Properties of Lithium Trivanadate Film Electrodes Formed on Garnet-Type Oxide Solid Electrolyte by Aerosol Deposition
Abstract
:1. Introduction
2. Materials and Methods
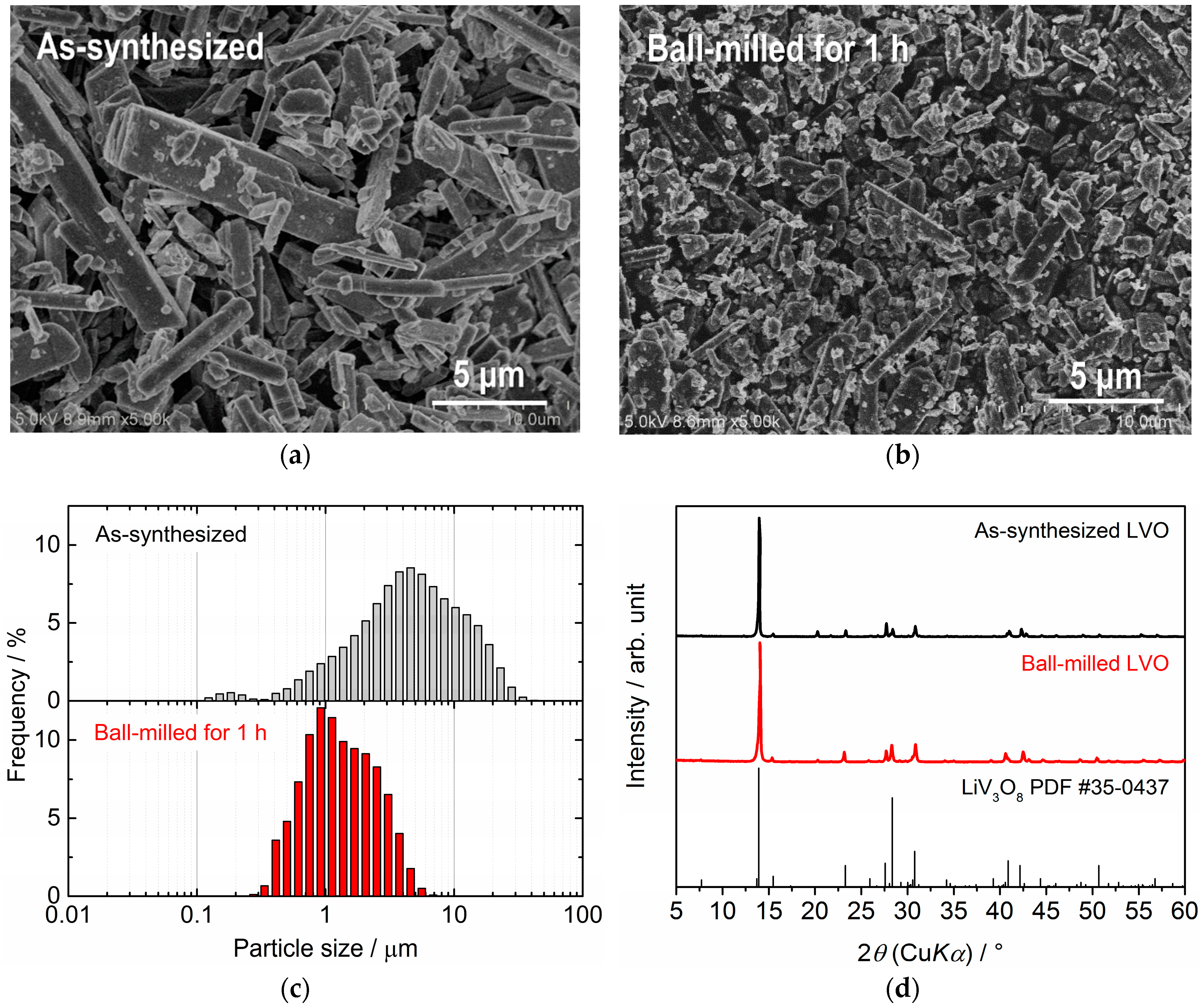
2.1. Synthesis and Characterization of LVO Powder Used for Film Fabrication
2.2. Synthesis and Characterization of Garnet-Type LLZT Pellet
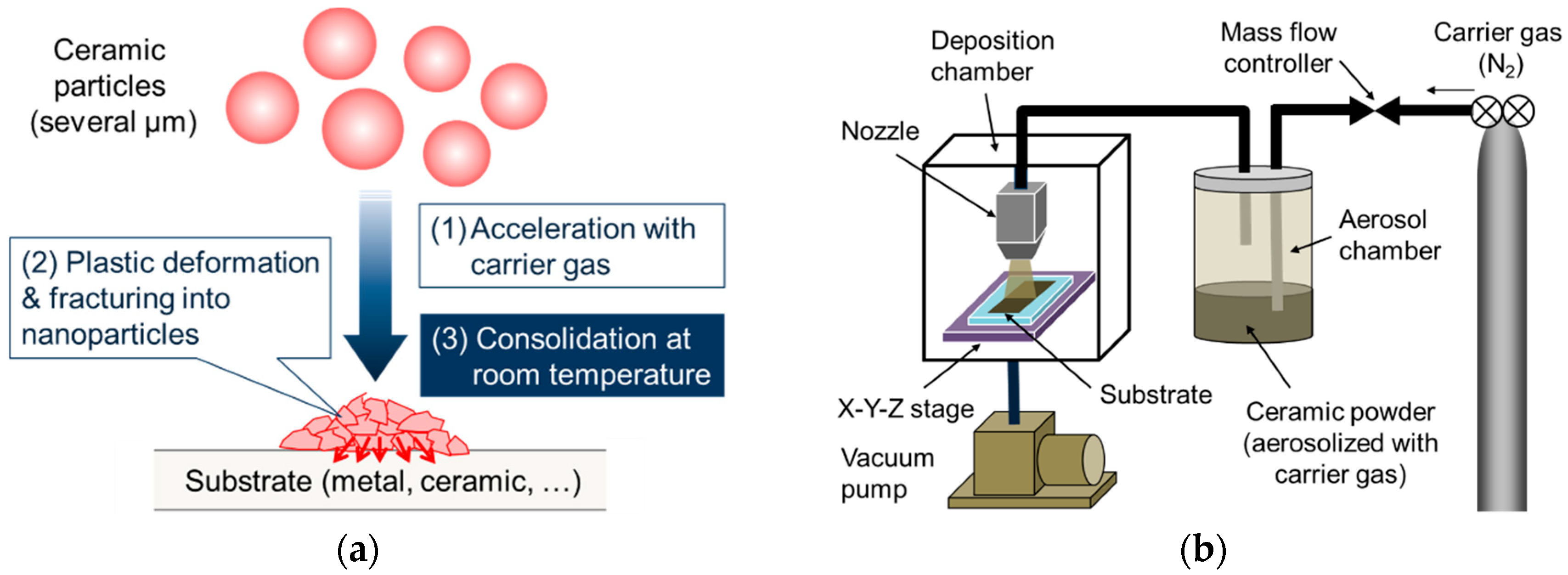
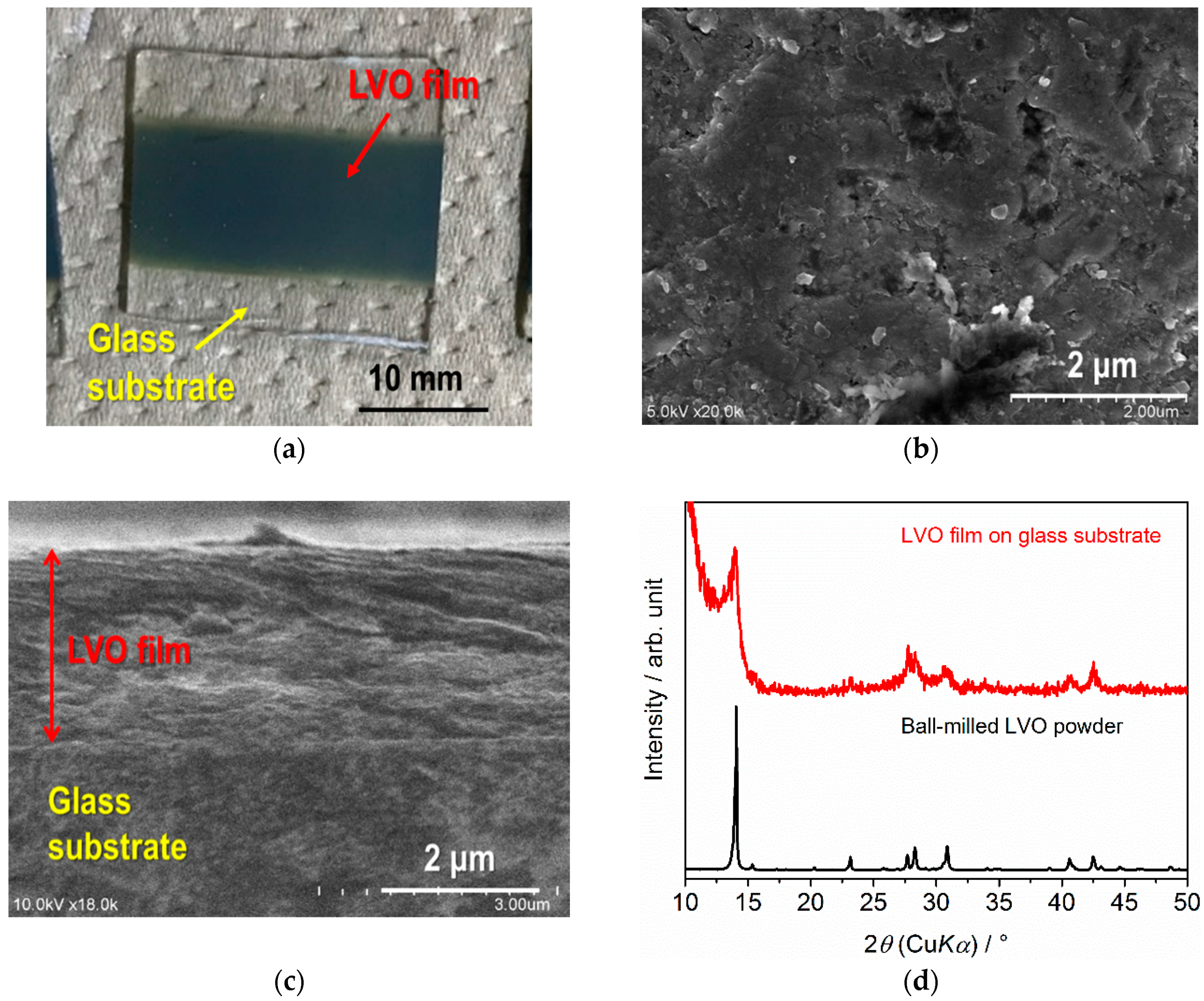
2.3. Fabrication and Characterization of LVO Films by AD on Glass, SUS316L and LLZT
2.4. Electrochemical Characterization for LVO Film Formed on LLZT Solid Electrolyte
3. Results and Discussion
3.1. Characterization of LVO Powders and Films on Glass and SUS316L Plates
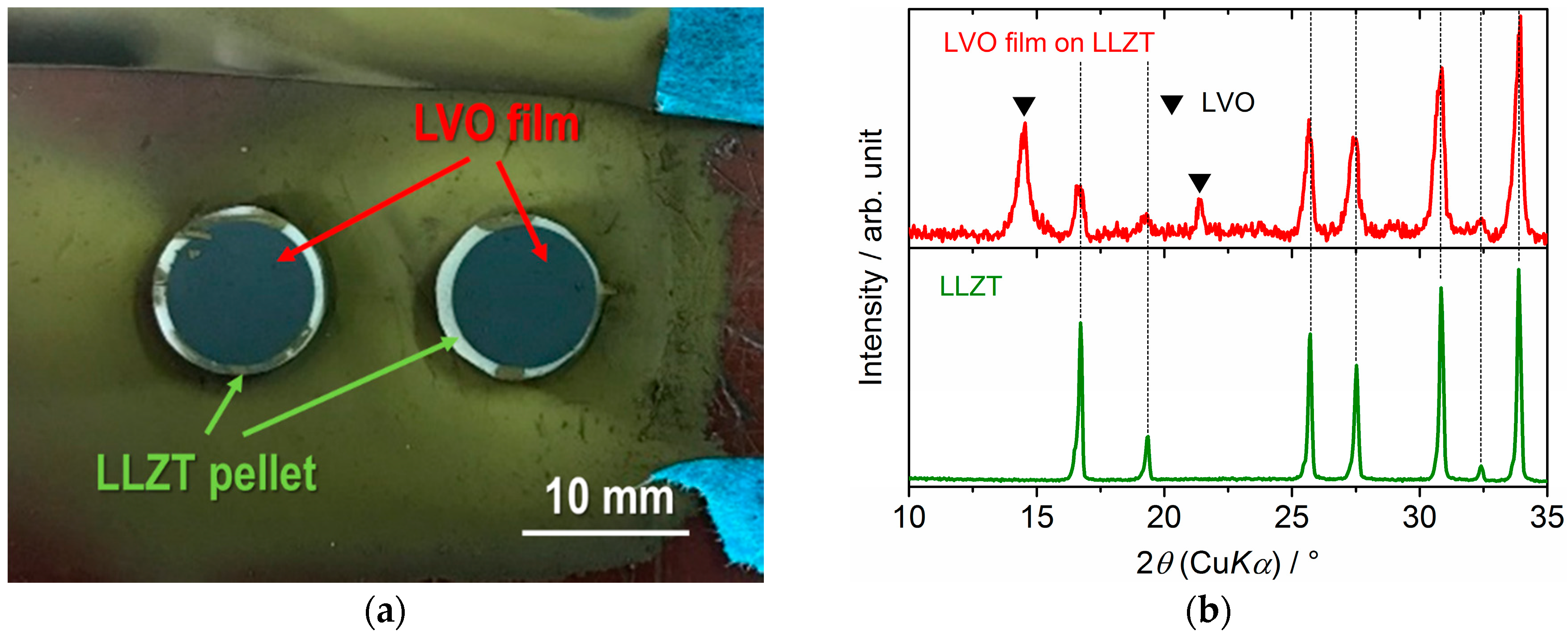
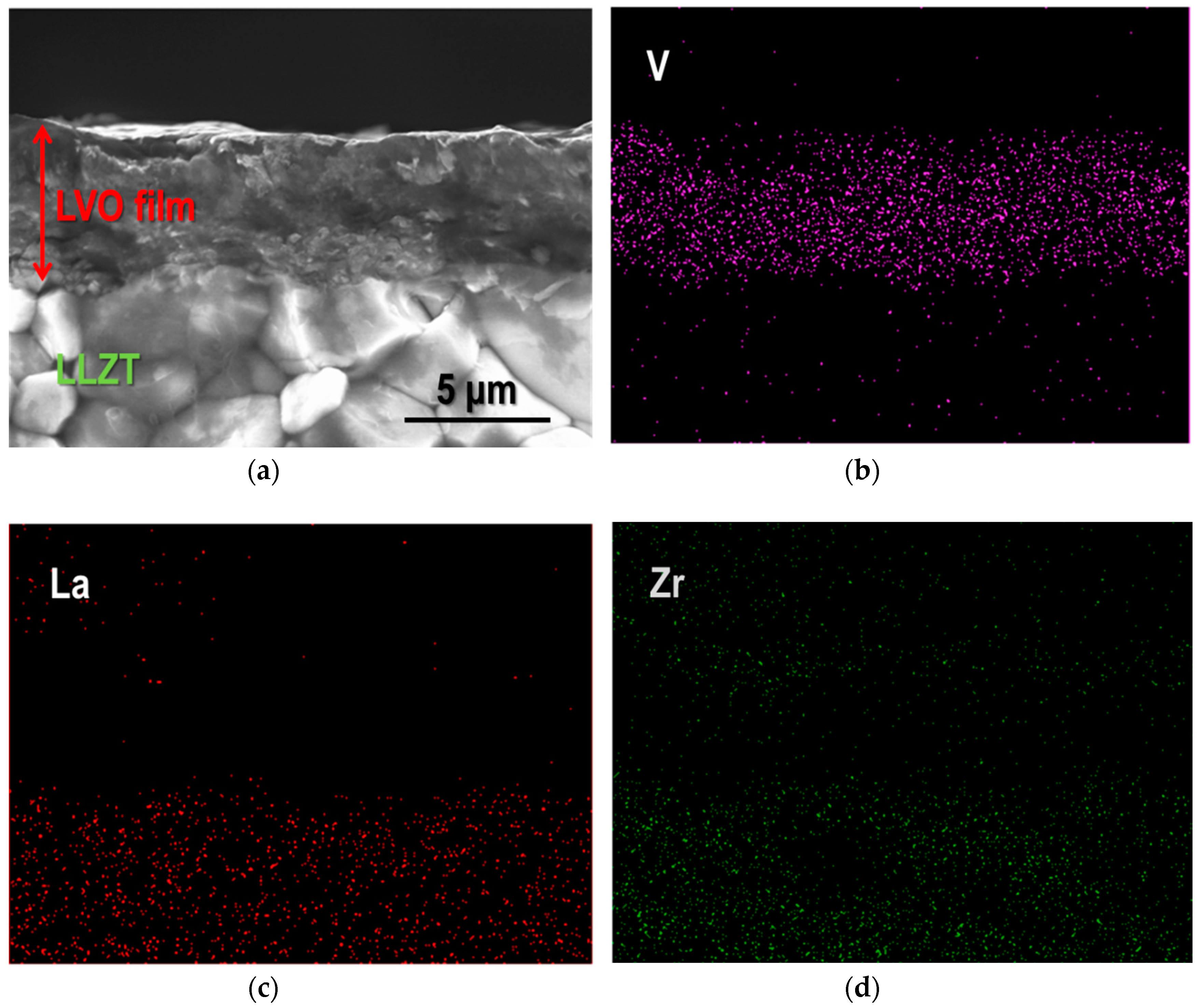
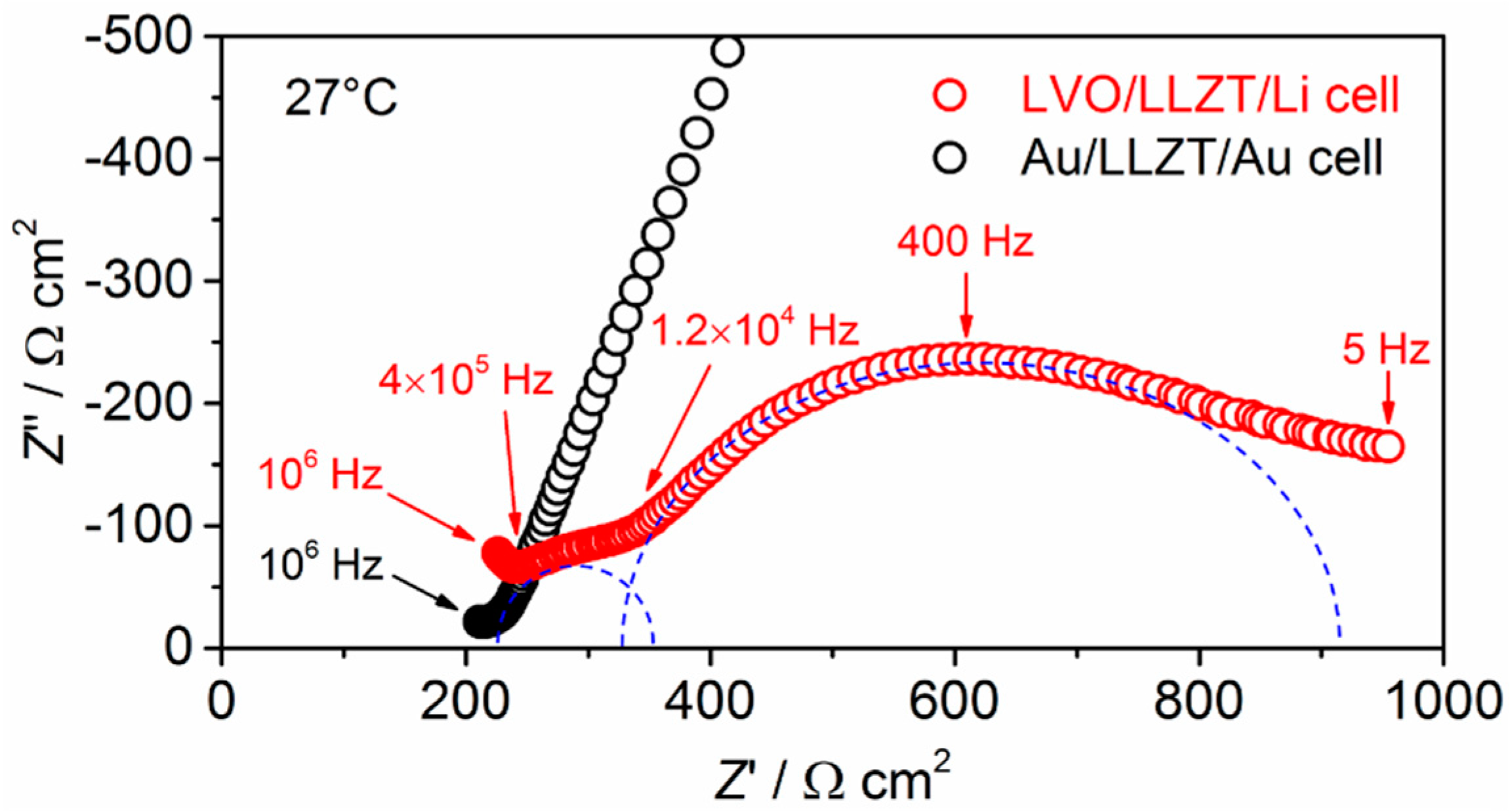
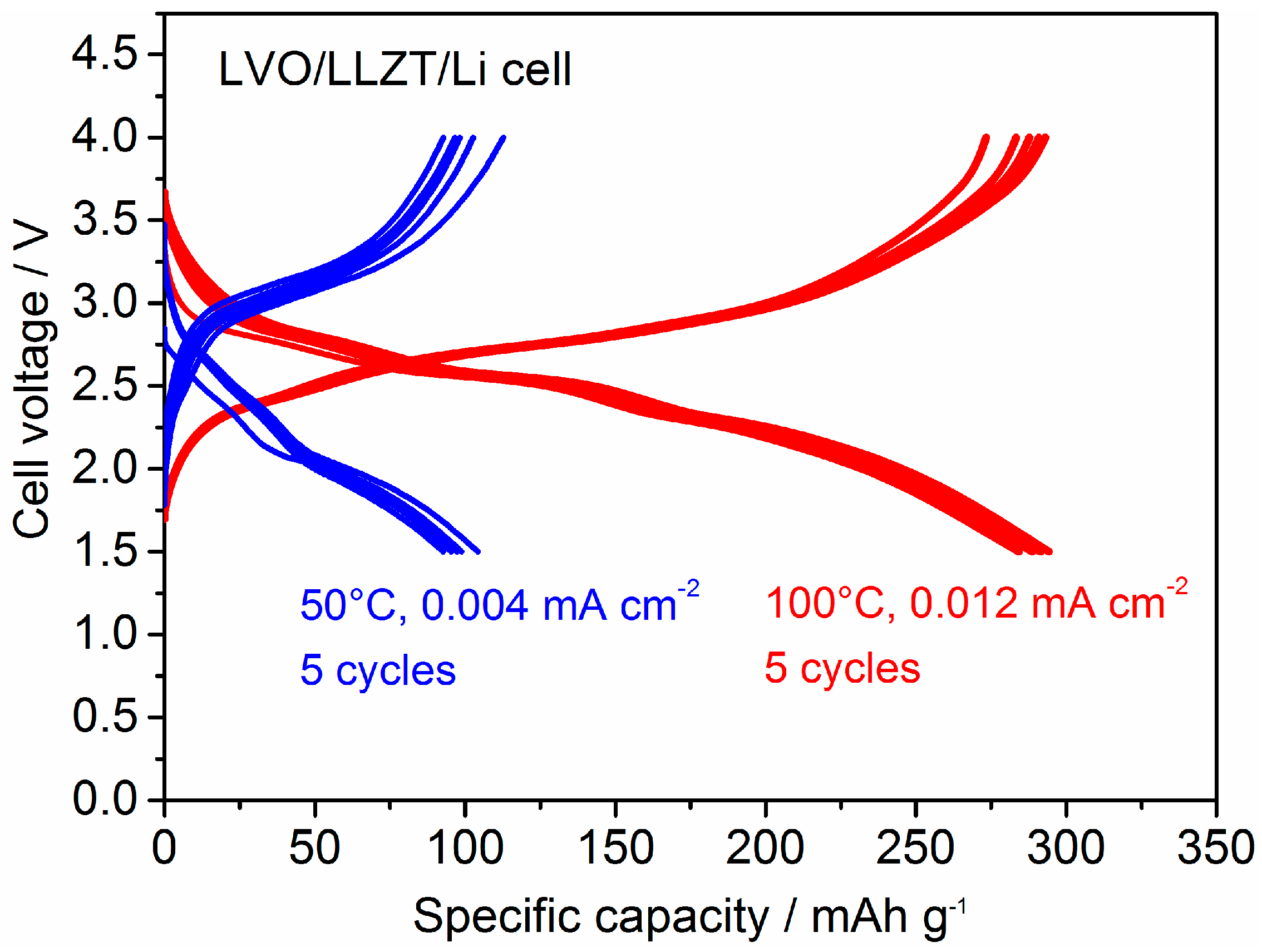
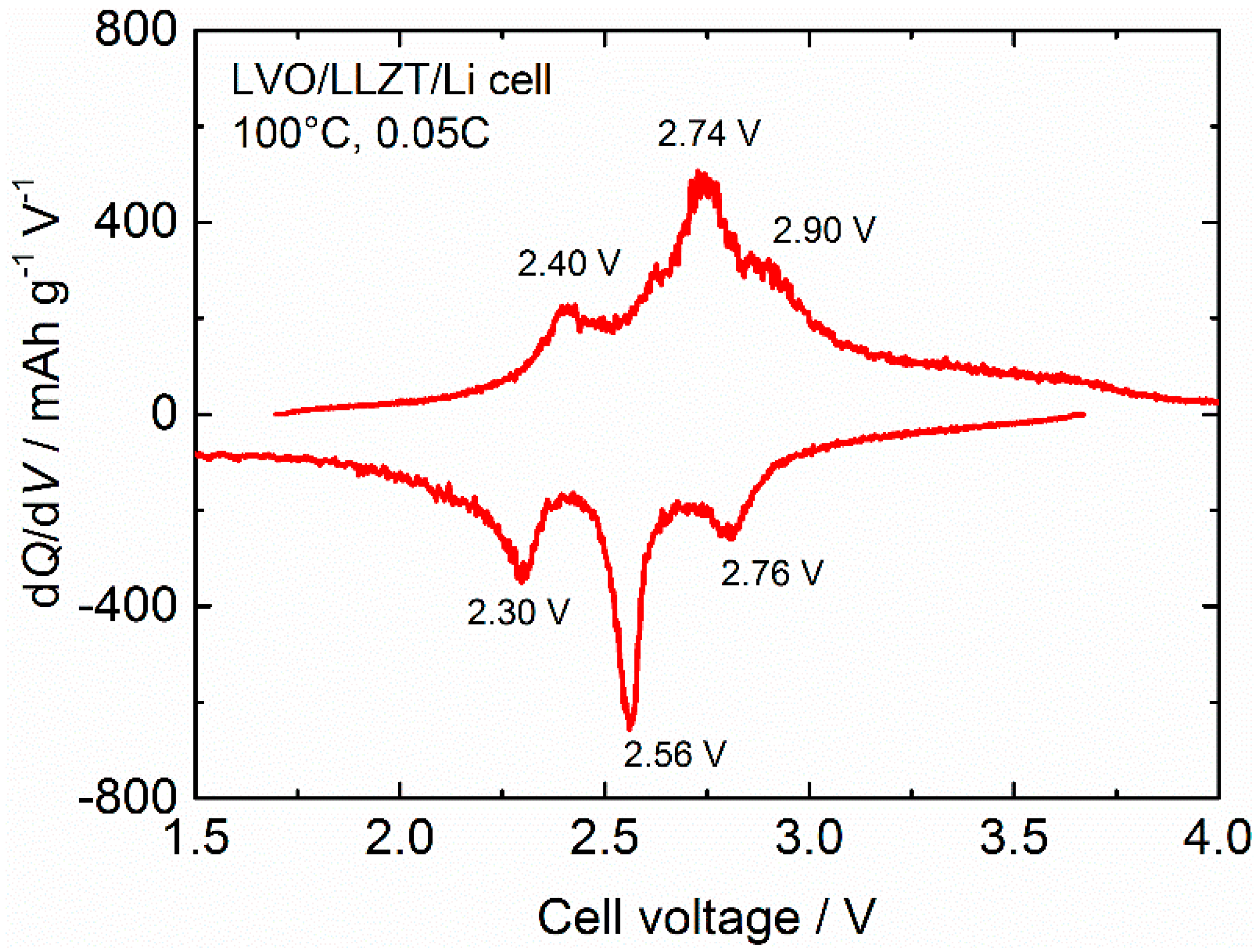
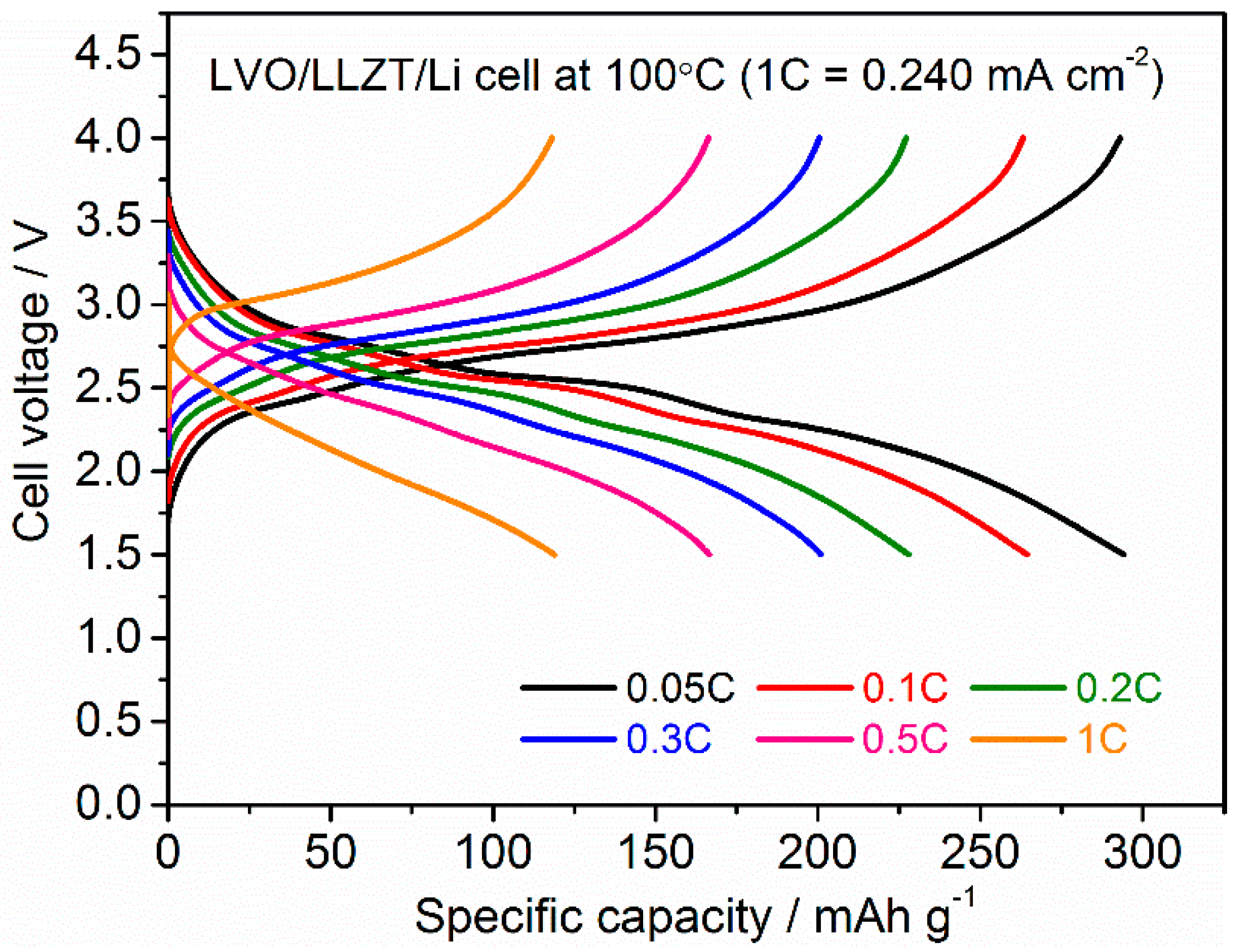
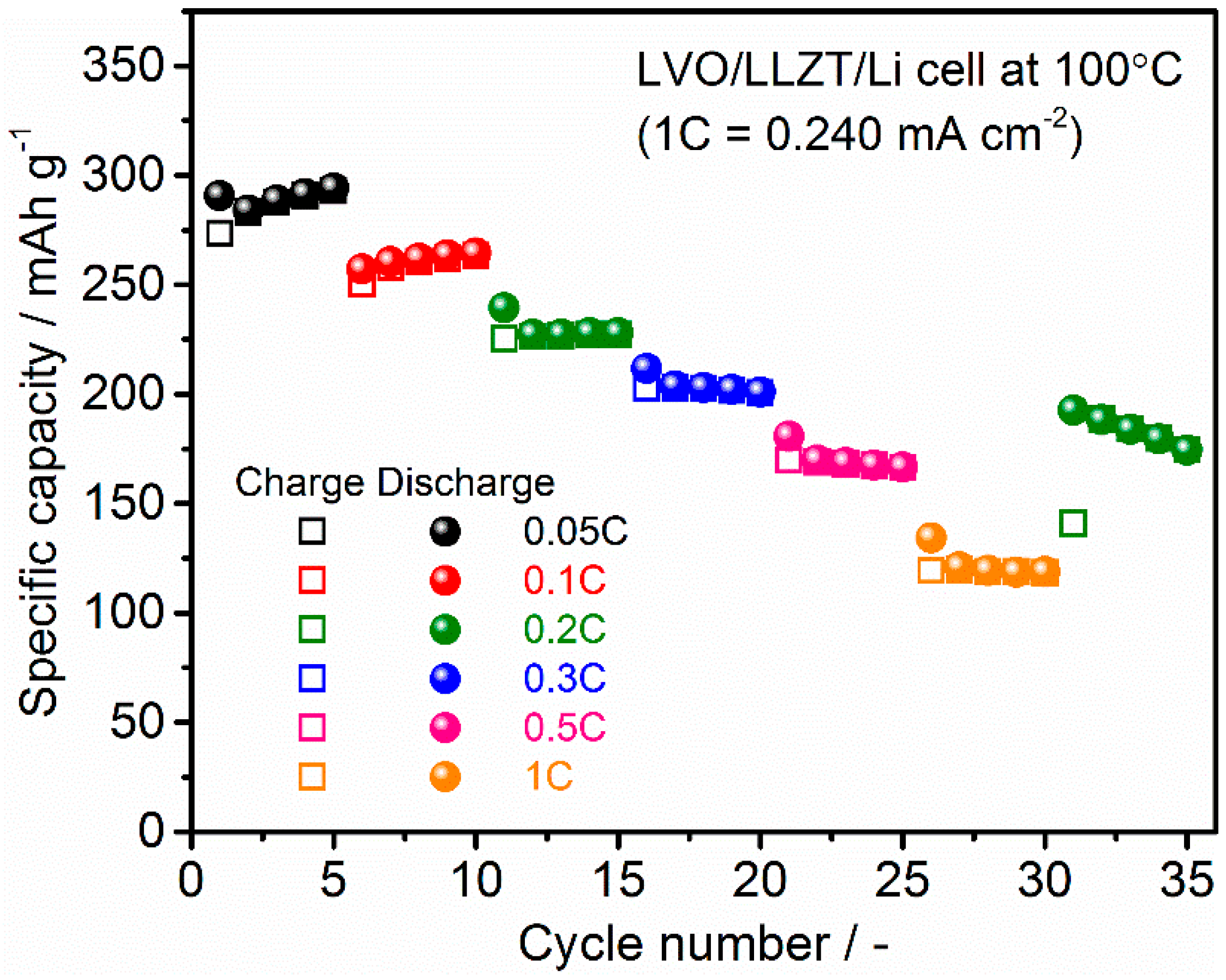
3.2. Characterization for LVO Film Electrode Formed on LLZT Solid Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2012, 61, 759–770. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Nagao, M.; Hayashi, A. Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable lithium batteries. J. Asian Ceram. Soc. 2013, 1, 117–125. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic solid Li ion conductors: An overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Cao, C.; Li, Z.-B.; Wang, X.-L.; Zhao, X.-B.; Han, W.-Q. Recent advances in inorganic solid electrolytes for lithium batteries. Front. Energy Res. 2014, 2, 25. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, K.; Chen, R.; Liu, T.; Zhang, Y.; Nan, C.-W. Oxide electrolytes for lithium batteries. J. Am. Ceram. Soc. 2015, 98, 3603–3623. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Awaka, J.; Takashima, A.; Kataoka, K.; Kijima, N.; Idemoto, Y.; Akimoto, J. Crystal structure of fast lithium-ion-conducting cubic Li7La3Zr2O12. Chem. Lett. 2011, 40, 60–62. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Hayakawa, H.; Akimoto, J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J. Solid State Chem. 2009, 182, 2046–2052. [Google Scholar] [CrossRef]
- Geiger, C.A.; Alekseev, E.; Lazic, B.; Fisch, M.; Armbruster, T.; Langner, R.; Fechtelkord, M.; Kim, N.; Pettke, T.; Weppner, W. Crystal chemistry and stability of “Li7La3Zr2O12” garnet: A fast lithium-ion conductor. Inorg. Chem. 2011, 50, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Kobayashi, T.; Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7−XLa3(Zr2−X, NbX)O12 (X = 0–2). J. Power Sources 2011, 196, 3342–3345. [Google Scholar] [CrossRef]
- Kihira, Y.; Ohta, S.; Imagawa, H.; Asaoka, T. Effect of simultaneous substitution of alkali earth metals and Nb in Li7La3Zr2O12 on lithium-ion conductivity. ECS Electrochem. Lett. 2013, 2, A56–A59. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.-T.; Wang, C.-A.; Xie, H.; Goodenough, J.B. Optimizing Li+ conductivity in a garnet framework. J. Mater. Chem. 2012, 22, 15357–15361. [Google Scholar] [CrossRef]
- Logéat, A.; Köhler, T.; Eisele, U.; Stiaszny, B.; Harzer, A.; Tovar, M.; Senyshyn, A.; Ehrenberg, H.; Kozinsky, B. From order to disorder: The structure of lithium-conducting garnets Li7-xLa3TaxZr2-xO12 (x = 0–2). Solid State Ion. 2012, 206, 33–38. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L. High ionic conductivity lithium garnet oxides of Li7−xLa3Zr2−xTaxO12 compositions. Electrochem. Solid State Lett. 2012, 15, A68–A71. [Google Scholar] [CrossRef]
- Thompson, T.; Sharafi, A.; Johannes, M.D.; Huq, A.; Allen, J.L.; Wolfenstine, J.; Sakamoto, J. Tale of two sites: On defining the carrier concentration in garnet-based ionic conductors for advanced Li batteries. Adv. Energy Mater. 2015, 5, 1500096. [Google Scholar] [CrossRef]
- Thompson, T.; Wolfenstine, J.; Allen, J.L.; Johannes, M.; Huq, A.; Davida, I.N.; Sakamoto, J. Tetragonal vs. cubic phase stability in Al-free Ta doped Li7La3Zr2O12 (LLZO). J. Mater. Chem. A 2014, 2, 13431–13436. [Google Scholar] [CrossRef]
- Inada, R.; Kusakabe, K.; Tanaka, T.; Kudo, S.; Sakurai, Y. Synthesis and properties of Al-free Li7-xLa3Zr2-xTaxO12 garnet related oxides. Solid State Ion. 2014, 262, 568–572. [Google Scholar] [CrossRef]
- Inada, R.; Yasuda, S.; Hosokawa, H.; Saito, M.; Tojo, T.; Sakurai, Y. Formation and stability of interface between garnet-type Ta-doped Li7La3Zr2O12 solid electrolyte and lithium metal electrode. Batteries 2018, 4, 26. [Google Scholar] [CrossRef]
- Nemori, H.; Matsuda, Y.; Mitsuoka, S.; Matsui, M.; Yamamoto, O.; Takeda, Y.; Imanishi, N. Stability of garnet-type solid electrolyte LixLa3A2-yByO12 (A = Nb or Ta, B = Sc or Zr). Solid State Ion. 2015, 282, 7–12. [Google Scholar] [CrossRef]
- Kim, Y.; Yoo, A.; Schmidt, R.; Sharafi, A.; Lee, H.; Wolfenstine, J.; Sakamoto, J. Electrochemical Stability of Li6.5La3Zr1.5M0.5O12 (M = Nb or Ta) against Metallic Lithium. Front. Energy Res. 2016, 4, 20. [Google Scholar] [CrossRef]
- Ren, Y.; Ting, L.; Shem, Y.; Lin, Y.; Nan, C.-W. Chemical compatibility between garnet-like solid state electrolyte Li6.75La3Zr1.75Ta0.25O12 and major commercial lithium battery cathode materials. J. Materiomics 2016, 2, 256–264. [Google Scholar] [CrossRef]
- Ohta, S.; Komagata, S.; Seki, J.; Saeki, T.; Morishita, S.; Asaoka, T. All solid-state lithium ion battery using garnet-type oxide and Li3BO3 solid electrolytes fabricated by screen-printing. J. Power Sources 2013, 238, 53–56. [Google Scholar] [CrossRef]
- Ohta, S.; Seki, J.; Yagi, Y.; Kihira, Y.; Tani, T.; Asaoka, T. Co-sinterable lithium garnet-type oxide electrolyte with cathode for all-solid-state lithium ion battery. J. Power Sources 2014, 265, 40–44. [Google Scholar] [CrossRef]
- Akedo, J. Aerosol deposition of ceramic thick films at room temperature: Densification mechanism of ceramic layers. J. Am. Ceram. Soc. 2006, 89, 1834–1839. [Google Scholar] [CrossRef]
- Akedo, J. Room temperature impact consolidation (RTIC) of fine ceramic powder by aerosol deposition method and applications to microdevices. J. Thermal Splay Technol. 2008, 17, 181–198. [Google Scholar] [CrossRef]
- Hanft, D.; Exner, J.; Schubert, M.; Thomas, S.; Fuierer, P.; Moos, R. An overview of the aerosol deposition method: Process fundamentals and new trends in materials applications. J. Ceram. Sci. Technol. 2015, 6, 147–182. [Google Scholar]
- Takai, S.; Sakaguchi, H.; Tanaka, K.; Nagao, Y.; Esaka, T. Cathode performance of LiMn2O4 thick films prepared by gas-deposition for lithium rechargeable battery. Electrochemistry 2008, 76, 293–296. [Google Scholar] [CrossRef]
- Usui, H.; Shibata, M.; Nakai, K.; Sakaguchi, H. Anode properties of thick-film electrodes prepared by gas deposition of Ni-coated Si particles. J. Power Sources 2011, 196, 2143–2148. [Google Scholar] [CrossRef]
- Kim, I.; Park, J.; Nam, T.-H.; Kim, K.-W.; Ahn, J.-H.; Park, D.-S.; Ahn, C.-W.; Wang, G.; Ahn, H.-J. Electrochemical properties of an as-deposited LiFePO4 thin film electrode prepared by aerosol deposition. J. Power Sources 2013, 244, 646–651. [Google Scholar] [CrossRef]
- Inada, R.; Shibukawa, K.; Masada, C.; Nakanishi, Y.; Sakurai, Y. Characterization of as-deposited Li4Ti5O12 thin film electrode prepared by aerosol deposition method. J. Power Sources 2014, 253, 181–186. [Google Scholar] [CrossRef]
- Iwasaki, S.; Hamanaka, T.; Yamakawa, T.; West, W.C.; Yamamoto, K.; Motoyama, M.; Hirayama, T.; Iriyama, Y. Preparation of thick-film LiNi1/3Co1/3Mn1/3O2 electrodes by aerosol deposition and its application to all-solid-state batteries. J. Power Sources 2014, 272, 1086–1090. [Google Scholar] [CrossRef]
- Kato, T.; Iwasaki, S.; Ishii, Y.; Motoyama, M.; West, W.C.; Yamamoto, Y.; Iriyama, Y. Preparation of thick-film electrode-solid electrolyte composites on Li7La3Zr2O12 and their electrochemical properties. J. Power Sources 2016, 303, 65–72. [Google Scholar] [CrossRef]
- Ahn, C.-W.; Choi, J.-J.; Ryu, J.; Hahn, B.-D.; Kim, J.-W.; Yoon, W.-H.; Choi, J.-H.; Park, D.-S. Microstructure and electrochemical properties of iron oxide film fabricated by aerosol deposition method for lithium ion battery. J. Power Sources 2015, 273, 336–340. [Google Scholar] [CrossRef]
- Inada, R.; Yasuda, S.; Tojo, T.; Sakurai, Y. Development of lithium-stuffed garnet-type oxide solid electrolytes with high ionic conductivity for application to all-solid-state batteries. Front. Energy Res. 2016, 4, 28. [Google Scholar] [CrossRef]
- Iriyama, Y.; Wadaguchi, M.; Yoshida, K.; Yamamoto, Y.; Motoyama, M.; Yamamoto, T. 5V-class bulk-type all-solid-state rechargeable lithium batteries with electrode-solid electrolyte composite electrodes prepared by aerosol deposition. J. Power Sources 2018, 385, 55–61. [Google Scholar] [CrossRef]
- Popovici, D.; Nagai, H.; Fujishima, S.; Akedo, J. Preparation of lithium aluminum titanium phosphate electrolytes thick films by aerosol deposition method. J. Am. Ceram. Soc. 2011, 94, 3847–3850. [Google Scholar] [CrossRef]
- Inada, R.; Ishida, K.; Tojo, M.; Okada, T.; Tojo, T.; Sakurai, Y. Properties of aerosol deposited NASICON-type Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte thin films. Ceram. Int. 2015, 41, 11136–11142. [Google Scholar] [CrossRef]
- Choi, J.-J.; Ahn, C.-W.; Ryu, J.; Hahn, B.-D.; Kim, J.-W.; Yoon, W.-H.; Park, D.-S. Li-ion conducting Li0.35La0.55TiO3 electrolyte thick films fabricated by aerosol deposition. J. Korean Phys. Soc. 2016, 68, 12–16. [Google Scholar] [CrossRef]
- Inada, R.; Okada, T.; Bando, A.; Tojo, T.; Sakurai, Y. Properties of garnet-type Li6La3ZrTaO12 solid electrolyte films fabricated by aerosol deposition method. Prog. Nat. Sci. Mater. Int. 2017, 27, 350–355. [Google Scholar] [CrossRef]
- Hanft, D.; Exner, J.; Moos, R. Thick-films of garnet-type lithium ion conductor prepared by the Aerosol Deposition Method: The role of morphology and annealing treatment on the ionic conductivity. J. Power Sources 2017, 361, 61–69. [Google Scholar] [CrossRef]
- West, K.; Zachau-Christiansen, B.; Skaarup, S.; Saidi, Y.; Barker, J.; Olsen, I.I.; Pynenburg, R.; Koksbang, R. Comparison of LiV3O8 cathode materials prepared by different methods. J. Electrochem. Soc. 1996, 143, 820–825. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, N.; Yu, A.; West, K. Li1−xNaxV3O8 as positive materials for secondary lithium batteries. J. Appl. Electrochem. 1997, 27, 953–958. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Wang, H.; Song, Z.-Q.; Wang, Y.-W.; Yan, H.; Yoshimura, M. Novel chemical method for synthesis of LiV3O8 nanorods as cathode materials for lithium ion batteries. Electrochim. Acta 2004, 49, 349–353. [Google Scholar] [CrossRef]
- Liu, L.; Jiao, L.; Sun, J.; Zhao, M.; Zhang, Y.; Yuan, H.; Wang, Y. Electrochemical performance of LiV3−2xNixMnxO8 cathode materials synthesized by the sol-gel method. Solid State Ion. 2008, 178, 1756–1761. [Google Scholar] [CrossRef]
- Liu, L.; Jiao, L.; Sun, J.; Zhang, Y.; Zhao, M.; Yuan, H.; Wang, Y. Electrochemical performance of LiV3−xNixO8 cathode materials synthesized by a novel low-temperature solid-state method. Electrochim. Acta 2008, 53, 7321–7325. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yang, W.; Zhou, H. A large capacity of LiV3O8 cathode material for rechargeable lithium-based batteries. Electrochim. Acta 2011, 56, 1392–1398. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yang, W.; Zhou, H. Synthesis and electrochemical performance of rod-like LiV3O8 cathode materials for rechargeable lithium batteries. J. Power Sources 2012, 198, 287–293. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ayyasamy, S.; Tok, E.-S.; Adams, S.; Reddy, M.V. Impact of electrical conductivity on the electrochemical performances of layered structure lithium trivanadate (LiV3−xMxO8, M = Zn/Co/Fe/Sn/Ti/Zr/Nb/Mo, x = 0.01−0.1) as cathode materials for energy storage. ACS Omega 2018, 3, 3036–3044. [Google Scholar] [CrossRef] [PubMed]
- Tatsumisago, M.; Takano, R.; Tadanaga, K.; Hayashi, A. Preparation of Li3BO3–Li2SO4 glass–ceramic electrolytes for all-oxide lithium batteries. J. Power Sources 2014, 270, 603–607. [Google Scholar] [CrossRef]
- Nagao, K.; Nose, M.; Kato, A.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Preparation and characterization of glass solid electrolytes in the pseudoternary system Li3BO3-Li2SO4-Li2CO3. Solid State Ion. 2017, 308, 68–76. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inada, R.; Okuno, K.; Kito, S.; Tojo, T.; Sakurai, Y. Properties of Lithium Trivanadate Film Electrodes Formed on Garnet-Type Oxide Solid Electrolyte by Aerosol Deposition. Materials 2018, 11, 1570. https://doi.org/10.3390/ma11091570
Inada R, Okuno K, Kito S, Tojo T, Sakurai Y. Properties of Lithium Trivanadate Film Electrodes Formed on Garnet-Type Oxide Solid Electrolyte by Aerosol Deposition. Materials. 2018; 11(9):1570. https://doi.org/10.3390/ma11091570
Chicago/Turabian StyleInada, Ryoji, Kohei Okuno, Shunsuke Kito, Tomohiro Tojo, and Yoji Sakurai. 2018. "Properties of Lithium Trivanadate Film Electrodes Formed on Garnet-Type Oxide Solid Electrolyte by Aerosol Deposition" Materials 11, no. 9: 1570. https://doi.org/10.3390/ma11091570




