Crystalline/Amorphous Blend Identification from Cobalt Adsorption by Layered Double Hydroxides
Abstract
1. Introduction
2. Materials and Methods
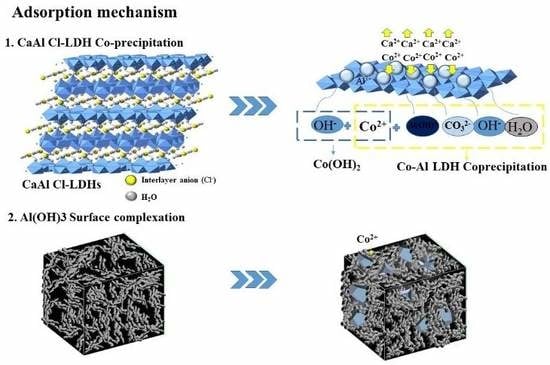
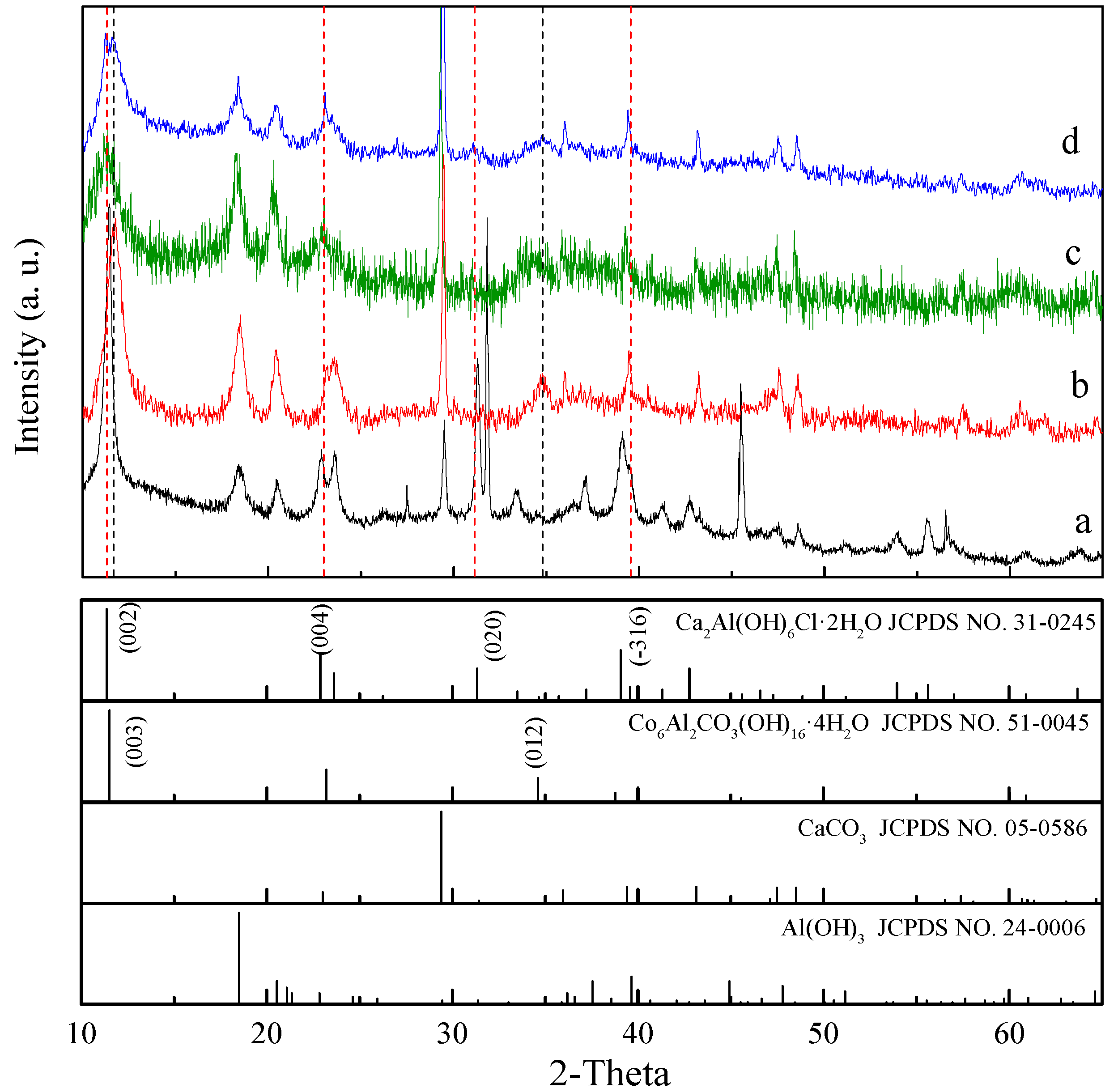
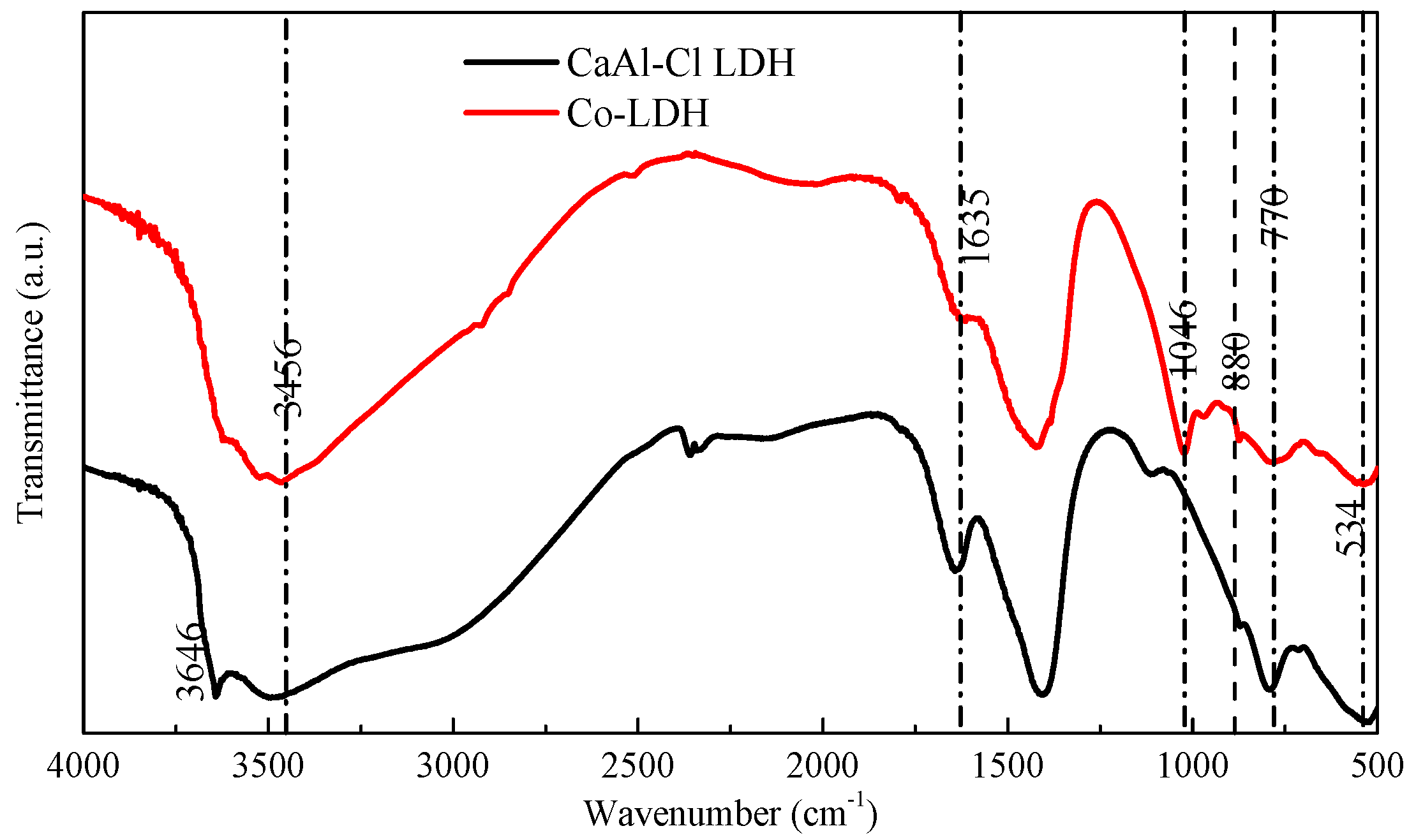
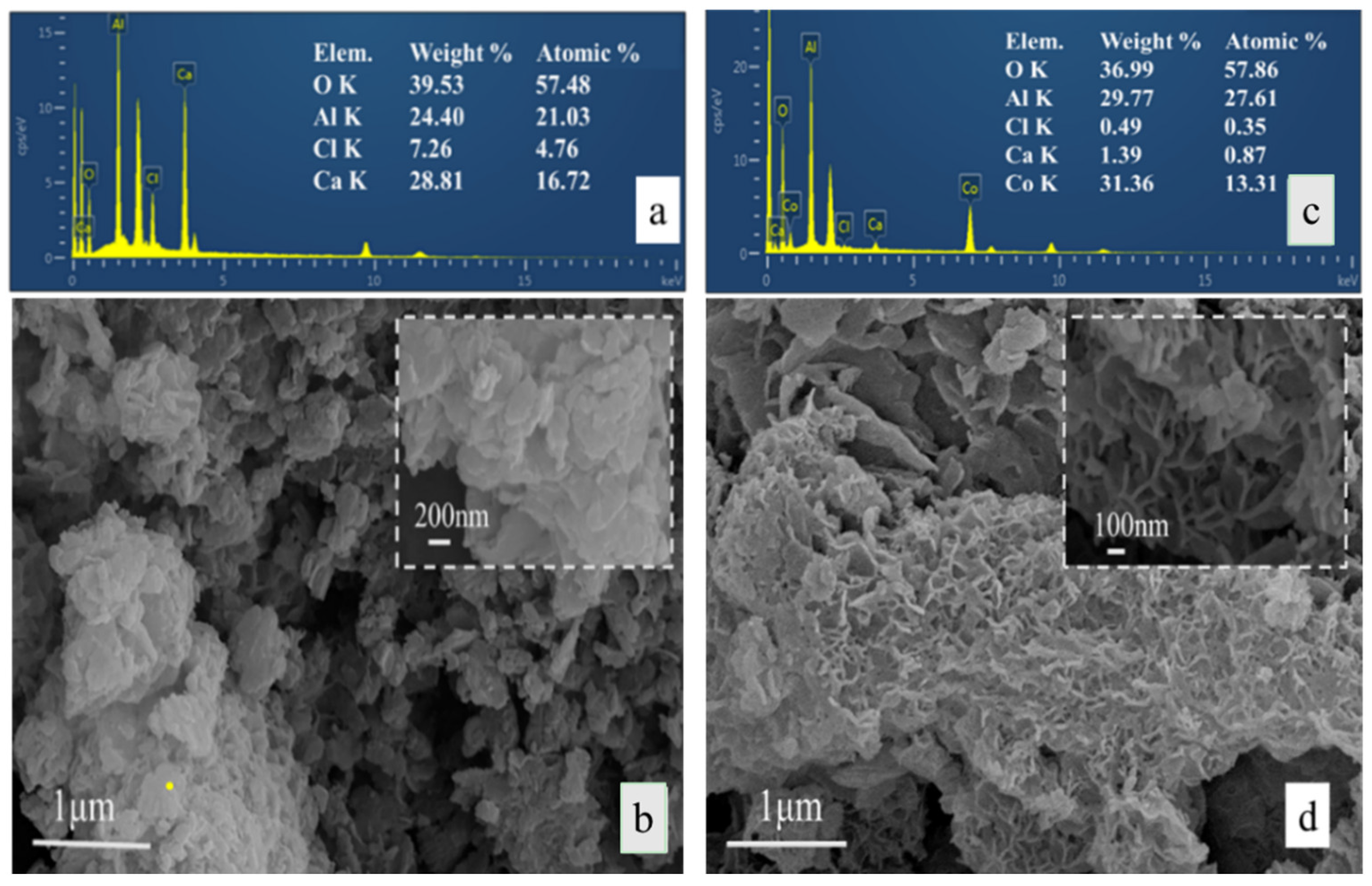
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Adsorption of cobalt(II) from aqueous solution onto hydroxyapatite/zeolite composite. Adv. Mater. Lett. 2011, 2, 309–312. [Google Scholar] [CrossRef]
- Tan, L.; Jin, Y.; Chen, J.; Cheng, X.; Wu, J.; Feng, L. Sorption of radiocobalt (II) from aqueous solutions to Na-attapulgite. J. Radioanal. Nucl. Chem. 2011, 289, 601–610. [Google Scholar] [CrossRef]
- Toth, V.; Sipiczki, M.; Pallagi, A.; Kukovecz, A.; Konya, Z.; Sipos, P.; Palinko, I. Synthesis and properties of CaAl-layered double hydroxides of hydrocalumite-type. Chem. Pap. 2014, 68, 633–637. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Layered Materials, 4th ed.; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Zhang, D.; Jia, Y.; Ma, J.; Li, Z. Removal of arsenic from water by Friedel’s salt (FS:3CaO·Al2O3·CaCl2·10H2O). J. Hazard Mater. 2011, 195, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Qian, G.; Cao, Y.; Chi, Y.; Xu, Y.; Zhou, J.; Liu, Q.; Xu, Z.P.; Qiao, S. Effective removal and fixation of Cr(VI) from aqueous solution with Friedel’s salt. J. Hazard Mater. 2009, 170, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, H.; Cao, H.; Li, H.; Li, Z. Removal of Cd2+ from water by Friedel’s salt (FS: 3CaO·Al2O3·CaCl2·10H2O): Sorption characteristics and mechanisms. J. Environ. Sci.-China 2013, 25, 1719–1725. [Google Scholar] [CrossRef]
- Wu, Y.; Chi, Y.; Bai, H.; Qian, G.; Cao, Y.; Zhou, J.; Xu, Y.; Liu, Q.; Xu, Z.P.; Qiao, S. Effective removal of selenate from aqueous solutions by the Friedel phase. J. Hazard Mater. 2010, 176, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Hatami, H.; Fotovat, A.; Halajnia, A. Comparison of adsorption and desorption of phosphate on synthesized Zn-Al LDH by two methods in a simulated soil solution. Appl. Clay Sci. 2018, 152, 333–341. [Google Scholar] [CrossRef]
- Milagres, J.L.; Bellato, C.R.; Vieira, R.S.; Ferreira, S.O.; Reis, C. Preparation and evaluation of the Ca-Al layered double hydroxide for removal of copper(II), nickel(II), zinc(II), chromium(VI) and phosphate from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 5469–5480. [Google Scholar] [CrossRef]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloid Surf. A 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Wu, K.; Shi, H.; Xu, L.; Guo, X.; De Schutter, G.; Xu, M. Influence of heavy metals on the early hydration of calcium sulfoaluminate. J. Therm. Anal. Calorim. 2014, 115, 1153–1162. [Google Scholar] [CrossRef]
- Luz, C.A.; Pera, J.; Cheriaf, M.; Rocha, J.C. Behaviour of calcium sulfoaluminate cement in presence of high concentrations of chromium salts. Cem. Concr. Res. 2007, 37, 624–629. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Zhao, M.; Yu, L.; Yang, K.; Zhu, X.; Jiang, X. Immobilization of Cr(VI) by hydrated Portland and without calcium sulfate. J. Hazard Mater. 2018, 342, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Pal, A.; Ghosh, S.K.; Mandal, M.; Pal, T. Removal of Arsenic from water using hardened paste of Portland cement. Environ. Technol. 2004, 25, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S. Amorphous solids: Their structure, lattice dynamics and elasticity. Phys. Rep. 1998, 296, 65–236. [Google Scholar] [CrossRef]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Co amorphous systems: A product development perspective. Int. J. Pharm. 2016, 515, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Granados-Reyes, J.; Salagre, P.; Cesteros, Y. CaAl-layered double hydroxides as active catalysts the transesterification of glycerol to glycerol carbonate. Appl. Clay Sci. 2016, 132, 216–222. [Google Scholar] [CrossRef]
- Zou, J.; Dai, Y.; Tian, C.; Pan, K.; Jiang, B.; Wang, L.; Zhou, W.; Tian, G.; Wang, X.; Xing, Z.; et al. Structure and properties of noncrystalline nano-Al(OH)3 reclaimed from carbonized residual wastewater treatment sludge. Environ. Sci. Technol. 2012, 46, 4560–4566. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, D.; Zhou, Q.; Liu, G.; Peng, Z. Concentration variation of aluminate ions during the seeded precipitation process of gibbsite from sodium aluminate solution. Hydrometallurgy 2011, 106, 93–98. [Google Scholar] [CrossRef]
- Xiao, Y.; Su, D.; Wang, X.; Wu, S.; Zhou, L.; Fang, S.; Li, F. Layered double hydroxides with larger interlayer distance for enhanced pseudocapacitance. Sci. China Mater. 2018, 61, 263–272. [Google Scholar] [CrossRef]
- Glasser, U.A.B.A. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 12, 1713–1723. [Google Scholar]
- Cai, J.; Zhao, X.; Zhang, Y.; Zhang, Q.; Pan, B. Enhanced fluoride removal by La-doped Li/Al layered double hydroxides. J. Colloid Interface Sci. 2018, 509, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Caravaggio, G.A.; Detellier, C.; Wronski, Z. Synthesis, stability and electrochemical properties of NiAl and NiV layered double hydroxides. J. Mater. Chem. 2001, 11, 912–921. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Q.; Liu, M.; Dai, Y.; Chen, J.; Huang, H.; Wen, Y.; Zhu, X.; Zhang, X.; Wei, Y. Synthesis of functionalized MgAl-layered double hydroxides via modified mussel inspired chemistry and their application in organic dye adsorption. J. Colloid Interface Sci. 2017, 505, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Liu, H.; Wei, G.; Xu, Z.; Liu, P.; Li, Y. Quantitative analysis of Fe and Co in Co-substituted magnetite using XPS: The application of non-linear least squares fitting (NLLSF). Appl. Surf. Sci. 2016, 389, 438–446. [Google Scholar] [CrossRef]
- Ranganatha, S.; Munichandraiah, N. Synthesis and performance evaluation of novel cobalt hydroxychlorides for electrochemical supercapacitors. J. Solid State Electr. 2017, 21, 939–946. [Google Scholar] [CrossRef]
- Wang, L.; Yu, J.; Chou, K.; Seetharaman, S. Effects of MgO and Al2O3 addition on redox state of chromium in CaO-SiO2-CrOx slag system by XPS method. Metall. Mater. Trans. B 2015, 46, 1802–1808. [Google Scholar] [CrossRef]
- Pavlovic, I.; Perez, M.R.; Barriga, C.; Ulibarri, M.A. Adsorption of Cu2+, Cd2+ and Pb2+ ions by layered double hydroxides intercalated with the chelating agents diethylenetriaminepentaacetate and meso-2,3-dimercaptosuccinate. Appl. Clay Sci. 2009, 43, 125–129. [Google Scholar] [CrossRef]
- Komarneni, S.; Kozai, N.; Roy, R. Novel function for anionic clays: selective transition metal cation uptake by diadochy. J. Mater. Chem. 1998, 8, 1329–1331. [Google Scholar] [CrossRef]
- Tokoro, C.; Yatsugi, Y.; Koga, H.; Owada, S. Sorption mechanisms of arsenate during coprecipitation with ferrihydrite in aqueous solution. Environ. Sci. Technol. 2010, 44, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Tokoro, C.; Sakakibara, T.; Suzuki, S. Mechanism investigation and surface complexation modeling of zinc sorption on aluminum hydroxide in adsorption/coprecipitation processes. Chem. Eng. J. 2015, 279, 86–92. [Google Scholar] [CrossRef]
- Haraguchi, D.; Tokoro, C.; Oda, Y.; Owada, S. Sorption mechanisms of arsenate in aqueous solution during coprecipitation with aluminum hydroxide. J. Chem. Eng. Jpn. 2013, 46, 173–180. [Google Scholar] [CrossRef]
- Izawa, S.; Maeda, M.; Tokoro, C.; Sasaki, K. Sorption mechanism of boron to magnesium hydroxide using co-precipitation process in aqueous solution. J. MMIJ 2014, 130, 155–161. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Wang, H.; Wang, X.; Zhou, L.; Liu, R.; Liang, Y. Laboratory study on the adsorption of Mn2+ on suspended and deposited amorphous Al(OH)3 in drinking water distribution systems. Water Res. 2012, 46, 4063–4070. [Google Scholar] [CrossRef] [PubMed]
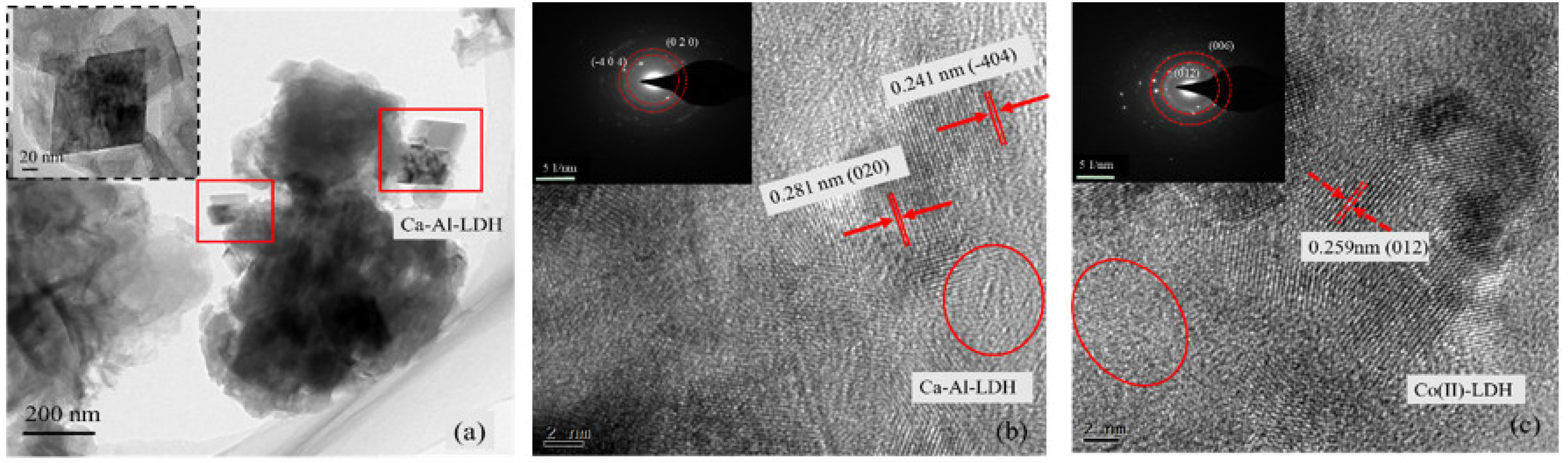
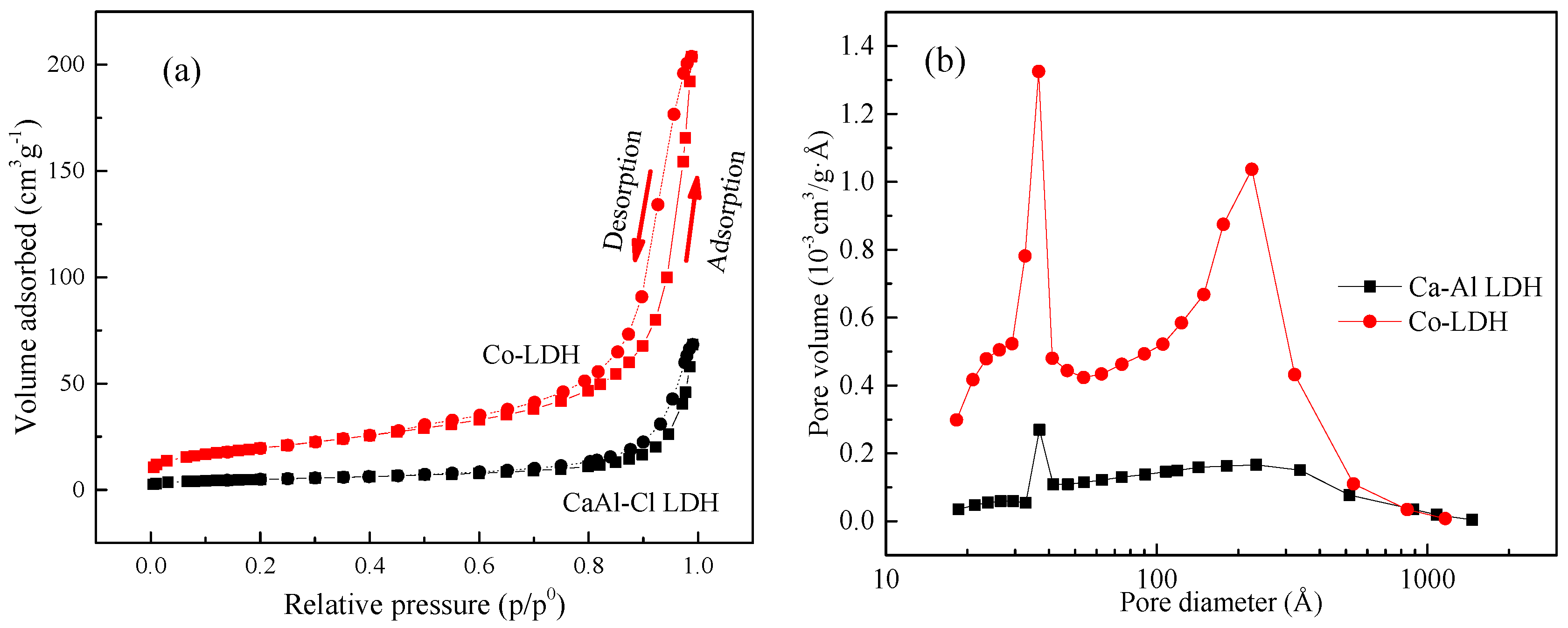
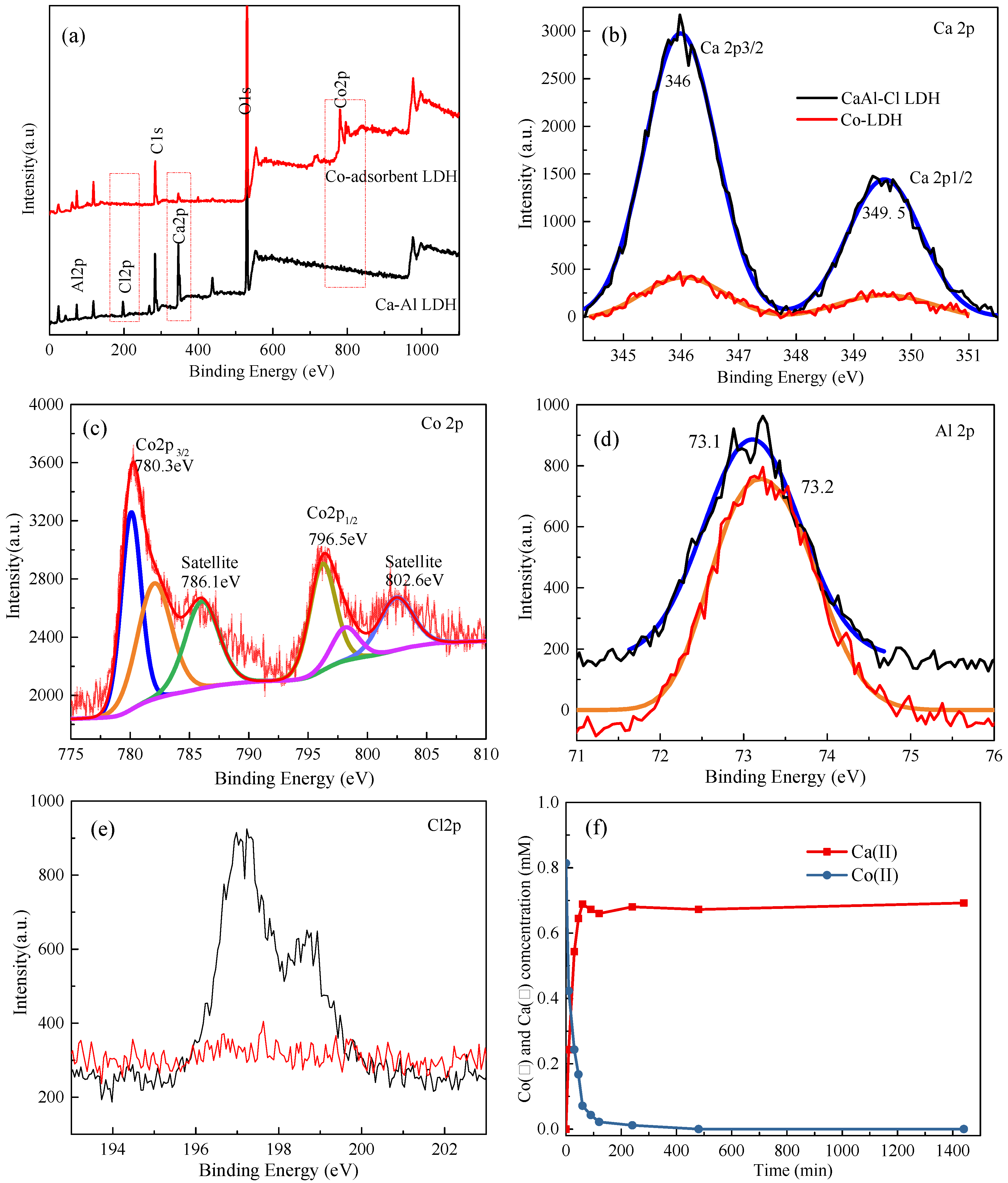
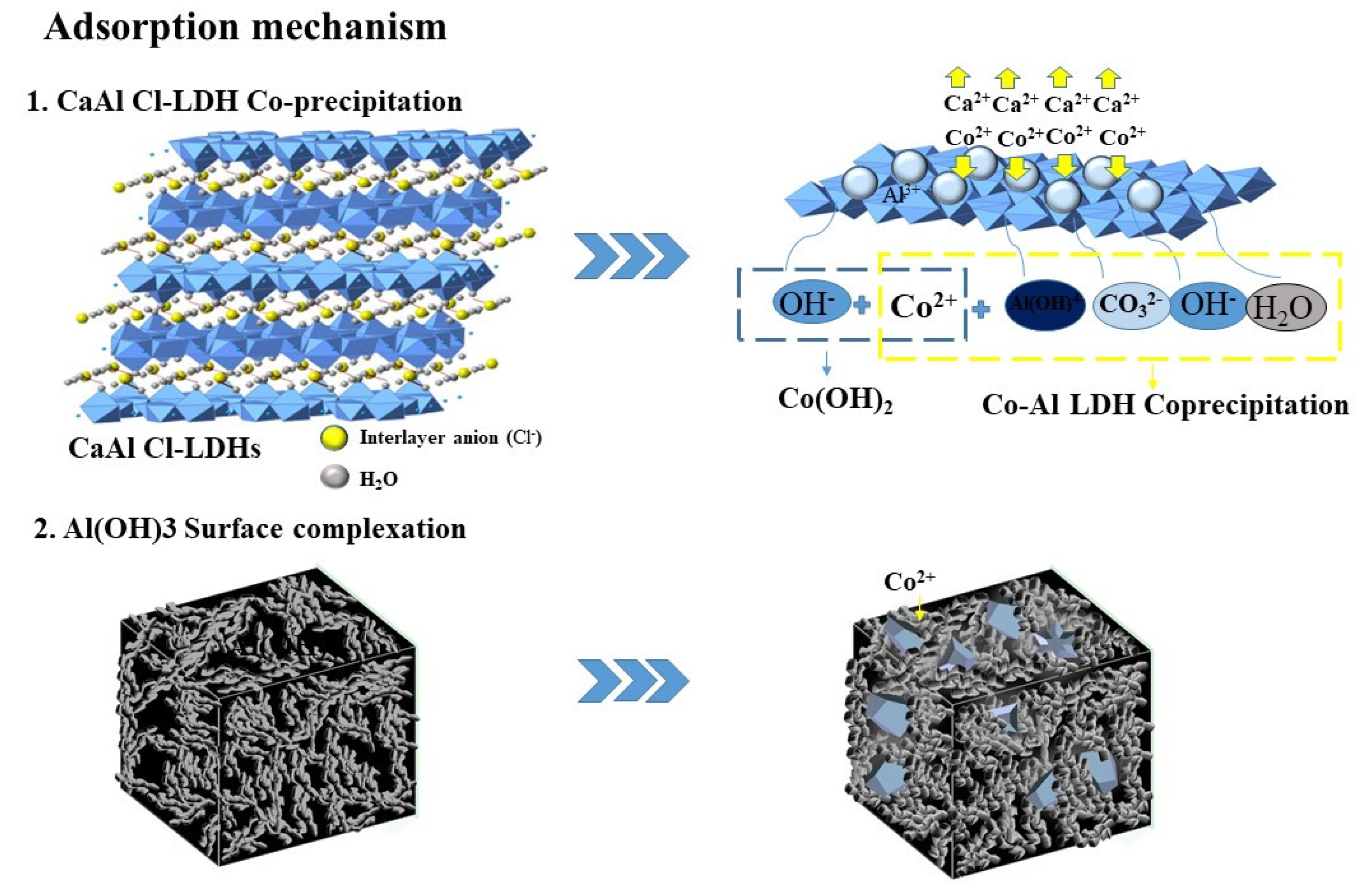
| Variables | Crystal Parameters | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | |||
|---|---|---|---|---|---|---|---|
| a (nm) | c (nm) | Crystal Symmetry | Space Groups | ||||
| CaAl- LDH | 0.985 | 1.690 | Monoclinic | P21/c | 17.604 | 0.106 | 14.230 |
| Co-LDH | 0.308 | 2.280 | Hexagonal | Rm | 70.747 | 0.317 | 13.507 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, L.; Wang, Z.; Sun, Y.; Lu, S.; Yao, Y. Crystalline/Amorphous Blend Identification from Cobalt Adsorption by Layered Double Hydroxides. Materials 2018, 11, 1706. https://doi.org/10.3390/ma11091706
Chi L, Wang Z, Sun Y, Lu S, Yao Y. Crystalline/Amorphous Blend Identification from Cobalt Adsorption by Layered Double Hydroxides. Materials. 2018; 11(9):1706. https://doi.org/10.3390/ma11091706
Chicago/Turabian StyleChi, Lin, Zheng Wang, Yuan Sun, Shuang Lu, and Yan Yao. 2018. "Crystalline/Amorphous Blend Identification from Cobalt Adsorption by Layered Double Hydroxides" Materials 11, no. 9: 1706. https://doi.org/10.3390/ma11091706
APA StyleChi, L., Wang, Z., Sun, Y., Lu, S., & Yao, Y. (2018). Crystalline/Amorphous Blend Identification from Cobalt Adsorption by Layered Double Hydroxides. Materials, 11(9), 1706. https://doi.org/10.3390/ma11091706