Deposition of Zinc Oxide on Different Polymer Textiles and Their Antibacterial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Applied Textile Materials
2.2. Textile Modification Process
3. Results and Discussion
3.1. Textile Characterization
3.2. Deposition and Characterization of Antibacterial Material
3.3. Antibacterial Characterization
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lee, Y.-H.; Kim, A.-L.; Park, Y.-G.; Hwang, E.-K.; Baek, Y.-M.; Cho, S.; Kim, H.-D. Colorimetric assay and deodorizing/antibacterial performance of natural fabrics dyed with immature pine cone extract. Text. Res. J. 2017, 88, 731–743. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, L.; Diao, H.; Li, F.; Zhang, Y.; Fu, F.; Liu, X. Antibacterial cotton fabric with enhanced durability prepared using silver nanoparticles and carboxymethyl chitosan. Carbohydr. Polym. 2017, 177, 187–193. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Gao, C.; Li, S.; Chung, C.T.W.; Xin, J.H. Non-leaching and durable antibacterial textiles finished with reactive zwitterionic sulfobetaine. J. Ind. Eng. Chem. 2017, 46, 373–378. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Willers, C.; Wentzel, J.F.; Du Plessis, L.H.; Gouws, C.; Hamman, J.H. Efflux as a mechanism of antimicrobial drug resistance in clinical relevant microorganisms: The role of efflux inhibitors. Expert Opin. Ther. Targets 2017, 21, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, W.; Li, J.; Wang, K.; Li, J.; Tan, H.; Fu, Q. Gemini quaternary ammonium salt waterborne biodegradable polyurethanes with antibacterial and biocompatible properties. Mater. Chem. Front. 2017, 1, 361–368. [Google Scholar] [CrossRef]
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Delezuk, J.A.; Ramírez-Herrera, D.E.; de Ávila, B.E.F.; Wang, J. Enhancement of thermal diffusivity in phase-separated bismaleimide/poly(ether imide) composite films containing needle-shaped ZnO particles. Nanoscale 2017, 9, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Mahbubul Hassan, M. Binding of a quaternary ammonium polymer-grafted-chitosan onto a chemically modified wool fabric surface: Assessment of mechanical, antibacterial and antifungal properties. RSC Adv. 2015, 5, 35497–35505. [Google Scholar] [CrossRef]
- Iyigundogdu, Z.U.; Demir, O.; Asutay, A.B.; Sahin, F. Developing Novel Antimicrobial and Antiviral Textile Products. Appl. Biochem. Biotechnol. 2017, 181, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, J.; Ji, D.; Qin, X.; Ge, Y.; Xie, S. A novel approach for fabricating antibacterial nanofiber/cotton hybrid yarns. Fibers Polym. 2017, 18, 987–992. [Google Scholar] [CrossRef]
- Milošević, M.; Krkobabić, A.; Radoičić, M.; Šaponjić, Z.; Radetić, T.; Radetić, M. Biodegradation of cotton and cotton/polyester fabrics impregnated with Ag/TiO2 nanoparticles in soil. Carbohydr. Polym. 2017, 158, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.F.; Moussa, M.; Sheta, A.M.F.; Taha, R.; Gamal, H. Synthesis of effective multifunctional textile based on silica nanoparticles. Prog. Org. Coat. 2017, 106, 41–49. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. Chem. Eng. J. 2013, 228, 596–613. [Google Scholar] [CrossRef]
- Sonia, S.; Ruckmani, K.; Sivakumar, M. Antimicrobial and antioxidant potentials of biosynthesized colloidal zinc oxide nanoparticles for a fortified cold cream formulation: A potent nanocosmeceutical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 581–589. [Google Scholar]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Fiedot, M.; Karbownik, I.; Maliszewska, I.; Rac, O.; Suchorska-Woźniak, P.; Teterycz, H. Deposition of one-dimensional zinc oxide structures on polypropylene fabrics and their antibacterial properties. Text. Res. J. 2015, 85, 1340–1354. [Google Scholar] [CrossRef]
- Fiedot, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Woźniak, P.; Lewińska, A.; Teterycz, H. The Relationship between the Mechanism of Zinc Oxide Crystallization and Its Antimicrobial Properties for the Surface Modification of Surgical Meshes. Materials 2017, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Textiles. Test Methods for Nonwovens. Part 6: Absorption. ISO 9073-6:2003; International Organization for Standardization (ISO): Geneva, Switzerland, 2001.
- Fiedot, M.; Rac, O.; Suchorska-Woźniak, P.; Karbownik, I.; Teterycz, H. Polymer-surfactant interactions and their influence on zinc oxide nanoparticles morphology. In Manufacturing Nanostructures; One Central Press (OCP): Manchester, UK, 2014. [Google Scholar]
- Textile fabrics—Determination of antibacterial activity. Agar diffusion plate test, ISO 20645:2004; International Organization for Standardization (ISO): Geneva, Switzerland, 2004.
- Vijayakumar, S.; Rajakumar, P.R. Infrared spectral analysis of waste pet samples. Int. Lett. Chem. Phys. Astron. 2012, 4, 58–65. [Google Scholar] [CrossRef]
- Shoushtari, A.M.; Malek, R.M.A.; Abdous, M. Effect of chemical oxidation treatment on dyeability of polypropylene. Dyes Pigme. 2004, 63, 95–100. [Google Scholar]
- Cheval, N.; Gindy, N.; Flowkes, C.; Fahmi, A. Polyamide 66 microspheres metallised with in situ synthesised gold nanoparticles for a catalytic application. Nanoscale Res. Lett. 2012, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D. Elements of X-ray Diffraction. Am. J. Phys. 1957, 25, 394–395. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray Line Broadening from Filed Al and W. Acta Met. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Curcio, E.; Fontananova, E.; Di Profio, G.; Drioli, E. Influence of the Structural Properties of Poly(vinylidene fluoride) Membranes on the Heterogeneous Nucleation Rate of Protein Crystals. J. Phys. Chem. B 2006, 110, 12438–12445. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fievet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Sonohara, R.; Muramatsu, N.; Ohshima, H.; Kondo, T. Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophys. Chem. 1995, 55, 273–277. [Google Scholar] [CrossRef]
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surf. Physicochem. Eng. Asp. 2011, 374, 1–8. [Google Scholar] [CrossRef]
- Russell, A.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003, 52, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.O.; Watson, S.P.; Foster, S.J. Characterization of the major superoxide dismutase of staphylococcus aureus and its role in starvation survival, stress resistance, and pathogenicity. J. Bacteriol. 1999, 181, 3898–3903. [Google Scholar] [PubMed]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal response to oxidative stress. Front. Cell. Infect. Microbiol. 2012, 2, 33. [Google Scholar] [CrossRef] [PubMed]
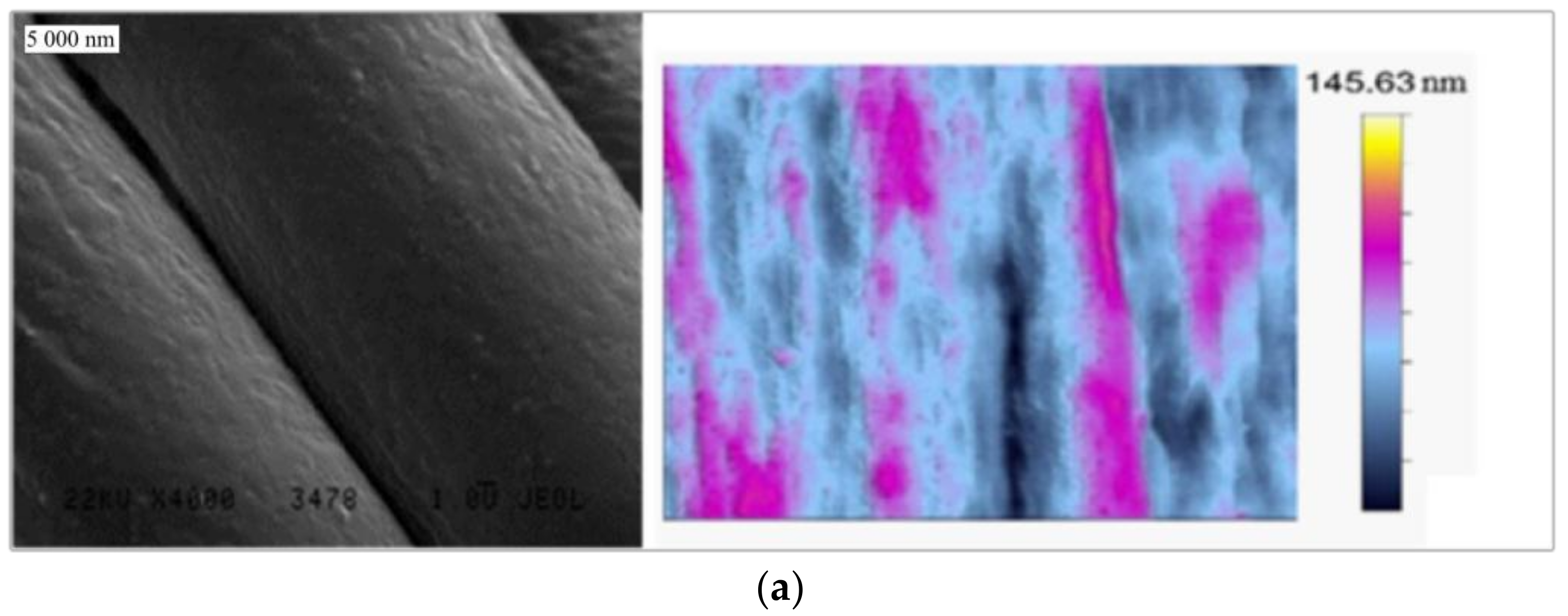
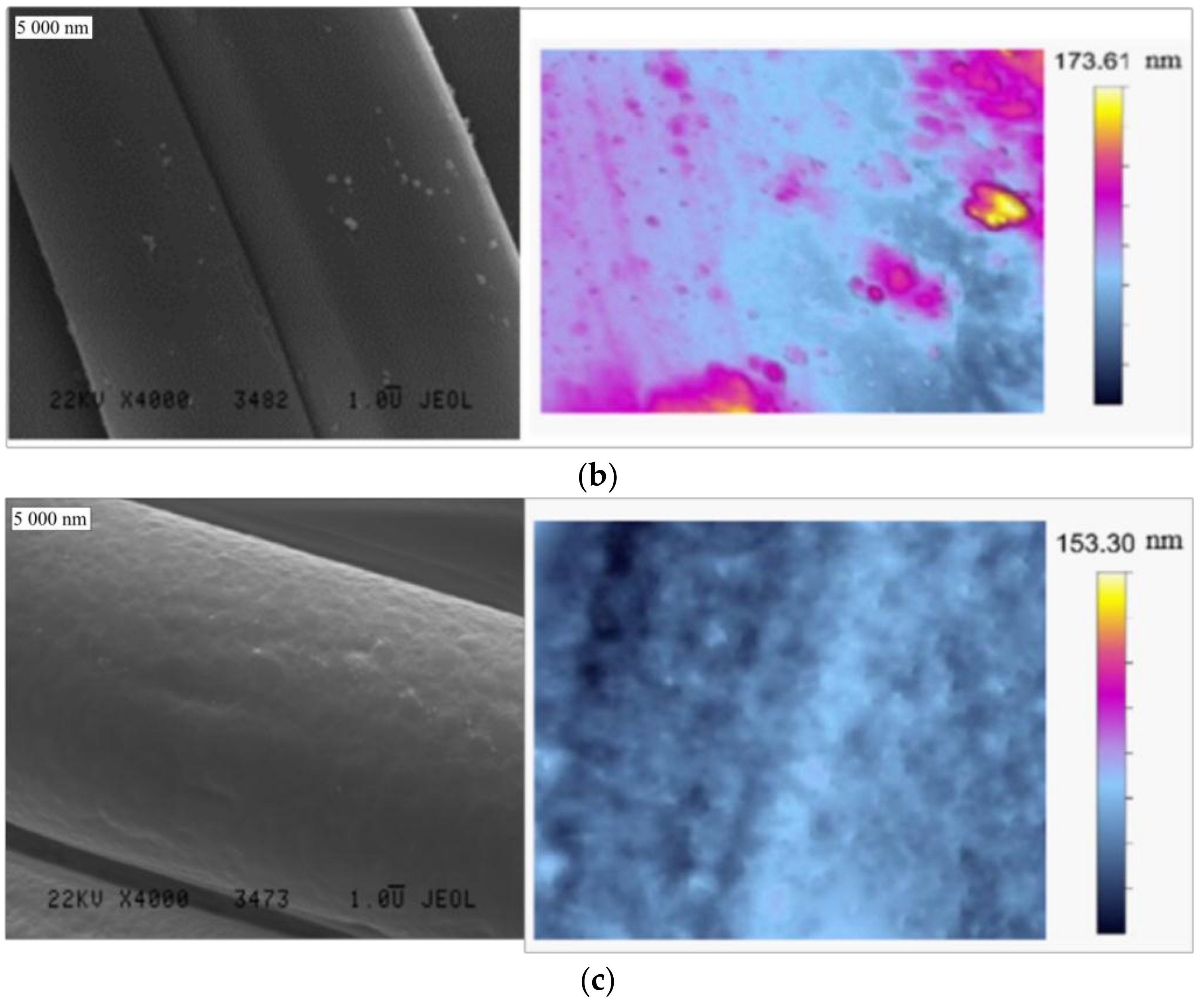

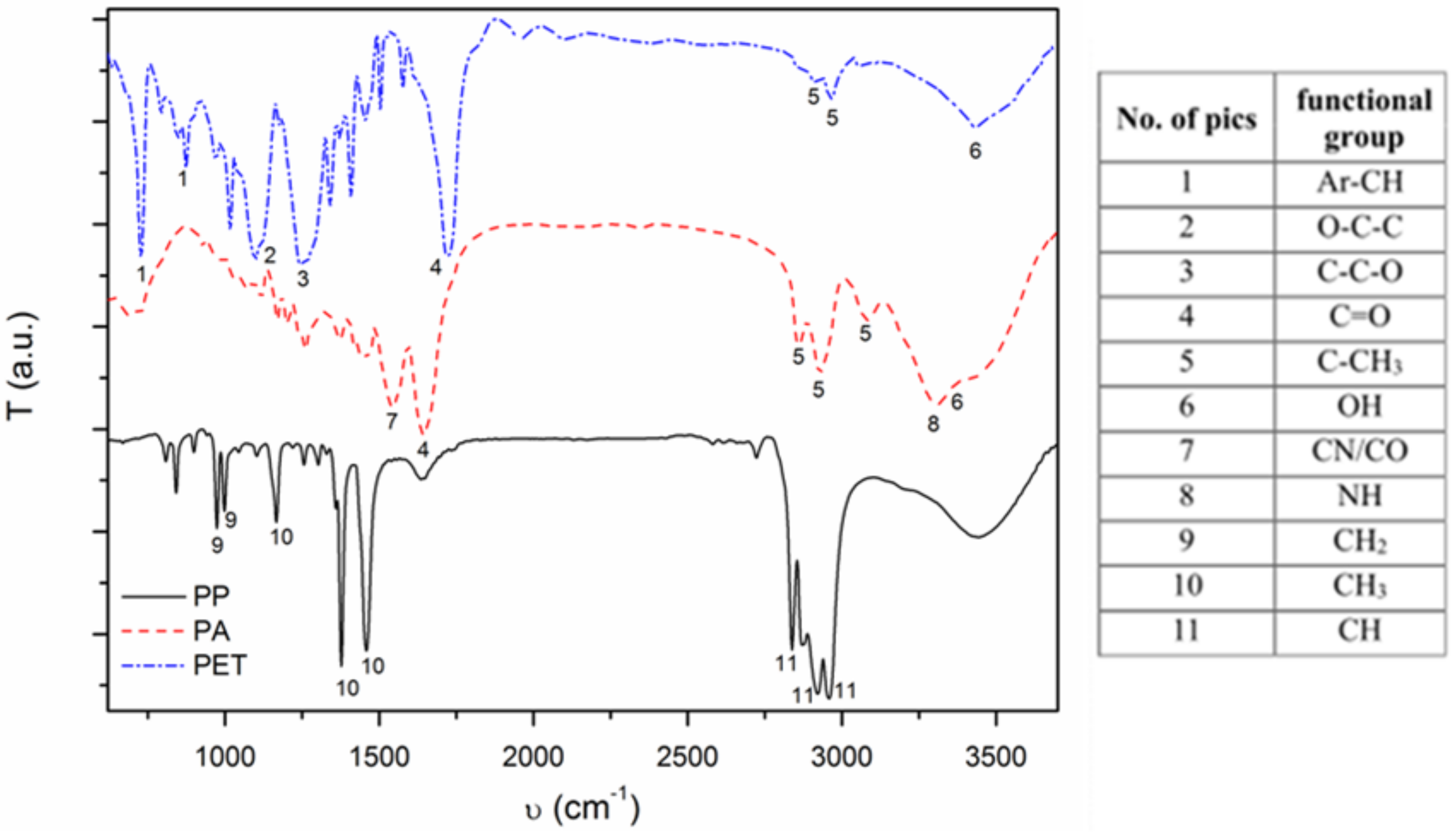
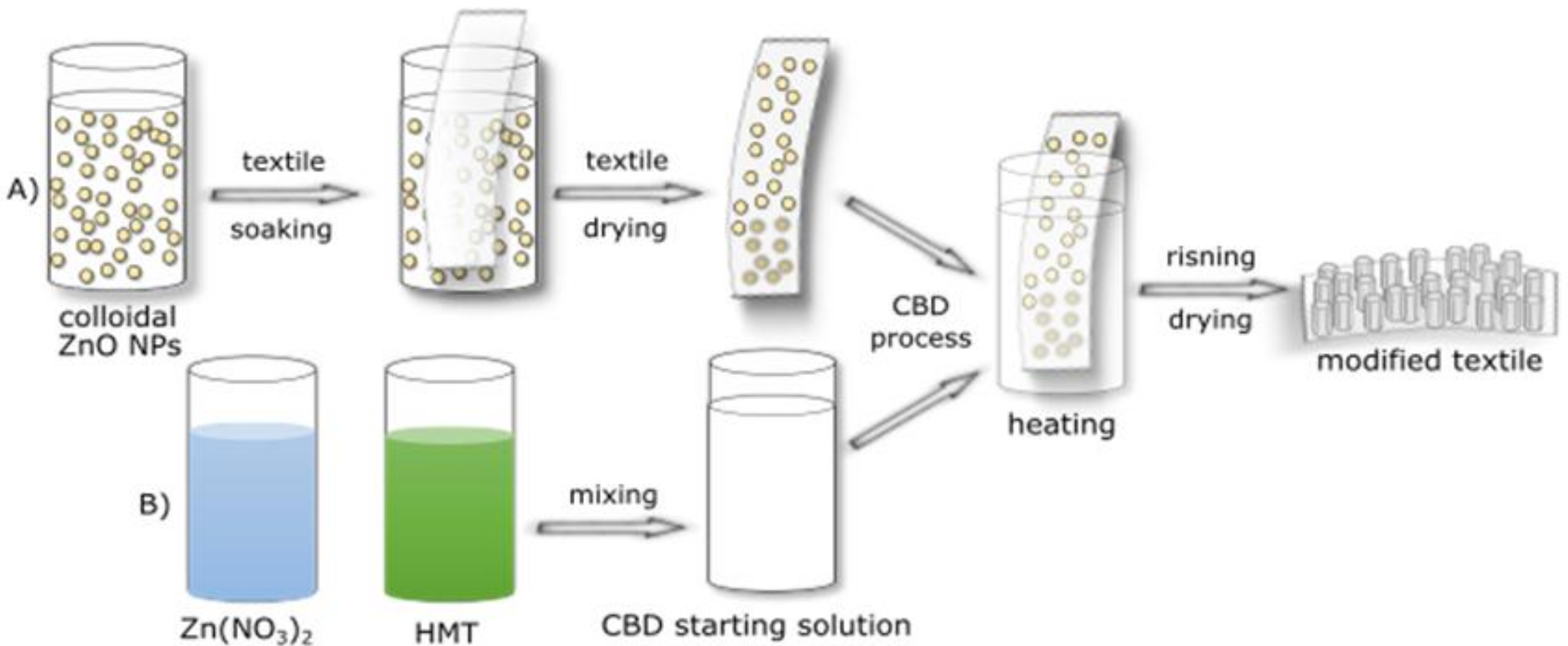


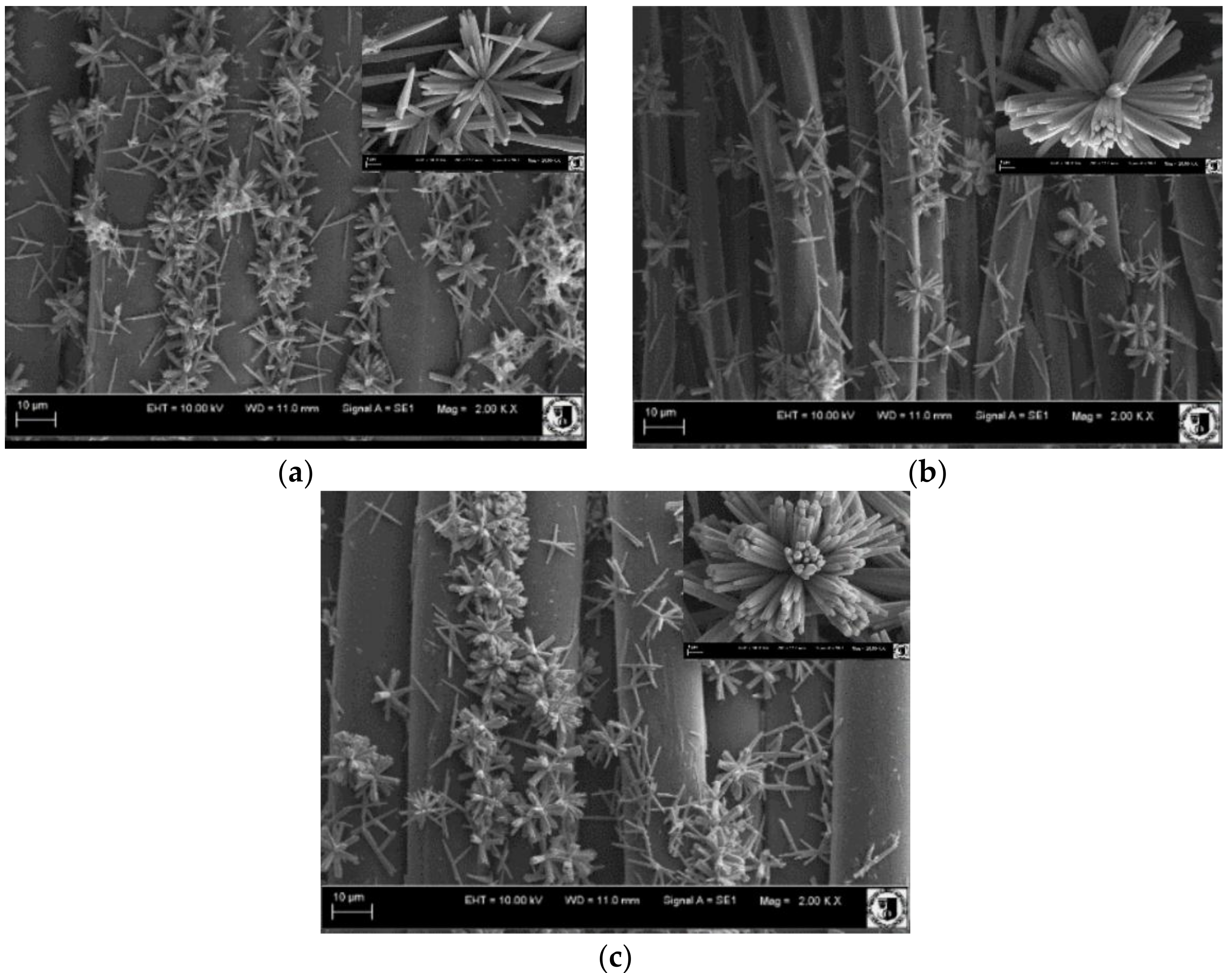


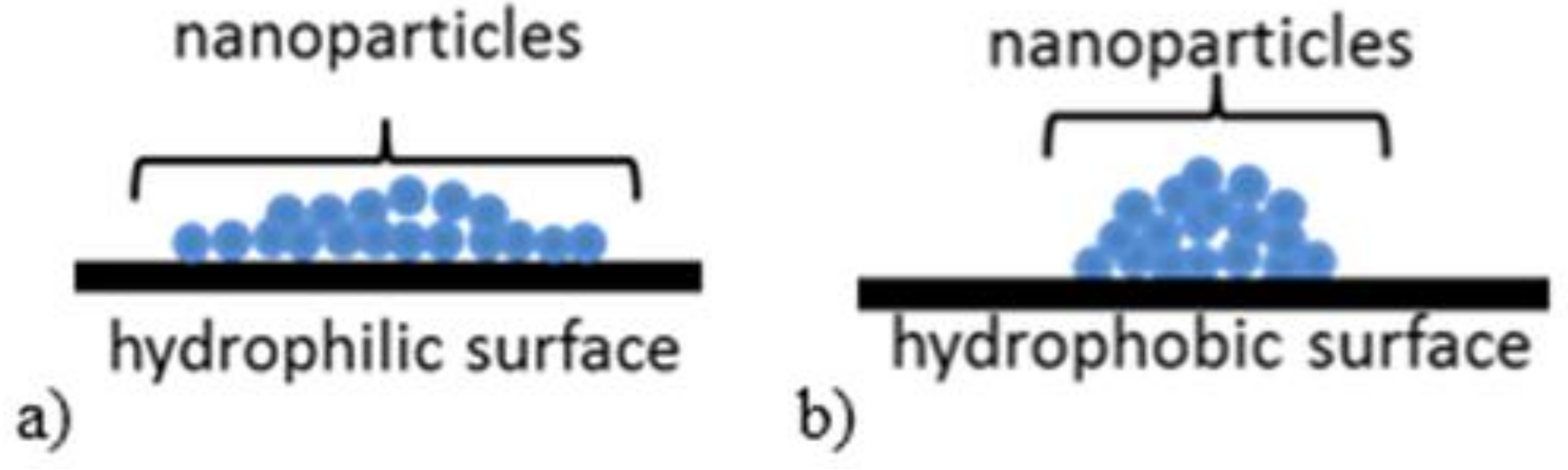
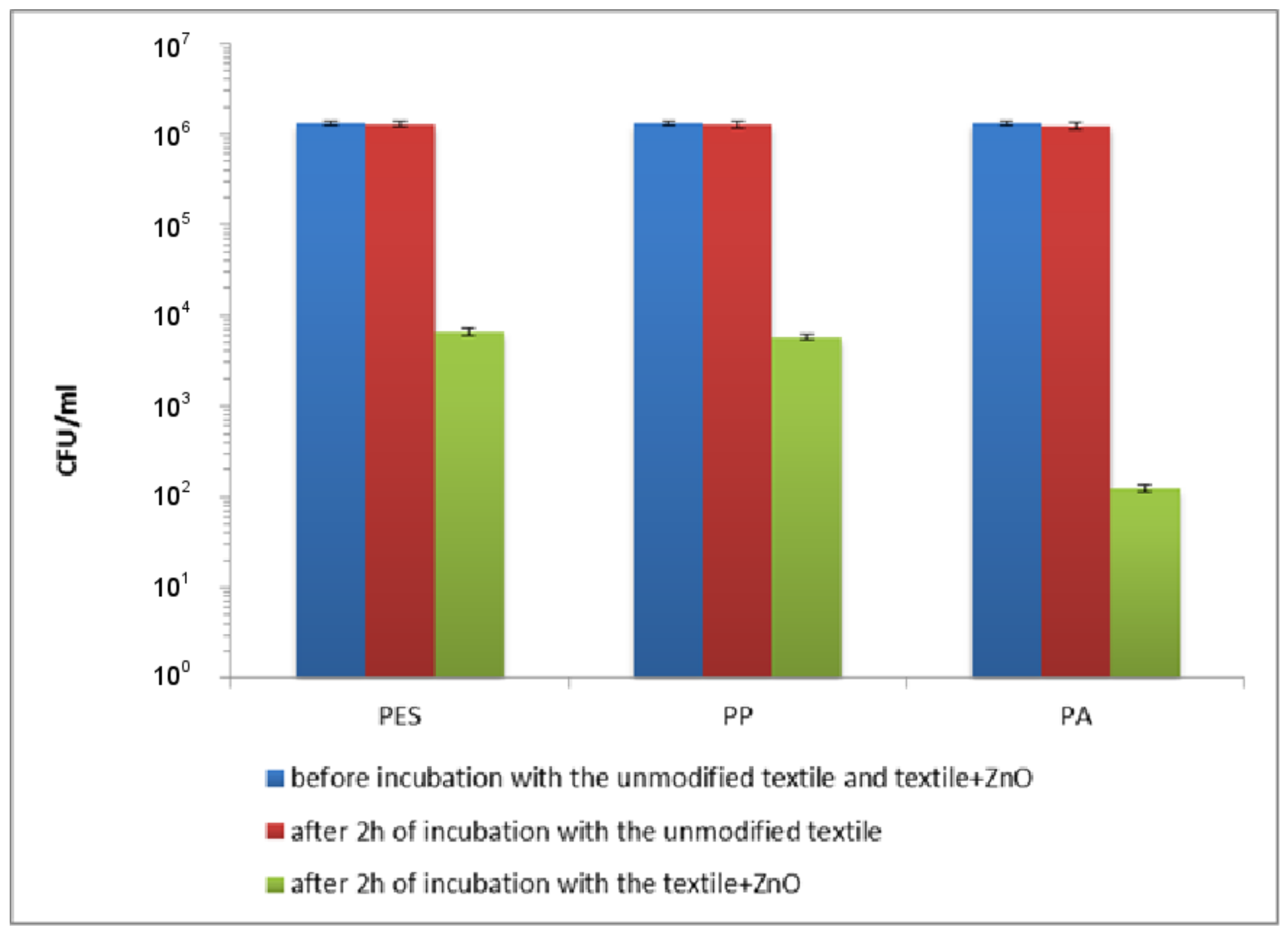

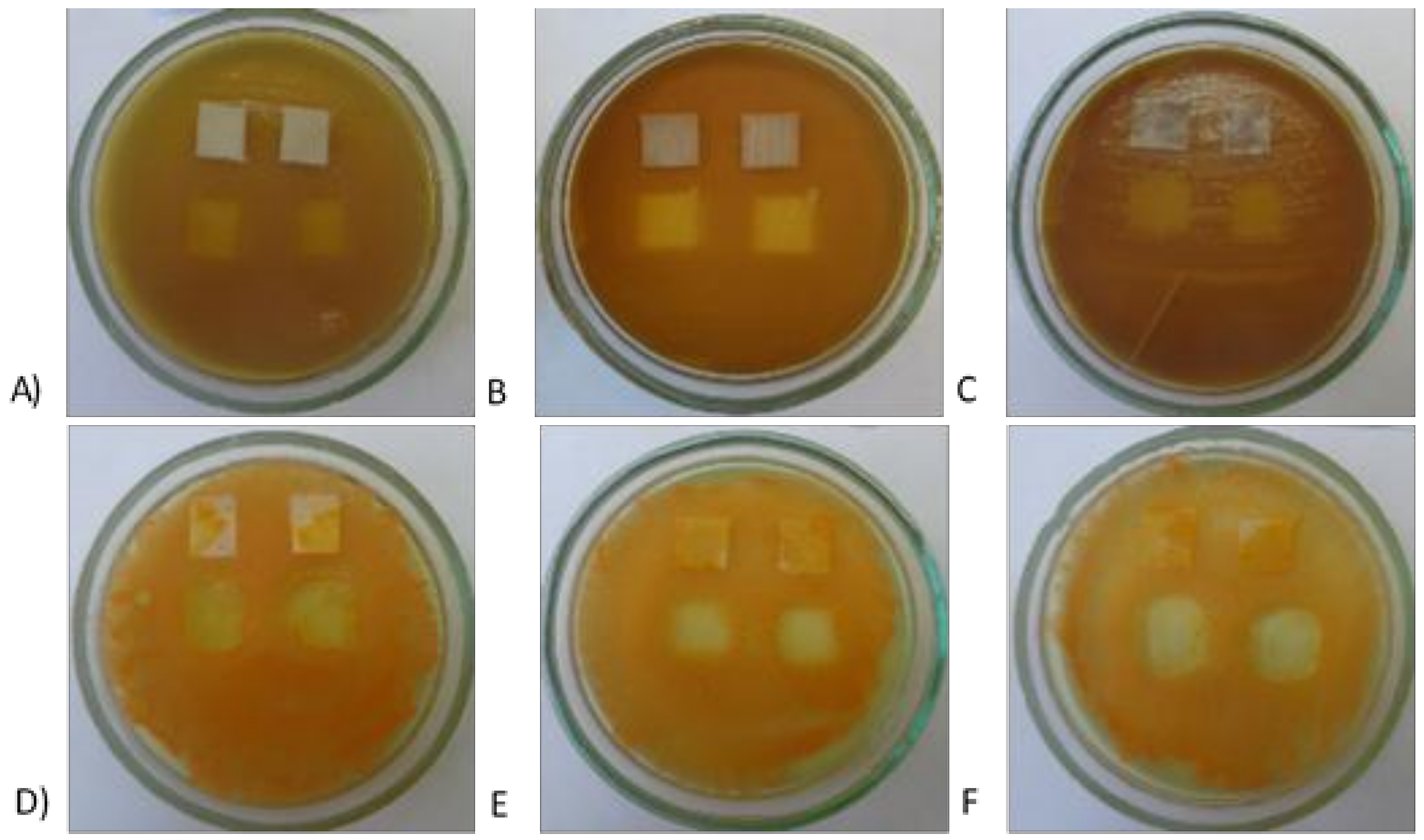
| Textile | Warp | Weft | Fabric Surface Mass (g/m2) | The Way of Fabric Preparation | ||
|---|---|---|---|---|---|---|
| Yarn Character | Number of Threads/10 cm | Yarn Character | Number of Threads/10 cm | |||
| PP | 84 dtexf33 | 460 | 84 dtexf33 | 330 | 72 | washing |
| PET | 84 dtexf48 | 390 | 150 dtexf216 | 320 | 79 | washing, thermal stabilization (20 s, 190 °C) |
| PA | 72 dtexF17 | 380 | 160 dtexF144 | 310 | 81 | washing, thermal stabilization (20 s, 185 °C) |
| Sample | ΔHm100 (J/g) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|
| PA | 230.1 | 116.2 | 50.5 |
| PET | 140.1 | 70.7 | 50.5 |
| PP | 207.1 | 106.1 | 51.2 |
| Sample | PA | PET | PP |
|---|---|---|---|
| LAC (%) | 185 ± | 169 ± 3 | 160 ± 5 |
| Sample | d(100) (Å) | d(002) (Å) | a (Å) | c (Å) | V (Å3) | ε | D (nm) |
|---|---|---|---|---|---|---|---|
| PA | 2.83 | 2.62 | 3.27 | 5.24 | 48.52 | 0.00069 | 23.3 |
| PET | 2.83 | 2.61 | 3.27 | 5.23 | 48.43 | 0.00061 | 22.8 |
| PP | 2.83 | 2.61 | 3.27 | 5.23 | 48.43 | 0.00099 | 24.6 |
| Standard | 2.81 | 2.60 | 3.25 | 5.21 | 47.60 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedot-Toboła, M.; Ciesielska, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Woźniak, P.; Teterycz, H.; Bryjak, M. Deposition of Zinc Oxide on Different Polymer Textiles and Their Antibacterial Properties. Materials 2018, 11, 707. https://doi.org/10.3390/ma11050707
Fiedot-Toboła M, Ciesielska M, Maliszewska I, Rac-Rumijowska O, Suchorska-Woźniak P, Teterycz H, Bryjak M. Deposition of Zinc Oxide on Different Polymer Textiles and Their Antibacterial Properties. Materials. 2018; 11(5):707. https://doi.org/10.3390/ma11050707
Chicago/Turabian StyleFiedot-Toboła, Marta, Magdalena Ciesielska, Irena Maliszewska, Olga Rac-Rumijowska, Patrycja Suchorska-Woźniak, Helena Teterycz, and Marek Bryjak. 2018. "Deposition of Zinc Oxide on Different Polymer Textiles and Their Antibacterial Properties" Materials 11, no. 5: 707. https://doi.org/10.3390/ma11050707
APA StyleFiedot-Toboła, M., Ciesielska, M., Maliszewska, I., Rac-Rumijowska, O., Suchorska-Woźniak, P., Teterycz, H., & Bryjak, M. (2018). Deposition of Zinc Oxide on Different Polymer Textiles and Their Antibacterial Properties. Materials, 11(5), 707. https://doi.org/10.3390/ma11050707









