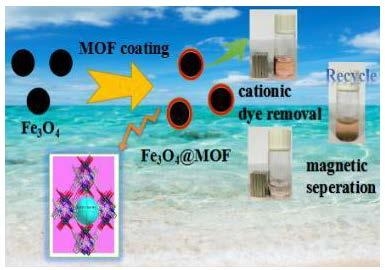
Facile Fabrication of Magnetic Metal-Organic Framework Composites for the Highly Selective Removal of Cationic Dyes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
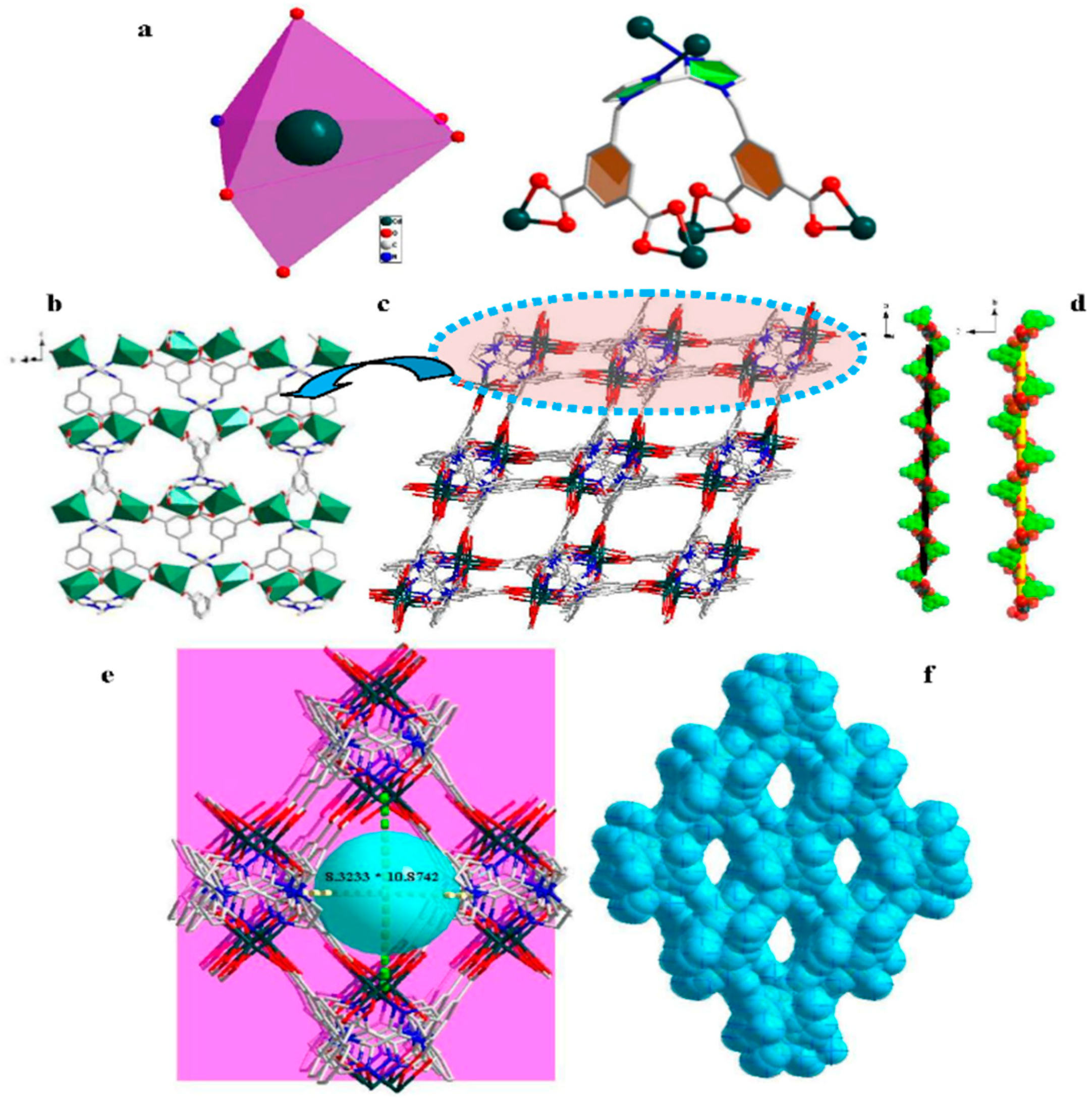
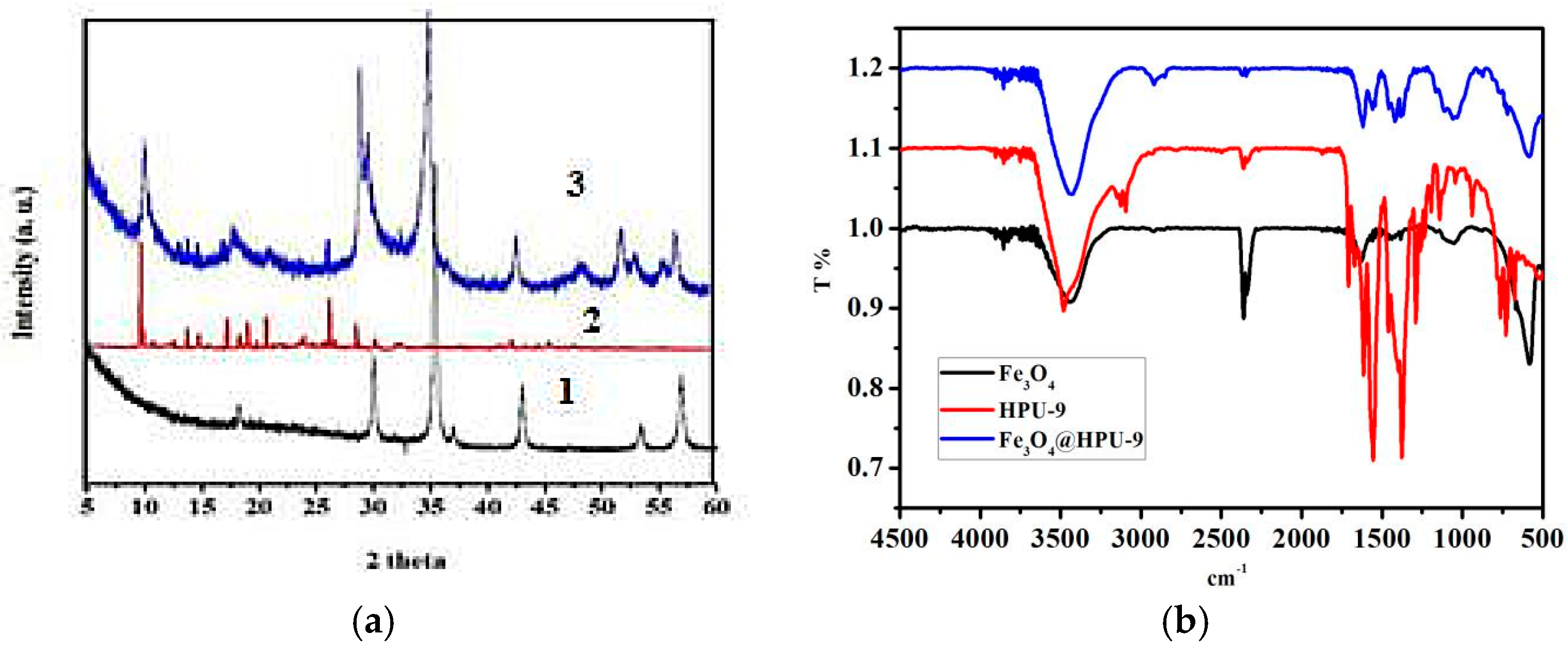
3.1. Structural Description
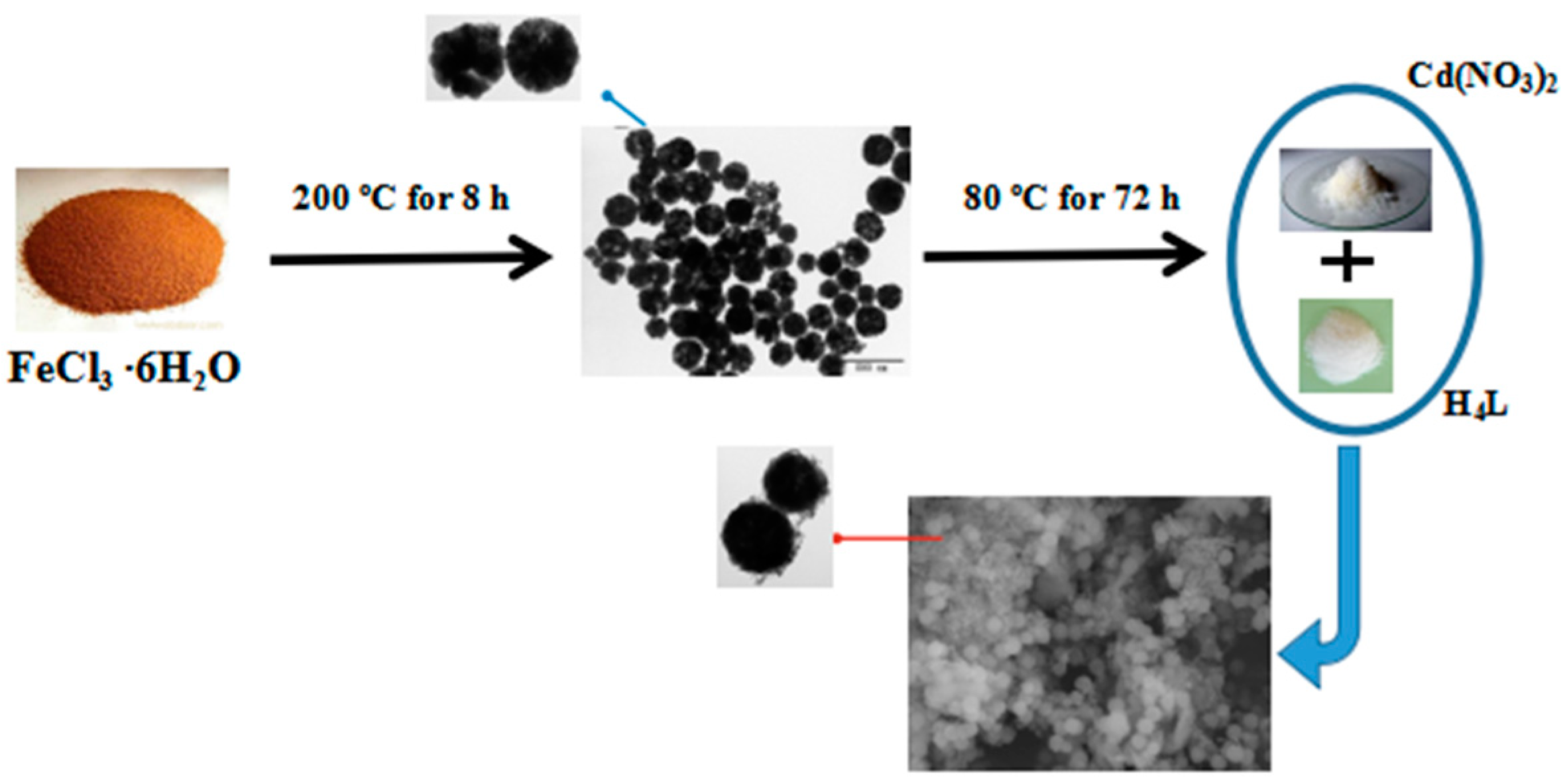
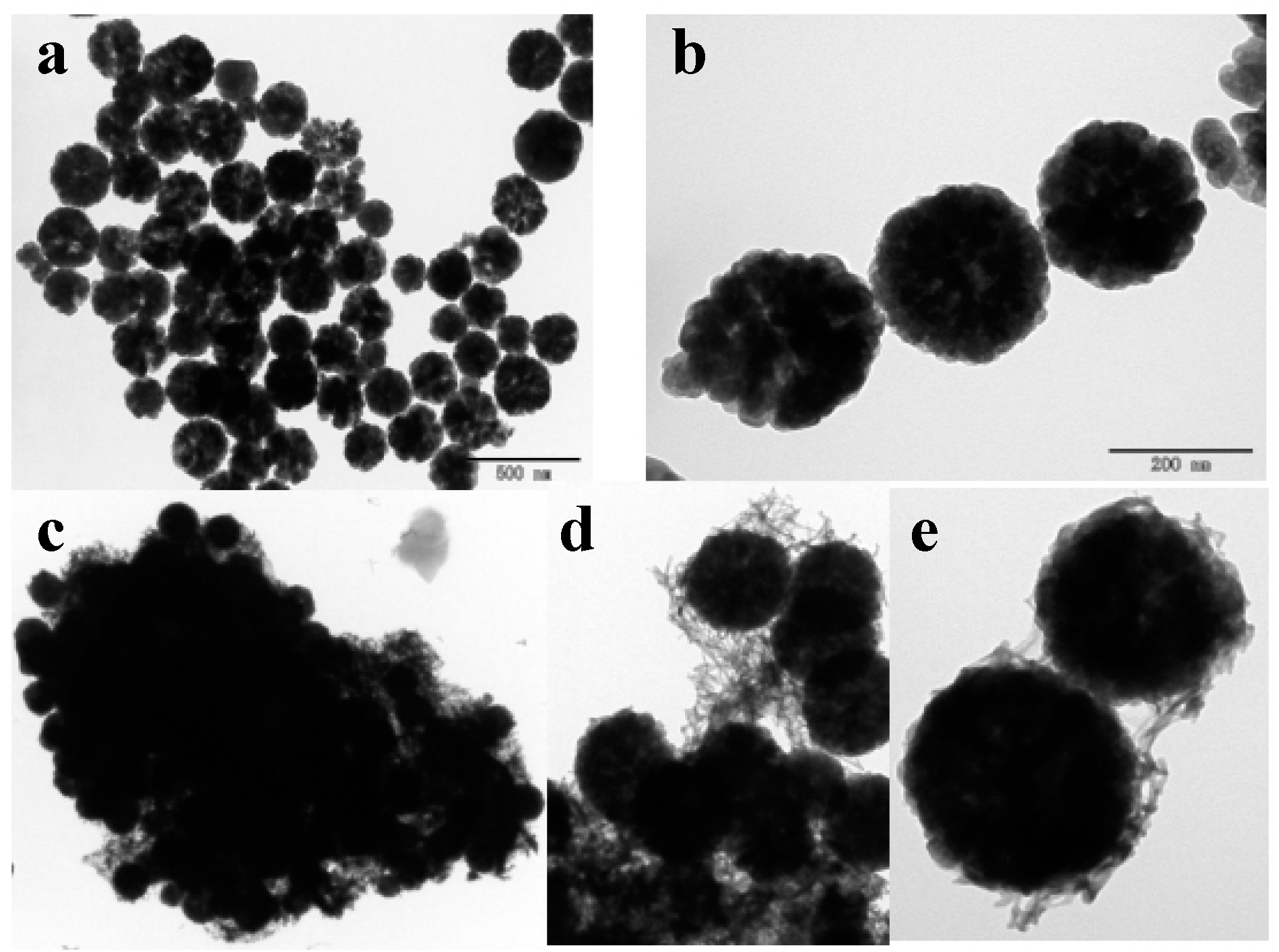
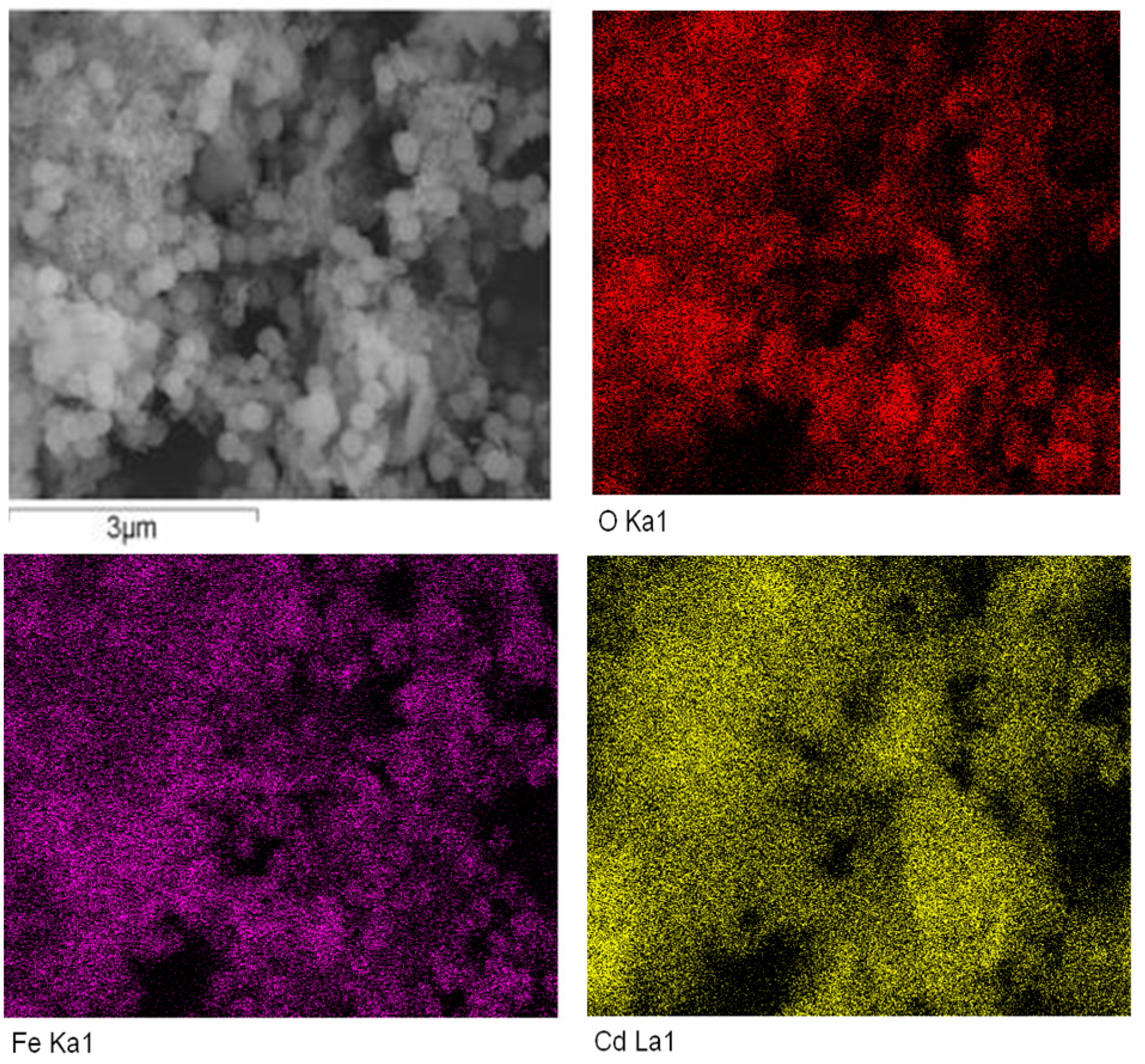
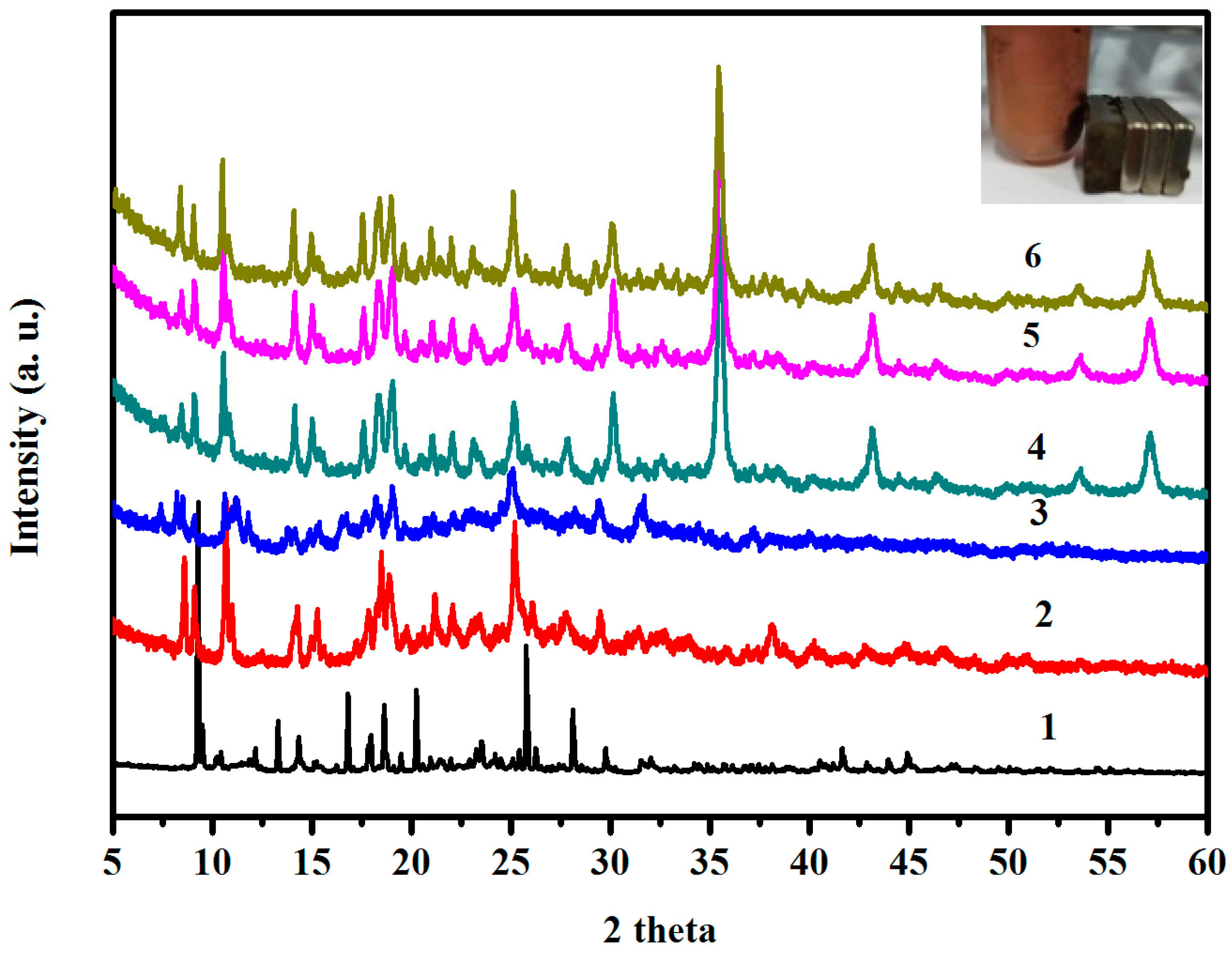
3.2. The Hybrization of HPU-9 and Fe3O4
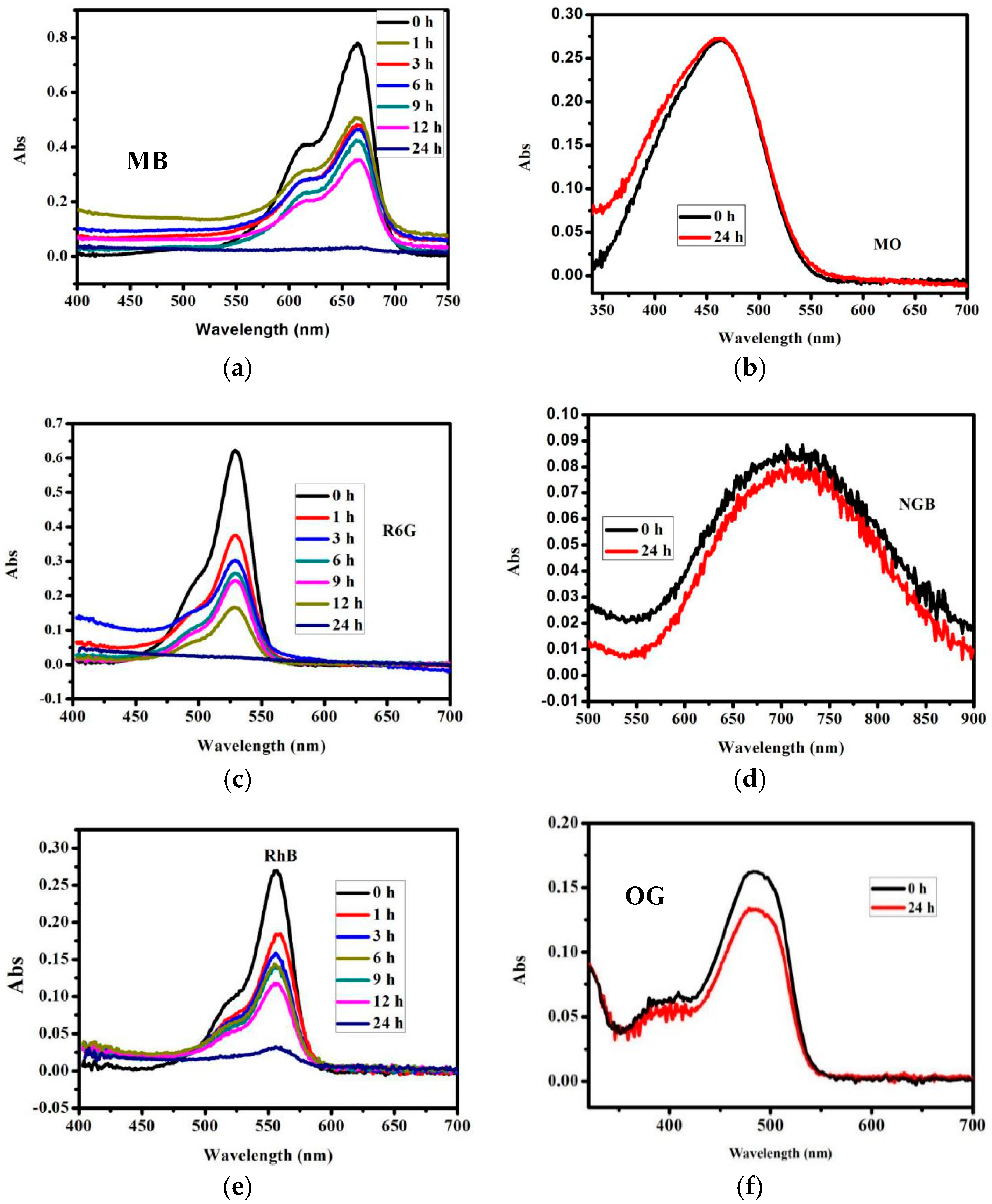
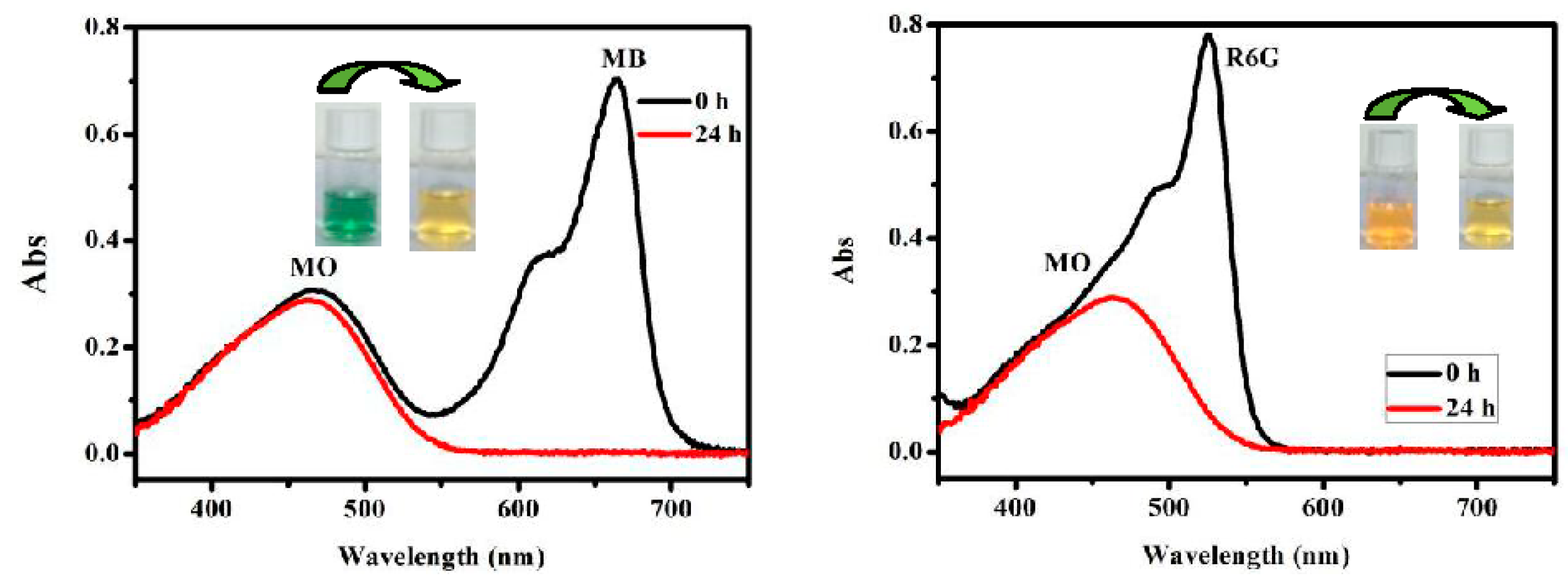
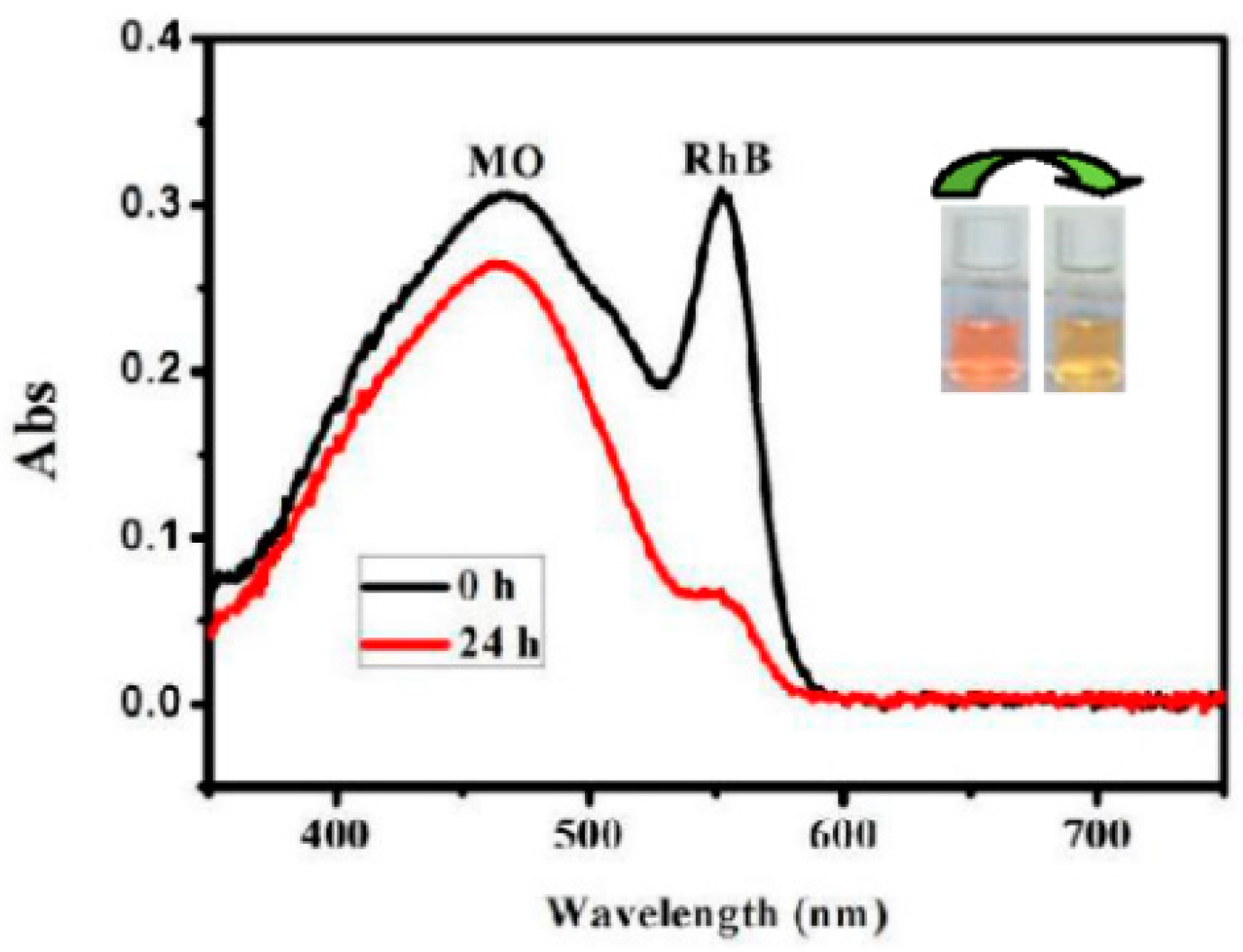
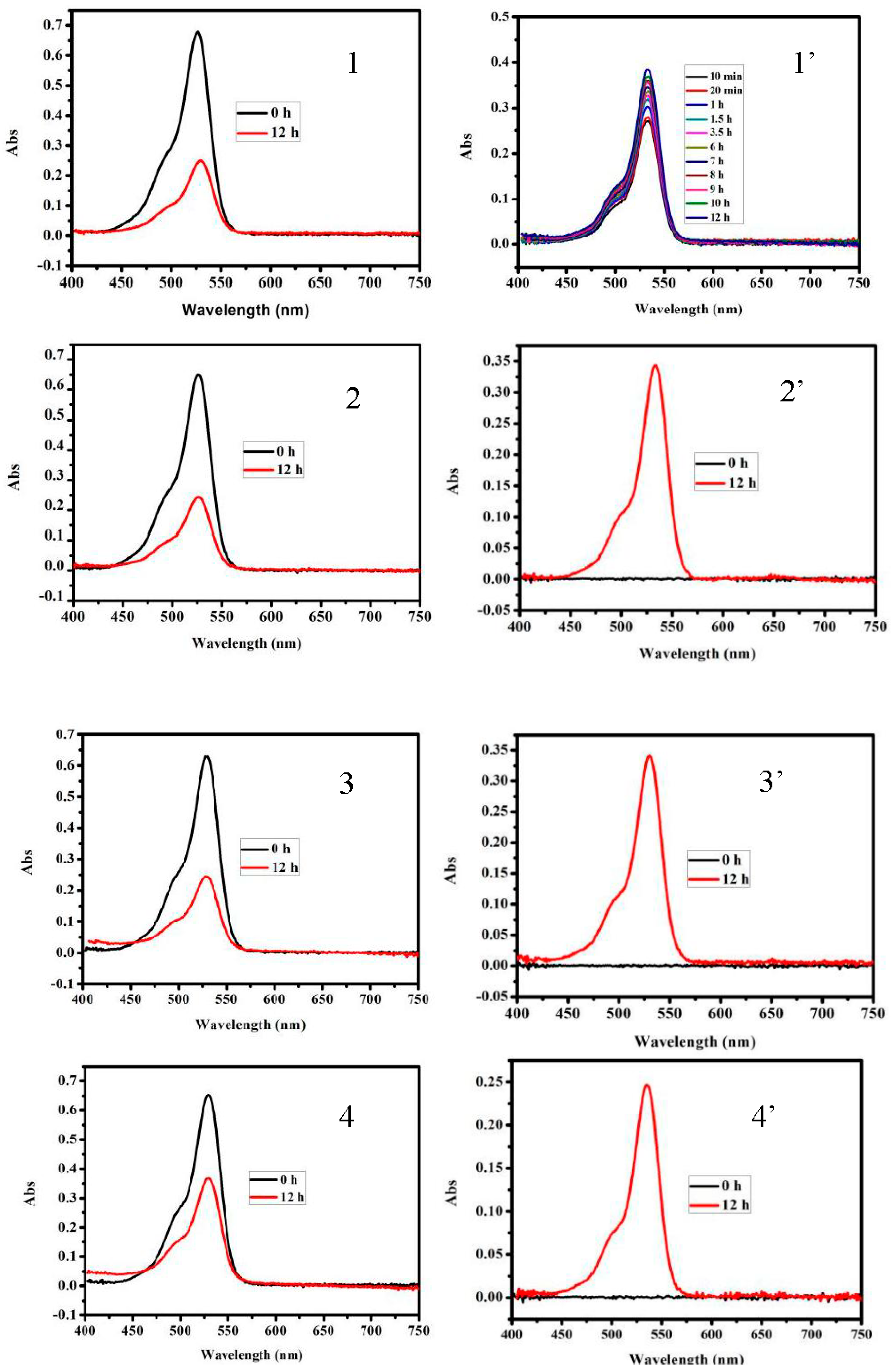
3.3. Dye Absorption and Separation
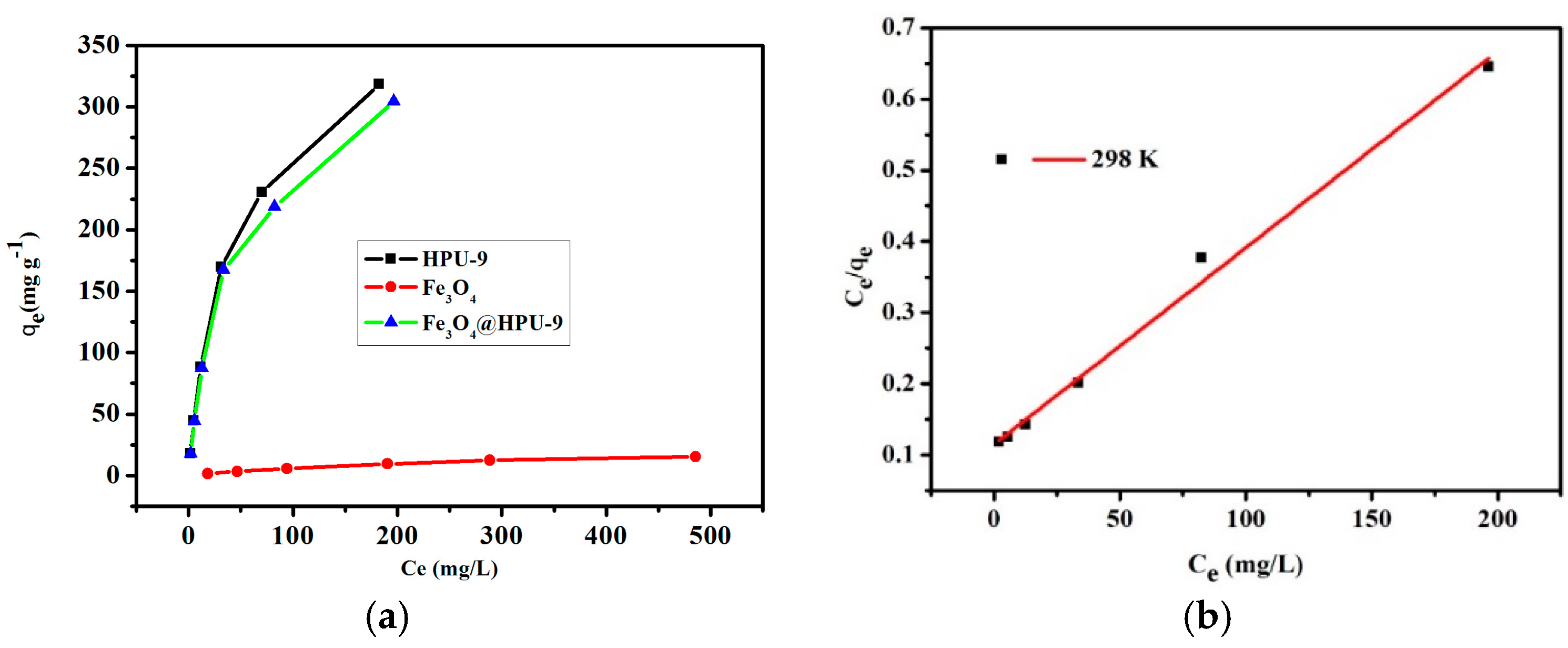
3.4. Absorption Kinetics and Absorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Polyethylenimine nanofibrous adsorbent for highly effective removal of anionic dyes from aqueous solution. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Y.; Ren, G.J.; Li, A.L.; Zhang, L.Z.; Feng, R.; Zhang, Y.H.; Bu, X.H. Temperature-Related Synthesis of Two Anionic Metal–Organic Frameworks with Distinct Performance in Organic Dye Absorption. Cryst. Growth Des. 2016, 16, 5593–5597. [Google Scholar] [CrossRef]
- Robson, K.C.D.; Hu, K.; Meyer, G.J.; Berlinguette, C.P. Atomic Level Resolution of Dye Regeneration in the Dye-Sensitized Solar Cell. J. Am. Chem. Soc. 2013, 135, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Y.; Zhang, M.; Wu, H.; Yang, L.; Li, R.Z.; Wang, P. Donor/Acceptor Indenoperylene Dye for Highly Efficient Organic Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2015, 137, 3799–3802. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.L.; Wang, W.J.; Liu, L.; Han, Z.B.; Wei, N.; Cao, X.M.; Yuan, D.Q. Microporous Hexanuclear Ln(III) Cluster-Based Metal–OrganicFrameworks: Color Tunability for Barcode Application and Selective Removal of Methylene Blue. Inorg. Chem. 2017, 56, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Sharifzade, G.; Asghari, A.; Rajabi, M. Highly effective adsorption of xanthene dyes (rhodamine B and erythrosine B) from aqueous solutions onto lemon citrus peel active carbon: Characterization, resolving analysis, optimization and mechanistic studies. RSC Adv. 2017, 7, 5362–5371. [Google Scholar] [CrossRef]
- Takeshita, J.; Hasegawa, Y.; Yanai, K.; Yamamoto, A.; Ishii, A.; Hasegawa, M.; Yamanaka, M. Organic Dye Absorption by Amphiphilic Tris-Urea Supramolecular Hydrogel. Chem. Asian J. 2017, 12, 2029–2032. [Google Scholar] [CrossRef] [PubMed]
- Das, P.P.; Agarkar, S.A.; Mukhopadhyay, S.; Manju, U.; Ogale, S.B.; Devi, P.S. Defects in Chemically Synthesized and Thermally Processed ZnO Nanorods: Implications for Active Layer Properties in Dye-Sensitized Solar Cells. Inorg. Chem. 2014, 53, 3961–3972. [Google Scholar] [CrossRef] [PubMed]
- Sonu, K.P.; Pavan Kumar, B.V.V.S.; George Subi, J.; Eswaramoorthy, M. Simple and Facile Approach to Create Charge Reversible Pores via Hydrophobic Anchoring of Ionic Amphiphiles. ACS Appl. Mater. Interfaces 2017, 9, 9136–9142. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Y.; Sun, Y.Z.S.; Chu, J.; Zhang, X.Z.; Deng, H.X. Multivariate Metal–Organic Frameworks for Dialing-in the Binding and Programming the Release of Drug Molecules. J. Am. Chem. Soc. 2017, 139, 14209–14216. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.L.; Yang, J.; Zhang, H.M.; Liu, Y.Y.; Ma, J.F. Metal-Ion Exchange, Small-Molecule Sensing, Selective Dye Absorption, and Reversible Iodine Uptake of Three Coordination Polymers Constructed by a New Resorcin[4]arene-Based Tetracarboxylate. Inorg. Chem. 2015, 54, 1744–1755. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Huang, Z.H.; El-Zohry, A.M.; Zheng, H.Q.; Zou, X.D. A Fast and Scalable Approach for Synthesis of Hierarchical Porous Zeolitic Imidazolate Frameworks and One-Pot Encapsulation of Target Molecules. Inorg. Chem. 2017, 56, 9139–9146. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Yan, B. A lanthanide metal-organic framework (MOF-76) for adsorbing dyes and fluorescence detecting aromatic pollutants. RSC Adv. 2016, 6, 11570–11576. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zou, Q.; Sun, S.K.; Yu, C.S.; Zhang, X.J.; Li, R.J.; Fu, Y.Y. Theranostic Metal-Organic Framework Core-Shell Composites for Magnetic Resonance Imaging and Drug Delivery. Chem. Sci. 2016, 7, 5294–5301. [Google Scholar] [CrossRef]
- Dolatkhah, A.; Wilson, L.D. Magnetite/Polymer Brush Nanocomposites with Switchable Uptake Behavior toward Methylene Blue. ACS Appl. Mater. Interfaces 2016, 8, 5595–5607. [Google Scholar] [CrossRef] [PubMed]
- An, H.Y.; Hu, Y.; Wang, L.; Zhou, E.L.; Fei, F.; Su, Z.M. 3D Racemic Microporous Frameworks and 3D Chiral Supramolecular Architectures Based on Evans–Showell-Type Polyoxometalates Controlled by the Temperature. Cryst. Growth Des. 2015, 15, 164–175. [Google Scholar] [CrossRef]
- Du, P.Y.; Gu, W.; Liu, X. Multifunctional Three-Dimensional Europium Metal–Organic Framework for Luminescence Sensing of Benzaldehyde and Cu2+ and Selective Capture of Dye Molecules. Inorg. Chem. 2016, 55, 7826–7828. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Konavarapu, S.K.; Sasmal, H.S.; Biradha, K. Porous Coordination Polymers Containing Pyridine-3,5-Bis(5-azabenzimidazole): Exploration of Water Sorption, Selective Dye Absorption, and Luminescent Properties. Cryst. Growth Des. 2016, 16, 5976–5984. [Google Scholar] [CrossRef]
- Song, Y.; Fan, R.Q.; Xing, K.; Du, X.; Su, T.; Wang, P.; Yang, Y.L. Insight into the Controllable Synthesis of Cu(I)/Cu(II) Metal–Organic Complexes: Size-Exclusive Selective Dye Absorption and Semiconductor Properties. Cryst. Growth Des. 2017, 17, 2549–2559. [Google Scholar] [CrossRef]
- Lu, L.; Wu, J.; Wang, J.; Liu, J.Q.; Li, B.H.; Singh, A.; Kumard, A.; Batten, S.R. An uncommon 3D 3, 3, 4, 8-c Cd(II) metal-organic framework for highly efficient luminescent sensing and organic dye absorption: Experimental and theoretical insight. CrystEngComm 2017, 19, 7057–7067. [Google Scholar] [CrossRef]
- Ke, F.; Yuan, Y.P.; Qiu, L.G.; Shen, Y.H.; Xie, A.J.; Zhu, J.F.; Tian, X.Y.; Zhang, L.D. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011, 21, 3843–3848. [Google Scholar] [CrossRef]
- Xiong, Y.H.; Ye, F.G.; Zhang, C.; Shen, S.F.; Su, L.J.; Zhao, S.L. Synthesis of magnetic porous γ-Fe2O3/C@HKUST-1 composites for efficient removal of dyes and heavy metal ions from aqueous solution. RSC Adv. 2015, 5, 5164–5172. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.L.; Gao, M.; Hong, W.; Liu, G.Z.; Fan, L.; Hu, B.; Xia, Q.H.; Liu, L.; Song, G.W.; et al. The absorption on magnetic hybrid Fe3O4/HKUST-1/GO of methylene blue from water solution. J. Mater. Chem. A 2014, 2, 1795–1801. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, X.M.; Deng, C.H. Rational synthesis of novel recyclable Fe3O4@MOF nanocomposites for enzymatic digestion. Chem. Commun. 2015, 51, 8116–8119. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Yan, X. Metal–organic framework MIL-100(Fe) for the absorption of malachite green from aqueous solution. J. Mater. Chem. 2012, 22, 7449–7455. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D.; Huang, H.; Zhang, W.; Yang, Q.; Zhong, C. The stability and defluoridation performance of MOFs in fluoride solutions. Microporous Mesoporous Mater. 2014, 185, 72–78. [Google Scholar] [CrossRef]
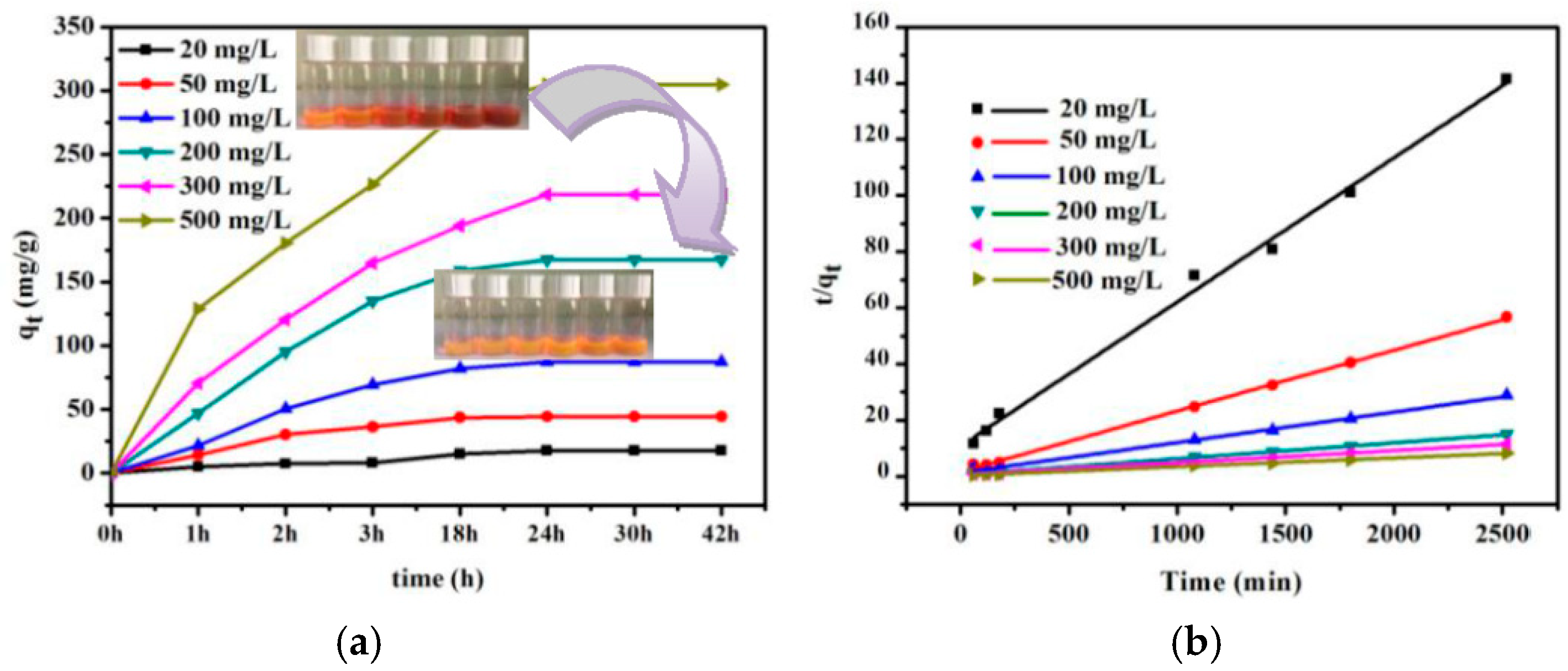
| Pseudo-Second-Order | 20 M | 50 M | 100 M | 200 M | 300 M | 500 M |
|---|---|---|---|---|---|---|
| qe (mg·g−1) | 19.48 | 46.18 | 92.42 | 175.74 | 229.35 | 316.45 |
| R2 | 0.99504 | 0.99887 | 0.99724 | 0.99825 | 0.99745 | 0.99941 |
| K2 (g·mg−1·min−1) | 2.43 × 10−4 | 2.86 × 10−4 | 9.26 × 10−5 | 5.45 × 10−5 | 3.87 × 10−5 | 3.74 × 10−5 |
| Analyte | qexp | Langmuir Constants | ||
|---|---|---|---|---|
| Qm | KL | R2 | ||
| HPU-9 | 317.273 | 384.615 | 0.025 | 0.99736 |
| Fe3O4 | 15.377 | 25.013 | 0.0032 | 0.99159 |
| Fe3O4@HPU-9 | 304.536 | 362.318 | 0.024 | 0.99151 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, Q.; He, Y.; Zhang, N.; Xu, Z.; Wang, Y. Facile Fabrication of Magnetic Metal-Organic Framework Composites for the Highly Selective Removal of Cationic Dyes. Materials 2018, 11, 744. https://doi.org/10.3390/ma11050744
Li H, Li Q, He Y, Zhang N, Xu Z, Wang Y. Facile Fabrication of Magnetic Metal-Organic Framework Composites for the Highly Selective Removal of Cationic Dyes. Materials. 2018; 11(5):744. https://doi.org/10.3390/ma11050744
Chicago/Turabian StyleLi, Huijun, Qingqing Li, Yaling He, Ning Zhang, Zhouqing Xu, and Yuan Wang. 2018. "Facile Fabrication of Magnetic Metal-Organic Framework Composites for the Highly Selective Removal of Cationic Dyes" Materials 11, no. 5: 744. https://doi.org/10.3390/ma11050744
APA StyleLi, H., Li, Q., He, Y., Zhang, N., Xu, Z., & Wang, Y. (2018). Facile Fabrication of Magnetic Metal-Organic Framework Composites for the Highly Selective Removal of Cationic Dyes. Materials, 11(5), 744. https://doi.org/10.3390/ma11050744