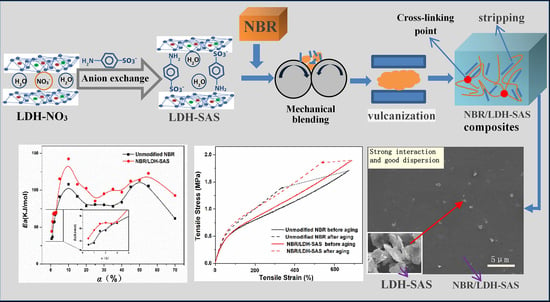
Aging-Resistant Functionalized LDH–SAS/Nitrile-Butadiene Rubber Composites: Preparation and Study of Aging Kinetics/Anti-Aging Mechanism
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of LDH–NO3
2.3. Preparation of LDH-SAS by an Organic Anion
2.4. Preparation of NBR/LDH Composites
2.5. Aging Test of NBR
2.6. Characterization and Measurements
3. Results and Discussion
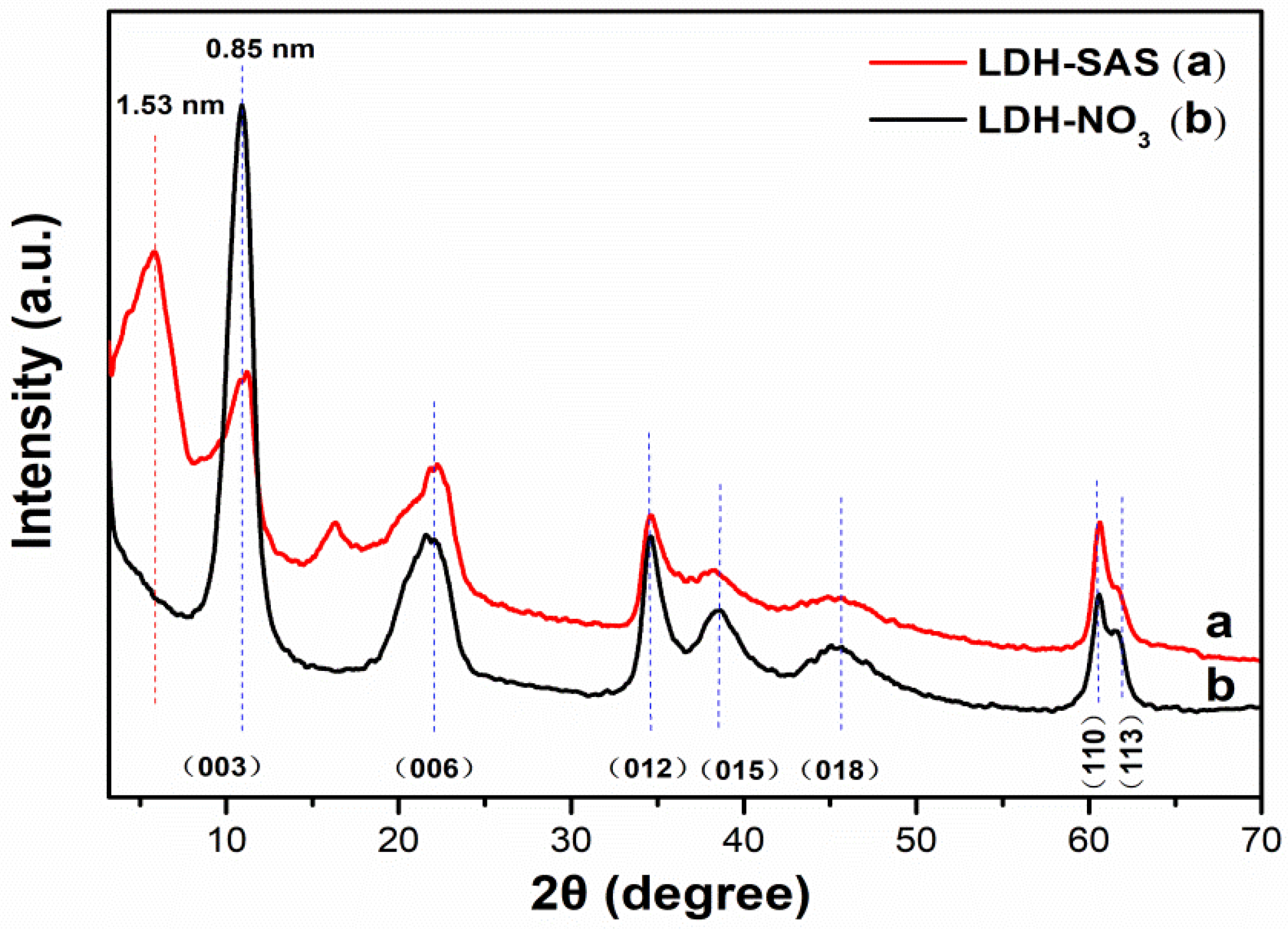
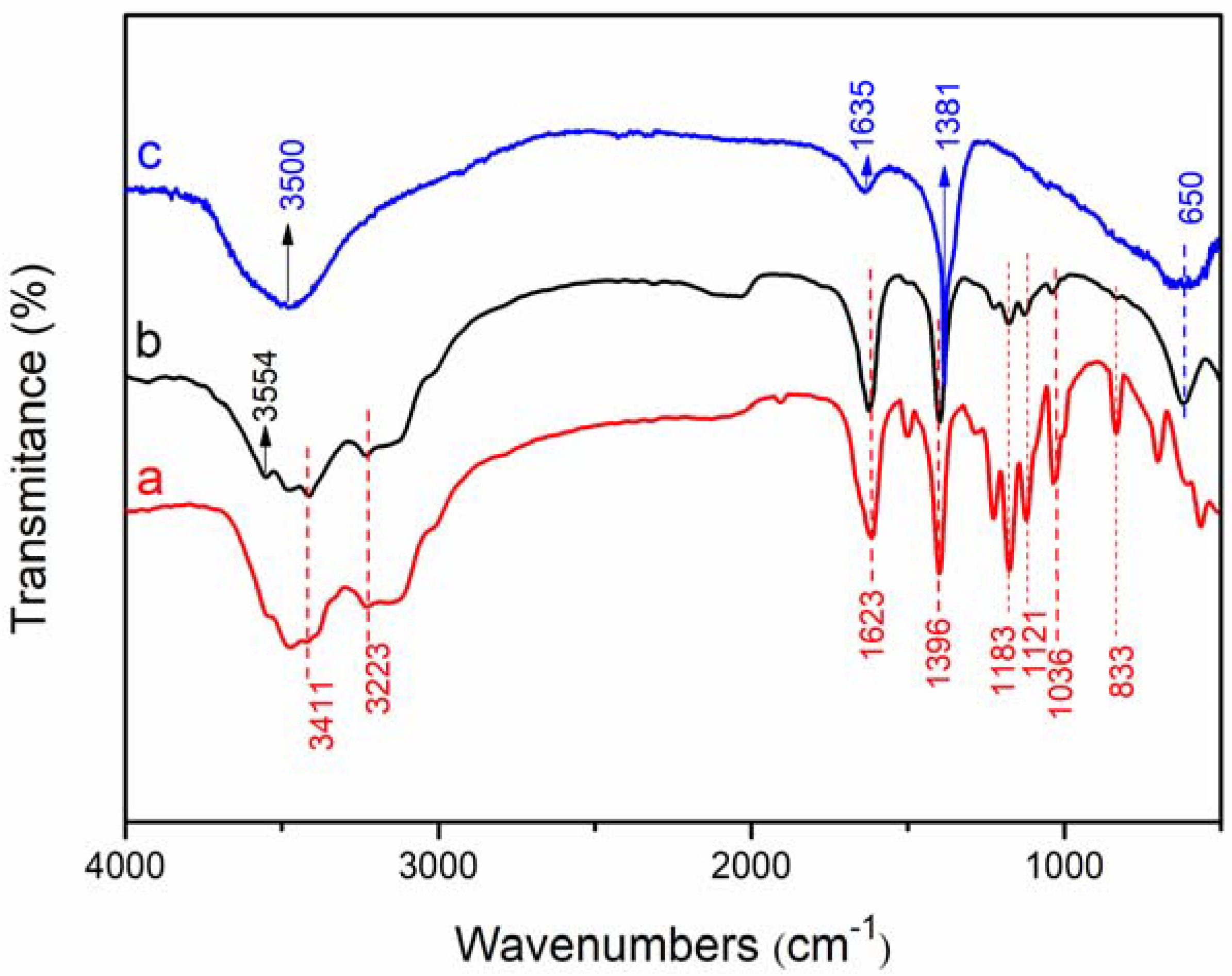
3.1. Structural and Chemical Properties of LDHs
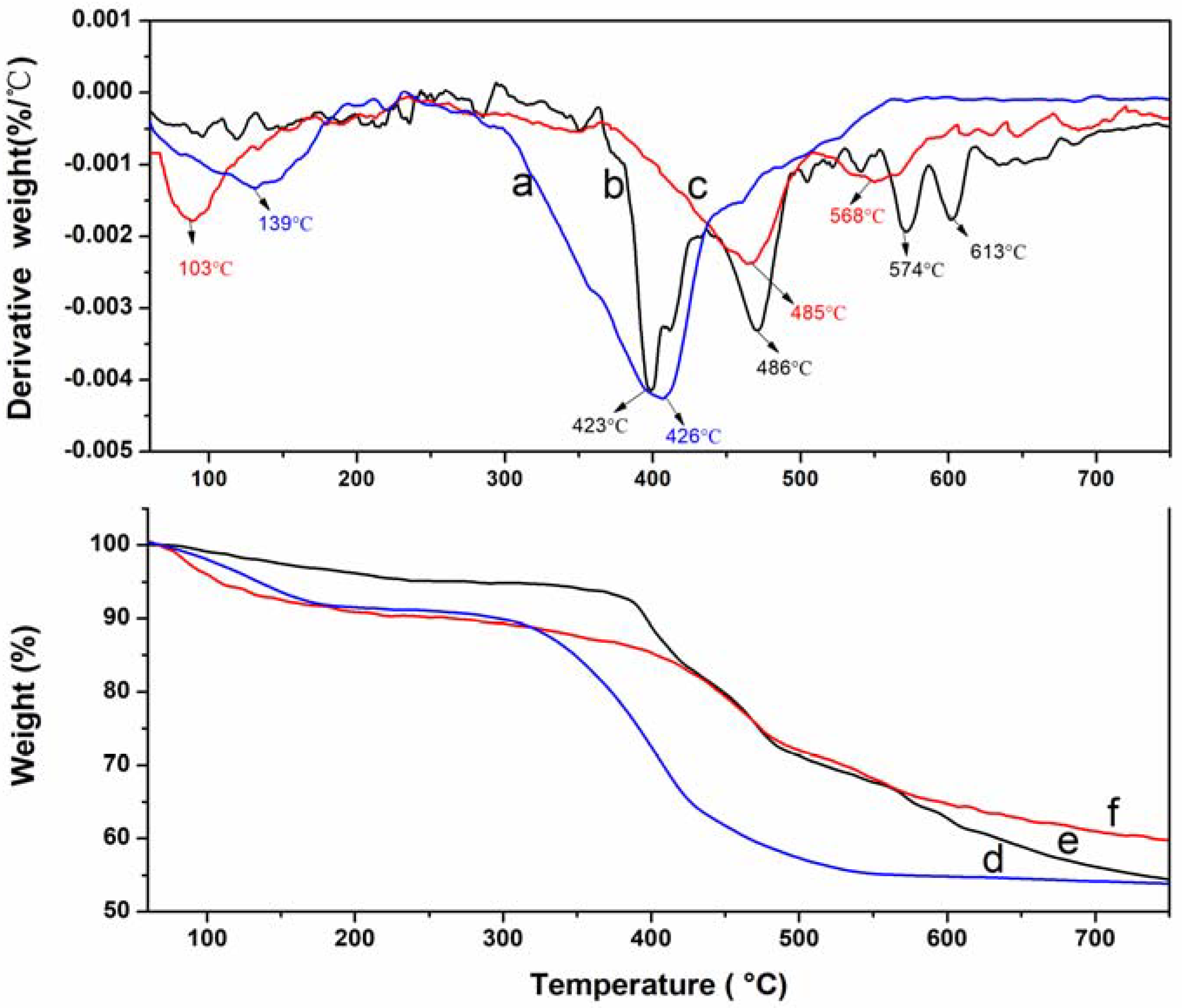
3.2. Thermal Stability of LDHs
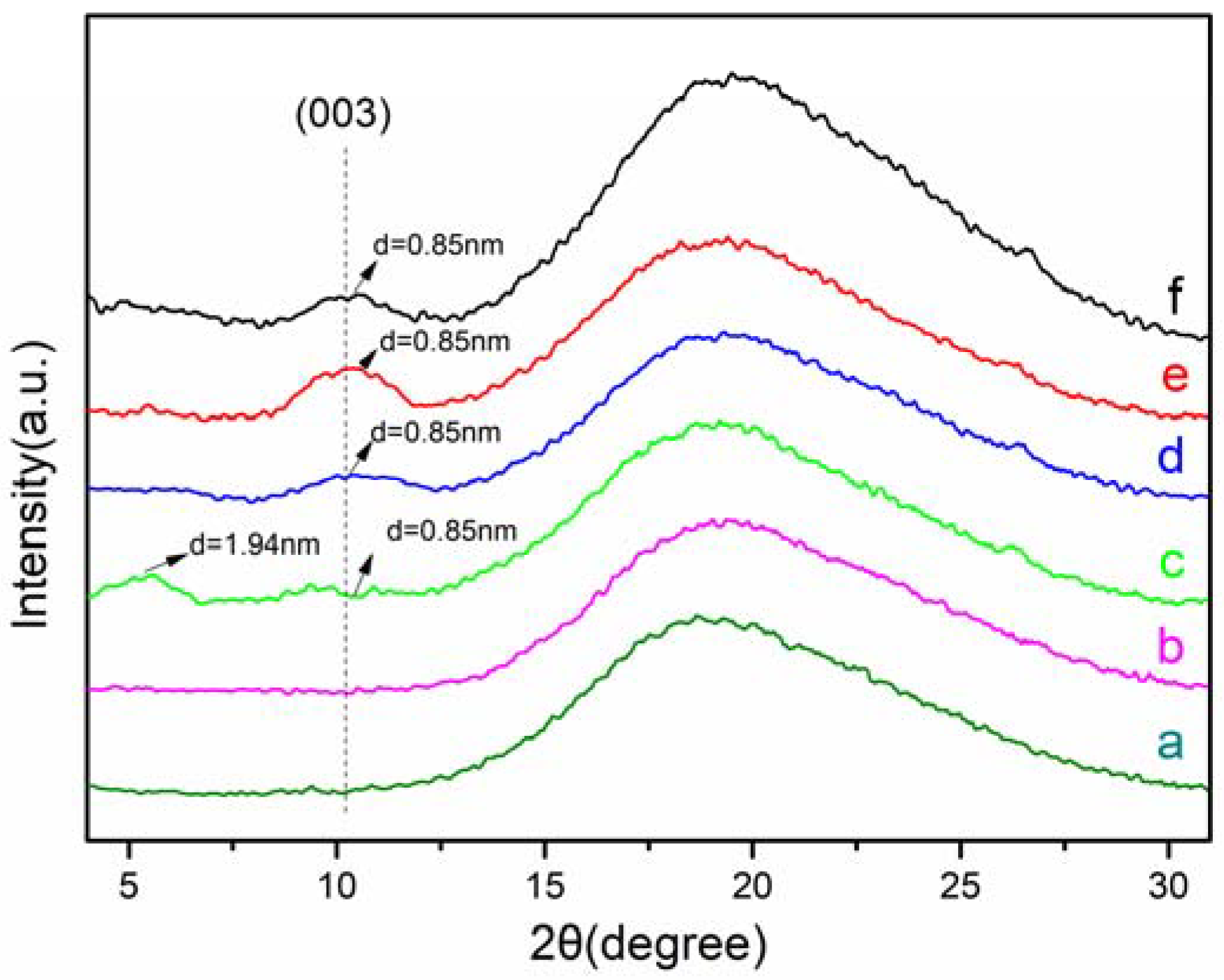
3.3. XRD Analysis of NBR Composites
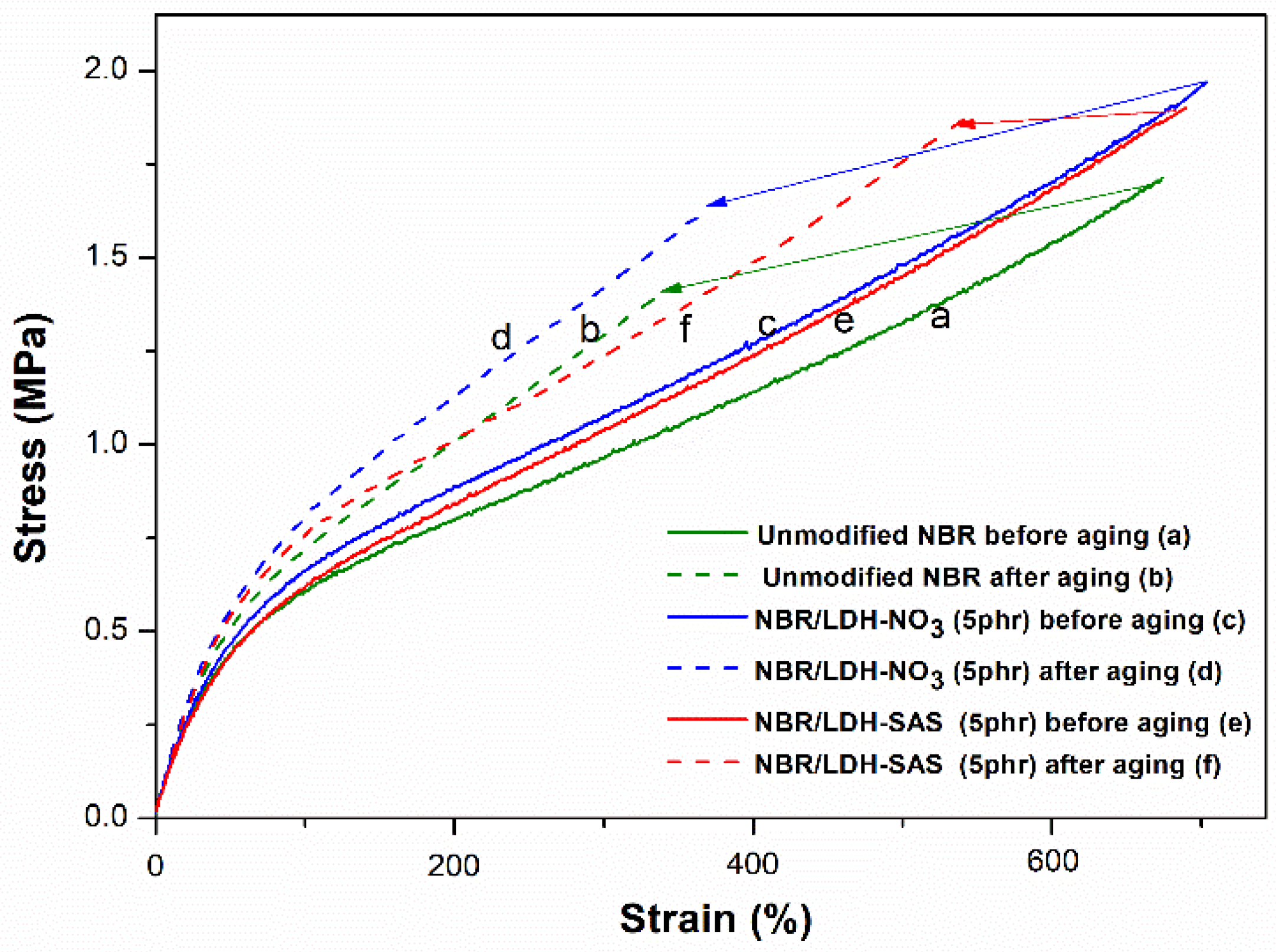
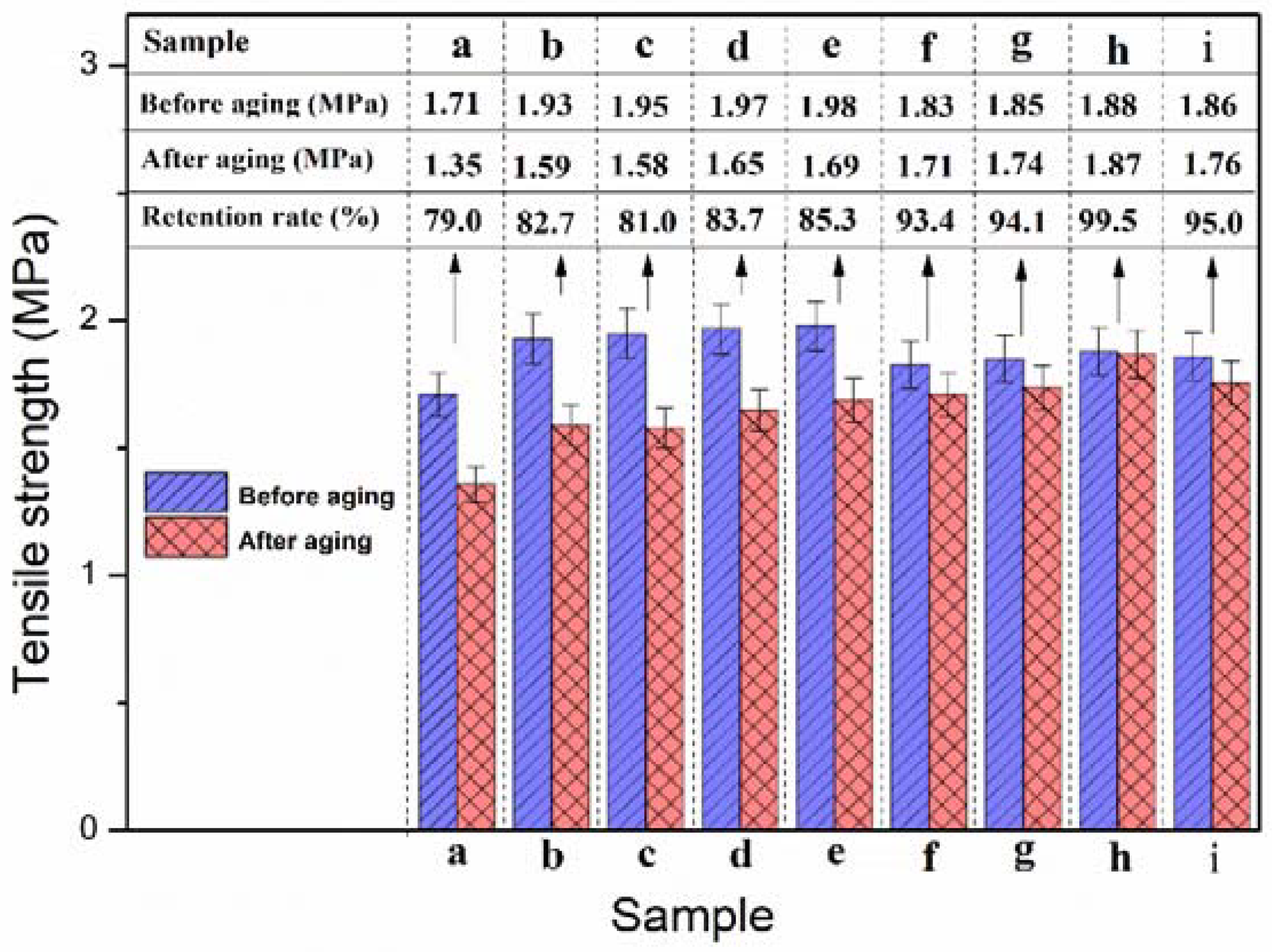
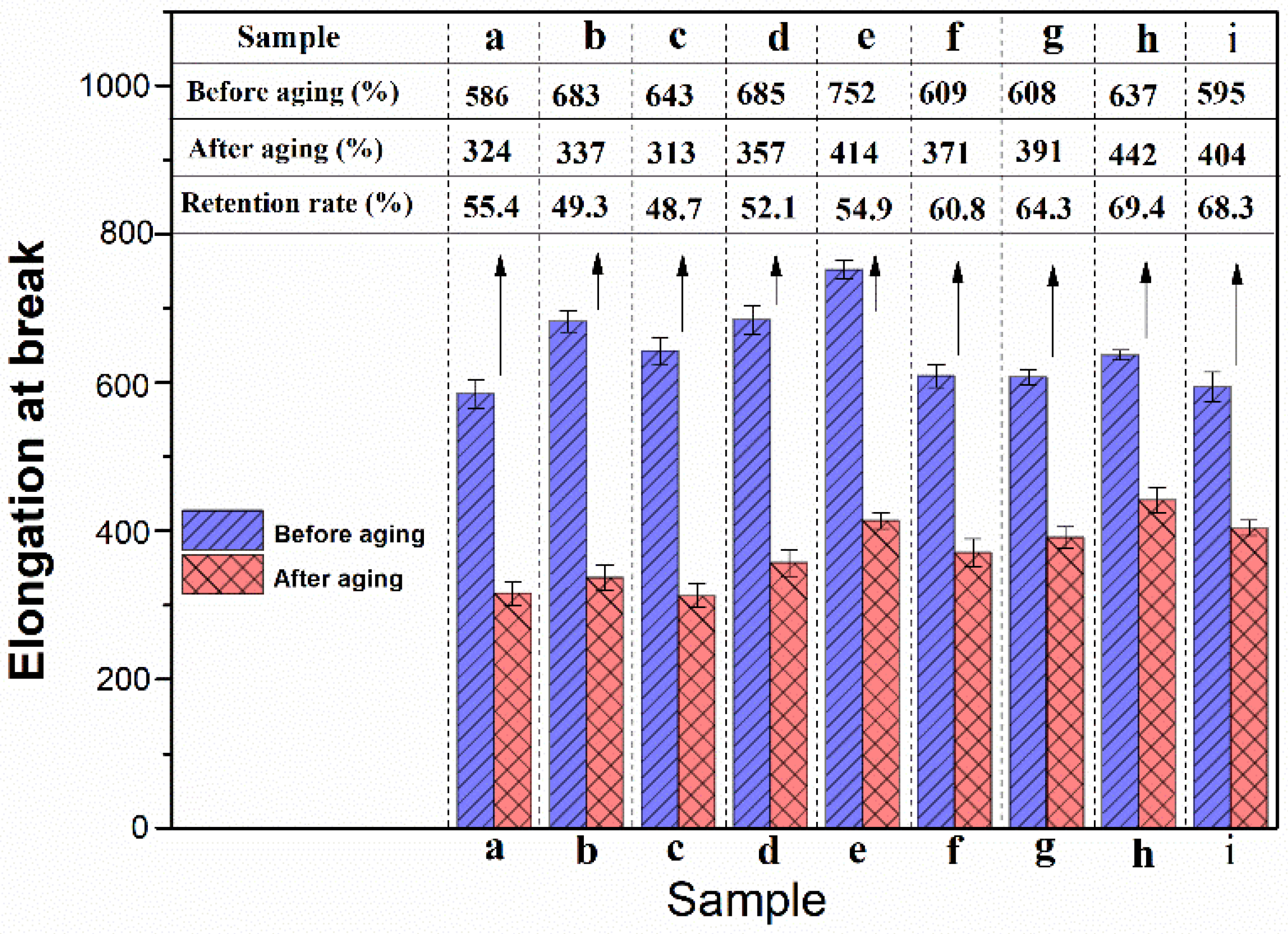
3.4. Mechanical Properties of NBR Composites
3.5. Micromorphology of LDHs and Fractured Surface of NBR Composites
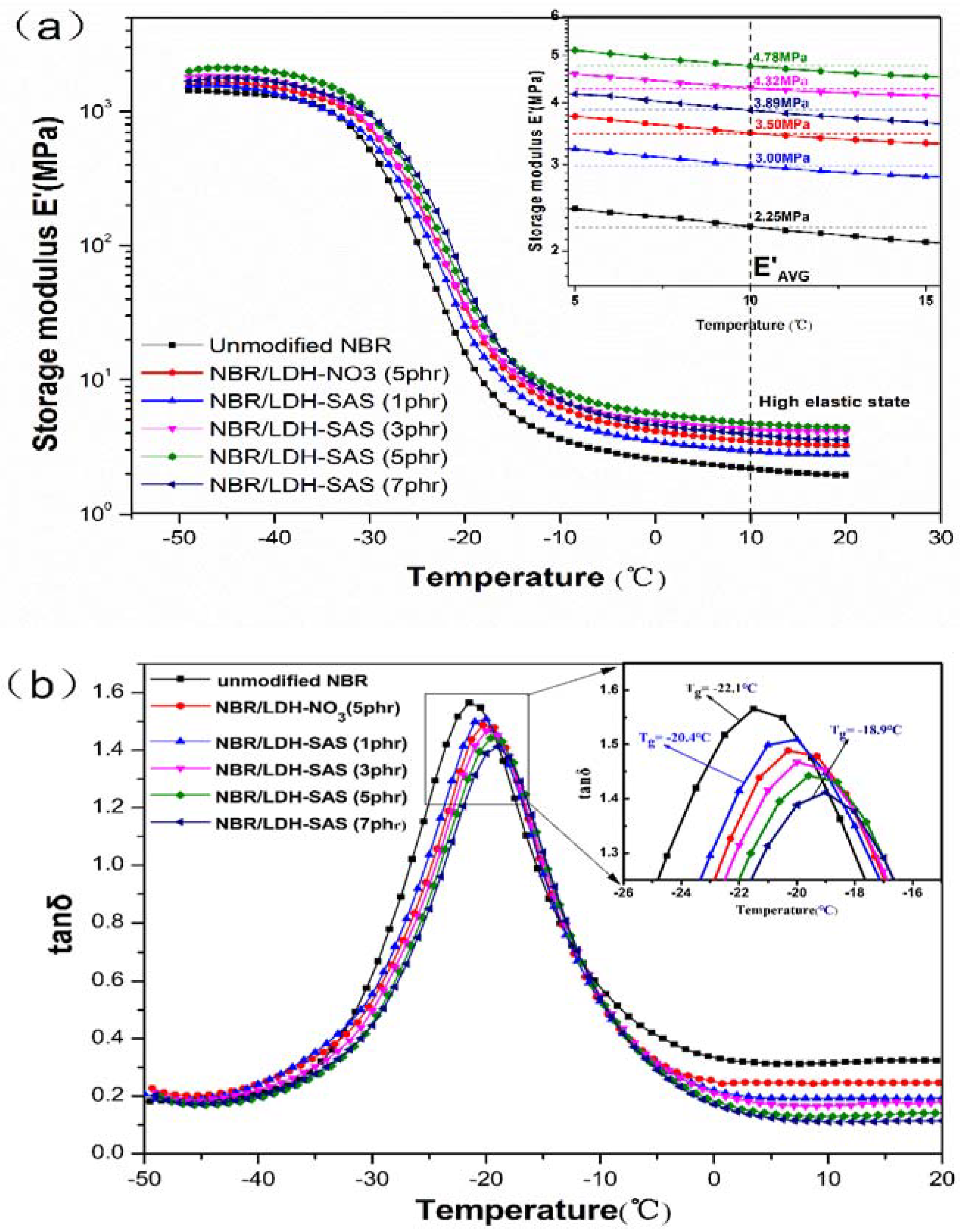
3.6. Dynamic Mechanical Analysis (DMA) of NBR Composites
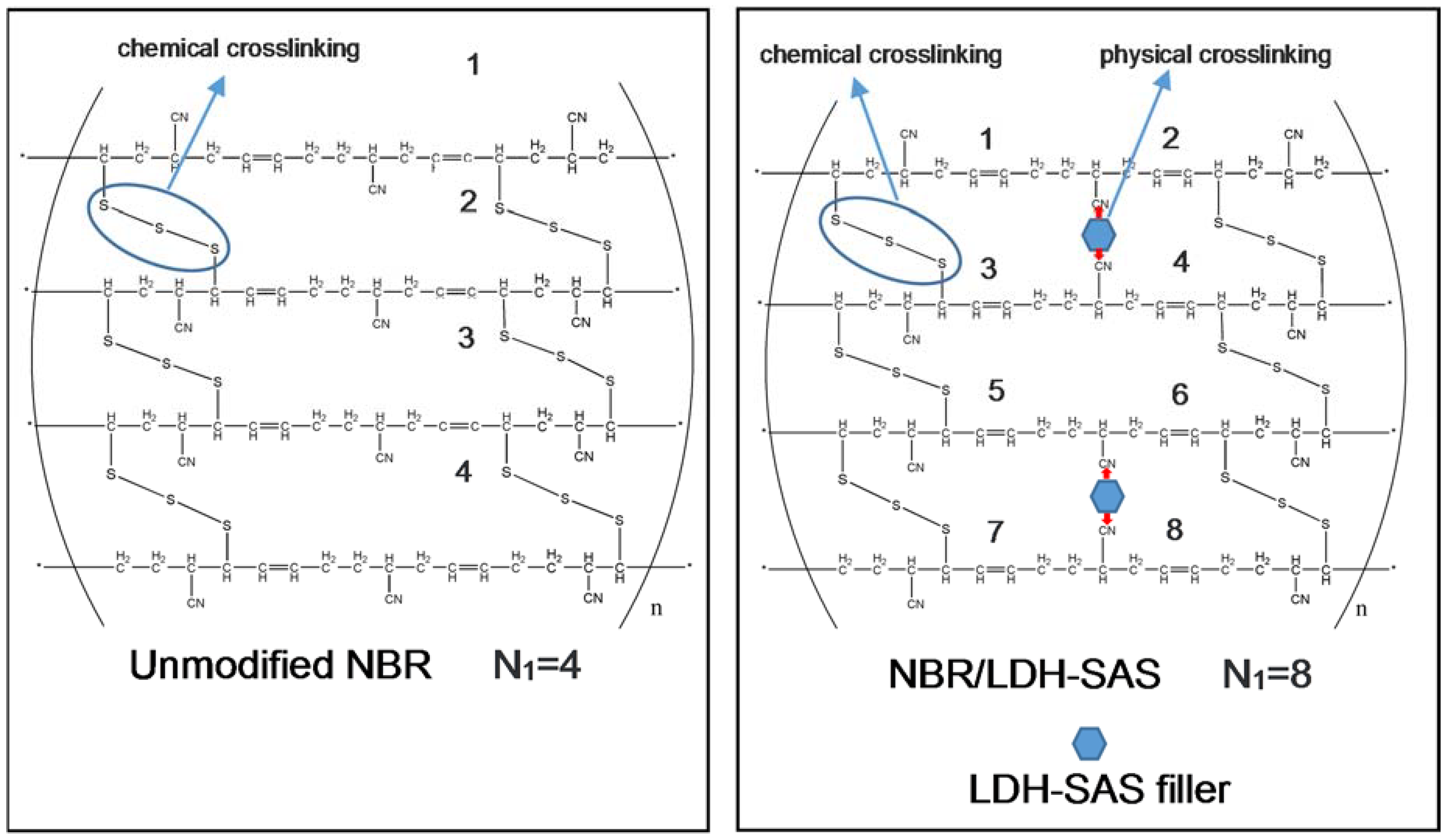
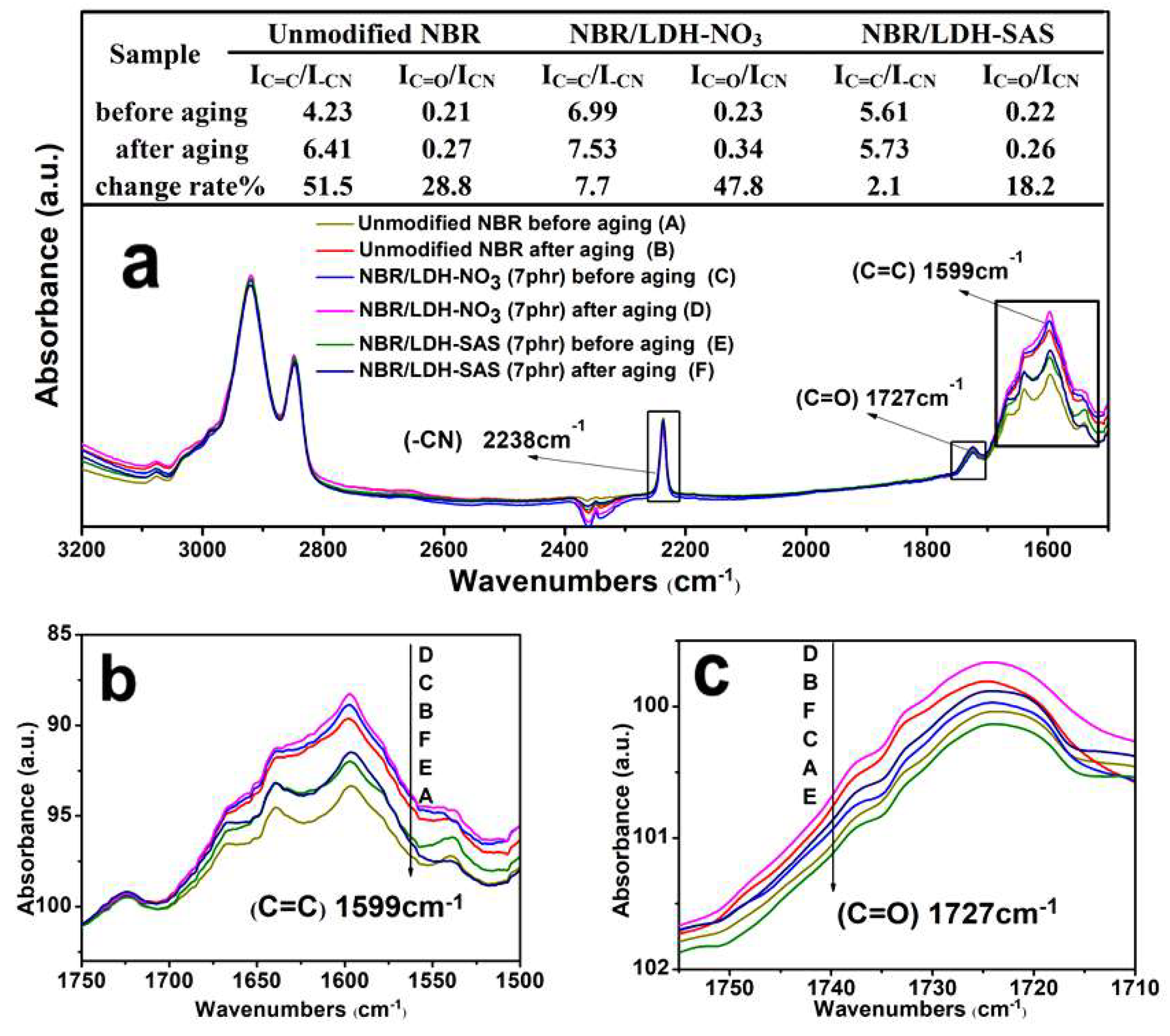
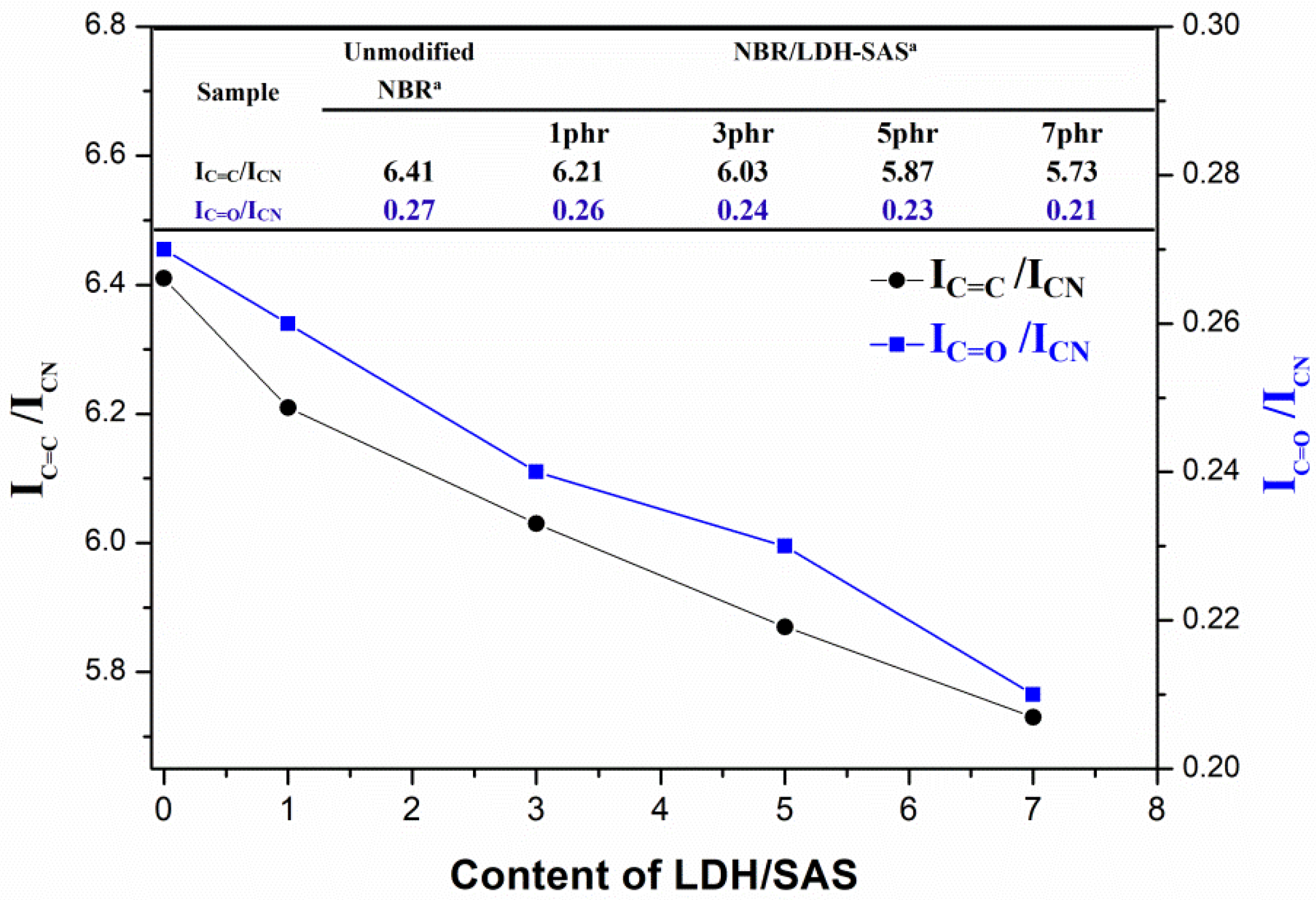
3.7. Aging Mechanism of the NBR Composites
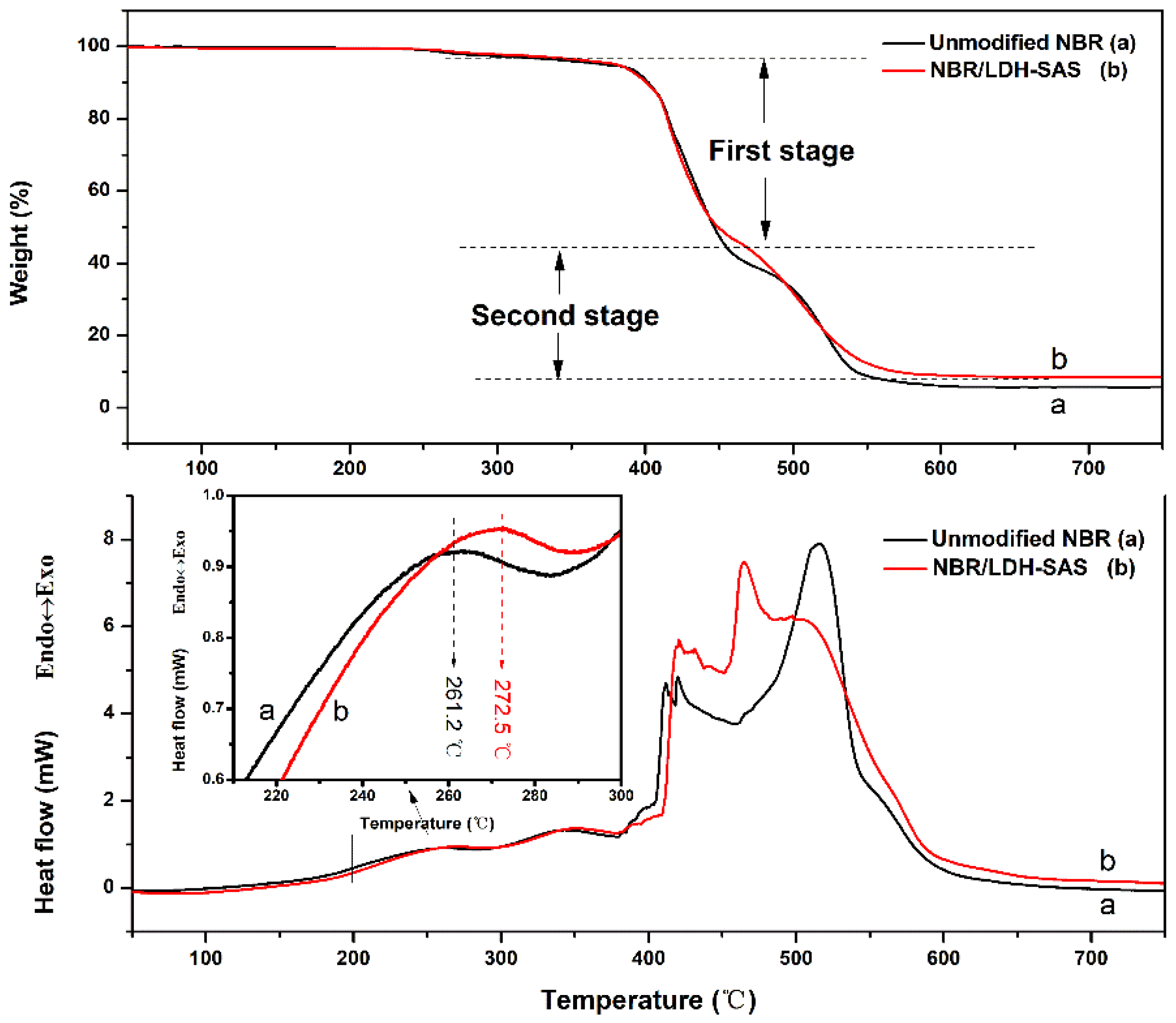
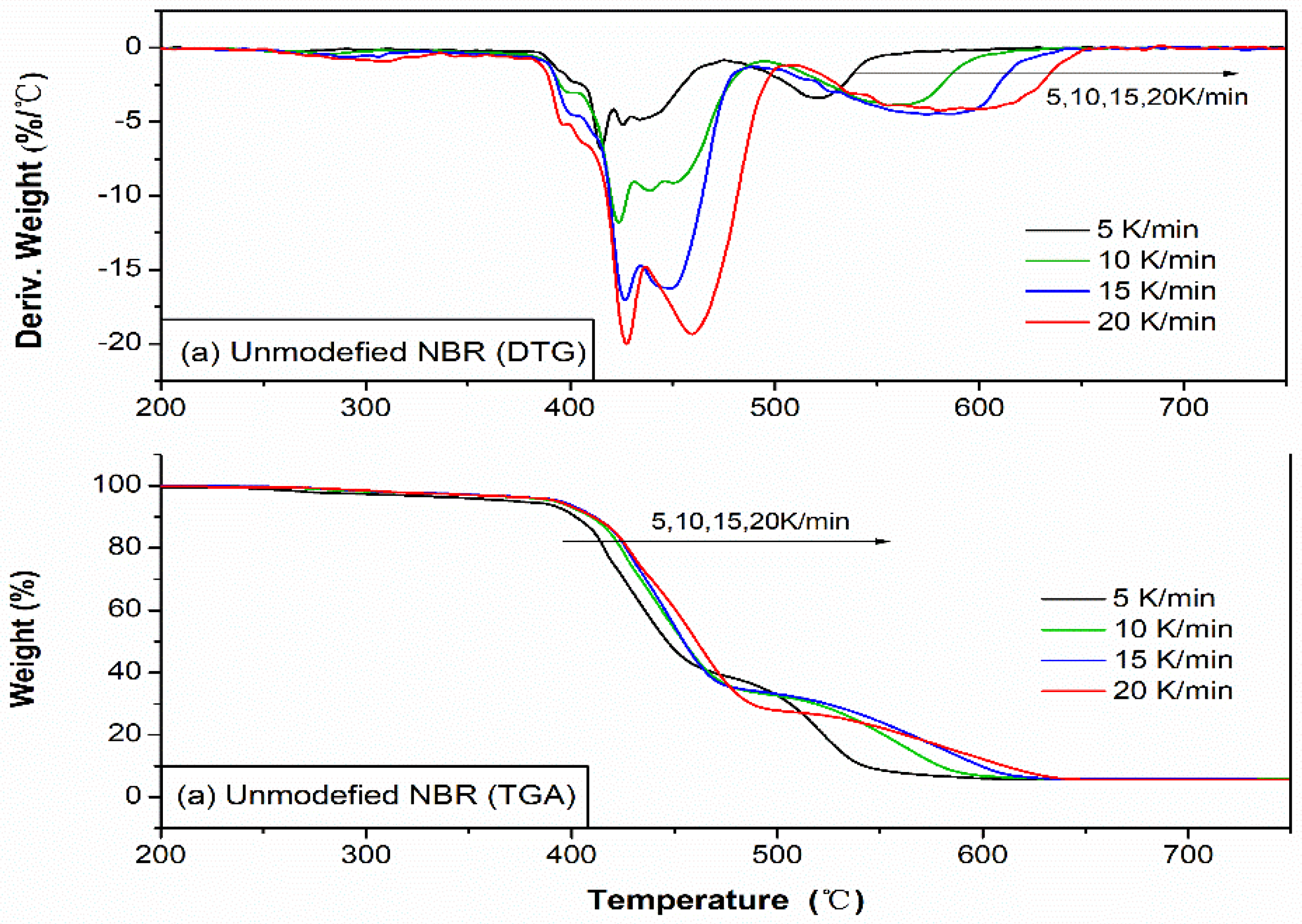
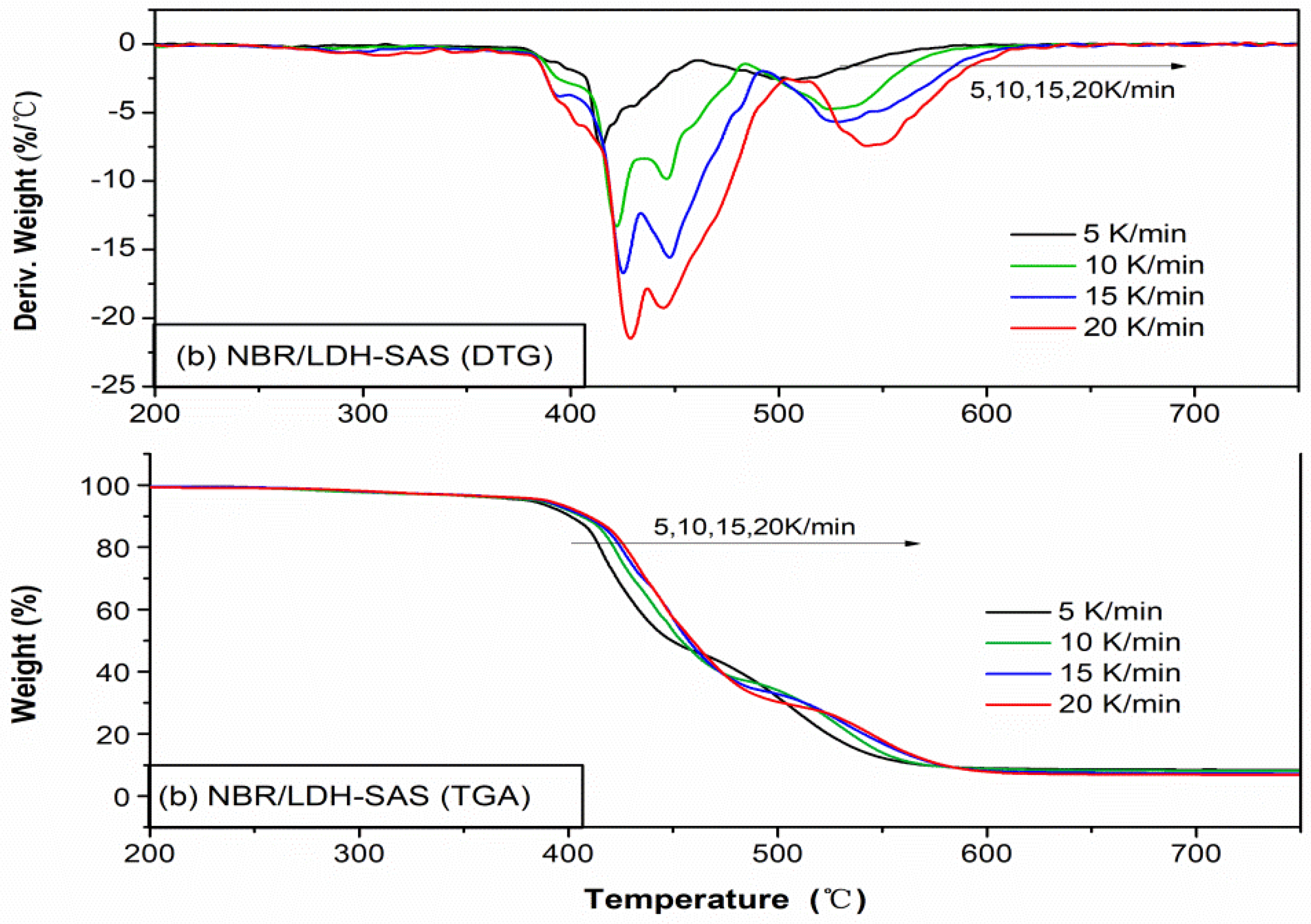
3.8. Thermal Properties of NBR Composites
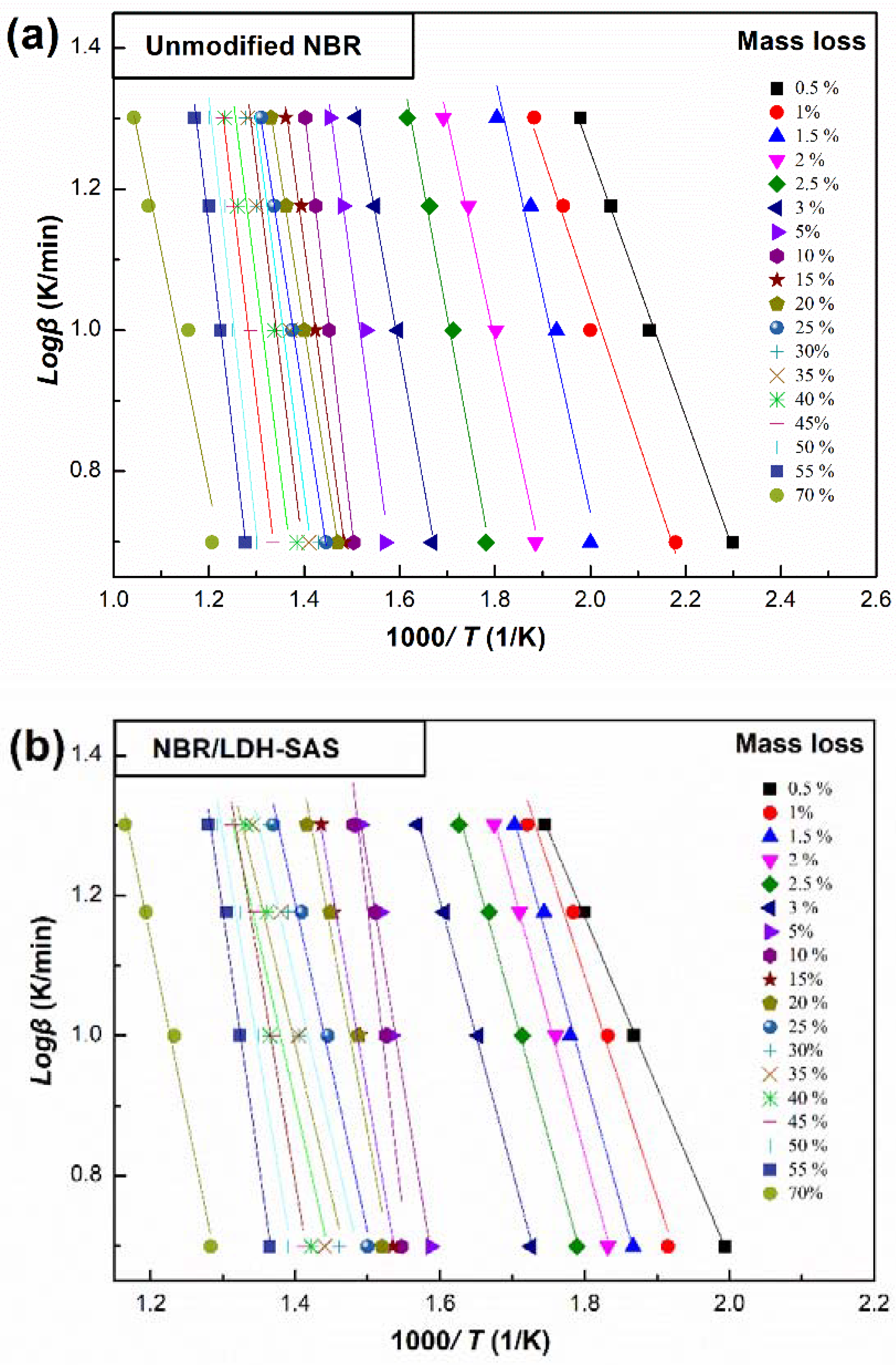
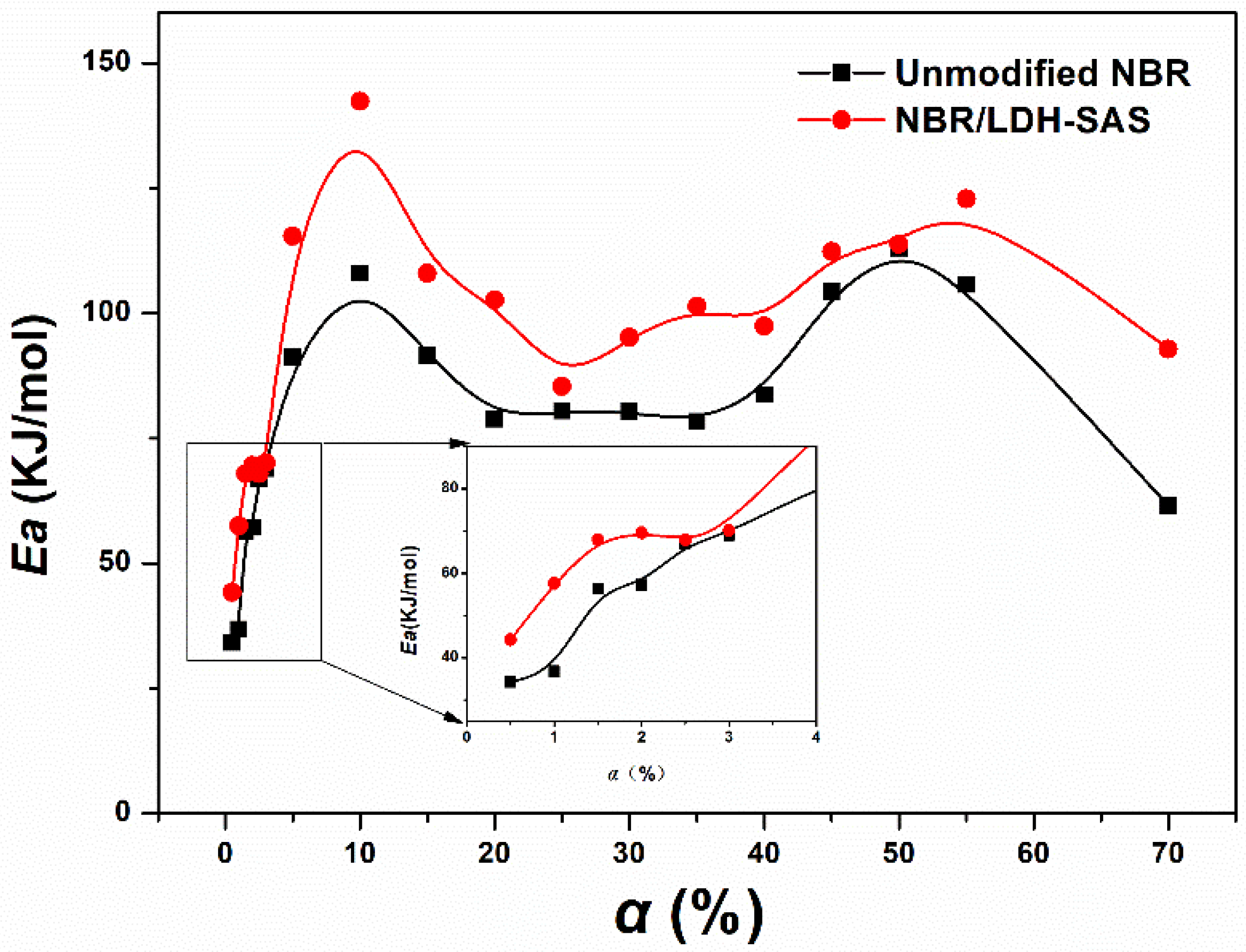
3.9. Kinetic Analysis of Thermal Oxidative Degradation NBR
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davydova, M.L.; Sokolova, M.D.; Haldeeva, A.R.; Djakonov, A.A. Modification of sealing rubber based on nitrile butadiene rubber by thermoexpanded graphite. J. Frict. Wear 2015, 36, 23–28. [Google Scholar] [CrossRef]
- Wang, P.; Su, Z.T.; Lai, L.Q.; Jiang, H.G.; Wang, J.H. Engine isolate mount elastomers. In Proceedings of the International Conference on Structural, Mechanical and Materials Engineering, Dalian, China, 6–8 November 2015. [Google Scholar]
- Gao, S.; Wang, R.; Fang, B.; Kang, H.; Mao, L.; Zhang, L. Preparation and properties of a novel bio-based and non-crystalline engineering elastomer with high low-temperature and oil resistance. J. Appl. Polym. Sci. 2015, 133, 42855. [Google Scholar] [CrossRef]
- Pradhan, B.; Srivastava, S.K.; Bhowmick, A.K.; Saxena, A. Effect of bilayered stearate ion-modified Mg-Al layered double hydroxide on the thermal and mechanical properties of silicone rubber nanocomposites. Polym. Int. 2012, 61, 458–465. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, C. Study of UV Irradiation on Behavior of NBR Rubber from Oil Seal. Adv. Mater. Res. 2014, 1082, 42–45. [Google Scholar] [CrossRef]
- Lee, Y.S.; Hwang, K.S.; Lee, J.C.; Kim, T.G.; Ha, K.R. Effect of TESPT Silane Coupling Agent on Mechanical Properties of Precipitated Silica Filled NBR Compound for Oil Seal. Elastom. Compos. 2011, 46, 45–53. [Google Scholar]
- He, X.; Li, T.; Shi, Z.; Wang, X.; Xue, F.; Wu, Z. Thermal-oxidative aging behavior of nitrile-butadiene rubber/functional LDHs composites. Polym. Degrad. Stab. 2016, 133, 219–226. [Google Scholar] [CrossRef]
- Yuan, W.N.; Zhang, Y.X.; Xiao, F.L. Application of Tackifier Ricobond 1756HS in HNBR Framework Oil Seal. China Rubber Ind. 2014, 61, 166–170. [Google Scholar]
- Dato, J.E.; Campomizzi, E.C.; Achten, D. HNBR for use in oilfield applications. Rubber World 2007, 236, 28. [Google Scholar]
- Molinari, N.; Sutton, A.; Stevens, J.; Mostofi, A. An atomistic model for cross-linked HNBR elastomers used in seals. In Proceedings of the APS March Meeting, San Antonio, TX, USA, 2–6 March 2015. [Google Scholar]
- Li, B.; Zhang, X.Y.; Fu, Z.Y.; Wang, P.Y.; Quan, W.X. The Improvement of Modified Filler on the Anti-Aging Properties of Conductive Silicone Rubber. Appl. Mech. Mater. 2014, 488, 178–184. [Google Scholar] [CrossRef]
- Liang, Z. Study on the structure and dynamic mechanics performanceof nano montmorillonoid/styren-butadiene rubber composite. China Rubber Plast. Technol. Equip. 2006, 32, 29–33. [Google Scholar]
- Gallo, E.; Schartel, B.; Schmaucks, G.; Ehe, K.V.D.; Böhning, M. Effect of well dispersed amorphous silicon dioxide in flame retarded styrene butadiene rubber. Plast. Rubber Compos. 2013, 42, 34–42. [Google Scholar] [CrossRef]
- Wang, X.; Dou, W. Preparation of graphite oxide (GO) and the thermal stability of silicone rubber/GO nanocomposites. Thermochim. Acta 2012, 529, 25–28. [Google Scholar] [CrossRef]
- Peng, Y.L.; Su, Z.T.; Liu, J.; Jiao, D.S. Effect of Fe2O3 on thermal stability of HTV silicone rubber. Silicone Mater. 2005, 4, 14–16. [Google Scholar]
- Costa, F.R.; Pradhan, S.; Wagenknecht, U.; Bhowmick, A.K.; Heinrich, G. XNBR/LDH Nanocomposites: Effect of vulcanization and organic modifier on nanofiller dispersion and strain-induced crystallization. J. Polym. Sci. Polym. Phys. 2010, 48, 2302–2311. [Google Scholar] [CrossRef]
- Kaushik, A.K.; Podsiadlo, P.; Qin, M.; Shaw, C.M.; Waas, A.M.; Kotov, N.A. The Role of Nanoparticle Layer Separation in the Finite Deformation Response of Layered Polyurethane-Clay Nanocomposites. Macromolecules 2010, 42, 6588–6595. [Google Scholar] [CrossRef]
- Saad, G.R.; Naguib, H.F.; Elmenyawy, S.A. Effect of organically modified montmorillonite filler on the dynamic cure kinetics, thermal stability, and mechanical properties of brominated epoxy/aniline formaldehyde condensates system. J. Therm. Anal. Calorim. 2013, 111, 1409–1417. [Google Scholar] [CrossRef]
- Jin, H.L.; Bae, J.W.; Kim, J.S.; Choi, Y.S.; Jo, N.J. Effect of Organically Modified Layered Silicate on Degradation of Chloroprene Rubber. Asian J. Chem. 2013, 25, 5159. [Google Scholar]
- Zhang, S.P.; Song, H.O. Preparation of dispersible graphene oxide as a filler to increase the thermal stability of a flame retarding polymer. Carbon Mater. 2013, 56, 61–65. [Google Scholar] [CrossRef]
- Costa, F.R.; Leuteritz, A.; Meinl, J.; Wagenknecht, U.; Heinrich, G. LDH as Nanofiller: Organic Modification and Dispersion in Polymers. Macromol. Symp. 2011, 301, 46–54. [Google Scholar] [CrossRef]
- Ahmad Rasyid, M.F.; Hazizan, M.A.; Sharif, J.M. Effect of Organic Modification of Muscovite on the Polypropylene Layered Silicate Nanocomposite. Key Eng. Mater. 2011, 471, 623–645. [Google Scholar] [CrossRef]
- He, X.R.; Zhang, R.; Chen, Q.; Rong, Y.Q.; Yang, Z.Q. Different surface functionalized nano-Fe3O4 particles for EVA composite adhesives. Int. J. Adhes. Adhes. 2014, 50, 128–135. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Chen, Q.; Feng, C.; Meng, L. Crystallization Kinetics of Functionallized FeO/Ethylene-vinyl Acetate Copolymer Nanocomposites Adhesives. J. Macromol. Sci. Part B 2015, 55, 55–72. [Google Scholar] [CrossRef]
- He, X.; Lu, X.; Chen, Q.; Zhang, R. Adhesive and viscoelastic performance of surface functionalized nano-Fe3O4 induced orientated ethylene vinyl-acetate composite hot melt adhesives. J. Appl. Polym. Sci. 2016, 133, 43931. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Zhang, J.; Lu, X.; Yang, C.; Liu, T. Influence of graphene/ferriferrous oxide hybrid particles on the properties of nitrile rubber. RSC Adv. 2016, 6, 91798. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Lai, Z.; Yang, D. Effect of some inorganic particles on the softening dispersion of the dynamics of butyl rubber. Polym. Bull. 2016, 74, 1031–1043. [Google Scholar] [CrossRef]
- Becker, C.M.; Gabbardo, A.D.; Wypych, F.; Amico, S.C. Mechanical and flame-retardant properties of epoxy/Mg–Al LDH composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 196–202. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, P.; Qu, B. Flammable, thermal, and mechanical properties of intumescent flame retardant PP/LDH nanocomposites with different divalent cations. Polym. Compos. 2009, 30, 1000–1006. [Google Scholar] [CrossRef]
- Chiang, M.F.; Chen, E.C.; Wu, T.M. Preparation, mechanical properties and thermal stability of poly(l-lactide)/γ-polyglutamate-modified layered double hydroxide nanocomposites. Polym. Degrad. Stab. 2012, 97, 995–1001. [Google Scholar] [CrossRef]
- Lebaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate Nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Stöckelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in layered double hydroxide (LDH)-based elastomer composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- Chibwe, K.; Jones, W. Synthesis of polyoxometalate pillared layered double hydroxides via calcined precursors. Chem. Mater. 1989, 1, 489–490. [Google Scholar] [CrossRef]
- Hsueh, H.B.; Chen, C.Y. Preparation and properties of LDHs/epoxy nanocomposites. Polymer 2003, 44, 5275–5283. [Google Scholar] [CrossRef]
- Costa, F.R.; Wagenknecht, U.; Heinrich, G. LDPE/Mg–Al layered double hydroxide nanocomposite: Thermal and flammability properties. Polym. Degrad. Stab. 2007, 92, 1813–1823. [Google Scholar] [CrossRef]
- Ven, L.V.D.; Gemert, M.L.M.V.; Batenburg, L.F.; Keern, J.J.; Gielgens, L.H.; Koster, T.P.M. On the action of hydrotalcite-like clay materials as stabilizers in polyvinylchloride. Appl. Clay Sci. 2000, 17, 25–34. [Google Scholar]
- Lin, Y.; Wang, J.; Evans, D.G.; Li, D. Layered and intercalated hydrotalcite-like materials as thermal stabilizers in PVC resin. J. Phys. Chem. Solids 2006, 67, 998–1001. [Google Scholar] [CrossRef]
- Nyambo, C.; Wang, D.; Wilkie, C.A. Will layered double hydroxides give nanocomposites with polar or non-polar polymers. Polym. Adv. Technol. 2010, 20, 332–340. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Heinrich, G. Layered Double Hydroxide (LDH)-Based Rubber Nanocomposites. Encycl. Polym. Nanomater. 2014, 55, 1–7. [Google Scholar]
- Braga, F.C.F.; Furtado, C.R.G.; Oliveira, M.G. Influence of the Surfactant on the Hydrotalcite Dispersion in NBR/LDH Composites Produced by Coagulation. Macromol. Symp. 2015, 343, 70–77. [Google Scholar] [CrossRef]
- Feng, J.; Liao, Z.; Zhu, J.; Su, S. Comparison of morphology and mechanical properties of peroxide-cured acrylonitrile butadiene rubber/LDH composites prepared from different organically modified LDHs. J. Appl. Polym. Sci. 2013, 127, 3310–3317. [Google Scholar] [CrossRef]
- Thakur, V.; Das, A.; Mahaling, R.N.; Rooj, S.; Gohs, U.; Wagenknecht, U. Influence of Layered Double Hydroxides on the Curing of Carboxylated Nitrile Rubber with Zinc Oxide. Macromol. Mater. Eng. 2009, 294, 561–569. [Google Scholar] [CrossRef]
- Xiao, S.; Tan, Y.; Xu, J.; Xiong, C.; Wang, X.; Su, S. Lignosulfonate as dispersant for layered double hydroxide in nitrile–butadiene rubber composites. Appl. Clay Sci. 2014, 97, 91–95. [Google Scholar] [CrossRef]
- Roy, S.; Srivastava, S.K.; Mittal, V. Facile noncovalent assembly of MWCNT-LDH and CNF-LDH as reinforcing hybrid fillers in thermoplastic polyurethane/nitrile butadiene rubber blends. J. Polym. Res. 2016, 23, 1–11. [Google Scholar] [CrossRef]
- Long-Chao, D.U.; Zhang, Y.C.; Chen, J.Y.; Tian, Y.F. Synergism between intumescent flame retardants modified anion and cation clays in rubber. J. Anhui Univ. 2011, 2, 81–88. [Google Scholar]
- Carlino, S. The intercalation of carboxylic acids into layered double Hydroxides: A critical evaluation and review of the different methods. Solid State Ion. 1997, 98, 73–84. [Google Scholar] [CrossRef]
- Martins, M.A.; Moreno, R.M.B.; Mcmahan, C.M.; Brichta, J.L.; Gonçalves, P.D.S.; Mattoso, L.H.C. Thermooxidative study of raw natural rubber from Brazilian IAC 300 series clones. Thermochim. Acta 2008, 474, 62–66. [Google Scholar] [CrossRef]
- Troutier-Thuilliez, A.L.; Taviot-Guého, C.; Cellier, J.; Hintze-Bruening, H.; Leroux, F. Layered particle-based polymer composites for coatings: Part I. Evaluation of layered double hydroxides. Prog. Org. Coat. 2009, 64, 182–192. [Google Scholar] [CrossRef]
- Zhang, P.; Sago, S.; Yamaguchi, T.; Anilkumar, G.M. Mg–Al layered double hydroxides containing glycine betaine as low humidity-dependent anion conducting electrolyte material for Solid State Alkaline Fuel Cell (SAFC). J. Power Sources 2013, 230, 225–229. [Google Scholar] [CrossRef]
- Kwon, E.; Castaldi, M.J. Fundamental Understanding of the Thermal Degradation Mechanisms of Waste Tires and Their Air Pollutant Generation in a N2 Atmosphere. Environ. Sci. Technol. 2009, 43, 5996–6002. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, A.; Zaborski, M.; Boiteux, G.; Gain, O.; Marzec, A.; Maniukiewicz, W. Effects of unmodified layered double hydroxides MgAl-LDHs with various structures on the properties of filled carboxylated acrylonitrile–butadiene rubber XNBR. Eur. Polym. J. 2014, 60, 172–185. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Crystallization behavior of nylon 6 nanocomposites. Polymer 2003, 44, 3945–3961. [Google Scholar] [CrossRef]
- Rajasekar, R.; Pal, K.; Heinrich, G.; Das, A.; Das, C.K. Development of nitrile butadiene rubber–nanoclay composites with epoxidized natural rubber as compatibilizer. Mater. Des. 2009, 30, 3839–3845. [Google Scholar] [CrossRef]
- Kotal, M.; Srivastava, S.K.; Manu, S.K. Layered double hydroxide as nanofiller in the development of polyurethane nanocomposites. J. Nanosci. Nanotechnol. 2010, 10, 5730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; He, X.; Yu, H. Why tanδ of poly (butyl acrylate) and poly (ethyl acrylate) with little double bonds are becoming asymmetric? Polymer 2014, 55, 4720–4727. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Yang, D.; Lai, Z. Non-isothermal crystallization kinetics and segmental dynamics of high density polyethylene/butyl rubber blends. Polym. Int. 2015, 64, 1252–1261. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Huang, G. A review of the slow relaxation processes in the glass–rubber transition region of amorphous polymers. Phase Transit. 2015, 88, 843–858. [Google Scholar] [CrossRef]
- Zhang, R.; He, X. Crystallization and molecular dynamics of ethylene-vinyl acetate copolymer/butyl rubber blends. RSC Adv. 2014, 5, 130. [Google Scholar] [CrossRef]
- Das, A.; George, J.J.; Kutlu, B.; Leuteritz, A.; Wang, D.Y.; Rooj, S. A novel thermotropic elastomer based on highly-filled LDH-SSB composites. Macromol. Rapid Commun. 2012, 33, 337–342. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yu, H.; Zhang, R.; Yang, C. Enhance Slower Relaxation Process of Poly(ethyl acrylate) through Internal Plasticization. Int. Polym. Process. 2014, 29, 419–424. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons: New York, NY, USA, 1986; pp. 155–156. ISBN 9780471706069. [Google Scholar]
- Zhao, J.; Yang, R.; Iervolino, R.; Barbera, S. Investigation of crosslinking in the thermooxidative aging of nitrile–butadiene rubber. J. Appl. Polym. Sci. 2015, 132, 41319–51323. [Google Scholar] [CrossRef]
- Wei, Z.; Li, L.; Zhao, X.; He, J.; Ao, W.; Chan, T.W. Data for effects of lanthanum complex on the thermo-oxidative aging of natural rubber. Data Brief 2015, 5, 789–795. [Google Scholar]
- Mei, C.; Ao, N.J.; Zhang, B.L.; Den, C.M.; Qian, H.L.; Zhou, H.L. Comparison and evaluation of the thermooxidative stability of medical natural rubber latex products prepared with a sulfur vulcanization system and a peroxide vulcanization system. J. Appl. Polym. Sci. 2010, 98, 591–597. [Google Scholar]
- Wei, Z.; Li, L.; Zhao, X.; He, J.; Ao, W.; Chan, T.W. Effects of lanthanum complex on the thermo-oxidative aging of natural rubber. Polym. Degrad. Stab. 2015, 120, 377–383. [Google Scholar]
- Gu, A.; Liang, G. Thermal stability and kinetics analysis of rubber-modified epoxy resin by high-resolution thermogravimetric analysis. J. Appl. Polym. Sci. 2003, 89, 3594–3600. [Google Scholar] [CrossRef]
- Marques, P.T.; Lima, A.M.F.; Bianco, G.; Laurindo, J.B.; Borsali, R.; Meins, J.F.L. Thermal properties and stability of cassava starch films cross-linked with tetraethylene glycol diacrylate. Polym. Degrad. Stab. 2006, 91, 726–732. [Google Scholar] [CrossRef]
- Xie, C.; Jia, Z.; Jia, D.; Luo, Y.; You, C. The Effect of Dy(III) Complex with 2-Mercaptobenzimidazole on the Thermo-Oxidation Aging Behavior of Natural Rubber Vulcanizates. Int. J. Polym. Mater. 2010, 59, 663–679. [Google Scholar] [CrossRef]
- Xie, C.; Jia, Z.; Luo, Y.; Jia, D. Antioxidant effect of SM(Ⅲ) complex with 2-mercaptobenzimidazole in natural rubber vulcanizates. Acta Polym. Sin. 2011, 32, 320–326. [Google Scholar]
- Xu, J.; Zhang, A.; Zhou, T.; Cao, X.; Xie, Z. A study on thermal oxidation mechanism of styrene–butadiene–styrene block copolymer (SBS). Polym. Degrad. Stab. 2007, 92, 1682–1691. [Google Scholar] [CrossRef]

| Material | phr a |
|---|---|
| NBR | 100 |
| LDH–NO3/LDH–SAS | 0, 1, 3, 5, 7 |
| ZnO | 5 |
| Antioxidant D | 1 |
| Stearic acid | 1 |
| DM | 0.5 |
| Sulfur | 1.5 |
| DOP | 1.5 |
| Compound | Scorch Time/t10 (min) | Cure Time/t90 (min) |
|---|---|---|
| Unmodified NBR | 10.58 | 27.87 |
| NBR/LDH–SAS (1 phr) | 10.57 | 27.43 |
| NBR/LDH–SAS (3 phr) | 10.87 | 27.94 |
| NBR/LDH–SAS (5 phr) | 11.43 | 28.46 |
| NBR/LDH–SAS (7 phr) | 11.96 | 28.13 |
| NBR/LDH–NO3 (5 phr) | 9.47 | 27.12 |
| Sample | Unmodified NBR | NBR/LDH–NO3 (5 phr) | NBR/LDH–SAS (1 phr) | NBR/LDH–SAS (3 phr) | NBR/LDH–SAS (5 phr) | NBR/LDH–SAS (7 phr) |
|---|---|---|---|---|---|---|
| Tg (°C) | −22.1 | −20.3 | −20.9 | −19.9 | −19.4 | −18.9 |
| E′AVG (MPa) | 2.25 | 3.50 | 3.00 | 4.32 | 4.78 | 3.89 |
| N1 (1014) | 1.92 | 2.99 | 2.56 | 3.69 | 4.08 | 3.32 |
| ΔN1 (1014) | 0 | 1.07 | 0.64 | 1.77 | 2.16 | 1.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Shi, Z.; He, X.; Jiang, P.; Lu, X.; Zhang, R.; Wang, X. Aging-Resistant Functionalized LDH–SAS/Nitrile-Butadiene Rubber Composites: Preparation and Study of Aging Kinetics/Anti-Aging Mechanism. Materials 2018, 11, 836. https://doi.org/10.3390/ma11050836
Li T, Shi Z, He X, Jiang P, Lu X, Zhang R, Wang X. Aging-Resistant Functionalized LDH–SAS/Nitrile-Butadiene Rubber Composites: Preparation and Study of Aging Kinetics/Anti-Aging Mechanism. Materials. 2018; 11(5):836. https://doi.org/10.3390/ma11050836
Chicago/Turabian StyleLi, Tianxiang, Zhengren Shi, Xianru He, Ping Jiang, Xiaobin Lu, Rui Zhang, and Xin Wang. 2018. "Aging-Resistant Functionalized LDH–SAS/Nitrile-Butadiene Rubber Composites: Preparation and Study of Aging Kinetics/Anti-Aging Mechanism" Materials 11, no. 5: 836. https://doi.org/10.3390/ma11050836
APA StyleLi, T., Shi, Z., He, X., Jiang, P., Lu, X., Zhang, R., & Wang, X. (2018). Aging-Resistant Functionalized LDH–SAS/Nitrile-Butadiene Rubber Composites: Preparation and Study of Aging Kinetics/Anti-Aging Mechanism. Materials, 11(5), 836. https://doi.org/10.3390/ma11050836