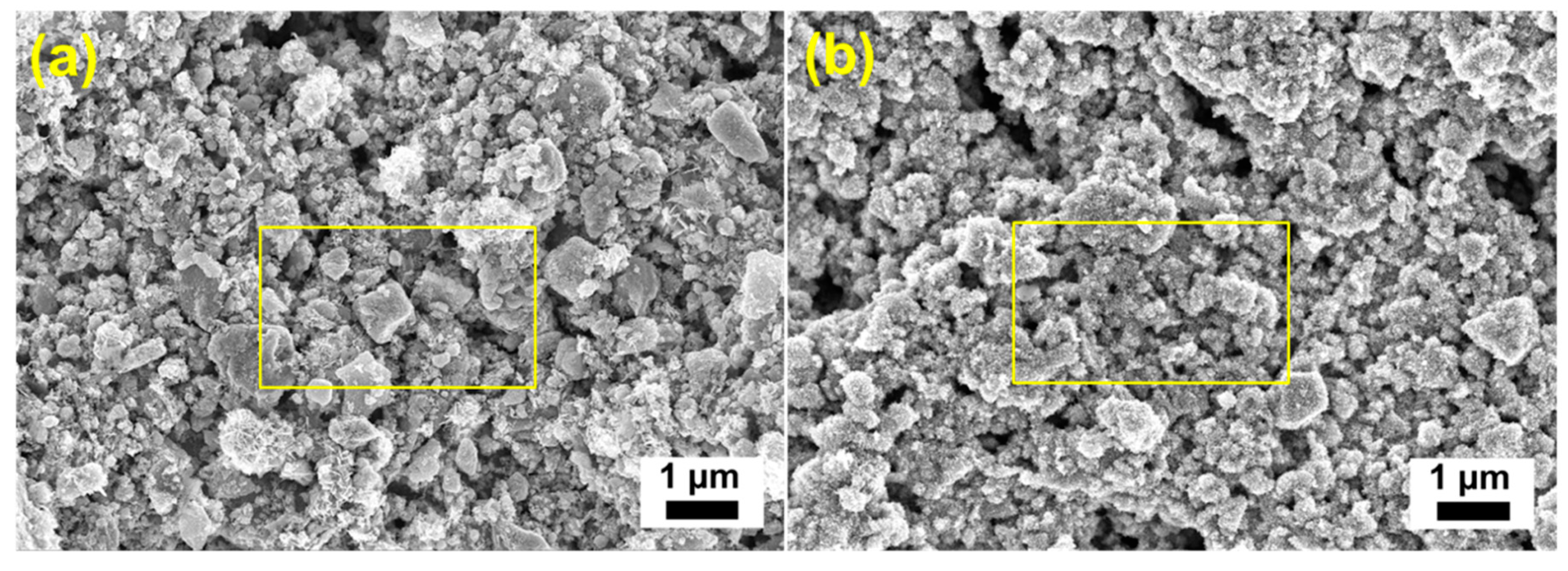
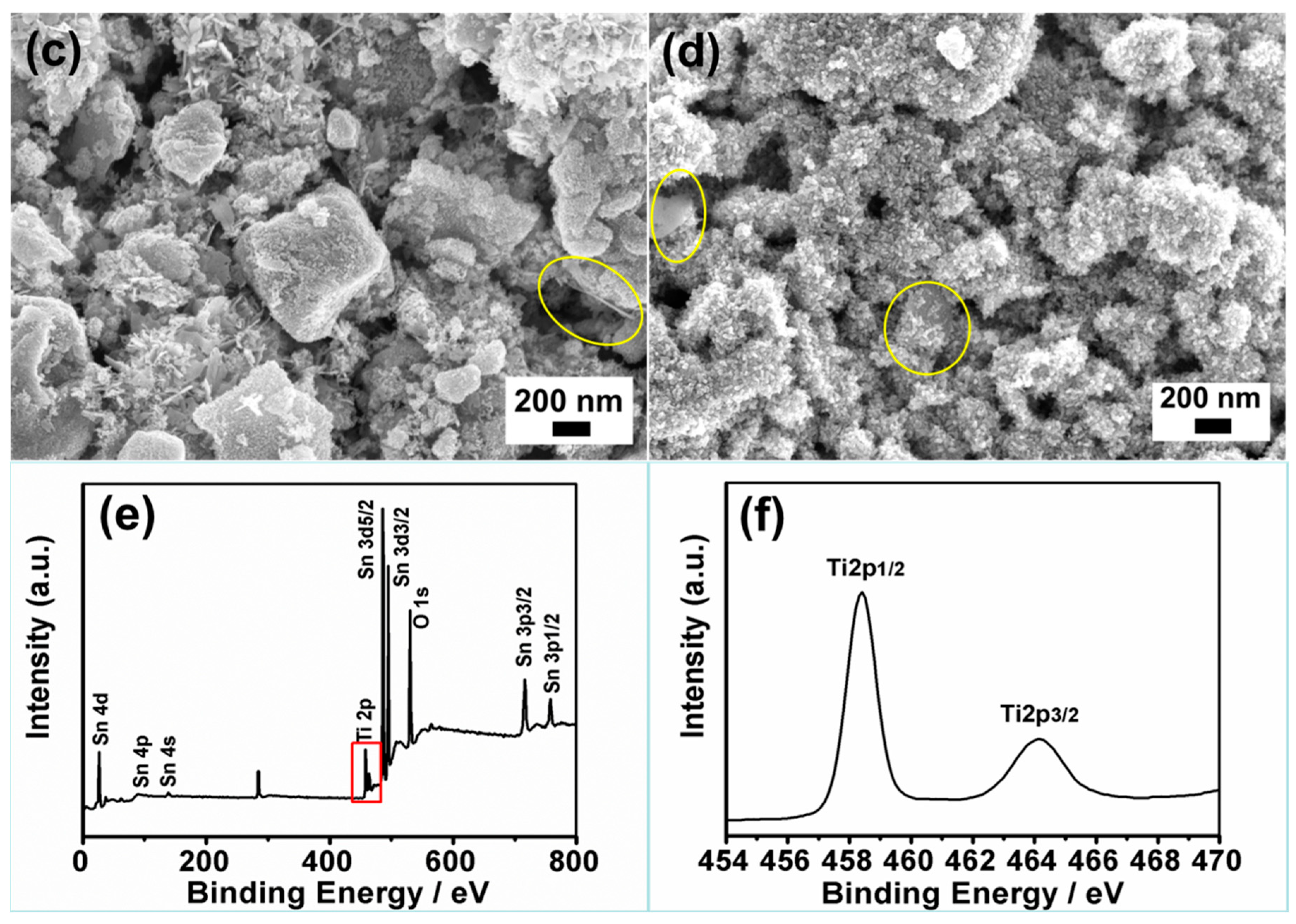
3.2. Morphological and Composition Characterization
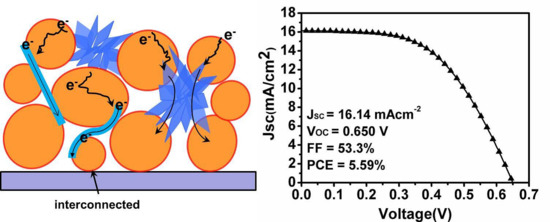
3.3. Photocurrent Density−Voltage Characteristics
3.4. Dye Absorption and Diffuse Reflectivity
3.5. EIS Analysis
3.6. IPCE Spectra
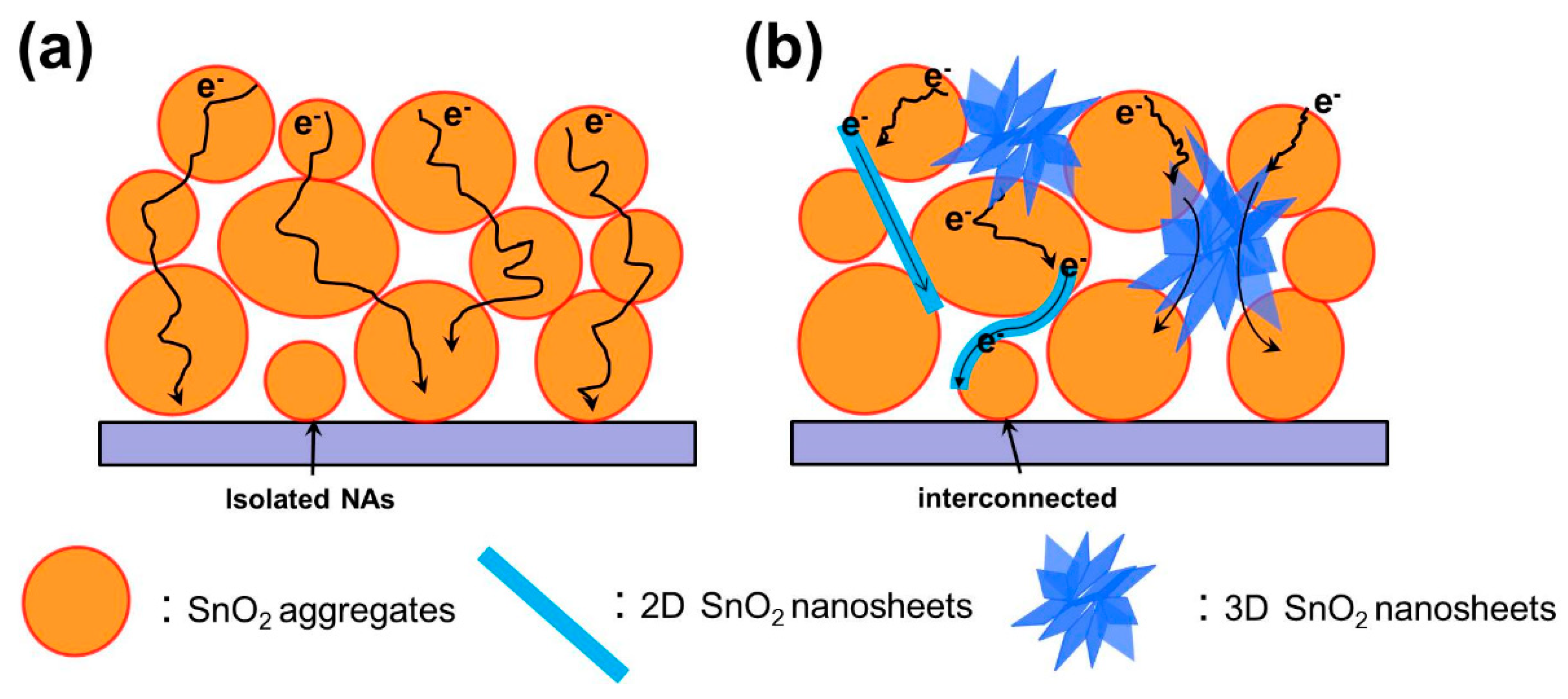
3.7. Schematic Views of Electron Transfer and Recombination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Song, D.D.; Cui, P.; Wang, T.Y.; Xie, B.X.; Jiang, Y.J.; Li, M.C.; Li, Y.Y.; Du, S.; He, Y.; Liu, Z.H.; et al. Bunchy TiO2 Hierarchical Spheres with Fast Electron Transport and Large Specific Surface Area for Highly Efficient Dye-Sensitized Solar Cells. Nano Energy 2016, 23, 122–128. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Kang, M.G.; Jun, Y. Metal Substrate based Electrodes for Flexible Dye-sensitized Solar Cells: Fabrication Methods, Progress and Challenges. Chem. Commun. 2013, 49, 11457–11475. [Google Scholar] [CrossRef] [PubMed]
- Balasingam, K.S.; Jun, Y. Recent Progress on Reduced Graphene Oxide-Based Counter Electrodes for Cost-Effective Dye-Sensitized Solar Cells. Isr. J. Chem. 2015, 55, 955–965. [Google Scholar] [CrossRef]
- Ko, K.W.; Lee, M.; Sekhon, S.S.; Balasingam, S.K.; Han, C.-H.; Jun, Y. Efficiency Enhancement of Dye-Sensitized Solar Cells by the Addition of an Oxidizing Agent to the TiO2 Paste. ChemSusChem 2013, 6, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Kim, D.J.; Chi, W.S.; Kim, J.H. Hierarchical Double-Shell Nanostructures of TiO2 Nanosheets on SnO2 Hollow Spheres for High-Efficiency, Solid-State, Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2014, 24, 5037–5044. [Google Scholar] [CrossRef]
- Rui, Y.C.; Xiong, H.; Su, B.; Wang, H.Z.; Zhang, Q.H.; Xu, J.L.; Buschbaum, P.M. Liquid-liquid interface assisted synthesis of SnO2 nanorods with tunable length for enhanced performance in dye-sensitized solar cells. Electrochim. Acta 2017, 227, 49–60. [Google Scholar] [CrossRef]
- Dadkhah, M.; Salavati, N.M. Controlled Synthesis of Tin Dioxide Nanostructures via Two Simple Methods and the Influence on Dye Sensitized Solar Cell. Electrochim. Acta 2014, 129, 62–68. [Google Scholar] [CrossRef]
- Shalan, A.E.; Rasly, M.; Osama, I.; Rashad, M.M.; Ibrahim, I.A. Photocurrent Enhancement by Ni2+ and Zn2+ Ion Doped in SnO2 Nanoparticles in Highly Porous Dye-Sensitized Solar Cells. Ceram. Int. 2014, 40, 11619–11626. [Google Scholar] [CrossRef]
- Wijeratne, K.; Alilavasan, J.; Thelakkat, M.; Bandara, J. Enhancing the Solar Cell Efficiency through Pristine 1-Dimentional SnO2 Nanostructures: Comparison of Charge Transport and Carrier Lifetime of SnO2 Particles vs. Nanorods. Electrochim. Acta 2012, 72, 192–198. [Google Scholar] [CrossRef]
- Xing, J.; Fang, W.Q.; Li, Z.; Yang, G.H. TiO2-Coated Ultrathin SnO2 Nanosheets Used as Photoanodes for Dye-Sensitized Solar Cells with High Efficiency. Ind. Eng. Chem. Res. 2012, 51, 4247–4253. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, H.; Li, Z.; Amal, R.; Lu, G.Q.; Wang, L.Z. In Situ Growth of a ZnO Nanowire Network within a TiO2 Nanoparticle Film for Enhanced Dye-Sensitized Solar Cell Performance. Adv. Mater. 2012, 24, 5850–5856. [Google Scholar] [CrossRef] [PubMed]
- Balasingam, S.K.; Lee, M.; Kang, M.G.; Jun, Y. Improvement of Dye-Sensitized Solar Cells toward the Broader Light Harvesting of the Solar Spectrum. Chem. Commun. 2013, 49, 1471–1487. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.H.; Ren, H.; Hessel, C.M.; Wang, J.Y.; Yu, R.B.; Jin, Q.; Yang, M.; Hu, Z.D.; Chen, Y.F.; Tang, Z.Y.; et al. Quintuple-shelled SnO2 Hollow Microspheres with Superior Light Scattering for High-Performance Dye-Sensitized Solar Cells. Adv. Mater. 2014, 26, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.C.; Sabba, D.; Mathews, N.; Wong, L.H.; Lam, Y.M.; Mhaisalkar, S. Hydrothermal Synthesis of High Electron Mobility Zn-doped SnO2 Nanoflowers as Photoanode Material for Efficient Dye-Sensitized Solar Cells. Chem. Mater. 2011, 23, 3938–3945. [Google Scholar] [CrossRef]
- Cho, C.Y.; Baek, S.; Kim, K.; Moon, J.K. 3D bicontinuous SnO2/TiO2 Core/Shell Structures for Highly Efficient Organic Dye-Sensitized Solar Cell Electrodes. RSC Adv. 2016, 6, 74003–74008. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Zhao, J.X.; Bi, S.Q.; Tao, X.; Huang, M.L.; Chen, J.F. Dual Interfacial Modifications of Hierarchically Structured Iodine-doped ZnO Photoanodes for High-Efficiency Dye-sensitized Solar Cells. Electrochim. Acta 2015, 157, 258–265. [Google Scholar] [CrossRef]
- Wali, Q.; Fakharuddin, A.; Ahmed, I.; Rahim, M.H.A.; Ismail, J.; Jose, R. Multiporous nanofibers of SnO2 by electrospinning for high efficiency dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 17427–17434. [Google Scholar] [CrossRef]
- Bai, Y.; Xing, Z.; Yu, H.; Li, Z.; Amal, R.; Wang, L.Z. Porous titania nanosheet/nanoparticle hybrids as photoanodes for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 12058–12065. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.S.; Yuan, Z.H.; Duan, Y.Q.; Bie, L.J. TiO2 coated SnO2 Nanosheet Flms for Dye-Sensitized Solar Cells. Thin Solid Films 2011, 519, 5645–5648. [Google Scholar] [CrossRef]
- Bhande, S.S.; Shinde, D.V.; Tehare, K.K.; Patil, S.A.; Mane, R.S.; Naushad, M.; Alothman, Z.; Hui, K.N.; Han, S.H. DSSCs Synergic Effect in Thin Metal Oxide Layer-Functionalized SnO2 Photoanodes. J. Photochem. Photobiol. A Chem. 2014, 295, 64–69. [Google Scholar] [CrossRef]
- Wang, Y.J.; Tian, J.J.; Fei, C.B.; Lv, L.L.; Liu, X.G.; Zhao, Z.X.; Cao, G.Z. Microwave-Assisted Synthesis of SnO2 Nanosheets Photoanodes for Dye-Sensitized Solar Cells. Phys. Chem. C 2014, 118, 25931–25938. [Google Scholar] [CrossRef]
- Yang, S.; Hou, Y.; Xing, J.; Zhang, B.; Tian, F.; Yang, X.H.; Yang, H.G. Ultrathin SnO2 scaffolds for TiO2-based Heterojunction Photoanodes in Dye-Sensitized Solar Cells: Oriented Charge Transport and Improved Light Scattering. Chem. Eur. J. 2013, 19, 9366–9370. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.H.; Zheng, Y.Z.; Bi, S.Q.; Wang, Y.; Tao, X.; Dai, L.M.; Chen, J.F. Multidimensional ZnO Architecture for Dye-Sensitized Solar Cells with High-Efficiency up to 7.35%. Adv. Energy Mater. 2014, 4, 1301802. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, J.W.; Hou, Y.F.; Yu, X.Y.; Su, C.Y.; Kuang, D.B. Hierarchical Tin Oxide Octahedra for Highly Efficient Dye-Sensitized Solar Cells. Chem. Eur. J. 2010, 16, 8620–8625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.R.; Xie, Y.; Dong, T.; Zhang, Z.G. Oxidation-Crystallization Process of Colloids: An Effective Approach for the Morphology Controllable Synthesis of SnO2 Hollow Spheres and Rod Bundles. J. Phys. Chem. C 2007, 111, 11598–11603. [Google Scholar] [CrossRef]
- Wang, D.T.; Liu, S.H.; Shao, M.F.; Li, Q.Y.; Gu, Y.K.; Zhao, J.H.; Zhang, X.X.; Zhao, J.S.; Fang, Y.Z. Aqueous Solution-Processed Multifunctional SnO2 Aggregates for Highly Efficient Dye-Sensitized Solar Cells. Ind. Eng. Chem. Res. 2018, 57, 7136–7145. [Google Scholar] [CrossRef]
- Roh, K.M.; Kim, S.K.; Choi, J.H. Synergetic Effect of Graphene Sheet and Three-Dimensional Crumpled Graphene on the Performance of Dye-Sensitized Solar Cells. AIChE J. 2016, 62, 574–579. [Google Scholar] [CrossRef]
- Xu, X.Q.; Qiao, F.J.; Dang, L.Y.; Lu, Q.Y.; Gao, F. Porous Tin Oxide Nanosheets with Enhanced Conversion Efficiency as Dye-Sensitized Solar Cell Electrode. J. Phys. Chem. C 2014, 118, 16856–16862. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, L.; Song, W.L.; Qin, Z.Y.; Zeng, D.W.; Xie, C.S. In situ synthesis of C-TiO2/g-C3N4 heterojunction nanocomposite as highly visible light active photocatalyst originated from effective interfacial charge transfer. Appl. Catal. B Environ. 2017, 202, 489–499. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.Y.; Chen, J.R.; Cheng, L.; Chang, J.; Sheng, W.C.; Hu, C.Y.; Cao, S.S. C-doped hollow TiO2 spheres: In situ synthesis, controlled shell thickness, and superior visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 715–722. [Google Scholar] [CrossRef]
- Gubbala, S.; Russell, H.B.; Shah, H.; Deb, B.; Jasinski, J.; Rypkemac, H.; Sunkara, M.K. Surface Properties of SnO2 Nanowires for Enhanced Performance with Dye-Sensitized Solar Cells. Energy Environ. Sci. 2009, 2, 1302–1309. [Google Scholar] [CrossRef]
- Gubbala, S.; Chakrapani, V.; Kumar, V.; Sunkara, M.K. Band-Edge Engineered Hybrid Structures for Dye-Sensitized Solar Cells Based on SnO2 Nanowires. Adv. Funct. Mater. 2008, 18, 2411–2418. [Google Scholar] [CrossRef]
- Cahen, D.; Hodes, G.; Grätzel, M.; Guillemoles, J.F.; Riess, I. Nature of Photovoltaic Action in Dye-Sensitized Solar Cells. J. Phys. Chem. B 2000, 104, 2053–2059. [Google Scholar] [CrossRef]
- Lee, M.; Balasingam, K.S.; Ko, Y.; Jeong, H.Y.; Min, B.K.; Yun, Y.J.; Jun, Y. Graphene Modified Vanadium pentoxide Nanobelts as an Efficient Counter Electrode for Dye-Sensitized Solar Cells. Synth. Met. 2016, 215, 110–115. [Google Scholar] [CrossRef]
- Adachi, M.; Sakamoto, M.; Jiu, J.; Ogata, Y.; Isoda, S. Determination of Parameters of Electron Transport in Dye-Sensitized Solar Cells Using Electrochemical Impedance Spectroscopy. J. Phys. Chem. B 2006, 110, 13872–13880. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Ding, H.Y.; Liu, Y.; Tao, X.; Cao, G.Z.; Chen, J.F. In situ Hydrothermal Growth of Hierarchical ZnO Nanourchin for High-Efficiency Dye-Sensitized Solar Cells. J. Power Sources 2014, 254, 153–160. [Google Scholar] [CrossRef]
- Wang, D.T.; Wang, W.X.; Ma, X.Y.; Zhang, C.; Zhao, J.S.; Zhang, X.X. Comparative Study on the Influence of TiO2 Precursors on ZnO-Based Dye-Sensitized Solar Cells. Ind. Eng. Chem. Res. 2015, 54, 12639–12645. [Google Scholar] [CrossRef]
- Tian, J.J.; Zhang, Q.F.; Zhang, L.L.; Gao, R.; Shen, L.F.; Zhang, S.G.; Qu, X.H.; Cao, G.Z. ZnO/TiO2 Nanocable Structured Photoelectrodes for CdS/CdSe Quantum Dot Co-Sensitized Solar Cells. Nanoscale 2013, 5, 4362–4369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.F.; Chou, T.P.; Russo, B.; Jenekhe, S.A.; Cao, G.Z. Aggregation of ZnO Nanocrystallites for High Conversion Efficiency in Dye-Sensitized Solar Cells. Angew. Chem. 2008, 120, 2436–2440. [Google Scholar] [CrossRef]
- Zhang, X.L.; Huang, W.C.; Gu, A.N.; Xiang, W.C.; Huang, F.Z.; Guo, Z.X.; Cheng, Y.B.; Spiccia, L. High Efficiency Solid-State Dye-Sensitized Solar Cells Using a Cobalt(II/III) Redox Mediator. J. Mater. Chem. C 2017, 5, 4875–4883. [Google Scholar] [CrossRef]
- Zhu, P.N.; Reddy, M.V.; Wu, Y.Z.; Peng, S.J.; Yang, S.Y.; Nair, A.S.; Lohd, K.P.; Chowdari, B.V.R.; Ramakrishna, S. Mesoporous SnO2 Agglomerates with Hierarchical Structures as An Efficient Dual-Functional Material for Dye-Sensitized Solar Cells. Chem. Commun. 2012, 48, 10865–10867. [Google Scholar] [CrossRef] [PubMed]


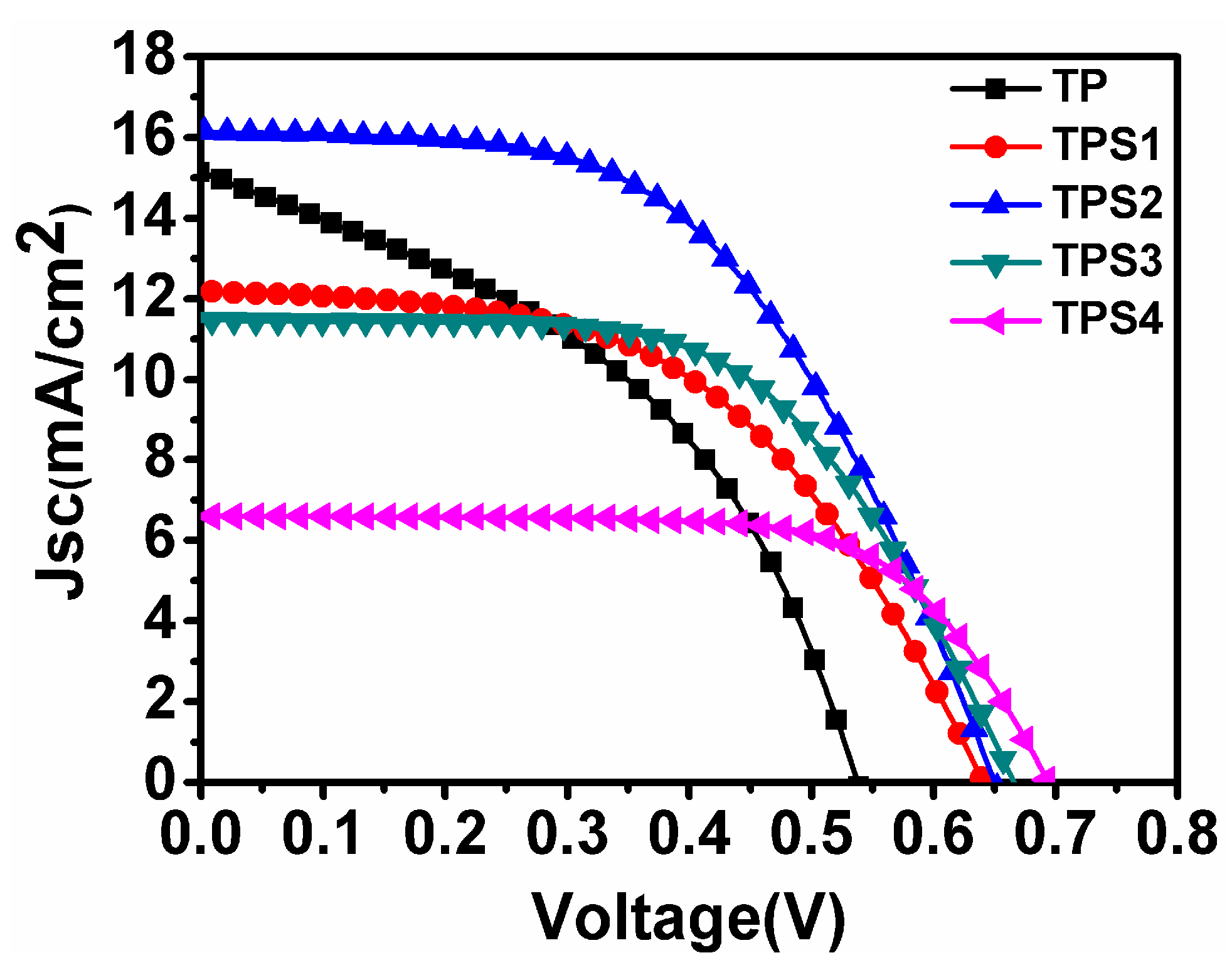
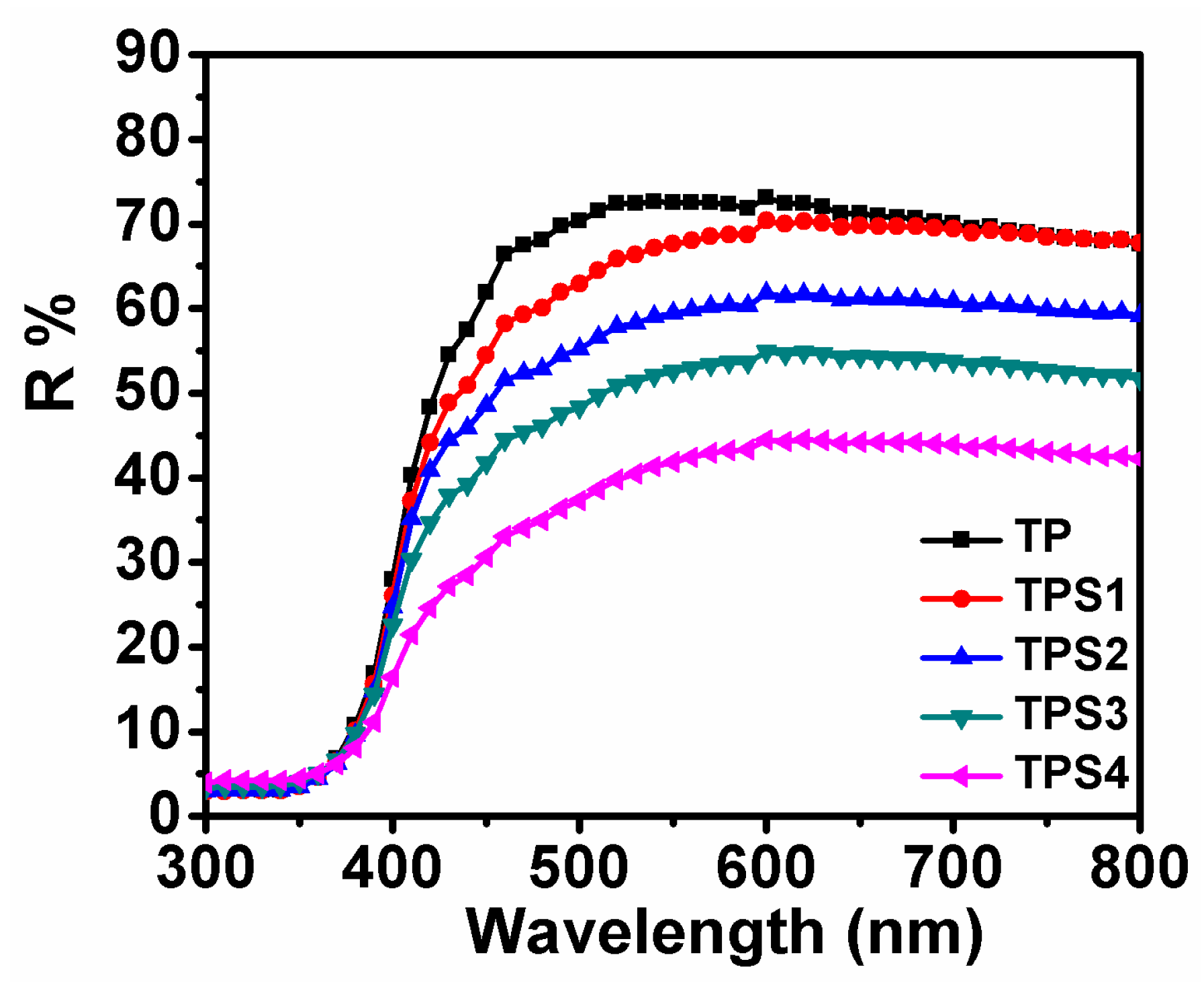
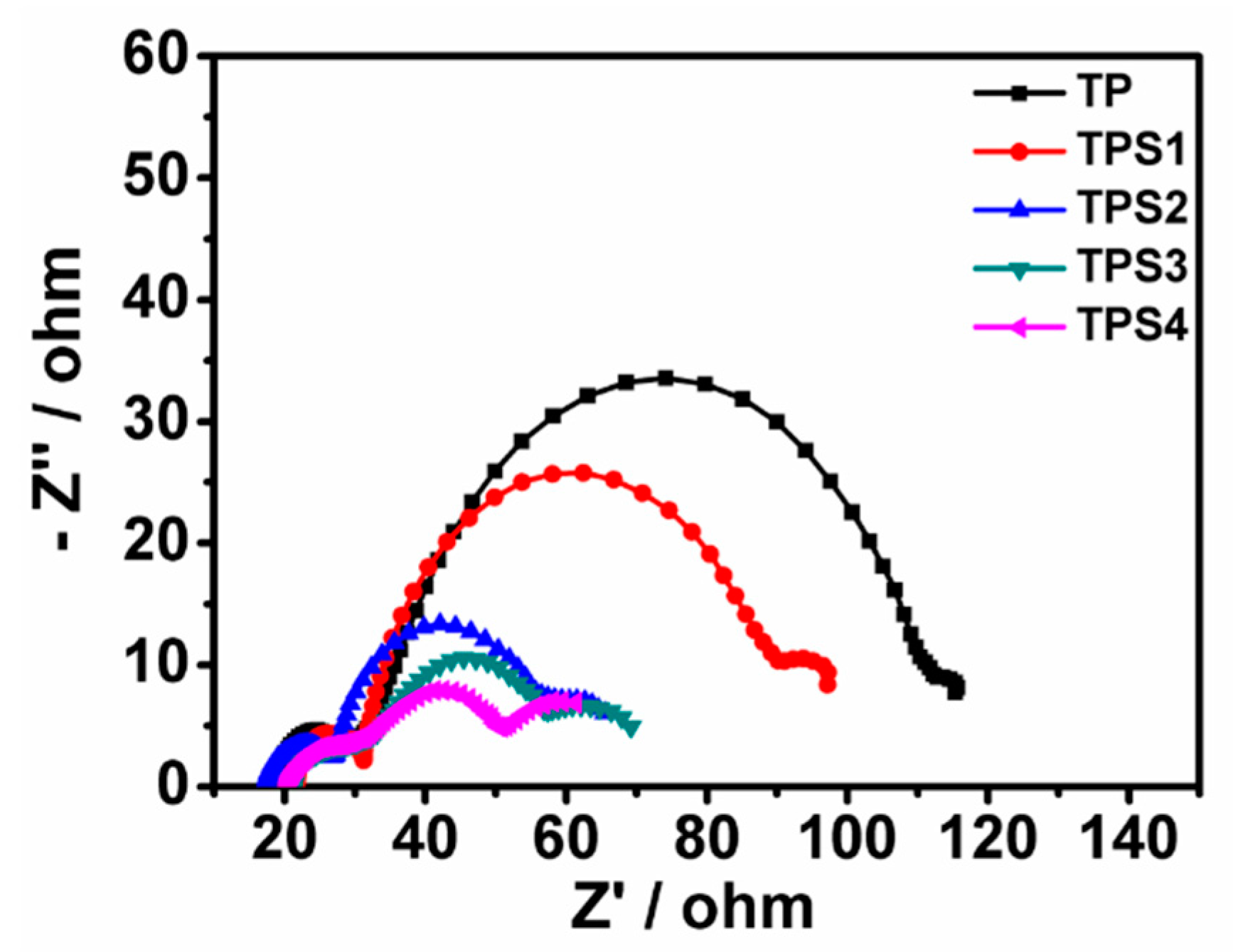
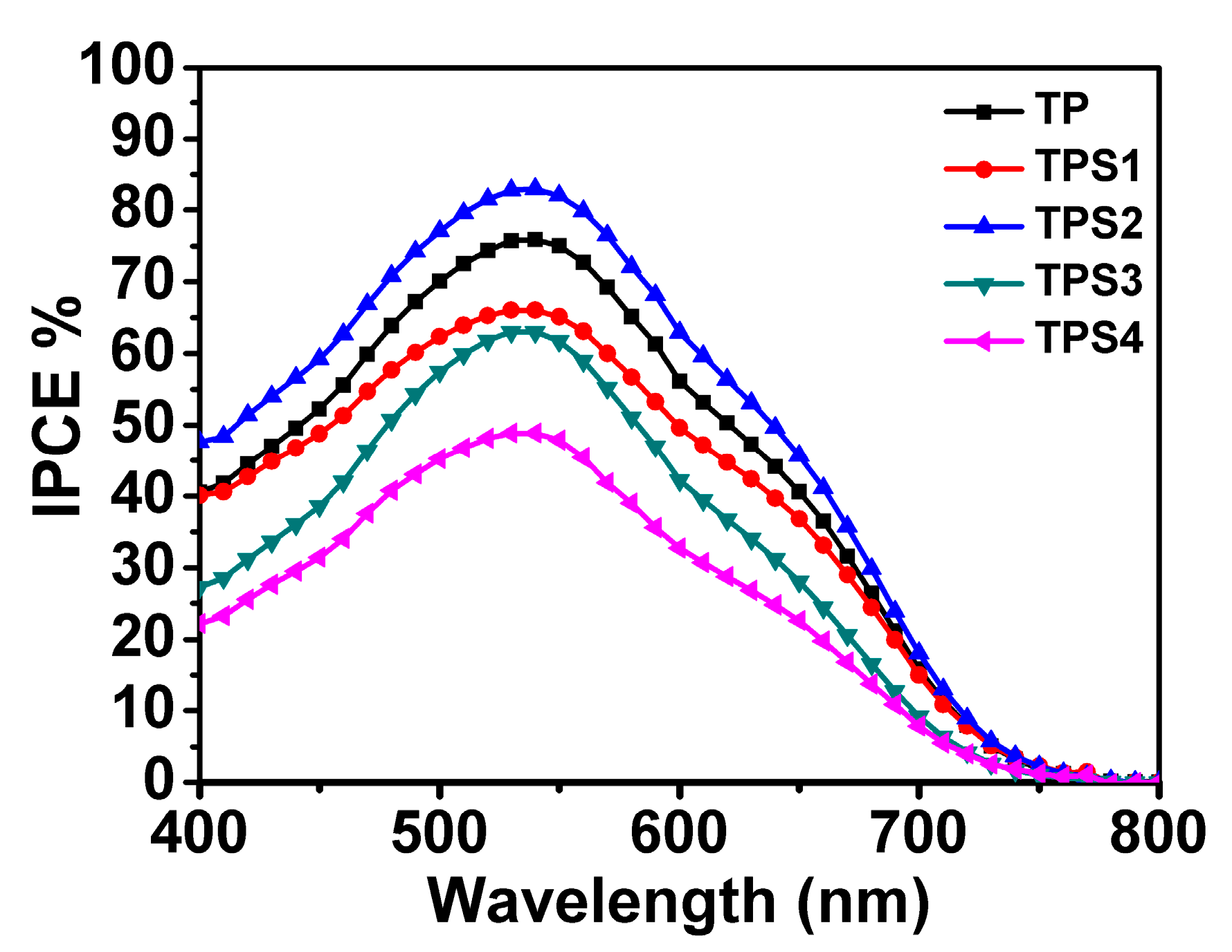
| Sample | JSC (mA/cm²) | VOC (V) | FF | PCE | Adsorbed Dye (×10−7 mol·cm−2) | Rct |
|---|---|---|---|---|---|---|
| TP | 15.12 | 0.537 | 43.1 | 3.50 | 1.94 | 38.9 |
| TPS1 | 14.61 | 0.640 | 51.6 | 4.85 | 2.23 | 27.8 |
| TPS2 | 16.14 | 0.650 | 53.3 | 5.59 | 2.07 | 18.5 |
| TPS3 | 11.46 | 0.665 | 58.8 | 4.48 | 1.76 | 11.6 |
| TPS4 | 6.59 | 0.693 | 68.1 | 3.11 | 1.15 | 9.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, S.; Shao, M.; Zhao, J.; Gu, Y.; Li, Q.; Zhang, X.; Zhao, J.; Fang, Y. Design of SnO2 Aggregate/Nanosheet Composite Structures Based on Function-Matching Strategy for Enhanced Dye-Sensitized Solar Cell Performance. Materials 2018, 11, 1774. https://doi.org/10.3390/ma11091774
Wang D, Liu S, Shao M, Zhao J, Gu Y, Li Q, Zhang X, Zhao J, Fang Y. Design of SnO2 Aggregate/Nanosheet Composite Structures Based on Function-Matching Strategy for Enhanced Dye-Sensitized Solar Cell Performance. Materials. 2018; 11(9):1774. https://doi.org/10.3390/ma11091774
Chicago/Turabian StyleWang, Dongting, Shangheng Liu, Mingfa Shao, Jinghan Zhao, Yukun Gu, Qiuyi Li, Xianxi Zhang, Jinsheng Zhao, and Yuzhen Fang. 2018. "Design of SnO2 Aggregate/Nanosheet Composite Structures Based on Function-Matching Strategy for Enhanced Dye-Sensitized Solar Cell Performance" Materials 11, no. 9: 1774. https://doi.org/10.3390/ma11091774
APA StyleWang, D., Liu, S., Shao, M., Zhao, J., Gu, Y., Li, Q., Zhang, X., Zhao, J., & Fang, Y. (2018). Design of SnO2 Aggregate/Nanosheet Composite Structures Based on Function-Matching Strategy for Enhanced Dye-Sensitized Solar Cell Performance. Materials, 11(9), 1774. https://doi.org/10.3390/ma11091774