3.1. Results of the Graphite Measurements
The first step of the material characterization included determination of the crystallite diameter (La), crystalline height (Lc) and interplanar distance (d002).
As shown in
Table 2, the analysed graphites are characterized by similar interplanar distances. Moreover, the crystallites have a large diameter (50–82 nm), which is desirable for subsequent synthetic processes. The crystalline height is from 20 to 29 nm, and the interplanar distance is in the range from 0.3362 to 0.3379 nm.
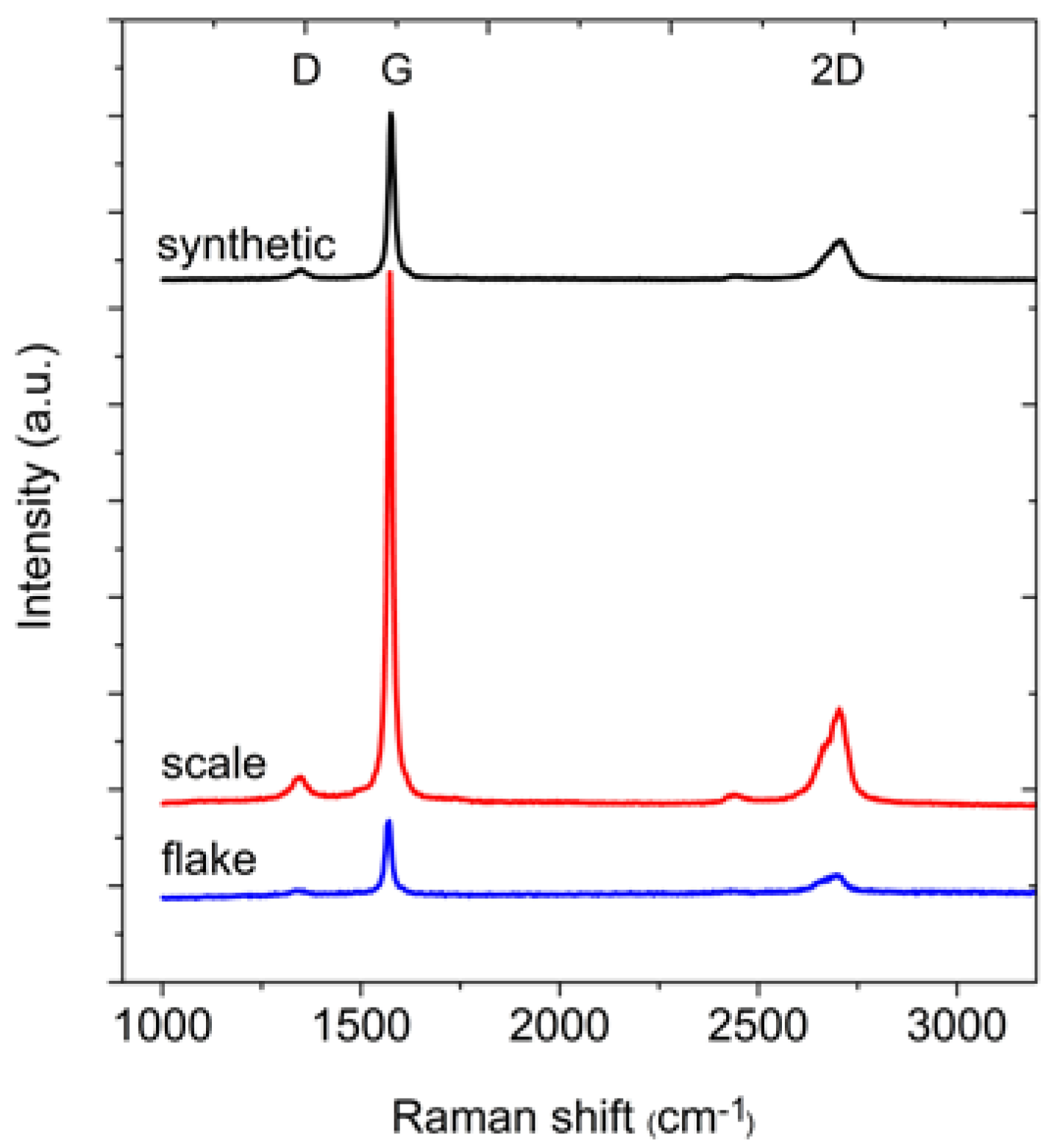
In the next step of the measurements, the Raman spectra of the graphite samples were collected (
Figure 3). From these spectra, the position and full width at half maximum (FWHM) of the D, G and 2D bands were determined (
Table 3). The differences between the positions of the individual bands are small. The FWHM of the G peak is in the range 16–19 cm
−1, and this small value indicates that the peak originates from the stretching vibrations of carbon bonds in the rings of the sp
2 hybridized graphene layers. Meanwhile, the FWHM of the D band is in the range from 38 to 52 cm
−1. This large FWHM means that the structure is perturbed, for example by the presence of heteroatoms or point dislocations in the structure (this band should not be observed in the case of perfect graphite [
30]). However, the structures of all graphites are quite well-ordered, as confirmed by the A
G/A
D ratio (where A represents the area under the peak). The area under the G peak is approximately 7–8 times larger than the area under the D peak. For all analysed graphites, the intensity of the G peak is much larger than the intensity of the D peak.
3.2. Results of the Graphite Oxide Measurements
The first stage of measurements of the graphite oxides included analysis using the XRD method. Based on the obtained data (
Table 4), the largest interplanar distances (d
001) are obtained for the GROFs. The smallest crystallite diameter, L
a, is observed for the graphite oxides obtained from synthetic graphite, regardless of the oxidation method. The crystalline height (L
c) is similar for all the GROs.
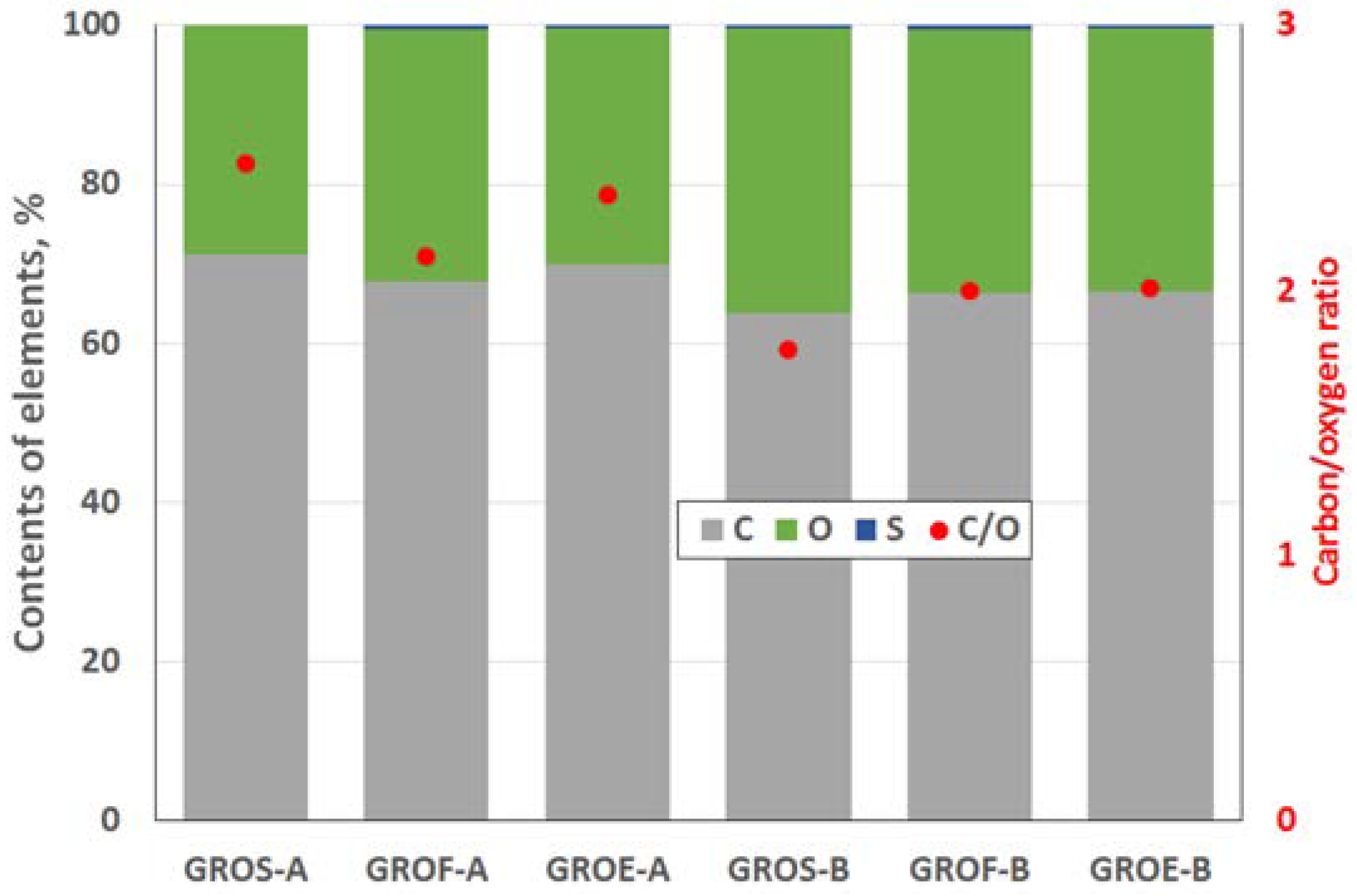
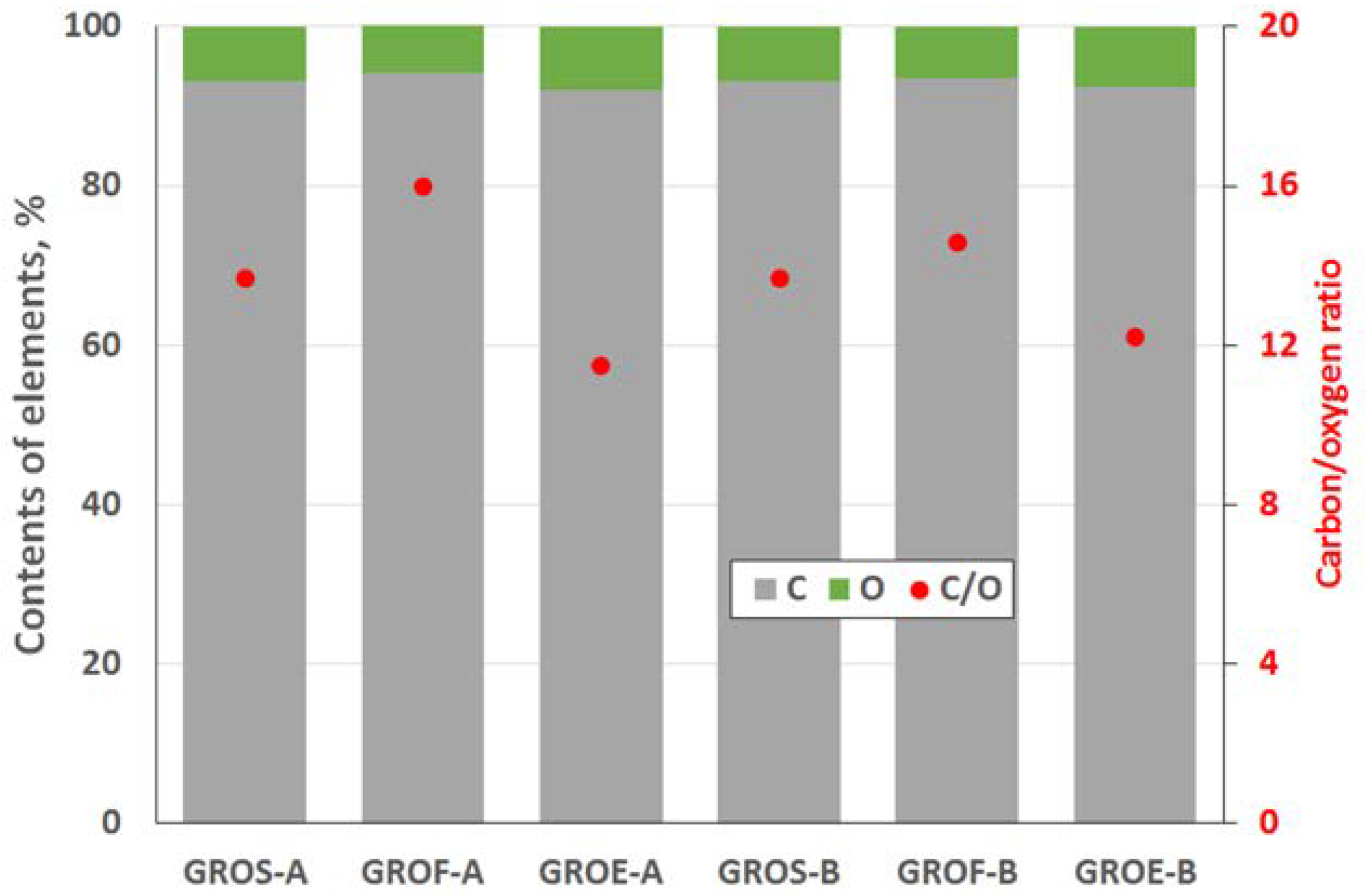
The GROs were also analysed using the XPS method (
Figure 4 and
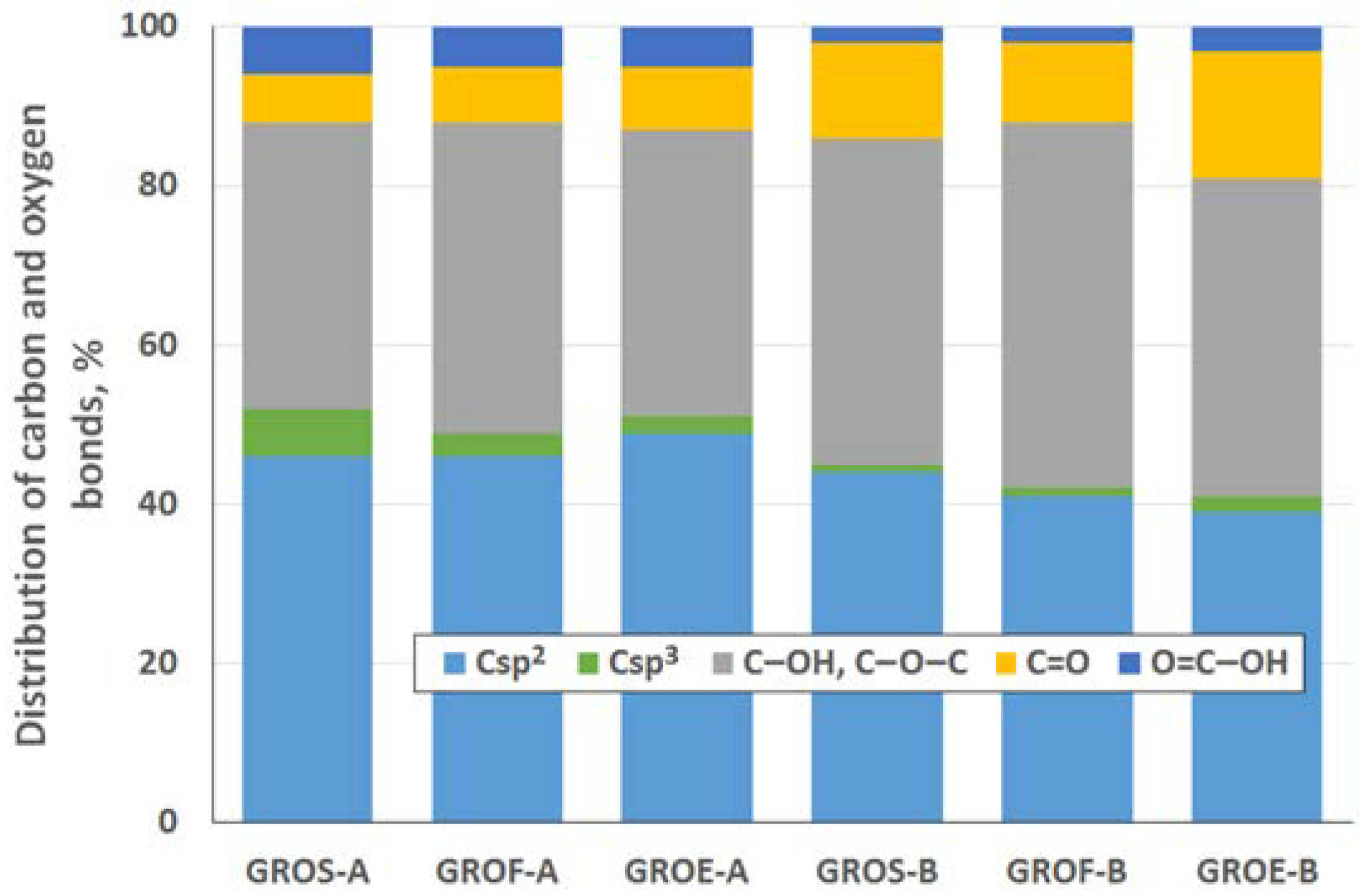
Figure 5). All the graphite oxides are composed mainly of carbon and oxygen. The amount of carbon (>63%) in the GROs is much smaller than that in the graphites, regardless of the graphite type. The amount of oxygen is above 28%. The C/O ratio determined for the GROs oxidized by method A is larger than that for the GROs oxidized by method B. This result indicates that the B method was more effective for introducing oxygen into the graphite structure. The content of sulphur is low—below 0.5 at %.
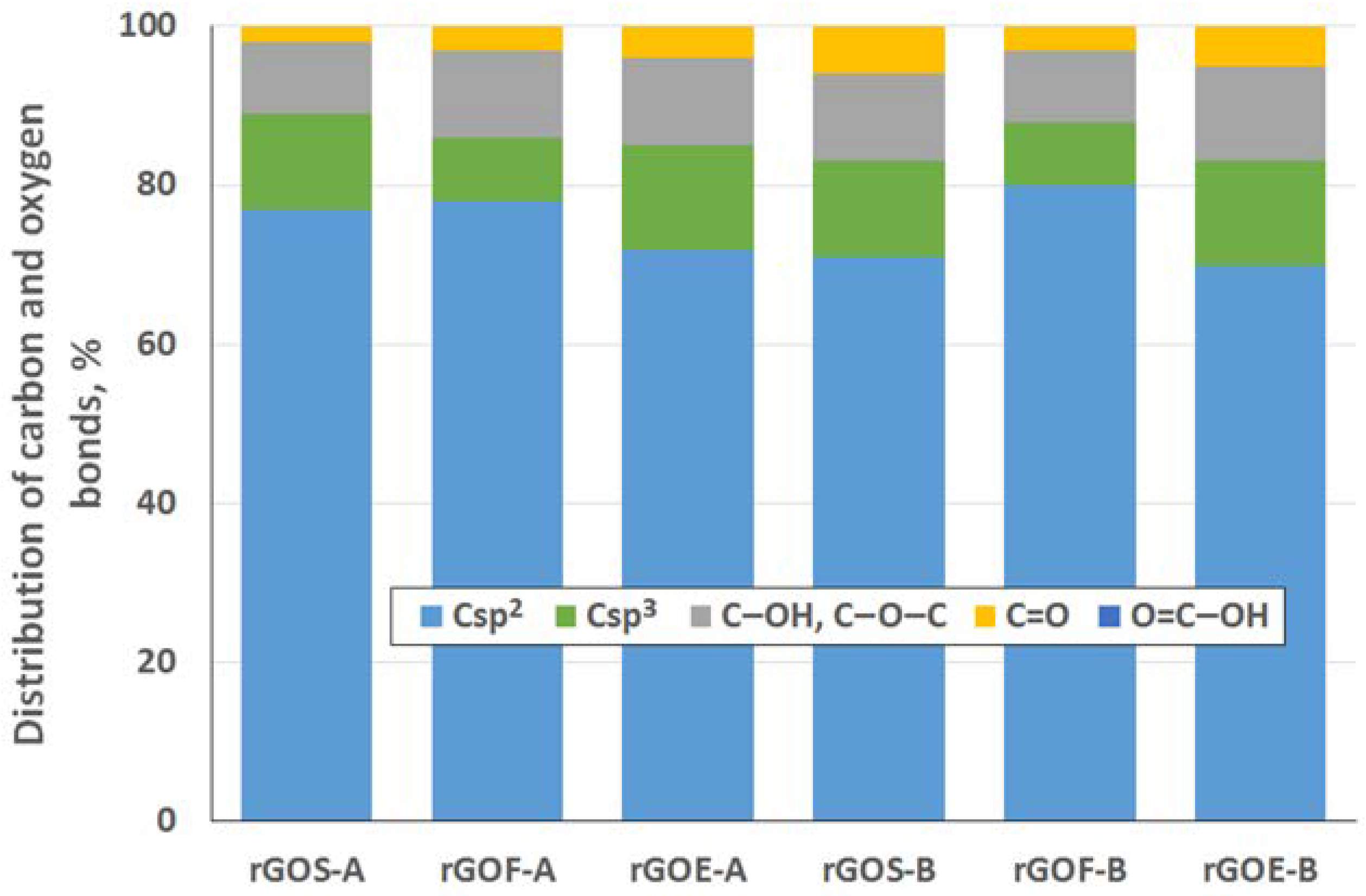
To determine the distribution of carbon and oxygen bonds, the C 1s XPS spectra of the graphite oxides were deconvoluted into five components. After deconvolution, two peaks appear at energies (284.5 ± 0.1 eV and 285.4 ± 0.2 eV) that correspond to the sp2 and sp3 bonds of carbon. Moreover, peaks corresponding to carbon bonded to oxygen dominate the spectra of the GROs: three peaks (286.5 ± 0.3 eV, 287.6 ± 0.2 eV and 288.9 ± 0.3 eV) that correspond to hydroxyl/epoxy (C–OH, C–O–C), carbonyl/quinone (C=O) and carboxyl (O=C–OH) groups are identified. Based on the obtained data, the hydroxyl and epoxy groups are the most abundant. Smaller amounts of carbonyl and quinone groups are present, and the carboxyl group content is the smallest (more carbonyl and quinone groups and fewer carboxyl groups are present in the GROs oxidized by method B). Furthermore, the lowest degree of graphite oxidation is observed for the GROSs.
All of the experiments whose results were described above (XRD and XPS) were performed to obtain information about the tested graphites and graphite oxides. This knowledge was helpful for interpreting the data obtained from Raman Spectroscopy (RS). The RS method enables much more detailed analysis of the changes related the introduction of oxygen atoms into the graphite structure, thus increasing the understanding of the unstructured phase.
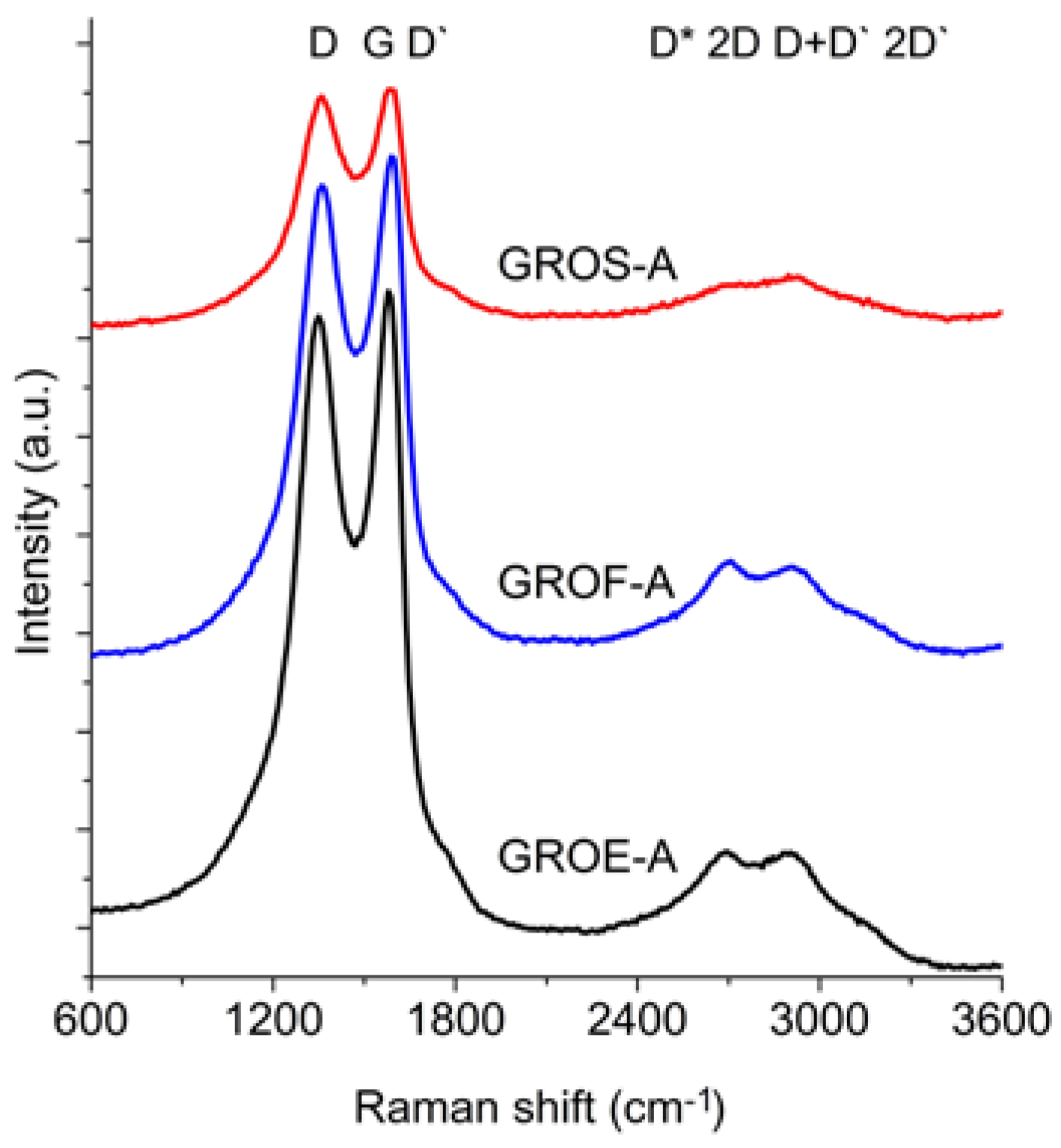
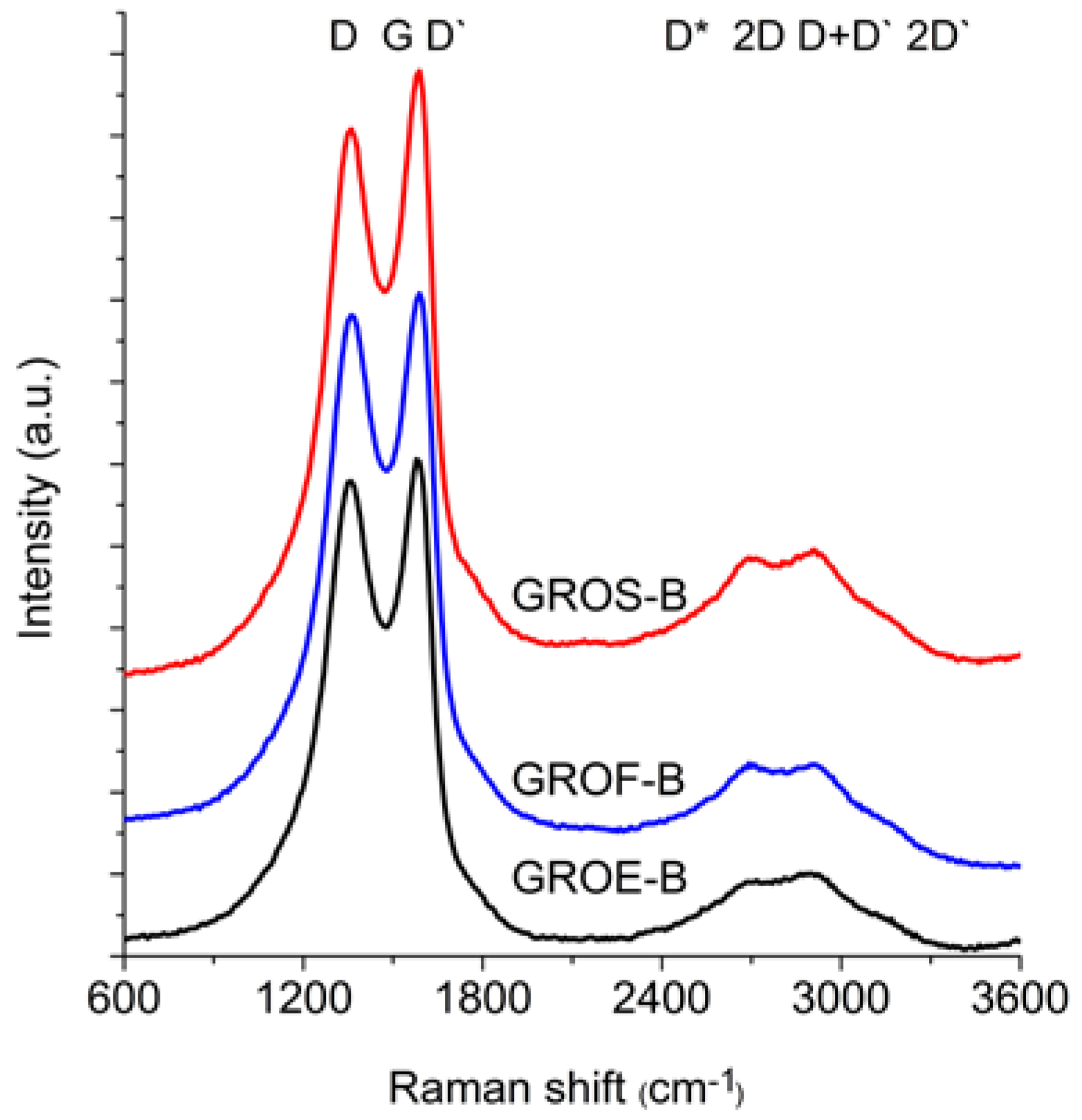
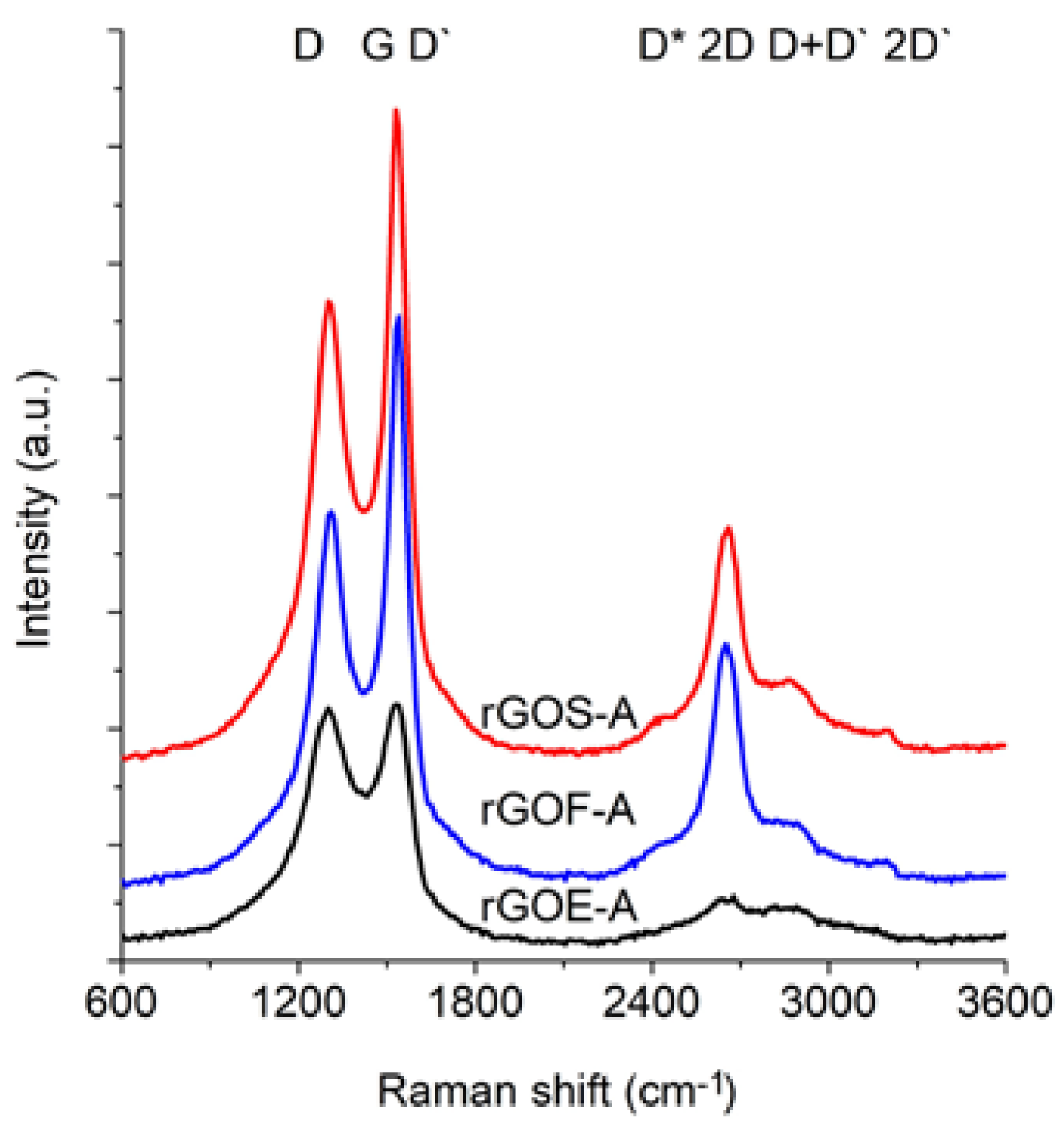
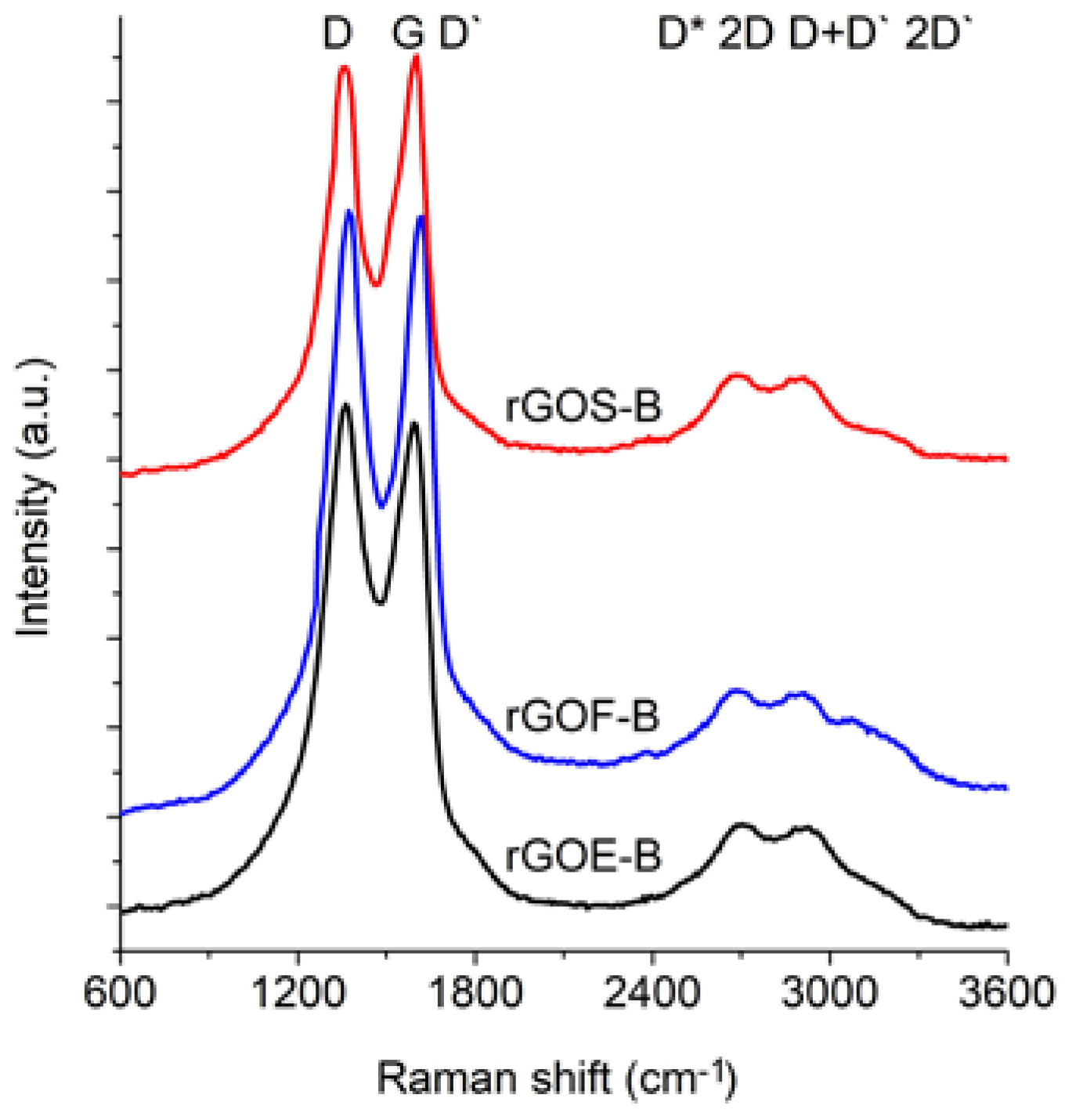
Figure 6 and
Figure 7 show the Raman spectra of the GROs oxidized by methods A and B, respectively. All spectra were deconvoluted according to the method in reference [
28] (for further analysis, the corresponding values were obtained from the deconvoluted spectra). The obtained data (FWHM and position of the peaks) are shown in
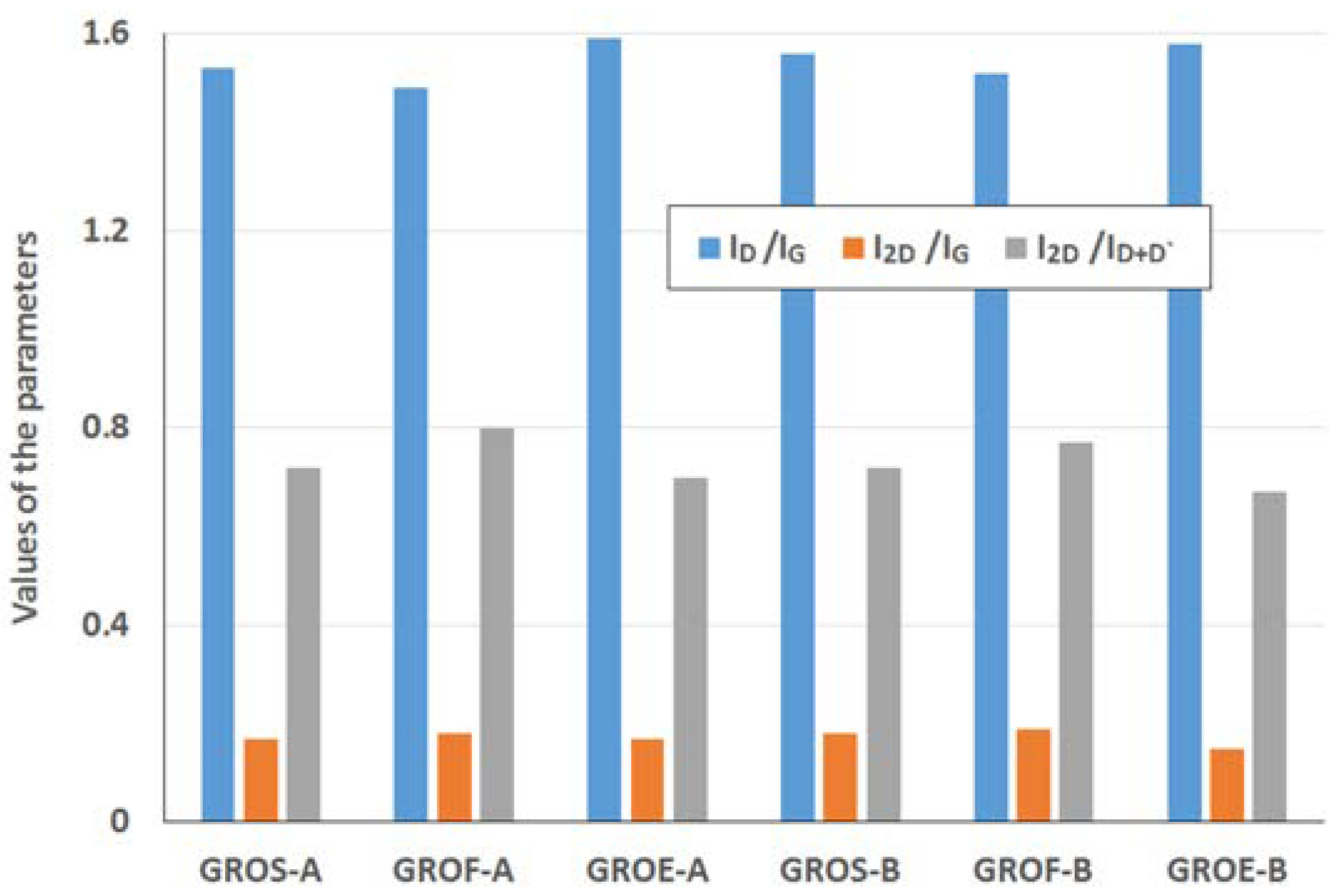
Table 5, and the results of the calculations (I
D/I
G, I
2D/I
G, and I
2D/I
D+D’) are shown in
Figure 8.
The spectra of GRO are characterized by intense D and G bands and a wide 2D band. Such an intense D band indicates the formation of defects in the structures. The increase in the intensity of the D band in relation to that of the G band indicates an increase in the amount of the disordered phase in the GROs. Unlike in the Raman spectra of the graphites, for the GROs, the D band is more intense than the G band, which is related to the formation of sp3 hybridized bonds as a result of the oxidation of graphite. Both the D and G bands of the GROs are wider than the D and G bands in the Raman spectra of graphite. Moreover, the G band of GRO is shifted towards lower wavenumbers, which confirms the presence of defects in the graphene layers.
The presence of oxygen atoms causes both an increase in the interplanar distance and changes in the characteristics of the vibrations in the material’s lattice. For this reason, the Raman spectra of oxidized graphites are characterized by more intense D peaks in comparison to the D peaks of the unoxidized graphites. If the degree of oxidation is larger, the intensity of this peak is also larger [
20,
31].
The FWHM values of the D and G bands are larger for the GROs than for the graphites. The wide G bands in the GROs are accompanied by a D’ band, the intensity of which is proportional to the number of defects. The FWHM of the D’ peak is in the range from 61 to 66 cm
−1. A D’ band is observed in the spectra of graphene materials obtained from the oxidation or direct exfoliation of graphites and subsequent reduction of the GROs, which confirms the formation of structural defects [
20,
22,
23,
31,
32]. Nevertheless, many researchers regard the wide G band in GROs as a single peak (not a sum of G and D’ peaks) [
20].
The 2D band is less intense than the D band. Based on the position, shape and intensity of the 2D band obtained from deconvolution of the Raman spectrum into components (Lorentz curves), the number of graphene layers can be determined [
33,
34,
35]. In the case of graphene monolayers, the 2D band occurs as a single peak near 2675 cm
−1 [
33]. In contrast, the 2D band of two graphene layers consists of four peaks: D*, 2D, D+D’ and 2D’. In this case, the D+D’ peak is more intense than the others. An increase in the number of layers reduces the intensities of the D* and 2D peaks and shifts the components towards higher wavenumbers.
In the spectra of the analysed GROs, four peaks: D* (2522–2558 cm
−1), 2D (2695–2703 cm
−1), D+D’ (2897–2910 cm
−1) and 2D’ (3090–3118 cm
−1), are obtained after deconvolution. The FWHM of the 2D peak is approximately 230 cm
−1, which is much larger than that of the 2D peak of graphene (approximately 30 cm
−1) [
30]. The shape of the spectra and the observation of D*, 2D, D+D’ and 2D’ peaks indicate that the GROs consist of two or more graphene layers in an aggregate. Moreover, the higher intensity of the 2D band in relation to that of the G band confirms the presence of more defects in the structure. Please note that when the material is composed of two or more layers in an aggregate, the 2D band consists of two peaks, 2D
1 and 2D
2 [
36] (marked according to the earlier designation as 2D and D+D’). In the case of the examined GROs, both the 2D and D+D’ peaks are observed.
The GROs obtained by method B are characterized by equal or lower I2D/ID+D’ ratios relative to those of the GROs prepared by method A. This difference suggests that more defects are present in the aggregates of the GROs prepared by method B. The largest ID/IG ratio and the smallest I2D/IG and I2D/ID+D’ ratios are observed for the oxides obtained from synthetic graphite, which may indicate that synthetic graphite is the most susceptible to oxidation and exfoliation.
3.3. Results of the Reduced Graphene Oxide Measurements
All reduced graphene oxides were examined by the same methods as those employed for the graphite oxides, and XRD measurements were performed first. The structural parameters of the rGOs are shown in
Table 6. Following thermal exfoliation and reduction, the (001) band completely disappears from the diffraction patterns of the rGOs. Simultaneously, a (00X) band appears due to the partial removal of oxygen groups. Moreover, a more intense (002) is observed, which indicates the progressive reconstruction of the crystalline structure. The oxidation of graphite by method B can be concluded to lead to a larger separation of graphene layers (larger values of d
00X and d
002) as a consequence of the greater degree of exfoliation that occurs during high-temperature processing. The values calculated on the basis of the 002 band are in the range (0.3373–0.3393 nm) for method A and in the range (0.3421–0.3440 nm) for method B. The largest values of the interplanar distances (d
00X) are observed for the rGOF materials, while the smallest ones are observed for rGOE. The maintenance of a large distance between graphene layers may be related to the presence of significant amounts of oxygen in the rGOs and a large degree of defect formation in the graphene layers. The crystallite sizes of the rGOs differ depending on the type of graphite used and the oxidation method. The smallest values of L
c and L
a are obtained for rGOs fabricated from synthetic graphite. Moreover, analysis of the obtained parameters reveals that higher values of L
c and L
a are obtained for the rGO-A series independent of the type of graphite. This result suggests that a lower degree of exfoliation occurs for the GROs obtained by method A. The resulting rGOs (method A) are characterized by a lower susceptibility to high-temperature exfoliation (larger number of layers).
Based on the data obtained using the XPS method (
Figure 9), a larger percentage of carbon and smaller percentage of oxygen are observed in the rGOs relative to those of the GROs, in which the amount of oxygen decreased 4–5 times. The degree of reduction of the rGO surface, represented by the C/O ratio, depends on the type of graphite used and increases in the order rGOE < rGOS < rGOF.
As was done for the GROs, the distribution of carbon and oxygen bonds was determined for rGO using the XPS method. Analysis of the XPS C 1s spectra after deconvolution (data presented in
Figure 10) confirms that the highest degree of reduction of the rGOs is obtained for the samples prepared from flake graphite. The highest percentage of sp
2 hybridized carbon (78% for A method and 80% for B method) occurs for these samples, and their percentage of sp
3 hybridized carbon is the smallest among all the rGOs. Meanwhile, the largest amount of sp
3 hybridized carbon in the rGOs is obtained for the samples prepared from synthetic graphite. As a reminder, the lowest degree of reduction, expressed as the C/O ratio, also occurs in the rGOEs.
Deconvolution of the C 1s spectra shows that the rGOs mainly contain hydroxyl and epoxy groups. The carbon in the carbonyl and quinone groups accounts for 2% to 6% of the total content, and only a trace content of carboxyl carbons is observed. Following thermal exfoliation/reduction to produce rGO, the amounts of hydroxyl and epoxy groups decreases almost four times (
Figure 10) compared to those in GRO (
Figure 5). Furthermore, fewer carbonyl and quinone groups are present in the structures of the rGOs.
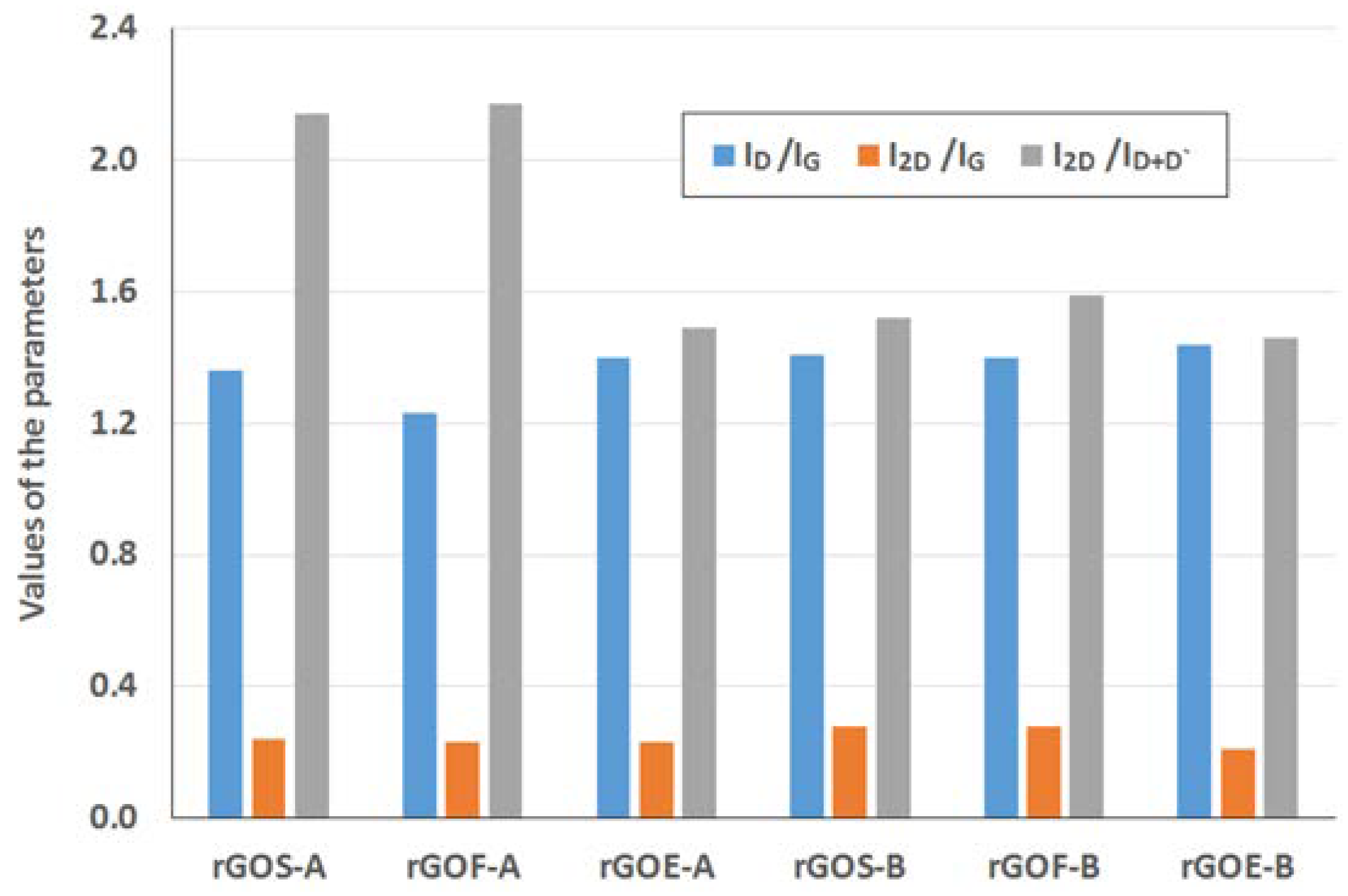
The Raman spectra of the reduced graphene oxides obtained by methods A and B are presented in
Figure 11 and
Figure 12, respectively. The position and the FWHM of the peaks are shown in
Table 7 while the average values of the parameters: I
D/I
G, I
2D/I
G, and I
2D/I
D+D’ are shown in
Figure 13. These spectra exhibit the same bands as those of the GROs. However, the G bands shift towards lower wavenumbers compared to the G bands in the Raman spectra of the GROs. This difference is associated with the lower content of oxygen groups in the rGOs and the regeneration of the graphite structure. At the same time, the intensity of the D band increases, which indicates the formation of new defects in the structures during the reduction process.
Reduced graphene oxides are made of graphene layers with residual oxygen groups attached to the planes and edges. The obtained materials exhibit intense G band and D bands, which confirms the presence of defects in the graphene layers. The FWHM values of the D and G peaks of the rGOs are smaller than those of the GROs, which indicates the presence of fewer defects in the structure (associated with a lower oxygen content). In the spectra of the rGOs, the intense G band is accompanied by a D’ band, the intensity of which is proportional to the amount of defects in the structure. The FWHM of this band is ~61–68 cm−1, which is comparable to the corresponding FWHM values of the GROs (~61–66 cm−1), thereby confirming that both the reduction and oxidation process contribute to the formation of defects in the graphene layers.
The rGO-Bs are characterized by D* and 2D bands with larger FWHM values compared to those of the rGO-As, which indicates that these samples have fewer graphene layers in their aggregates (as a reminder: the average number of layers determined using the XRD method is 22–28 for the rGO-As and 8–11 for the rGO-Bs). Simultaneously, the FWHM values obtained for the rGO-Bs are larger than those obtained for the GRO-Bs. This result indicates that the aggregates of the rGOs contain fewer graphene layers than the aggregates of the GROs (also, the intensities of these bands are weaker).
The intensity of the D peak is lower for the rGO-A samples than the rGO-B samples, confirming that these materials contain fewer defects. This feature further results in lower I
D/I
G ratios. The higher I
D/I
G ratios obtained for the rGO-B samples confirm that despite thermal reduction of these structures, more defects and imperfections are present in these samples than in the rGO-A structures. This difference can be explained by the higher oxygen content in the rGO-Bs, which is related to larger distances between the layers and a greater degree of exfoliation. The I
D/I
G values determined for the rGOs depend on the type of graphite used and increase in the order: flake (F) < scale (S) < synthetic (E) graphite (the lowest I
D/I
G values are obtained for the rGOs prepared from flake graphite, which indicates that these samples contain the fewest defects). This trend can be related to the size of the graphene layers (L
a) determined using the XRD method, in which the opposite trend is observed (synthetic < scale < flake graphite). Based on the measurements performed, smaller graphite crystallites lead to larger amounts of defects in the obtained rGO structures. This relationship was also confirmed in the papers [
1,
37], where the authors determined that graphene oxides containing the fewest defects were obtained from graphite precursors with the largest crystallites.
Analysis of the Raman spectra of the rGOs shows that the intensity of the 2D band is much lower than that of the G band. The I2D/IG ratio is related to the number of graphene layers, and larger values of this ratio are observed for the rGOs than for the corresponding GROs. The smallest values are obtained for rGOE-B, which indicates that this material has the fewest layers in its aggregates. Due to the type of graphite used, the I2D/ID+D’ ratio is the smallest for the GRO-Es. Please note that these materials are characterized by the smallest quantity of sp2 hybridized carbon and the largest quantity of sp3 hybridized carbon (determined by XPS measurements), indicating a high degree of destruction of the graphene layers. Furthermore, the I2D/ID+D’ ratios are the smallest in this case. Conversely, the rGO-Fs are characterized by the largest quantity of sp2 hybridized carbon and the smallest quantity of sp3 hybridized carbon, which indicates the presence of fewer defects in these graphene structures.