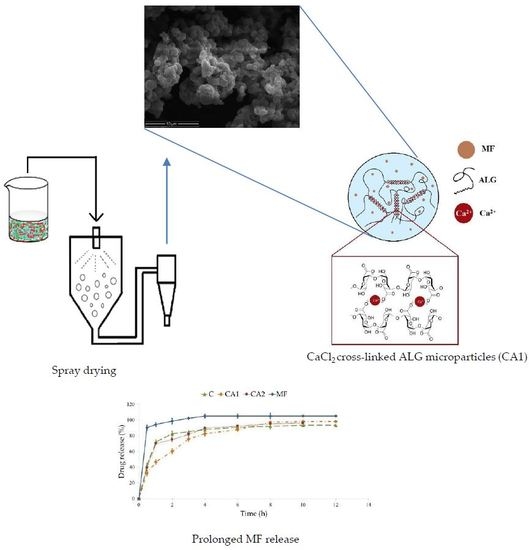
Calcium Chloride Modified Alginate Microparticles Formulated by the Spray Drying Process: A Strategy to Prolong the Release of Freely Soluble Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
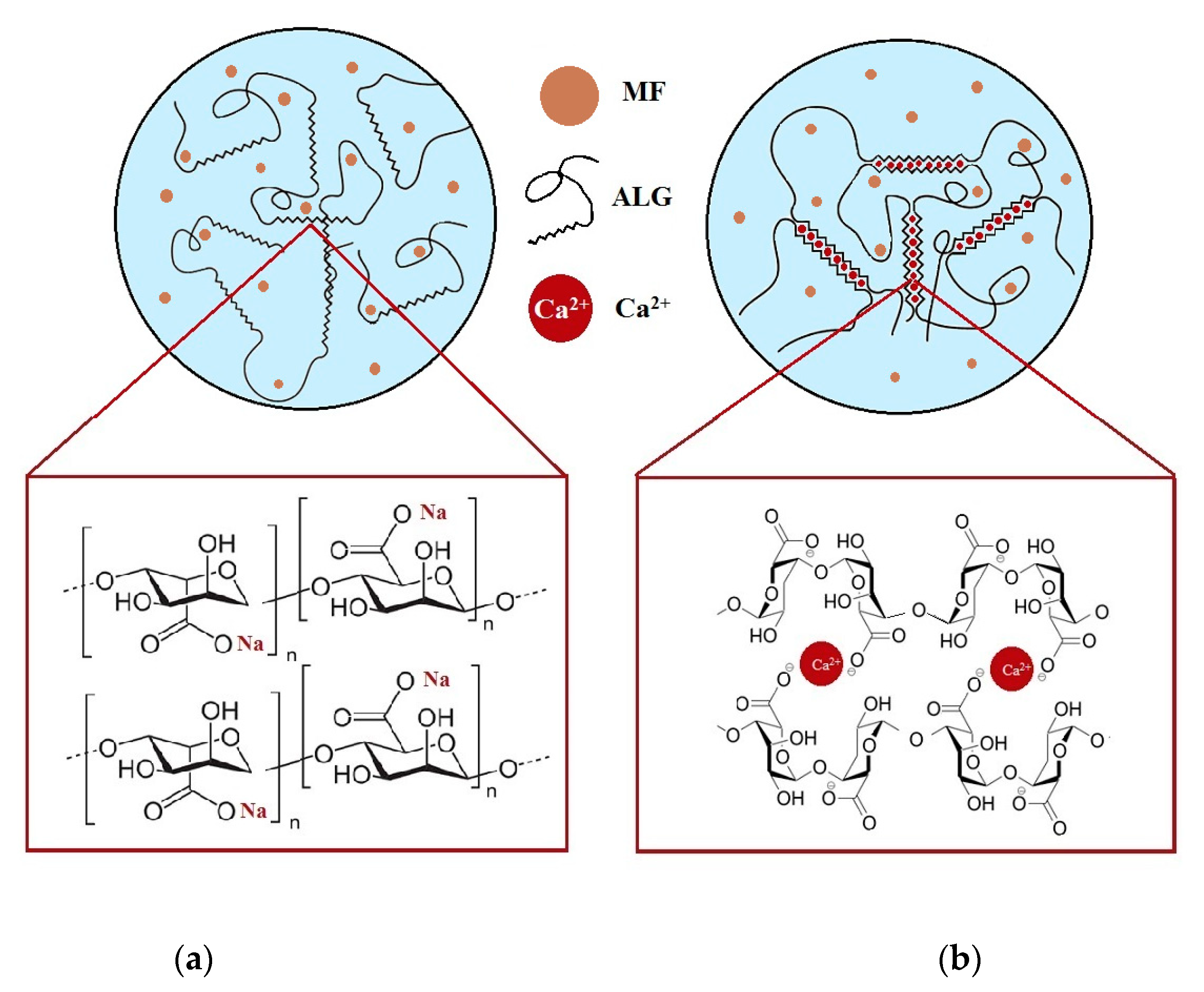
2.2. Formulation of CaCl2 Modified ALG Microparticles
2.3. Evaluation of Microparticles
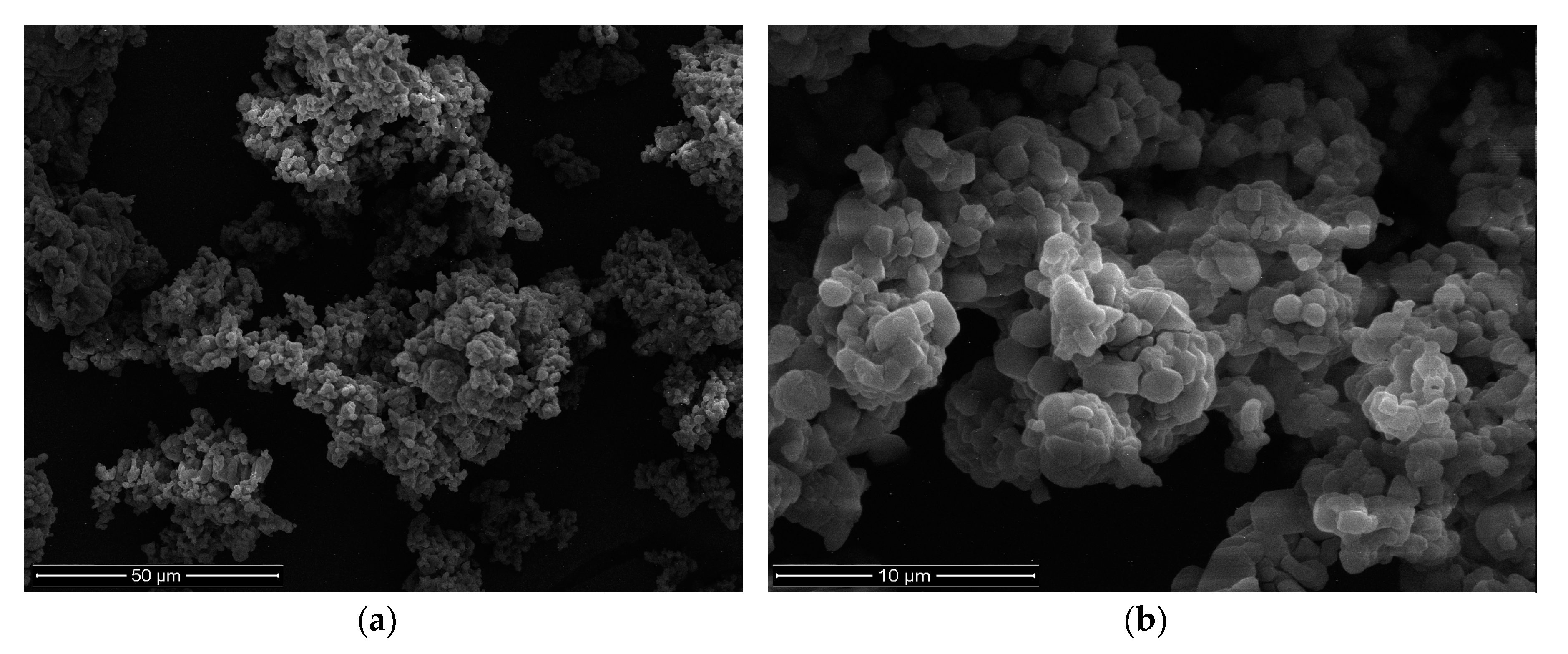
2.3.1. Shape and Size
2.3.2. High Performance Liquid Chromatography (HPLC) Assay
2.3.3. Drug Encapsulation
2.3.4. Zeta Potential
2.3.5. Swelling Characteristics
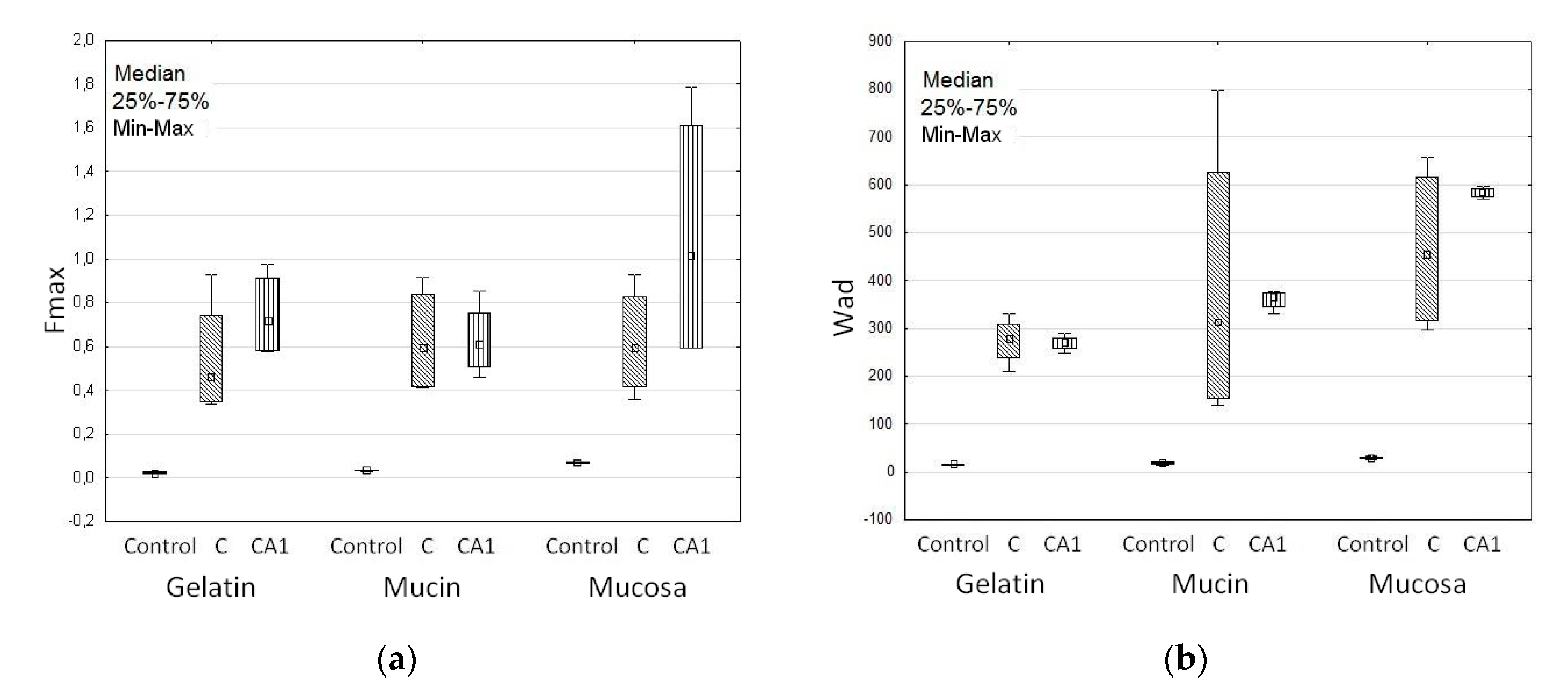
2.3.6. Mucoadhesiveness
2.4. MF Dissolution
2.5. Mathematical Modeling of the MF Release Profile
2.6. Index of Similarity and Dissimilarity
2.7. Differential Scanning Calorimetry (DSC)
2.8. Statistics
3. Results and Discussion
3.1. Microparticles Characteristics
3.2. Swelling and Mucoadhesive Properties
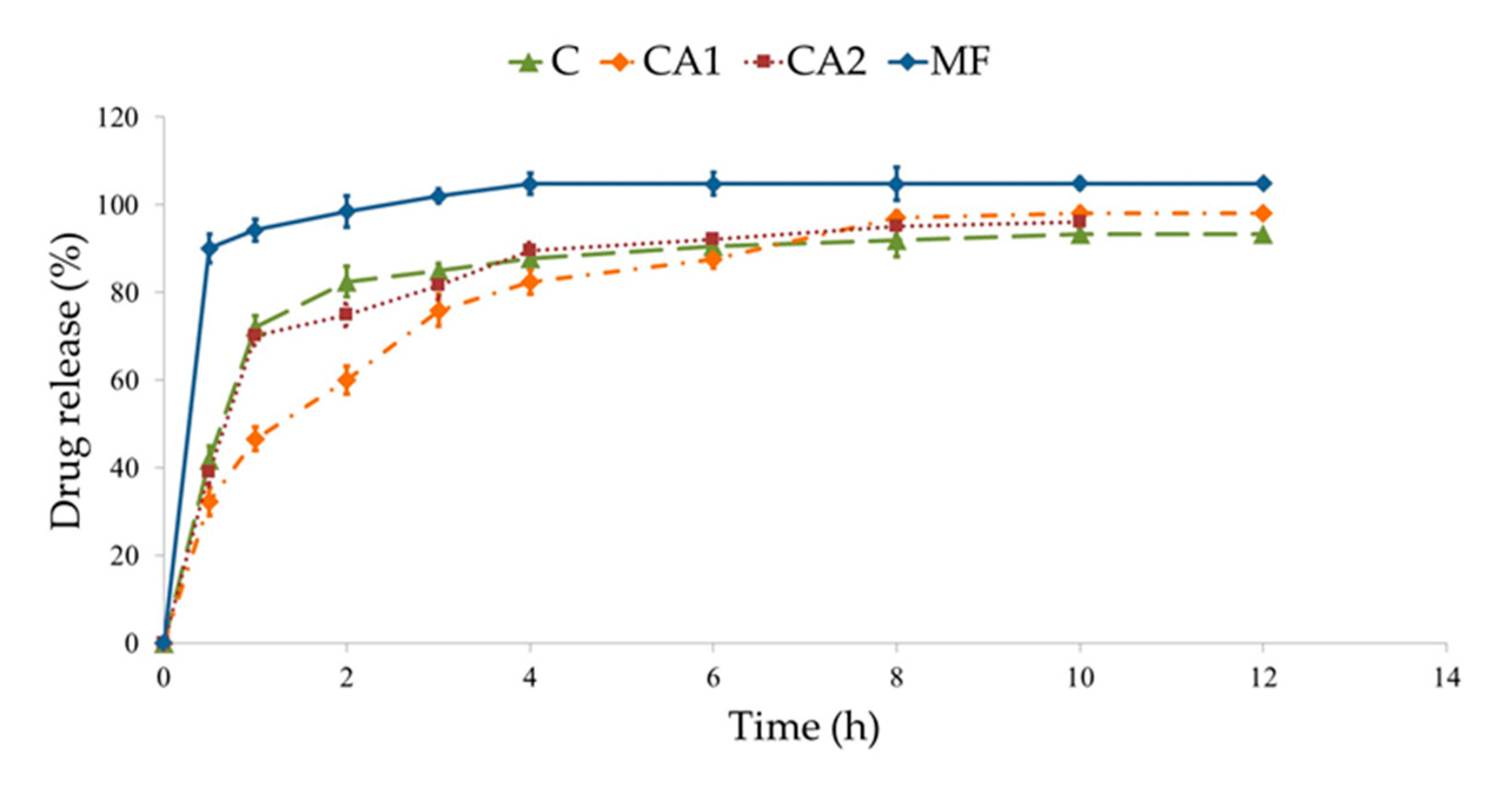
3.3. MF Dissolution
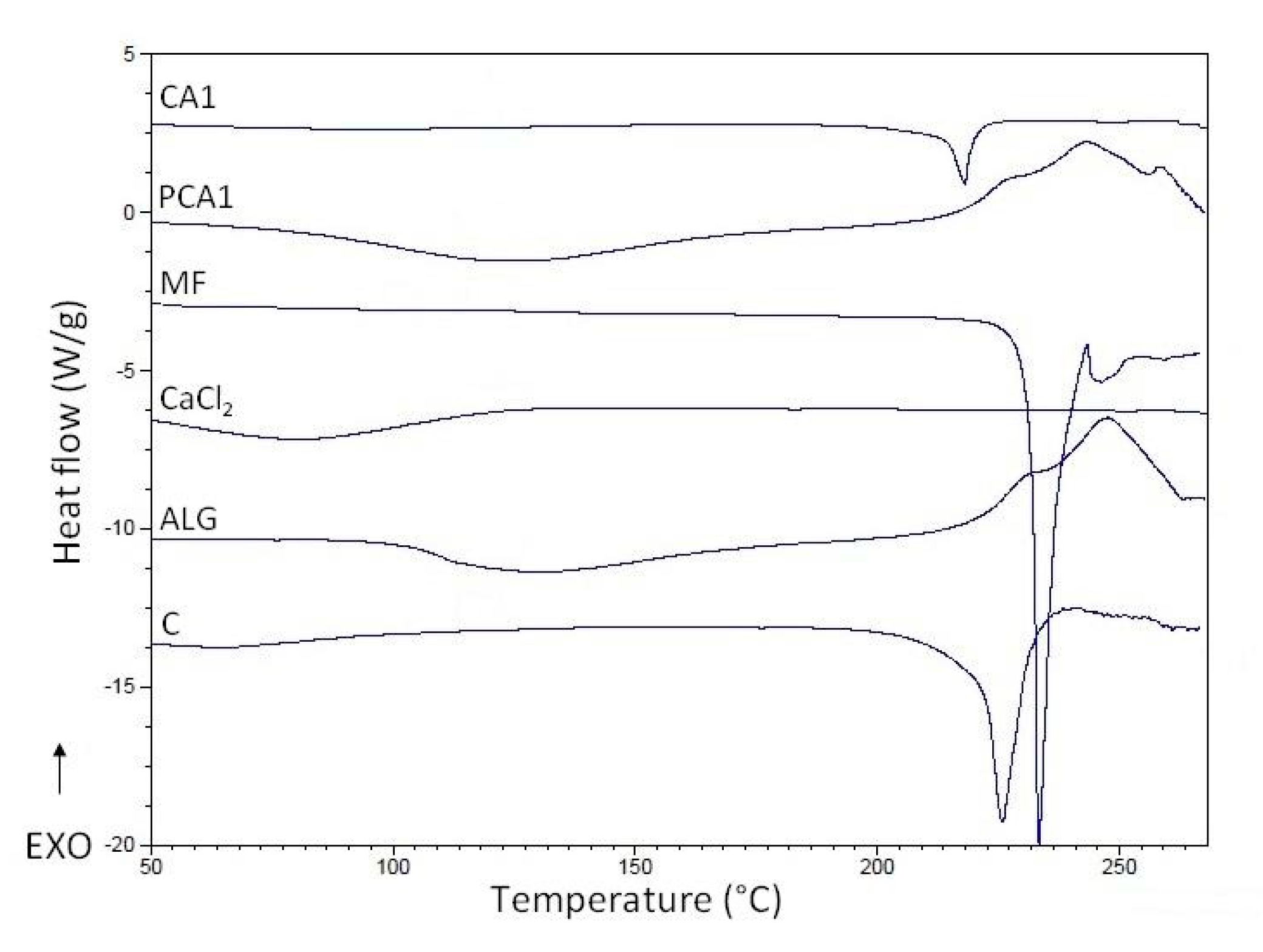
3.4. Differential Scanning Calorimetry (DSC)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, G.; Mei, X.; Xiao, F.; Chen, X.; Tang, Q.; Peng, D. Applications of important polysaccharides in drug delivery. Curr. Pharm. Des. 2015, 25, 3692–3696. [Google Scholar] [CrossRef]
- Asada, T.; Yoshihara, N.; Ochiai, Y.; Kimura, S.I.; Iwao, Y.; Itai, S. Formulation of a poorly water-soluble drug in sustained-release hollow granules with a high viscosity water-soluble polymer using a fluidized bed rotor granulator. Int. J. Pharm. 2018, 541, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Khandai, M.; Sharma, A.; Patra, C.N.; Patro, V.J.; Sen, K.K. Effects of drug solubility on the release kinetics of water soluble and insoluble drugs from HPMC based matrix formulations. Acta Pharm. 2009, 59, 313–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Mythri, G.; Kavitha, K.; Kumar, M.R.; Jagadeesh Singh, S.D. Novel mucoadhesive polymers—A review. J. App. Pharm. Sci. 2011, 1, 37–42. [Google Scholar]
- Patwekar, S.; Baramade, M.K. Controlled release approach to novel multiparticulate drug delivery system. Int. J. Pharm. Pharm. Sci. 2012, 4, 757–763. [Google Scholar]
- Dey, N.S.; Majumdar, S.; Rao, M.E.B. Multiparticulate drug delivery systems for controlled release. Trop. J. Pharm. Res. 2008, 7, 1067–1075. [Google Scholar] [CrossRef]
- Sachan, K.N.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199. [Google Scholar]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W. Alginate graft copolymers and alginate–co-excipient physical mixture in oral drug delivery. J. Pharm. Pharmacol. 2011, 63, 1497–1512. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Jinno, D.; Liu, D.; Isobe, T.; Kofuji, K.; Kawashima, S. The drug release profile from calcium-induced alginate gel beads coated with an alginate hydrolysate. Molecules 2007, 12, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Lee, J.S.; Lee, H.G. Calcium-alginate microparticles for sustained release of catechin prepared via an emulsion gelation technique. Food Sci. Biotechnol. 2016, 25, 1337–1343. [Google Scholar] [CrossRef]
- Lira, A.A.; Rossetti, F.C.; Nanclares, D.M.; Neto, A.F.; Bentley, M.V.; Marchetti, J.M. Preparation and characterization of chitosan-treated alginate microparticles incorporating all-trans retinoic acid. J. Microencapsul. 2009, 26, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Agüero, A.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sarei, F.; Dounighi, N.M.; Zolfagharian, H.; Khaki, P.; Bidhendi, S.M. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J. Pharm. Sci. 2013, 75, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Khanna, O.; Larson, J.C.; Moya, M.L.; Opara, E.C.; Brey, E.M. Generation of alginate microspheres for biomedical applications. J. Vis. Exp. 2012, 66, 3388. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, M.; Maheshwari, D.; Rakha, P.; Dureja, H.; Goyal, S.; Dhingra, G. Formulation development and evaluation of alginate microspheres of ibuprofen. J. Young Pharm. 2012, 4, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; El-Rasoul, S.A.; Auda, S.H.; Ibrahim, M.A. Emulsification/internal gelation as a method for preparation of diclofenac sodium–sodium alginate microparticles. Saudi Pharm. J. 2013, 21, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Jain, D.; Verma, K.; Verma, S. Formulation and in vitro characterization of alginate microspheres loaded with diloxanide furoate for colon-specific drug delivery. Asian J. Pharm. 2010, 6, 199–204. [Google Scholar]
- Suganya, V.; Anuradha, V. Microencapsulation and nanoencapsulation: A review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Giri, T.K.; Choudhary, C.; Alexander, A.; Badwaik, H.; Tripathi, D.K. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharm. J. 2013, 21, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interfacce Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Patel, J.K.; Chakraborty, S. Review of patents and application of spray drying in pharmaceutical, food and flavor industry. Recent Pat. Drug Deliv. Formul. 2014, 8, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, L.; Madadlou, A.; Yarmand, M.; Mousavi, M.E. Spray-dried alginate microparticles carrying caffeine-loaded and potentially bioactive nanoparticles. Food Res. Int. 2014, 62, 1113–1119. [Google Scholar] [CrossRef]
- Santa-Maria, M.; Scher, H.; Jeoh, T. Microencapsulation of bioactives in cross-linked alginate matrices by spray drying. J. Microencapsul. 2012, 29, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Wróblewska, M.; Sosnowska, K.; Winnicka, K. Influence of sodium alginate on hypoglycemic activity of metformin hydrochloride in the microspheres obtained by the spray drying. Int. J. Polym. Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate–chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- León, O.; Muñoz-Bonilla, A.; Soto, D.; Pérez, D.; Rangel, M.; Colina, M.; Fernández-García, M. Removal of anionic and cationic dyes with bioadsorbent oxidized chitosans. Carbohydr. Polym. 2018, 194, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K.; Amelian, A.; Cwalina, U. Vaginal chitosan tablets with clotrimazole-design and evaluation of mucoadhesive properties using porcine vaginal mucosa, mucin and gelatin. Chem. Pharm. Bull. 2014, 62, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. The European Pharmacopeia, 9th ed.; Council of Europe: Strasburg, France, 2016; Volume 1, p. 302. [Google Scholar]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Soni, T.; Nagda, C.; Gandhi, T.; Chotai, N.P. Development of discriminating method for dissolution of aceclofenac marketed formulations. Dissolut. Technol. 2008, 15, 31–35. [Google Scholar] [CrossRef]
- Diaz, D.D.; Colgan, S.T.; Langer, C.S.; Bandi, N.T.; Likar, M.D.; Alstine, L.V. Dissolution similarity requirements: How similar or dissimilar are the global regulatory expectations? AAPS J. 2016, 18, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gray, V.; Kelly, G.; Xia, M.; Butler, C.; Thomas, S.; Mayock, S. The Science of USP 1 and 2 dissolution: Present challenges and future relevance. Pharm. Res. 2009, 26, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Mazurek-Wądołkowska, E.; Winnicka, K.; Czajkowska-Kośnik, A.; Czyżewska, U.; Miltyk, W. Application of differential scanning calorimetry in evaluation of solid state interactions in tablets containing acetaminophen. Acta Pol. Pharm. 2013, 70, 787–793. [Google Scholar] [PubMed]
- Santana, A.A.; Kieckbusch, T.G. Physical evaluation of biodegradable films of calcium alginate plasticized with polyols. Braz. J. Chem. Eng. 2013, 30, 835–884. [Google Scholar] [CrossRef]
- Mandal, S.; Basu, S.K.; Sa, B. Sustained release of a water-soluble drug from alginate matrix tablets prepared by wet granulation method. AAPS PharmSciTech 2009, 10, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Bar-Shalom, D. Alginate drug delivery systems: Application in context of pharmaceutical and biomedical research. Drug Dev. Ind. Pharm. 2014, 40, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.; Bettini, R.; Santi, P.; Peppas, N.A. Swellable matrices for controlled drug delivery: Gel-layer behaviour, mechanisms and optimal performance. Pharm. Sci. Technol. Today 2000, 3, 198–204. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P.; Konthong, S.; Nunthanid, J. Fabrication of calcium pectinate microparticles from pomelo pectin by ionotropic gelation. JAASP. J. 2012, 1, 203–209. [Google Scholar]
- Li, H.; Hardy, R.J.; Gu, X. Effect of drug solubility on polymer hydration and drug dissolution from polyethylene oxide (PEO) matrix tablets. AAPS PharmSciTech 2008, 9, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Hu, H.; Luo, A. The conversion of calcium alginate fibers into alginic acid fibers and sodium alginate fibers. J. Appl. Polym. Sci. 2006, 101, 4216–4221. [Google Scholar] [CrossRef]
- Rojewska, M.; Olejniczak-Rabinek, M.; Bartkowiak, A.; Snela, A.; Prochaska, K.; Lulek, J. The wettability and swelling of selected mucoadhesive polymers in simulated saliva and vaginal fluids. Colloids Surf. B Biointerfaces 2017, 156, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Shaikh, R.; Raj Singh, T.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Aggarwal, S. Mucoadhesive polymeric platform for drug delivery; a comprehensive review. Curr. Drug Deliv. 2015, 12, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Perkins, A.C. In vitro assessment of the mucoadhesion of cholestyramine to porcine and human gastric mucosa. Eur. J. Pharm. Biopharm. 2001, 52, 121–127. [Google Scholar] [CrossRef]
- Abdelbary, A.; El-Gazayerly, O.N.; El-Gendy, N.A.; Ali, A.A. Floating tablet of trimetazidine dihydrochloride: An approach for extended release with zero-order kinetics. AAPS PharmSciTech 2010, 11, 1058–1067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Segale, L.; Giovannelli, L.; Mannina, P.; Pattarino, F. Calcium alginate and calcium alginate-chitosan beads containing celecoxib solubilized in a self-emulsifying phase. Scientifica 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Bianco-Peled, H. A quantitative analysis of alginate swelling. Carbohydr. Polym. 2010, 79, 1020–1027. [Google Scholar] [CrossRef]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; Abu El-Naaj, I.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Sun, Y.S.; Pang, H.; Munyendo, W.L.; Lv, H.X.; Zhu, S.L. Preparation and evaluation of berberine alginate beads for stomach-specific delivery. Molecules 2011, 14, 10347–10356. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Kansal, S.; Garg, G.; Awasthi, R.; Singodia, D.; Kulkarni, G.T. Gastroretentive dosage forms: A review with special emphasis on floating drug delivery systems. Drug Deliv. 2011, 18, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Lehr, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Mi, F.L.; Sung, H.W.; Shyu, S.S. Drug release from chitosan–alginate complex microcapsules reinforced by a naturally occurring cross-linking agent. Carbohydr. Polym. 2002, 48, 61–72. [Google Scholar] [CrossRef]
- Stevenson, C.L.; Bennett, D.B.; Lechuga-Ballesteros, D. Pharmaceutical liquid crystals: The relevance of partially ordered systems. J. Pharm. Sci. 2005, 94, 1861–1880. [Google Scholar] [CrossRef] [PubMed]
- Shirkhorshidi, A.S.; Aghabozorgi, S.; Wah, T.Y. A comparison study on similarity and dissimilarity measures in clustering continuous data. PLoS ONE 2015, 10, e0144059. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), U.S. Government Printing Office. Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations; Guidance for Industry; Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), U.S. Government Printing Office: Washington, DC, USA, 1997. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070239.pdf.U.S (accessed on 10 June 2018).
- European Medicines Agency. Investigation of Bioequivalence; European Medicines Agency: London, UK, 29 January 2010. [Google Scholar]
- Gohel, M.C.; Sarvaiya, K.G.; Mehta, N.R.; Soni, C.D.; Vyas, V.U.; Dave, R.K. Assessment of similarity factor using different weighting approaches. Dissolut. Technol. 2005, 12, 22–37. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Química 2004, 2, 53–56. [Google Scholar] [CrossRef]
- Mucha, M.; Pawlak, A. Thermal analysis of chitosan and its blends. Thermochim. Acta 2005, 427, 69–76. [Google Scholar] [CrossRef]

| Formulation | ALG 1 (%) | MF 2:ALG 1 Ratio | CaCl2 (%) |
|---|---|---|---|
| C | 2 | 2:1 | – |
| PCA1 | 2 | – | 0.1 |
| PCA2 | 2 | – | 0.05 |
| CA1 | 2 | 2:1 | 0.1 |
| CA2 | 2 | 2:1 | 0.05 |
| Solution | Viscosity (mPa∙s) 1 |
|---|---|
| 2% ALG | 132.6 ± 2.7 |
| 0.5% CaCl2 + 2% ALG | 4700.4 ± 14.5 |
| 0.1% CaCl2 + 2% ALG | 1600.6 ± 12.4 |
| 0.05% CaCl2 + 2% ALG | 791.7 ± 5.3 |
| Microparticles | Zeta Potential (mV) | Production Yield (%) | Encapsulation Efficiency (%) | Loading Percentage (%) | Particle Size (µm) |
|---|---|---|---|---|---|
| C | −1.3 ± 0.7 | 61.7 ± 2.1 | 113.4 ± 2.3 | 75.6 ± 1.5 | 3.0 ± 1.6 |
| CA1 | 2.6 ± 0.4 | 57.1 ± 1.6 | 91.5 ± 2.1 | 77.1 ± 1.7 | 3.5 ± 1.2 |
| CA2 | 2.6 ± 0.9 | 56.1 ± 2.4 | 93.9 ± 2.7 | 73.9 ± 3.4 | 3.4 ± 1.4 |
| Formulation | Kind of Adhesive Material | |||||
|---|---|---|---|---|---|---|
| Gelatine Disc | Mucin Gel | Porcine Stomach Mucosa | ||||
| Fmax (N) 1 | Wad (µJ) 2 | Fmax (N) 1 | Wad (µJ) 2 | Fmax (N) 1 | Wad (µJ) 2 | |
| Control 3 | 0.02 ± 0.01 | 15.2 ± 0.7 | 0.03 ± 0.01 | 18.1 ± 3.5 | 0.07 ± 0.01 | 29.4 ± 3.3 |
| C | 0.5 ± 0.2 | 283.3 ± 51.2 | 0.6 ± 0.3 | 342.3 ± 29.3 | 0.6 ± 0.2 | 467.5 ± 17.4 |
| PCA1 | 0.7 ± 0.1 | 291.3 ± 14.4 | 0.5 ± 0.2 | 362.9 ± 18.8 | 1.3 ± 0.3 | 519.7 ± 16.9 |
| PCA2 | 0.6 ± 0.2 | 272.3 ± 13.1 | 0.5 ± 0.1 | 353.4 ± 21.4 | 1.2 ± 0.1 | 504.6 ± 21.3 |
| CA1 | 0.7 ± 0.2 | 269.4 ± 14.1 | 0.6 ± 0.2 | 359.2 ± 18.5 | 1.1 ± 0.7 | 583.4 ± 15.7 |
| CA2 | 0.6 ± 0.1 | 254.6 ± 16.7 | 0.7 ± 0.3 | 347.1 ± 32.1 | 1.3 ± 0.2 | 500.5 ± 13.5 |
| Mathematical Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Formulation | Zero Order | First Order | Highuchi | Korsmeyer-Peppas | Hixson-Crowell | ||||||
| R2 | K | R2 | K | R2 | K | R2 | K | n | R2 | K | |
| C | 0.52 | 4.62 | 0.73 | 0.21 | 0.66 | 18.76 | 0.59 | 0.28 | 0.12 | 0.65 | 0.19 |
| CA1 | 0.86 | 1.68 | 0.96 | 0.55 | 0.95 | 29.37 | 0.88 | 0.34 | 0.08 | 0.94 | 0.58 |
| CA2 | 0.92 | 1.67 | 0.98 | 0.17 | 0.97 | 22.98 | 0.94 | 0.31 | 0.08 | 0.96 | 0.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szekalska, M.; Sosnowska, K.; Czajkowska-Kośnik, A.; Winnicka, K. Calcium Chloride Modified Alginate Microparticles Formulated by the Spray Drying Process: A Strategy to Prolong the Release of Freely Soluble Drugs. Materials 2018, 11, 1522. https://doi.org/10.3390/ma11091522
Szekalska M, Sosnowska K, Czajkowska-Kośnik A, Winnicka K. Calcium Chloride Modified Alginate Microparticles Formulated by the Spray Drying Process: A Strategy to Prolong the Release of Freely Soluble Drugs. Materials. 2018; 11(9):1522. https://doi.org/10.3390/ma11091522
Chicago/Turabian StyleSzekalska, Marta, Katarzyna Sosnowska, Anna Czajkowska-Kośnik, and Katarzyna Winnicka. 2018. "Calcium Chloride Modified Alginate Microparticles Formulated by the Spray Drying Process: A Strategy to Prolong the Release of Freely Soluble Drugs" Materials 11, no. 9: 1522. https://doi.org/10.3390/ma11091522
APA StyleSzekalska, M., Sosnowska, K., Czajkowska-Kośnik, A., & Winnicka, K. (2018). Calcium Chloride Modified Alginate Microparticles Formulated by the Spray Drying Process: A Strategy to Prolong the Release of Freely Soluble Drugs. Materials, 11(9), 1522. https://doi.org/10.3390/ma11091522