A Facile Synthesis Procedure for Sulfonated Aniline Oligomers with Distinct Microstructures
Abstract
1. Introduction
2. Results and Discussion
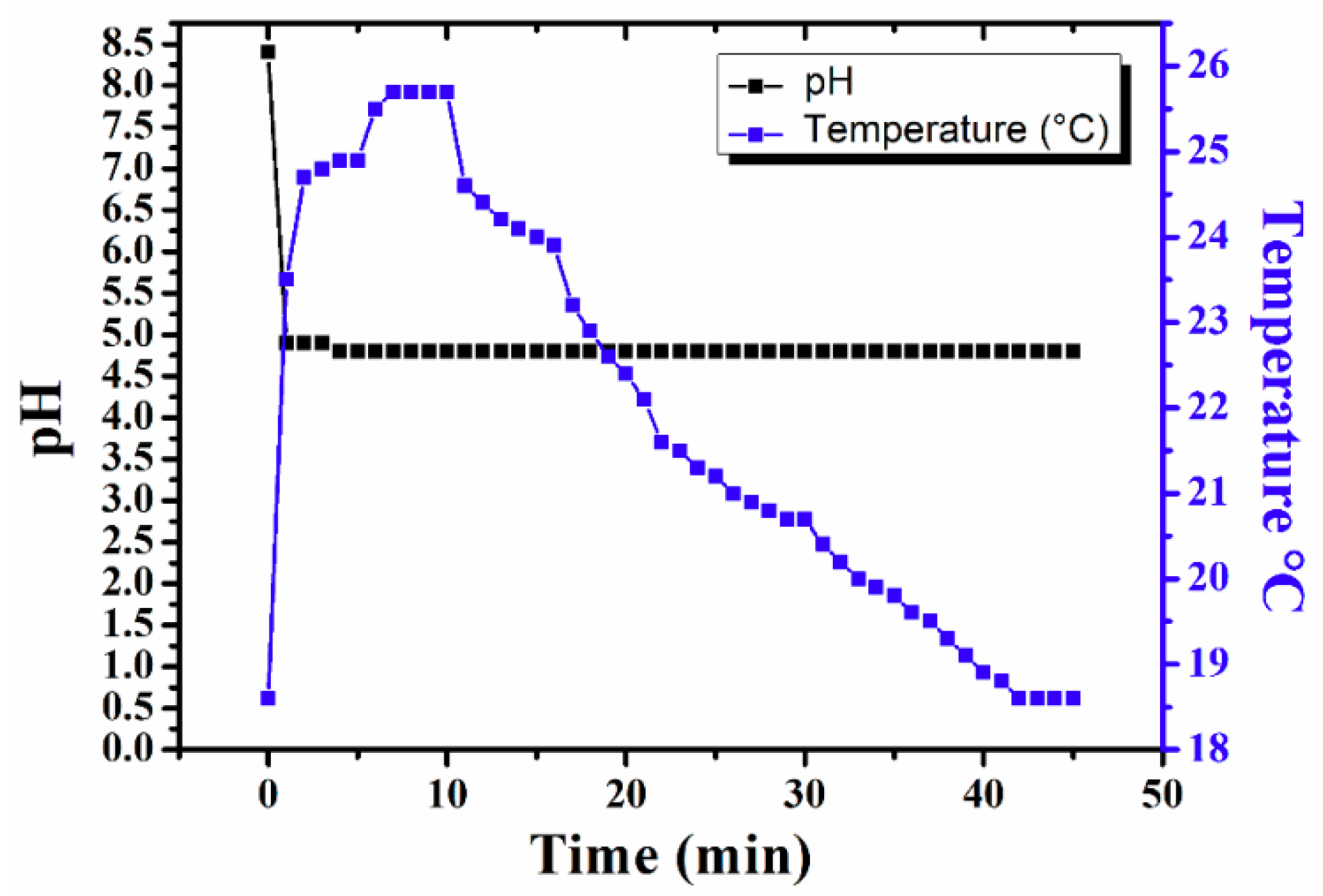
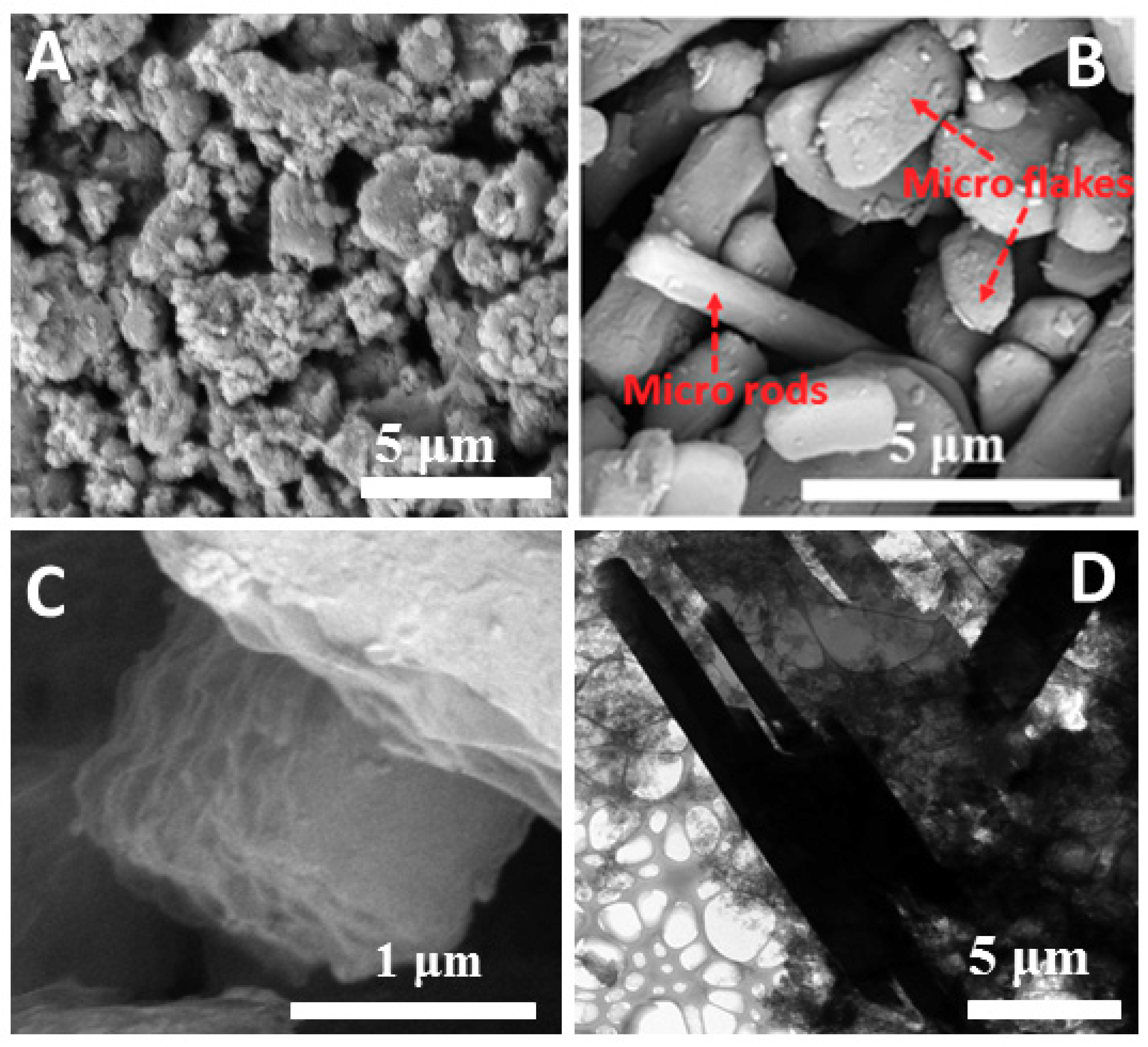
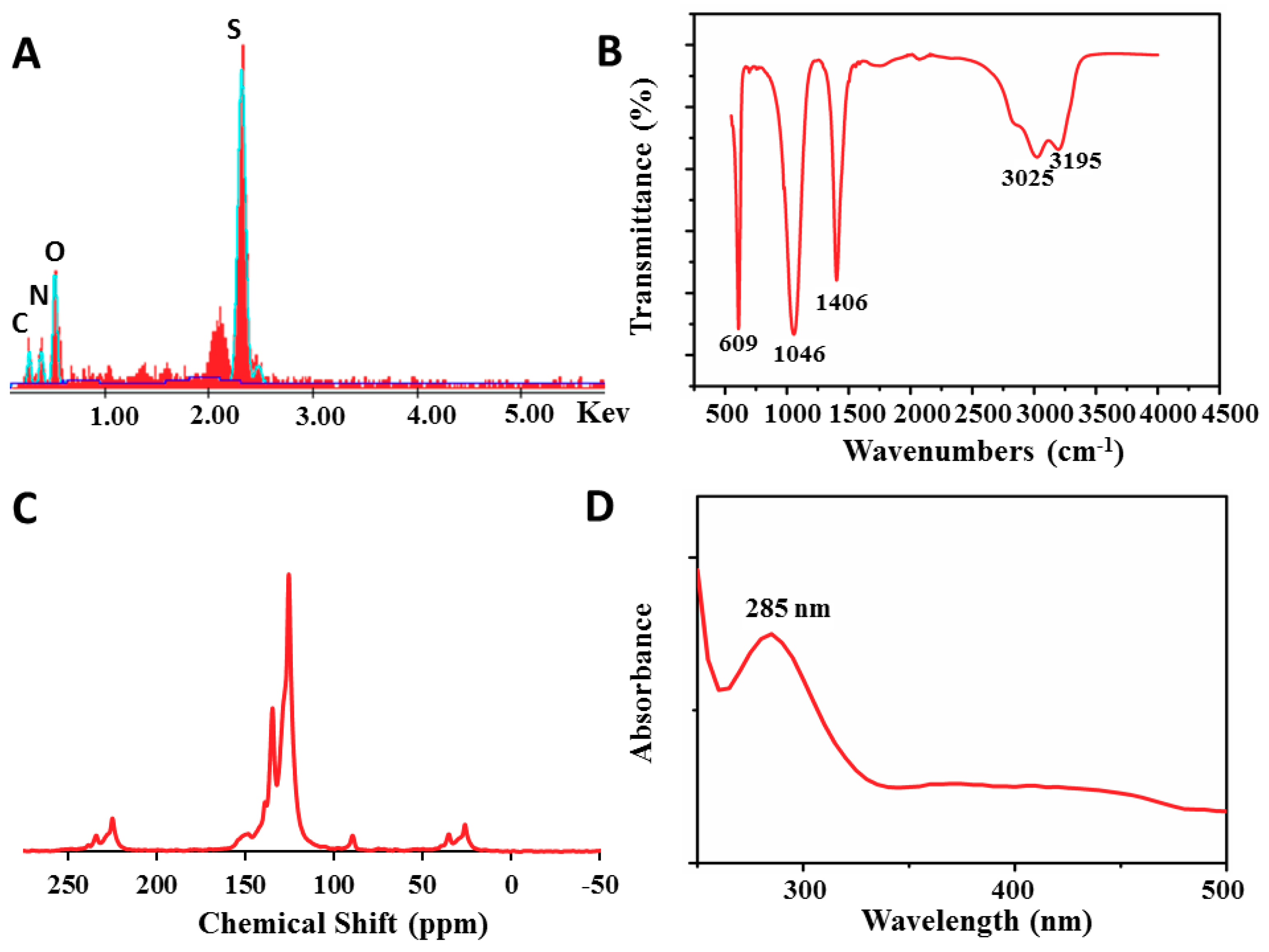
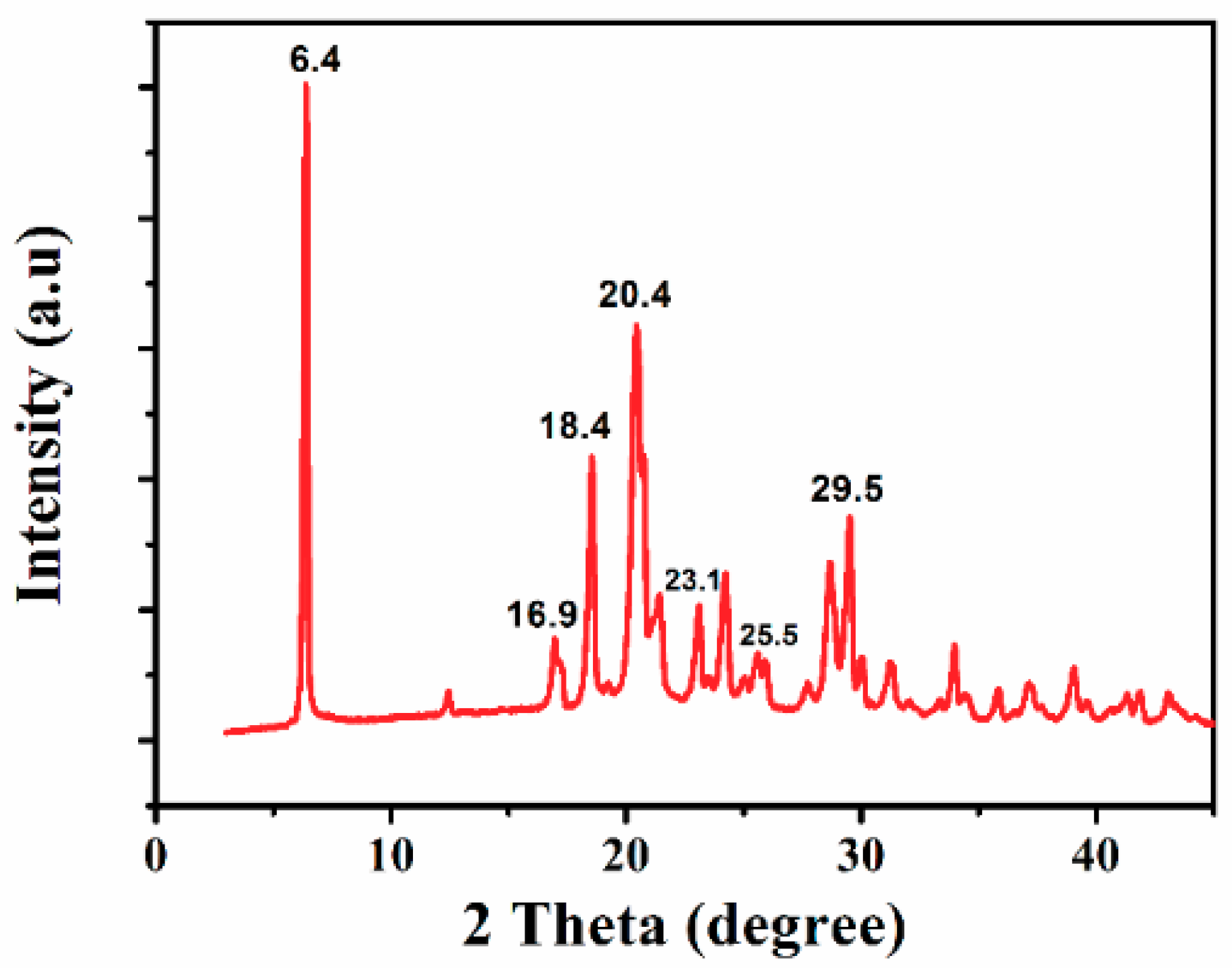
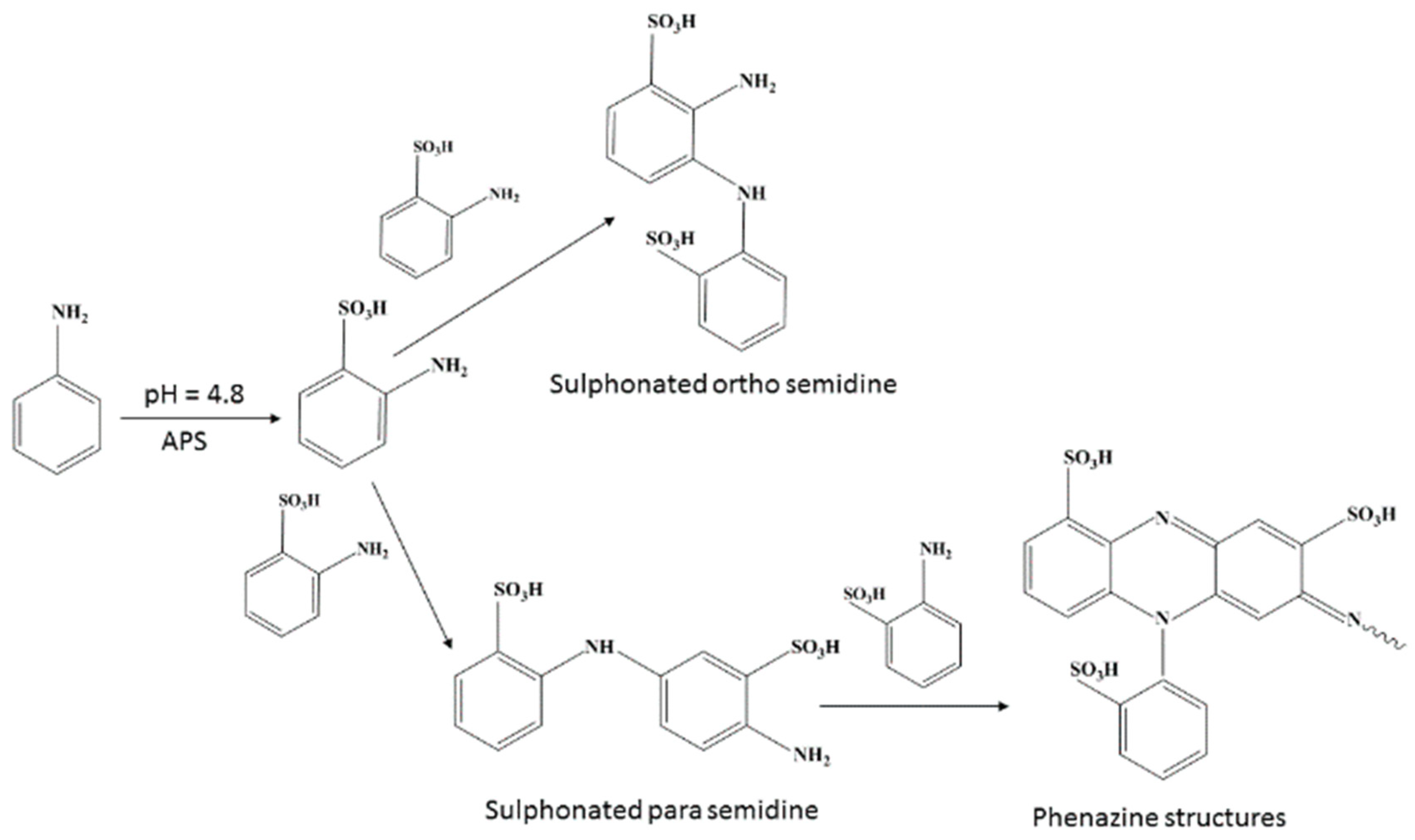
2.1. Formation of Sulfonated Aniline Oligomers (SAO)
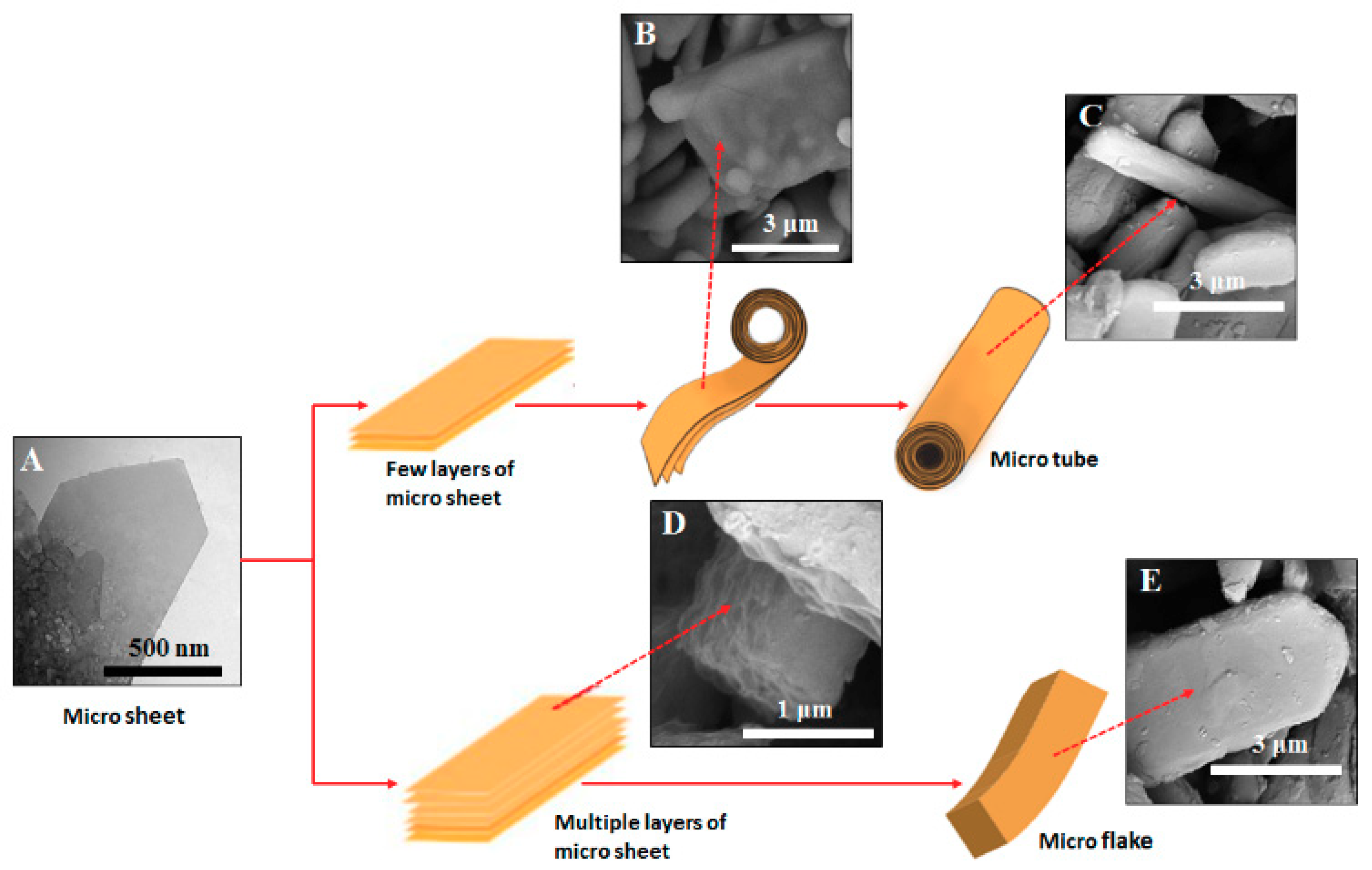
2.2. Mechanism of Microstructure Formation
3. Materials and Methods
3.1. Materials
3.2. Methods
Preparation of Sulfonated PANI Oligomers
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yue, J.; Epstein, A.J. Synthesis of self-doped conducting polyaniline. J. Am. Chem. Soc. 1990, 112, 2800–2801. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Dev. 2006, 1, 225–255. [Google Scholar]
- Epstein, A.J.; Ginder, J.M.; Zuo, F.; Woo, H.S.; Tanner, D.B.; Richter, A.F.; Angelopoulos, M.; Huang, W.S.; MacDiarmid, A.G. Insulator-to-metal transitionin polyaniline: Effect of protonation in emaraldine. Synth. Met. 1987, 21, 63–70. [Google Scholar] [CrossRef]
- Gvozdenović, M.; Jugović, B.; Jambrec, D.; Stevanović, J.; Grgur, B. Application of polyaniline in corrosion protection of metals. Zaš Mater. 2012, 53, 353–360. [Google Scholar]
- Ghanbari, K.; Mousavi, M.; Shamsipur, M. Preparation of plyaniline nanofibers and their use as a cathode of aquous rechargable batteries. Electrochim. Acta 2006, 52, 1514–1522. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Jussila, S.; Puustinen, M.; Hassinen, T.; Olkkonen, J.; Sandberg, H.G.; Solehmainen, K. Self-alligned patterning method of poly (aniline) for organic field-effect transistor gate electrode. Org. Electron. 2012, 13, 1308–1314. [Google Scholar] [CrossRef]
- Yang, Y.; Heeger, A. Polyaniline as a transparent electrode for polymer light-emiting didodes: Lower operating voltage and higher efficiency. Appl. Phys. Lett. 1994, 64, 1245–1247. [Google Scholar] [CrossRef]
- Lim, K.G.; Ahn, S.; Kim, H.; Choi, M.R.; Huh, D.H.; Lee, T.W. Self-Doped conducting polymer as a hole-extraction layer in organic-inorganic hybrid perovskite solar cells. Adv. Mater. Interfaces 2016, 3, 1500678. [Google Scholar] [CrossRef]
- Zujovic, Z.; Webber, A.L.; Travas-Sejdic, J.; Brown, S.P. Self-assembled oligoanilinic nanosheets: Molecular structure revealed by solid-state NMR spectroscopy. Macromolecules 2015, 48, 8838–8843. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Stejskal, J. The effect of pH on the oxidative polymerization of aniline and the morphology and the properties of the products. Russ. Chem. Rev. 2010, 79, 1123–1143. [Google Scholar] [CrossRef]
- Huang, Y.F.; Lin, C.W. Introduction of methanol in the formation of polyaniline nanotubes in an acid-free aquous solution through a self-curling process. Polymer 2009, 50, 775–782. [Google Scholar] [CrossRef]
- Konyushenko, E.; Trchová, M.; Stejskal, J.; Sapurina, I. The role of acidity profile in the nanotubular growth of polyaniline. Chem. Pap. 2010, 64, 56–64. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchová, M. Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog. Polym. Sci. 2010, 35, 1420–1481. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in the preparation, processing and application of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Coltevieille, D.; Le Méhauté, A.; Challioui, C.; Mirebeau, P.; Demay, J.N. Industrial applications of polyaniline. Synth. Met. 1999, 101, 703–704. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Hou, C.; Qiao, M.; Chen, Y.; Zhang, H.; Zhang, Q. Multidimensional polyaniline structures from micellar templates. J. Mater. Sci. 2017, 52, 2995–3002. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Z.H.; Cromack, K.R.; Epstein, A.J.; MacDiarmid, A.G. Effect of the sulfonic acid group on polyaniline backbone. J. Am. Chem. Soc. 1991, 113, 2665–2671. [Google Scholar] [CrossRef]
- Yue, J.; Epstein, A.; Zhong, Z.; Gallagher, P.; MacDiarmid, A. Thermal stabilities of polyanilines. Synth. Met. 1991, 41, 765–768. [Google Scholar] [CrossRef]
- Shimizu, S.; Saitoh, T.; Uzawa, M.; Yuasa, M.; Yano, K.; Maruyama, T.; Watanabe, K. Synthesis and applications of sulfonated polyaniline. Synth. Met. 1997, 85, 1337–1338. [Google Scholar] [CrossRef]
- Liao, Y.; Strong, V.; Chian, W.; Wang, X.; Li, X.G.; Kaner, R.B. Sulfonated polyaniline nanostructures synthesized via rapid initiated copolymerization with controllable morphology, size, and electrical properties. Macromolecules 2012, 45, 1570–1579. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Xue, Z.; Guo, D. Spectroscopic and electrical characterization of some aniline oligomers and polyaniline. Synth. Met. 1986, 16, 305–315. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, J.; MacDiarmid, A.; Epstein, A. Synthesis of oligomeric aniline. Synth. Met. 1997, 84, 119–120. [Google Scholar] [CrossRef]
- Wienk, M.; Janssen, R. High-spin cation radicals of meta-para aniline oligomers. J. Am. Chem. Soc. 1997, 119, 4492–4501. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M. Aniline oligomers versus polyaniline. Polym. Int. 2012, 61, 240–251. [Google Scholar] [CrossRef]
- Zujovic, Z.D.; Laslau, C.; Bowmaker, G.A.; Kilmartin, P.A.; Webber, A.L.; Brown, S.P.; Travas-Sejdic, J. Role of aniline oligomeric nanosheets in the formation of polyaniline nanotubes. Macromolecules 1990, 43, 662–670. [Google Scholar] [CrossRef]
- Zujovic, Z.D.; Laslau, C.; Travas-Sejdic, J. Lamella-structured nanoflakes comprised of stacked oligoaniline nanosheets. Chem. Asian J. 2011, 6, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.; Al-Saigh, Z.Y. Surface and thermodynamic characterization of conducting polymers by inverse gas chromotography: I. Polyaniline. J. Chromatogr. A 2002, 969, 229–243. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, T.; Kan, J. Effect of methanol on morphology of polyaniline. Eur. Polym. J. 2007, 43, 395–402. [Google Scholar] [CrossRef]
- Kan, J.; Lv, R.; Zhang, S. Effect of ethanol on properties of electrochemically synthesized polyaniline. Synth. Met. 2004, 145, 37–42. [Google Scholar] [CrossRef]
- Hussain, A.A.; Sharma, S.; Pal, A.R.; Bailung, H.; Chutia, J.; Patil, D.S. Role of plasma parameters on the conjugatd structure retention in polyaniline thin film. Plasma Chem. Plasma Process 2012, 32, 817–832. [Google Scholar] [CrossRef]
- Kumar, R.; Mamlouk, M.; Scott, K. Sulfonated polyether ether ketone-sulfonated graphene oxide composite membranes for polymer electrolyte fuel cells. RSC Adv. 2014, 4, 617–623. [Google Scholar] [CrossRef]
- Viva, F.; Andrade, E.; Molina, F.; Florit, M. Electropolymerization of 2-methoxy aniline. Electrochemical and spectroscopical product characterization. J. Electroanal. Chem. 1999, 471, 180–189. [Google Scholar] [CrossRef]
- Trchová, M.; Šedenková, I.; Konyushenko, E.N.; Stejskal, J.; Holler, P.; Ciric-Marjanovic, G. Evolution of polyaniline nanotubes: The oxidation of aniline in water. J. Phys. Chem. B 2006, 110, 9461–9468. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.; Phan, T.; Nguyen, D. Synthesis and characterization of polyaniline nanoparticles in phosphonic acid amphiphile aquous micellar solutions for waterborne corrosion protection coating. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1606–1616. [Google Scholar] [CrossRef]
- Júnior, A.; Santos, J.H.; Bertuol, D.A.; Meneguzzi, A.; Ferreira, C.A.; Amado, F.D.R. Cater oil and commercial thermoplastic polyurethane membranes modified with polyaniline: A comparative study. Mater. Res. 2013, 16, 860–866. [Google Scholar] [CrossRef]
- Rao, P.S.; Sathyanarayana, D.N.; Palaniappan, S. Polymerization of aniline in an organic peroxide system by the inverted emulsion process. Macromolecules 2002, 35, 4988–4996. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Lee, D. Preparation of a sulfonated carbonaceous material from lignosulfonate and its usefulness as an esterification catalysts. Molecules 2013, 18, 8168–8180. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Chong, J.H.; Majid, E.; Liu, Y.; Hrapovic, S.; Leung, A.C.W.; Luong, J.H.T. Carbocatalytic dehydration of xylose to furfural in water. Carbon 2012, 50, 1033–1043. [Google Scholar] [CrossRef]
- Díaz, F.R.; del Valle, M.A.; Borrego, E.; Gacitúa, M.A.; Camarada, M.; Antilén, M.P.; Del Río, R.; Arteaga, G.C. Electro-synthesis and characterization of O-anisidine Oligomers. Int. J. Electrochem. Sci. 2009, 7, 2552–2565. [Google Scholar]
- Saluja, P.; Chaudhary, A.; Khurana, J.M. Synthesis of novel fluorescent benzo [a] pyrano [2, 3-c] phenazine and benzo [a] chromeno [2, 3-c] phenazine derivatives via facile four-component domino protocol. Tetrahedron Lett. 2017, 55, 3431–3435. [Google Scholar] [CrossRef]
- Venancio, E.C.; Wang, P.C.; MacDiarmid, A. The azanes: A class of material incorporating nano/micro self-assembled hollow spheres obtained by aquous oxidative polymerization of aniline. Synth. Met. 2016, 156, 357–369. [Google Scholar] [CrossRef]
- Angélica del Valle, M.; Díaz, F.R.; Bodini, M.E.; Alfonso, G.; Soto, G.M.; Borrego, E.D. Electrosynthesis and characterization of O-phenylenediamine oligomers. Polym. Int. 2005, 54, 526–532. [Google Scholar] [CrossRef]
- Jana, T.; Chatterjee, J.; Nandi, A.K. Sulfonic acid doped thermoreversible polyaniline gels. 3. Structural investigation. Langmuir 2002, 18, 5720–5727. [Google Scholar] [CrossRef]
- Zhang, L.; Long, Y.; Chen, Z.; Wan, M. The effect of hydrogen bonding on self-assembled polyaniline nanostructures. Adv. Funct. Mater. 2004, 14, 693–698. [Google Scholar] [CrossRef]
- Liu, W.; Yan, X.; Chen, J.; Feng, Y.; Xue, Q. Novel and high-performance asymmetric micro- supercapacitors based on graphene quantum dots and polyaniline nanofibers. Nanoscale 2013, 5, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Liu, N.; Lu, X.; Zhang, W. Novel micro/nanostructures of polyaniline in the presence of different amino acids via a self-assembly process. Chem. Lett. 2007, 36, 1288–1289. [Google Scholar] [CrossRef]
- Prathap, M.U.A.; Srivastava, R. Morphological controlled synthesis of mocro-/nano-polyaniline. J. Polym. Res. 2011, 18, 2455–2467. [Google Scholar] [CrossRef]
- Belaabed, B.; Wojkiewicz, J.L.; Lamouri, El.-K.N.; Redon, N. Thermomechanical behaviors and dielectric properties of polyaniline-doped para-toluene sulfonic acid/epoxy resin composites. Polym. Adv. Technol. 2012, 23, 1194–1201. [Google Scholar] [CrossRef]
- Křiž, J.; Starovoytova, L.; Trchová, M.; Konyushenko, E.N.; Stejskal, J. NMR investigation of aniline oligomers produced in the early stages of oxidative polymerization of aniline. J. Phys. Chem. B 2009, 113, 6666–6673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zujovic, Z.D.; Peng, H.; Bowmaker, G.A.; Kilmartin, P.A.; Travas-Sejdic, J. Structural characterization of polyaniline nanotubes synthesized from different buffer solutions. J. Macromol. 2008, 41, 8877–8884. [Google Scholar] [CrossRef]
- Viva, F.; Andrade, E.; Florit, M.; Molina, F. Electropolymerization of 2-methoxyaniline. Polymerization kinetics and phenazineinsertion at low monomer concentration. Phys. Chem. Chem. Phys. 2002, 4, 2293–2300. [Google Scholar] [CrossRef]
| Aniline Concentration (mM) | APS Concentration (mM) | Products Formed | Medium | Structures Formed | Reference | ||
|---|---|---|---|---|---|---|---|
| Aggregates | Flakes (μm) | Rods/Tubes (μm) | |||||
| 0.20 | 0.25 | PANI | Water | Shapeless aggregates | - | - | [12] |
| 0.20 | 0.25 | PANI | Methanol | - | - | Tube OD = 0.2 | [12] |
| 200 | 25 | AO | Water | Shapeless aggregates | - | - | [13] |
| 24 | 24 | AO | Water | - | L = 1.5 W = 1.0 T = 0.01 | - | [27] |
| 50 | 5 | SAO | Water | Shapeless aggregates | - | - | This study |
| 50 | 5 | SAO | Ethanol | - | L = 3.0 W = 2.0 T = 0.4 | Rod L = 5.0 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunagaran, R.; Coghlan, C.; Tran, D.; Tung, T.T.; Burgun, A.; Doonan, C.; Losic, D. A Facile Synthesis Procedure for Sulfonated Aniline Oligomers with Distinct Microstructures. Materials 2018, 11, 1755. https://doi.org/10.3390/ma11091755
Karunagaran R, Coghlan C, Tran D, Tung TT, Burgun A, Doonan C, Losic D. A Facile Synthesis Procedure for Sulfonated Aniline Oligomers with Distinct Microstructures. Materials. 2018; 11(9):1755. https://doi.org/10.3390/ma11091755
Chicago/Turabian StyleKarunagaran, Ramesh, Campbell Coghlan, Diana Tran, Tran Thanh Tung, Alexandre Burgun, Christian Doonan, and Dusan Losic. 2018. "A Facile Synthesis Procedure for Sulfonated Aniline Oligomers with Distinct Microstructures" Materials 11, no. 9: 1755. https://doi.org/10.3390/ma11091755
APA StyleKarunagaran, R., Coghlan, C., Tran, D., Tung, T. T., Burgun, A., Doonan, C., & Losic, D. (2018). A Facile Synthesis Procedure for Sulfonated Aniline Oligomers with Distinct Microstructures. Materials, 11(9), 1755. https://doi.org/10.3390/ma11091755







