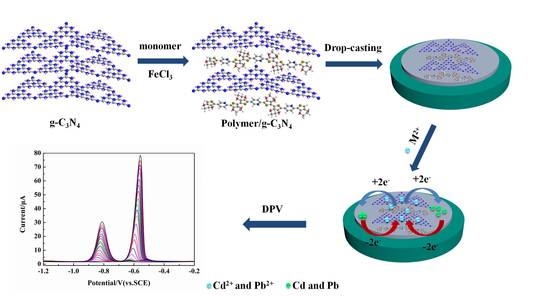
An Electrochemical Sensor of Poly(EDOT-pyridine-EDOT)/Graphitic Carbon Nitride Composite for Simultaneous Detection of Cd2+ and Pb2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments and Characterizations
2.3. Preparation of g-C3N4 and Poly(BPE)/g-C3N4 Composites
2.3.1. Preparation of g-C3N4
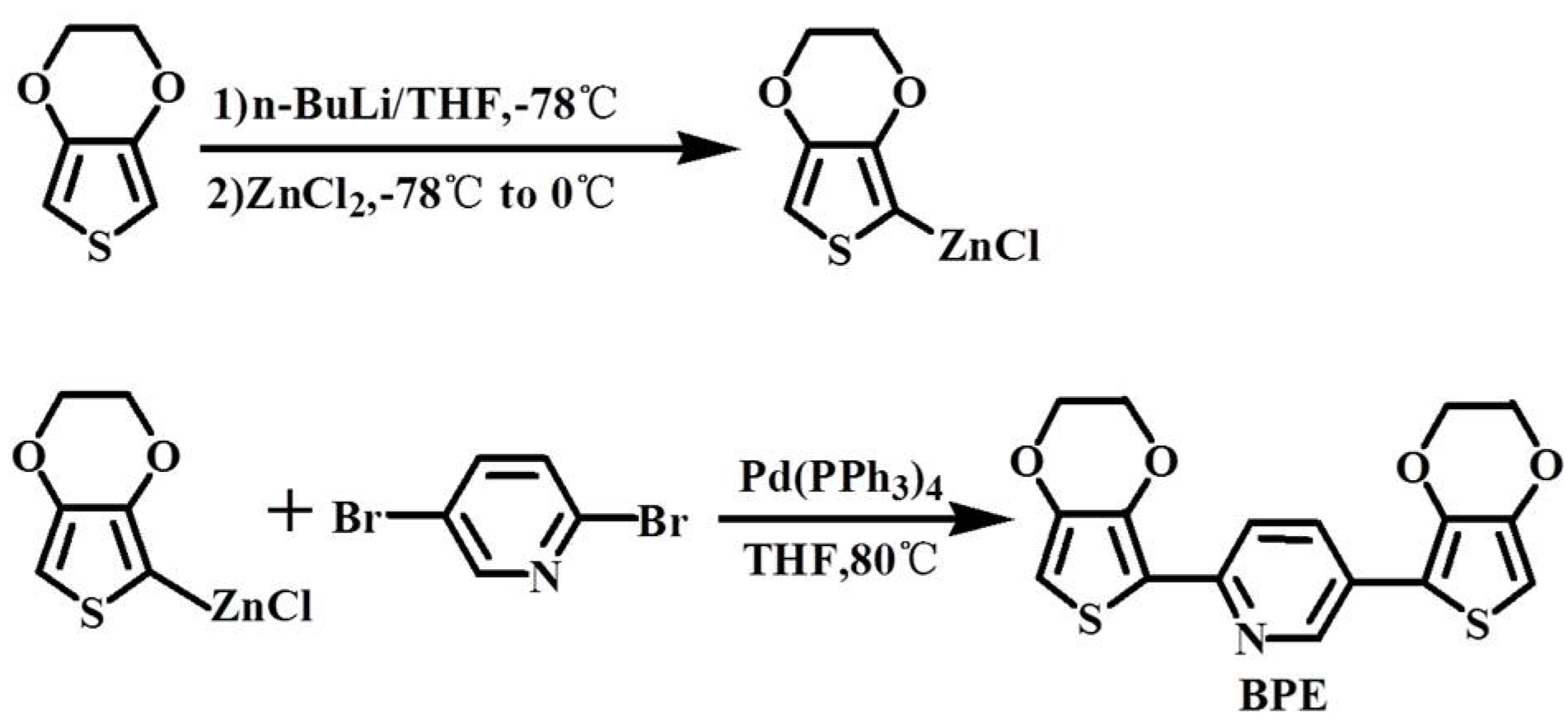
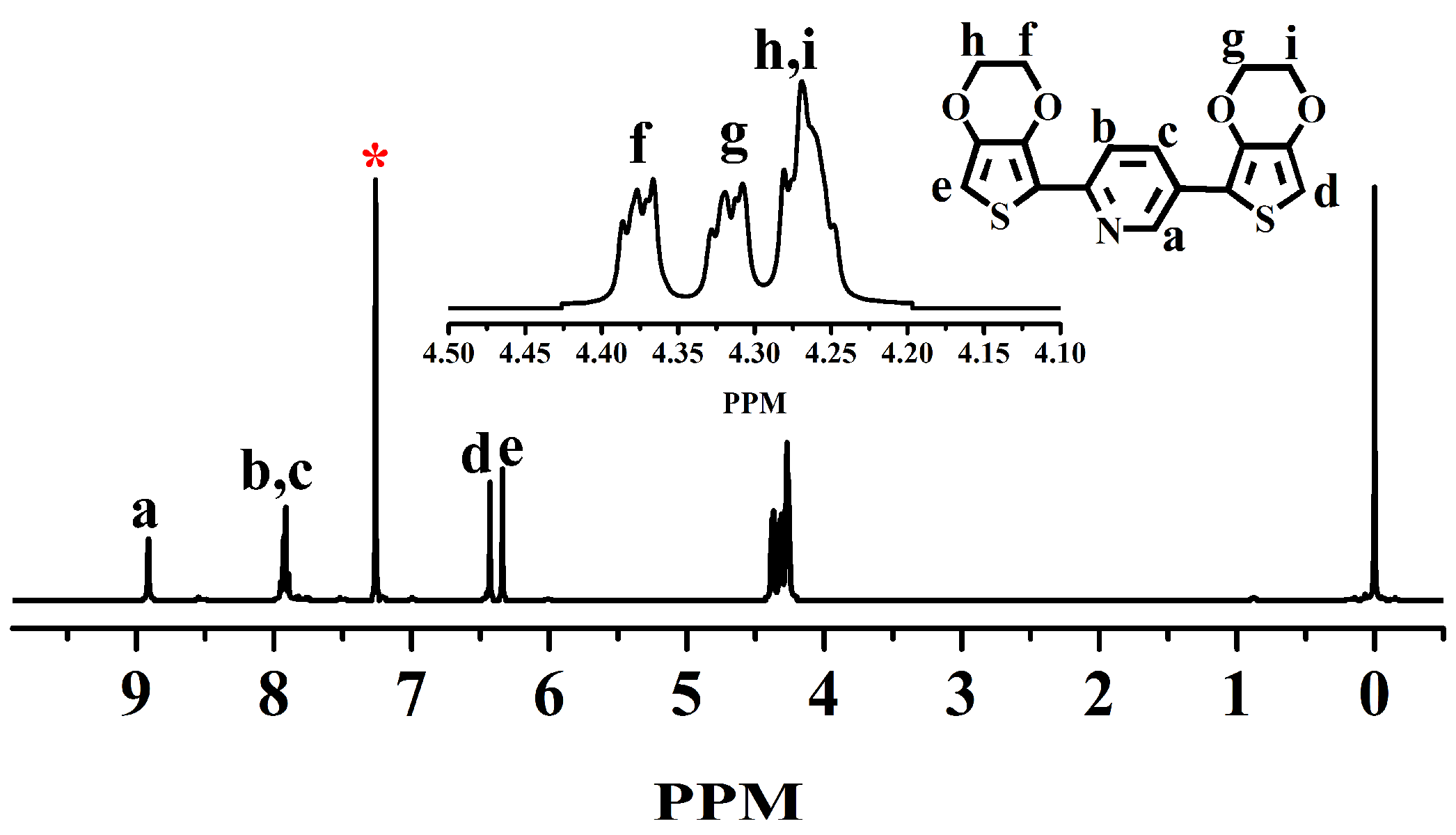
2.3.2. Preparation of Monomer BPE
2.3.3. Preparation of Poly(BPE)/g-C3N4 Composites
2.4. Preparation of Modified Electrodes
2.5. Electrochemical Measurements
3. Results and Discussion
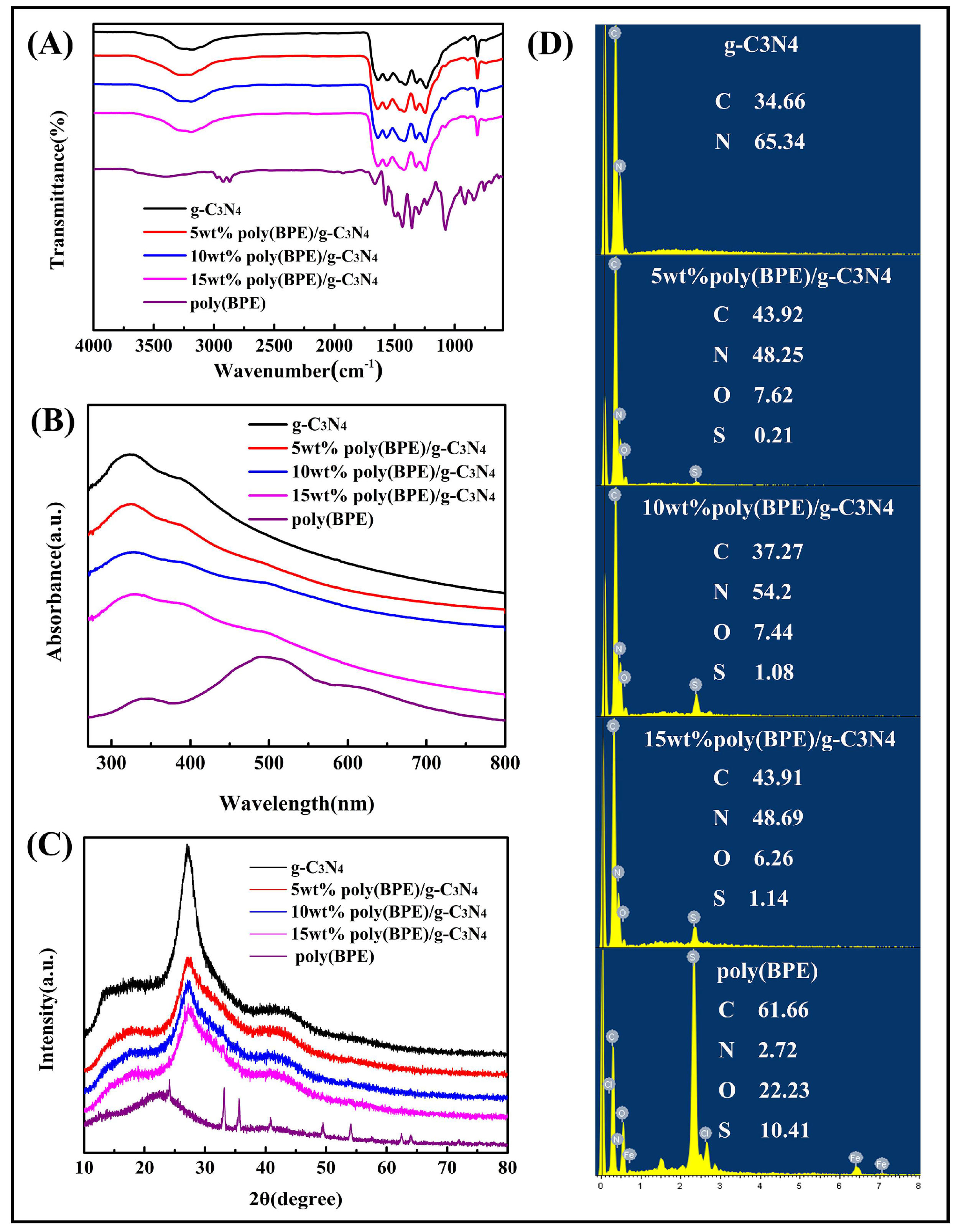
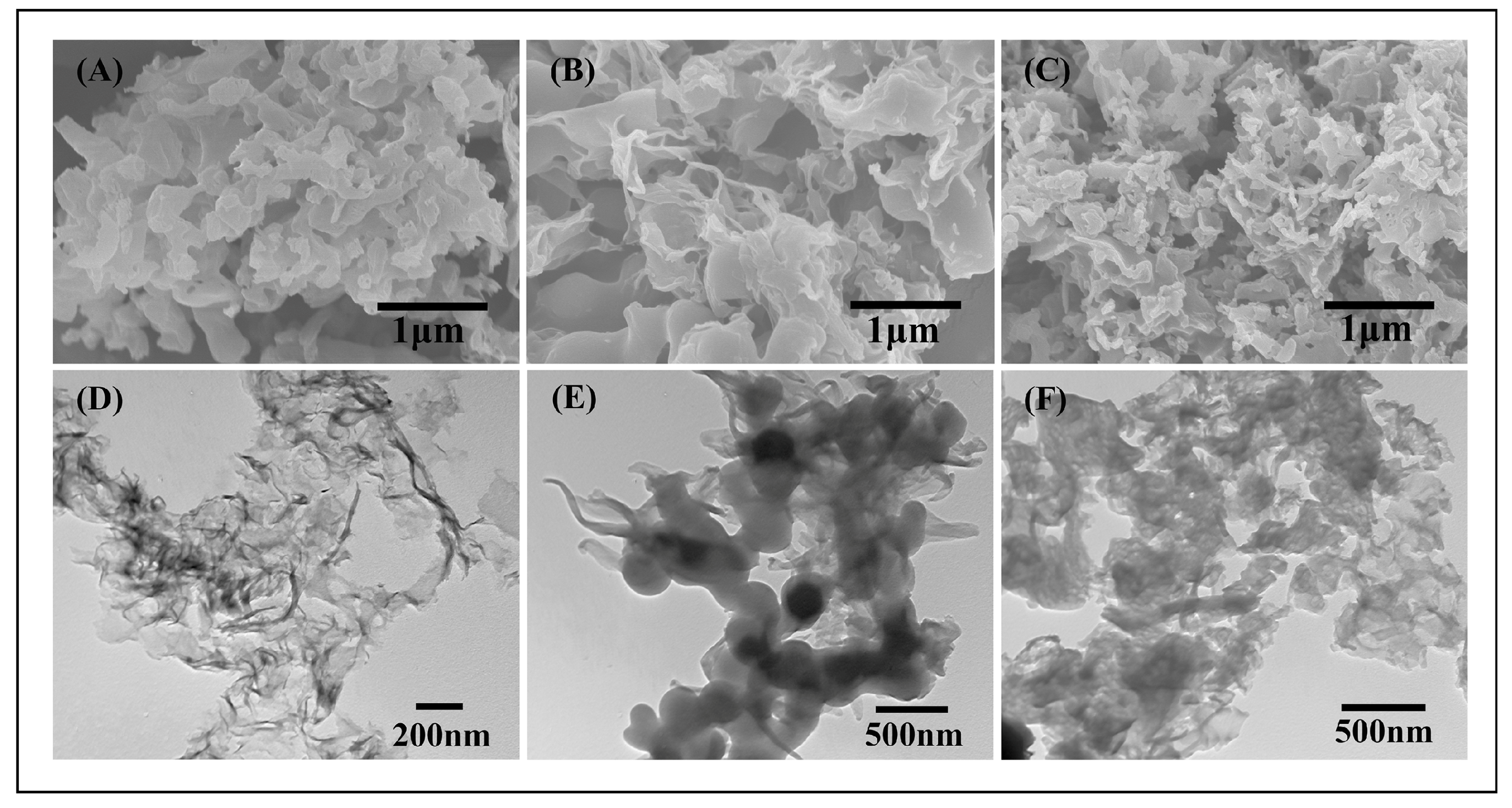
3.1. Structure Characterization of Poly(BPE)/g-C3N4 Composites
3.2. Electrochemical Characterization of Poly(BPE)/g-C3N4 Composites
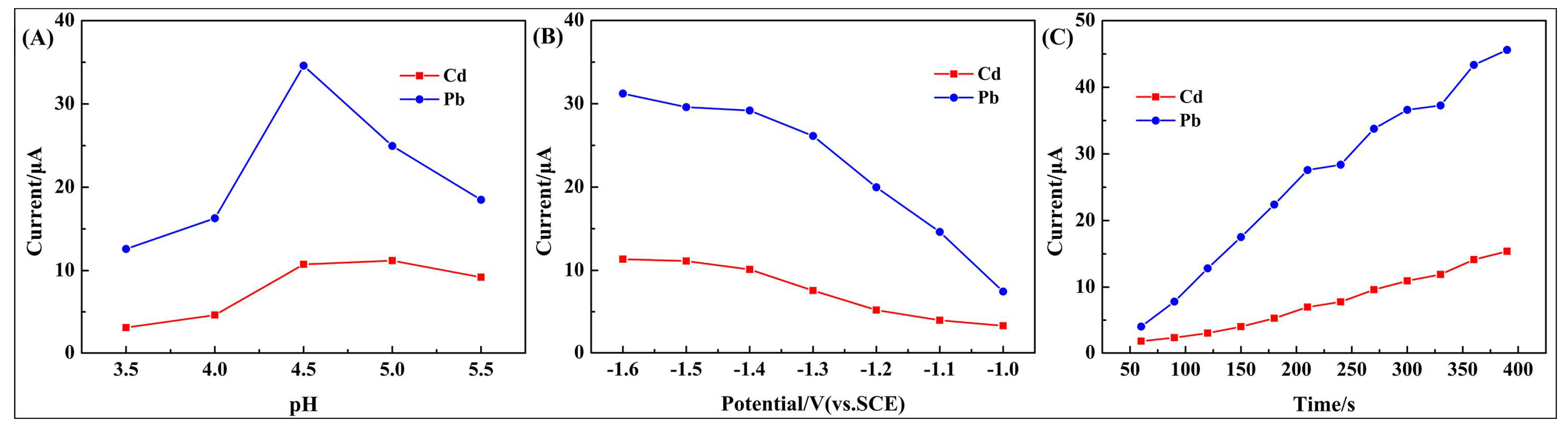
3.3. Optimization of Experimental Conditions
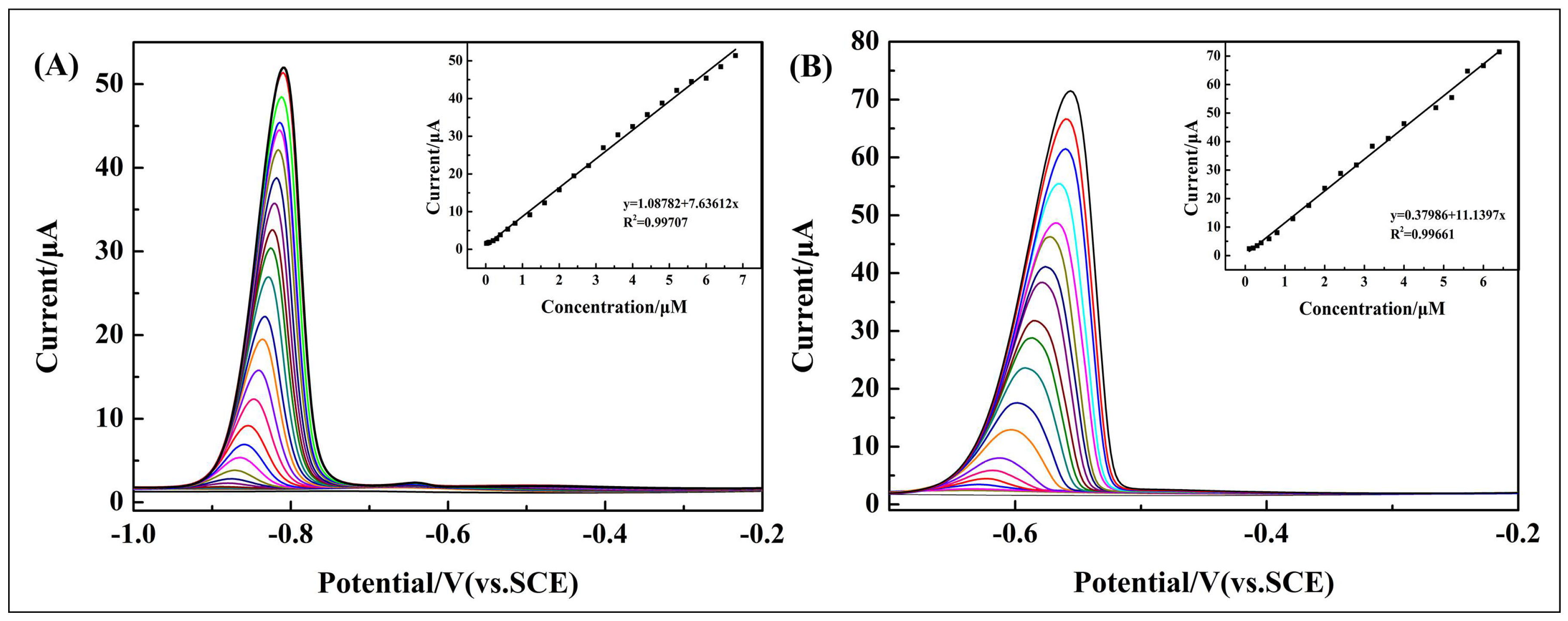
3.4. Individual Determination of Cd2+ and Pb2+
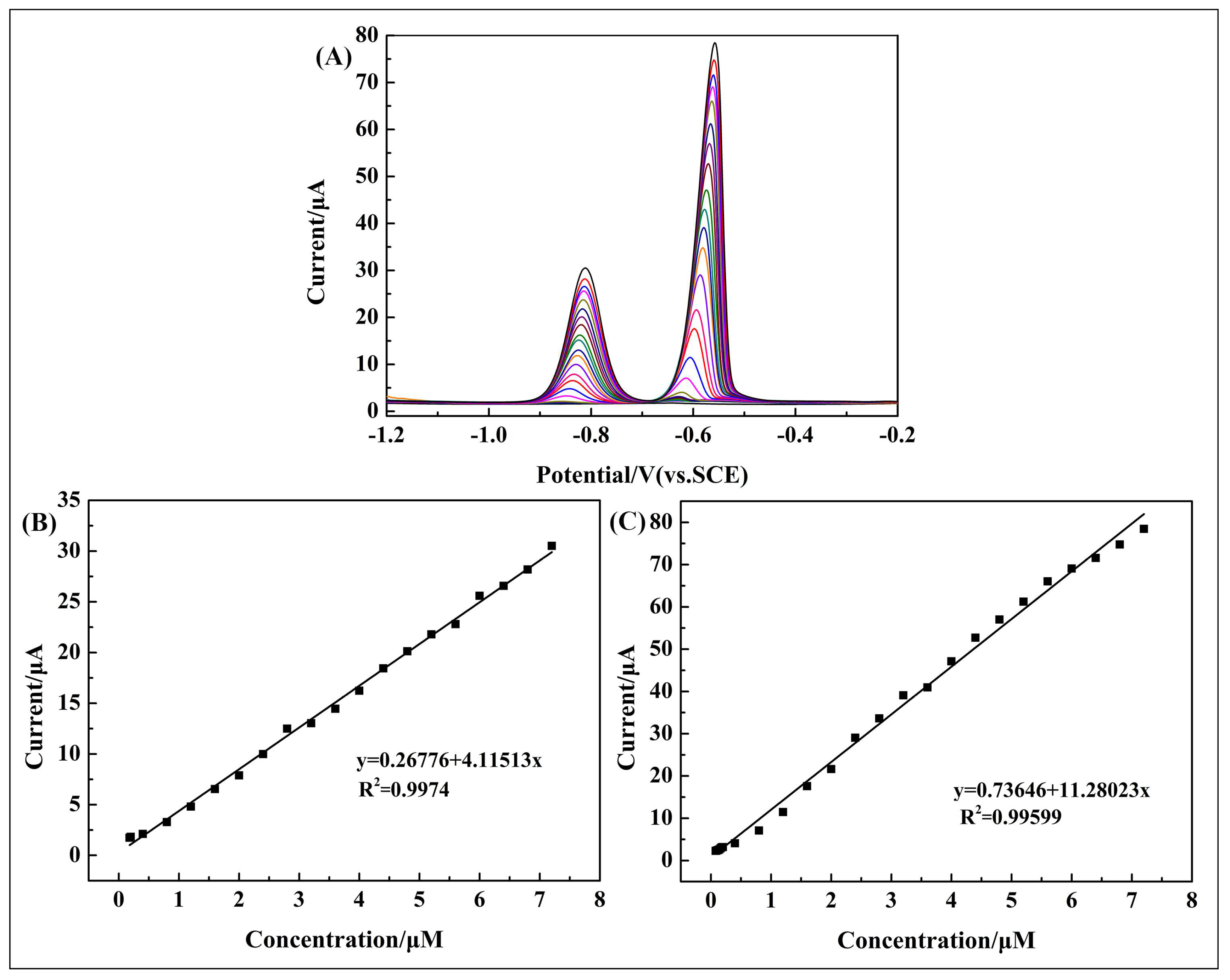
3.5. Simultaneous Determination of Cd2+ and Pb2+
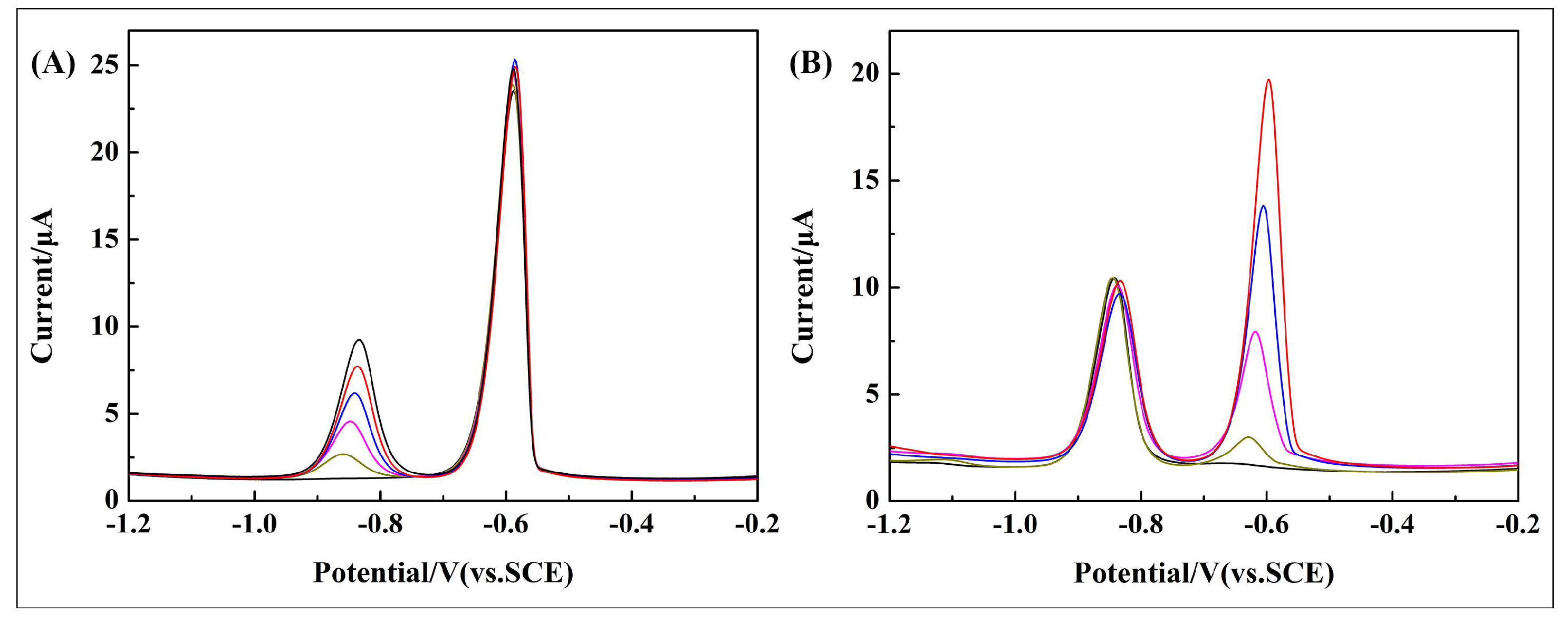
3.6. Interference Study
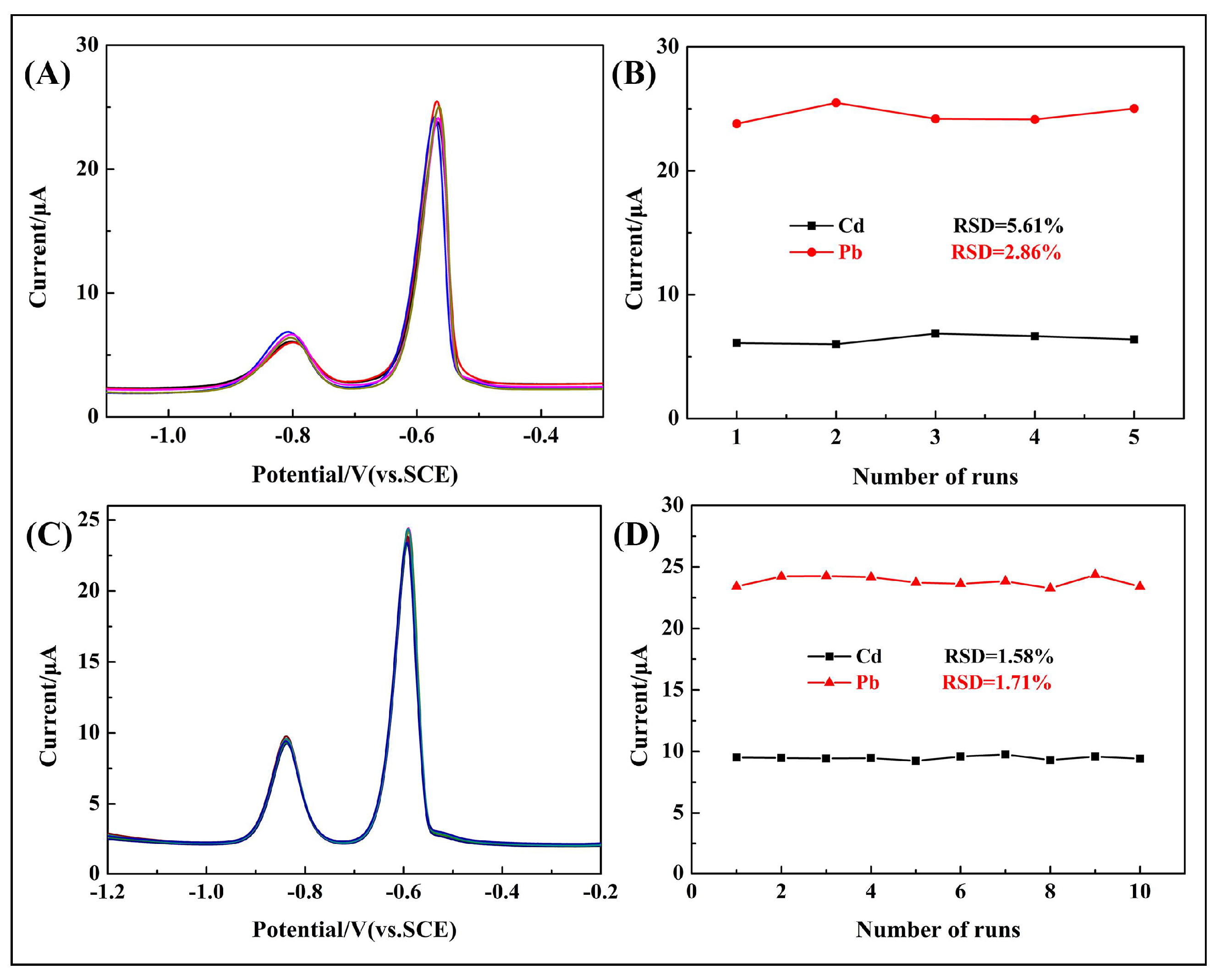
3.7. Reproducibility of Modified Electrode
3.8. Real Sample Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shahbazi, Y.; Ahmadi, F.; Fakhari, F. Voltammetric determination of Pb, Cd, Zn, Cu and Se in milk and dairy products collected from Iran: An emphasis on permissible limits and risk assessment of exposure to heavy metals. Food Chem. 2016, 192, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Pandit, U.J.; Wankar, S.; Limaye, S.N. Centrifugation assisted digestion for simultaneous voltammetric determination of ultra trace metal ions in water and milk samples. Environ. Nanotechnol. Monit. Manag. 2017, 7, 64–72. [Google Scholar] [CrossRef]
- Mahesar, S.A.; Sherazi, S.T.; Niaz, A.; Bhanger, M.I.; Uddin, S.; Rauf, A. Simultaneous assessment of Zinc, Cadmium, Lead and Copper in poultry feeds by differential pulse anodic stripping voltammetry. Food Chem. Toxicol. 2010, 48, 2357–2360. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, D.D.; Luo, X.K.; Li, Y.H.; Zhao, Q.N.; Li, M.M.; Zhao, Y.T.; Sun, T.S.; Ma, C. Simultaneous determination of trace Cd(II), Pb(II) and Cu(II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-l-lysine nanocomposite modified glassy carbon electrode. J. Colloid Interface Sci. 2016, 490, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2017, 178, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Koelmel, J.; Amarasiriwardena, D. Imaging of metal bioaccumulation in hay-scented fern (Dennstaedtia punctilobula) rhizomes growing on contaminated soils by laser ablation ICP-MS. Environ. Pollut. 2012, 168, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Massadeh, A.M.; Alomary, A.A.; Mir, S.; Momani, F.A.; Haddad, H.I.; Hadad, Y.A. Analysis of Zn, Cd, As, Cu, Pb, and Fe in snails as bioindicators and soil samples near traffic road by ICP-OES. Environ. Sci. Poll. Res. 2016, 23, 13424–13431. [Google Scholar] [CrossRef] [PubMed]
- Sánchezrodas, D.; Corns, W.T.; Chen, B.; Stockwell, P.B. Atomic Fluorescence Spectrometry: A suitable detection technique in speciation studies for arsenic, selenium, antimony and mercury. J. Anal. At. Spectrom. 2010, 25, 933–946. [Google Scholar] [CrossRef]
- Siraj, K.; Kitte, S.A. Analysis of Copper, Zinc and Lead using Atomic Absorption Spectrophotometer in ground water of Jimma town of Southwestern Ethiopia. Int. J. Chem. Anal. Sci. 2013, 4, 201–204. [Google Scholar] [CrossRef]
- Grasso, G.; D’Urso, L.; Messina, E.; Cataldo, F. A mass spectrometry and surface enhanced Raman spectroscopy study of the interaction between linear carbon chains and noble metals. Carbon 2009, 47, 2611–2619. [Google Scholar] [CrossRef]
- Caschera, D.; Federici, F.; Zane, D.; Focanti, F.; Curulli, A.; Padeletti, G. Gold nanoparticles modified GC electrodes: Electrochemical behaviour dependence of different neurotransmitters and molecules of biological interest on the particles size and shape. J. Nanopart. Res. 2008, 11, 1925–1936. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, R.; Yu, X.Y.; Wang, L.; Liu, J.H.; Huang, X.J. Stripping voltammetry study of ultra-trace toxic metal ions on highly selectively adsorptive porous magnesium oxide nanoflowers. Analyst 2012, 137, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guo, X.; Wang, T.; Alvarez, N.; Shanov, V.N.; Heineman, W.R. Simultaneous Detection of Heavy Metals by Anodic Stripping Voltammetry Using Carbon Nanotube Thread. Electroanalysis 2014, 26, 488–496. [Google Scholar] [CrossRef]
- Gadgil, B.; Damlin, P.; Dmitrieva, E.; Ääritalo, T.; Kvarnström, C. Exploring amide linkage in a polyviologen derivative towards simultaneous voltammetric determination of Pb(II), Cu(II) and Hg(II) ions. Electrochim. Acta 2016, 192, 482–488. [Google Scholar] [CrossRef]
- Xie, Y.L.; Zhao, S.Q.; Ye, H.L.; Yuan, J.; Song, P.; Hu, S.Q. Graphene/CeO2 hybrid materials for the simultaneous electrochemical detection of Cadmium(II), Lead(II), Copper(II), and Mercury(II). J. Electroanal. Chem. 2015, 757, 235–242. [Google Scholar] [CrossRef]
- Ruecha, N.; Rodthongkum, N.; Cate, D.M.; Volckens, J.; Chailapakul, O.; Henry, C.S. Sensitive electrochemical sensor using a graphene–polyaniline nanocomposite for simultaneous detection of Zn(II), Cd(II), and Pb(II). Anal. Chim. Acta 2015, 874, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, M.; Fei, Q.; Wang, D.; Sun, Z.; Geng, Z.; Xu, W.; Liu, F. Graphite-like g-C3N4-F127-Au Nanosheets Used for Sensitive Monitoring of Heat Shock Protein 90. Sens. Actuators B Chem. 2017, 256, 160–166. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Li, C. A comparison of graphitic carbon nitrides synthesized from different precursors through pyrolysis. J. Photochem. Photobiol. Chem. 2017, 332, 32–44. [Google Scholar] [CrossRef]
- Xu, L.; Xia, J.; Wang, L.; Ji, H.; Qian, J.; Xu, H.; Wang, K.; Li, H. Graphitic Carbon Nitride Nanorods for Photoelectrochemical Sensing of Trace Copper(II) Ions. Eur. J. Inorg. Chem. 2014, 2014, 3665–3673. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Sun, Q.; Jena, P. Functionalized Graphitic Carbon Nitride for Efficient Energy Storage. J. Phys. Chem. C 2013, 117, 6055–6059. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J. Graphene Supported Co-g-C3N4 as a Novel Metal–Macrocyclic Electrocatalyst for the Oxygen Reduction Reaction in Fuel Cells. Langmuir 2013, 29, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Shalom, M.; Inal, S.; Fettkenhauer, C.; Neher, D.; Antonietti, M. Improving carbon nitride photocatalysis by supramolecular preorganization of monomers. J. Am. Chem. Soc. 2013, 135, 7118–7121. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.M.; Shen, Y.Z.; Li, M.X.; Zhang, Z.L.; Zhao, W.; Xu, J.J.; Chen, H.Y. Dual-Wavelength Electrochemiluminescence Ratiometry Based on Resonance Energy Transfer between Au Nanoparticles Functionalized g-C3N4 Nanosheet and Ru(bpy)32+ for microRNA Detection. Anal. Chem. 2016, 88, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Q.; Asiri, A.M.; Alyoubi, A.O.; Sun, X. Ultrathin graphitic carbon nitride nanosheet: A highly efficient fluorosensor for rapid, ultrasensitive detection of Cu2+. Anal. Chem. 2013, 85, 5595–5599. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, X.; Li, P.; Wu, Q.; Wu, S.; Yu, Y.; Ding, K. Highly sensitive and selective detection of cadmium with a graphite carbon nitride nanosheets/Nafion electrode. RSC Adv. 2016, 6, 113570–113575. [Google Scholar] [CrossRef]
- Wang, D.P.; Tang, Y.; Zhang, W.D. A carbon nitride electrode for highly selective and sensitive determination of lead(II). Microchim. Acta 2013, 180, 1303–1308. [Google Scholar] [CrossRef]
- Amiri, M.; Salehniya, H.; Habibiyangjeh, A. Graphitic Carbon Nitride/Chitosan Composite for Adsorption and Electrochemical Determination of Mercury in Real Samples. Ind. Eng. Chem. Res. 2016, 55, 8114–8122. [Google Scholar] [CrossRef]
- Lu, X.; Yang, Y.; Liu, T. Synthesis, Al/Mg Intercalation and Structure Study of Graphite-like Carbon Nitride. J. Mater. Sci. Technol. 2011, 27, 245–251. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Liu, Y.; Wu, S.; Zou, J. A facile one-pot preparation of Co3O4/g-C3N4 heterojunctions with excellent electrocatalytic activity for the detection of environmental phenolic hormones. Appl. Surf. Sci. 2017, 430, 362–370. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Q.; Huang, Y.; Li, F.; Zhang, W.; Wei, C.; Chen, J.; Dai, P.; Huang, L.; Huang, Z. Graphitic carbon nitride nanosheets doped graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine and uric acid. Electrochim. Acta 2014, 142, 125–131. [Google Scholar] [CrossRef]
- Chidhambaram, N.; Ravichandran, K. Single step transformation of urea into metal-free g-C3N4 nanoflakes for visible light photocatalytic applications. Mater. Lett. 2017, 207, 44–48. [Google Scholar] [CrossRef]
- Dubois, C.J.; Reynolds, J.R. 3,4-Ethylenedioxythiophene–Pyridine-Based Polymers: Redox or n-Type Electronic Conductivity? Adv. Mater. 2010, 14, 1844–1846. [Google Scholar] [CrossRef]
- Boydis, M.J.; Müller, J.O.; Antonietti, M.; Arne, T. Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chemistry 2008, 14, 8177–8182. [Google Scholar]
- Martha, S.; Nashim, A.; Parida, K.M. Facile synthesis of highly active g-C3N4 for efficient hydrogen production under visible light. J. Mater. Chem. A 2013, 1, 7816–7824. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, B.; Li, L.; Peng, F.; Wang, H.; Yu, H.; Fang, Y. A carbon nitride/TiO2 nanotube array heterojunction visible-light photocatalyst: Synthesis, characterization, and photoelectrochemical properties. J. Mater. Chem. 2012, 22, 17900–17905. [Google Scholar] [CrossRef]
- Liu, F.; Jamal, R.; Wang, Y.; Wang, M.; Yang, L.; Abdiryim, T. Photodegradation of methylene blue by photocatalyst of D-A-D type polymer/functionalized multi-walled carbon nanotubes composite under visible-light irradiation. Chemosphere 2016, 168, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jamal, R.; Wang, M.; Yang, L.; Liu, F.; Abdiryim, T. A donor–acceptor–donor-type conjugated polymer-modified TiO2 with enhanced photocatalytic activity under simulated sunlight and natural sunlight. J. Mater. Sci. 2017, 52, 4820–4832. [Google Scholar] [CrossRef]
- Yang, L.; Jamal, R.; Liu, F.; Wang, Y.; Abdiryim, T. Structure and photocatalytic activity of a low band gap donor–acceptor–donor (D–A–D) type conjugated polymer: Poly(EDOT–pyridazine–EDOT). RSC Adv. 2017, 7, 1877–1886. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Ma, D.; Shang, J.K.; Li, Y.X. Simple preparation of MgO/g-C3N4 catalyst and its application for catalytic synthesis of dimethyl carbonate via transesterification. Catal. Commun. 2017, 95, 72–76. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, Y.; Wang, L.; Ai, S.; Ding, H.; Dong, Q.; Chen, Y.; Wang, L.; Ai, S.; Ding, H. Cu-modified alkalinized g-C3N4 as photocatalytically assisted heterogeneous Fenton-like catalyst. Appl. Surf. Sci. 2017, 426, 1133–1140. [Google Scholar] [CrossRef]
- Wang, L.; Ma, C.; Guo, Z.; Lv, Y.; Chen, W.; Chang, Z.; Yuan, Q.; Ming, H.; Wang, J. In-situ growth of g-C3N4 layer on ZnO nanoparticles with enhanced photocatalytic performances under visible light irradiation. Mater. Lett. 2017, 188, 347–350. [Google Scholar] [CrossRef]
- Dai, H.; Wang, N.; Wang, D.; Ma, H.; Lin, M. An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd(II) and Pb(II). Chem. Eng. J. 2016, 299, 150–155. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Wang, H.; Zou, C.E.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Ali, A.; Jamal, R.; Abdiryim, T.; Huang, X. Synthesis of monodispersed PEDOT/Au hollow nanospheres and its application for electrochemical determination of dopamine and uric acid. J. Electroanal. Chem. 2017, 787, 110–117. [Google Scholar] [CrossRef]
- Xu, F.; Fan, W.; Yang, D.; Yong, G.; Li, H. Electrochemical sensing platform for L-CySH based on nearly uniform Au nanoparticles decorated graphene nanosheets. Mater. Sci. Eng. C 2014, 38, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yamauchi, Y. Facile Synthesis of Three-Dimensional Dendritic Platinum Nanoelectrocatalyst. Chem. Mater. 2009, 21, 3562–3569. [Google Scholar] [CrossRef]
- Zhao, G.; Yin, Y.; Wang, H.; Liu, G.; Wang, Z. Sensitive stripping voltammetric determination of Cd(II) and Pb(II) by a Bi/multi-walled carbon nanotube-emeraldine base polyaniline-Nafion composite modified glassy carbon electrode. Electrochim. Acta 2016, 220, 267–275. [Google Scholar] [CrossRef]
- Tseliou, F.; Avgeropoulos, A.; Falaras, P.; Prodromidis, M.I. Low dimensional Bi2Te3-graphene oxide hybrid film-modified electrodes for ultra-sensitive stripping voltammetric detection of Pb(II) and Cd(II). Electrochim. Acta 2017, 231, 230–237. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanarat, P.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sens. Actuators B Chem. 2015, 207, 526–534. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.D.; Zhu, H.; Yang, T.T.; Zou, M.L.; Zhang, M.; Du, M.L. Facile and green fabrication of size-controlled AuNPs/CNFs hybrids for the highly sensitive simultaneous detection of heavy metal ions. Electrochim. Acta 2016, 196, 422–430. [Google Scholar] [CrossRef]
- Hu, C.; Wu, K.; Dai, X.; Hu, S. Simultaneous determination of lead(II) and cadmium(II) at a diacetyldioxime modified carbon paste electrode by differential pulse stripping voltammetry. Talanta 2003, 60, 17–24. [Google Scholar] [CrossRef]
- Gao, F.; Gao, N.; Nishitani, A.; Tanaka, H. Rod-like hydroxyapatite and Nafion nanocomposite as an electrochemical matrix for simultaneous and sensitive detection of Hg2+, Cu2+, Pb2+ and Cd2+. J. Electroanal. Chem. 2016, 775, 212–218. [Google Scholar] [CrossRef]
- Cesarino, I.; Marino, G.; Matos, J.R.; Cavalheiro, E.T. Evaluation of a carbon paste electrode modified with organofunctionalised SBA-15 nanostructured silica in the simultaneous determination of divalent lead, copper and mercury ions. Talanta 2008, 75, 15–21. [Google Scholar] [CrossRef] [PubMed]
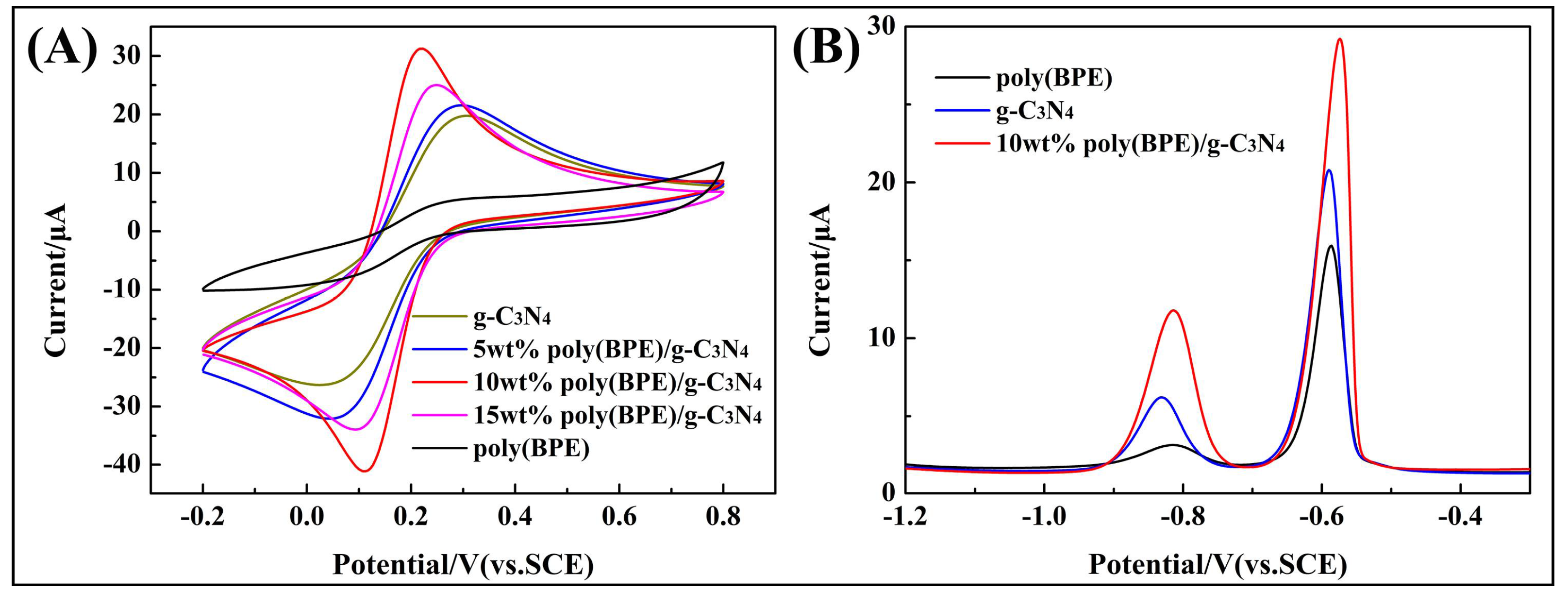
| Modified GCE | Eanodicpeak (mV) | Ecathodicpeak (mV) | ∆Ep (mV) |
|---|---|---|---|
| g-C3N4 | 305 | 225 | 280 |
| 5 wt % poly(BPE)/g-C3N4 | 295 | 48 | 247 |
| 10 wt % poly(BPE)/g-C3N4 | 220 | 110 | 110 |
| 15 wt % poly(BPE)/g-C3N4 | 249 | 94 | 155 |
| Types | Analytes | Linear Range (μM) | Linear Regression Equation | R2 | Detection Limit (μM) |
|---|---|---|---|---|---|
| Individual determination | Cd2+ | 0.1–6.8 | I (μA) = 1.08782 + 7.63612c (μM) | 0.99707 | 0.00970 |
| Pb2+ | 0.1–6.4 | I (μA) = 0.37986 + 11.1397c (μM) | 0.99661 | 0.00327 | |
| Simultaneous determination | Cd2+ | 0.12–7.2 | I (μA) = 0.26776 + 4.11513c (μM) | 0.99740 | 0.0180 |
| Pb2+ | 0.08–7.2 | I (μA) = 0.73646 + 11.28023c (μM) | 0.99599 | 0.00324 |
| Electrode | Methods | Analytes | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|---|
| PA/PPy/GO | DPV | Cd2+ | 0.045–1.335 | 0.019 | [43] |
| Pb2+ | 0.024–0.724 | 0.002 | |||
| l-cys/GR-CS/GCE | DPASV | Cd2+ | 0.005–0.6 | 0.0012 | [4] |
| Pb2+ | 0.005–0.3 | 0.0072 | |||
| GO/ex-Bi2Te3 modified GCEs | SWV | Cd2+ | 0.009–0.178 | 0.0018 | [49] |
| Pb2+ | 0.0024–0.0965 | 0.00048 | |||
| G/PANI/PS fiber/SPCE | SWASV | Cd2+ | 0.089–4.448 | 0.039 | [50] |
| Pb2+ | 0.048–2.413 | 0.016 | |||
| AuNPs/CNFs | SWASV | Cd2+ | 0.1–1.0 | 0.1 | [51] |
| Pb2+ | 0.1–1.0 | 0.1 | |||
| DCD-CPE | DPSV | Cd2+ | 0.25–25 | 0.04 | [52] |
| Pb2+ | 0.1–15 | 0.01 | |||
| Nafion-HAP | DPASV | Cd2+ | 0.1–10.0 | 0.035 | [53] |
| Pb2+ | 0.1–10.0 | 0.049 | |||
| BT-SBA-15/CPE | DPASV | Cd2+ | 2.0–10.0 | 0.4 | [54] |
| Pb2+ | 0.3–7.0 | 0.04 | |||
| Poly(BPE)/g-C3N4 | DPV | Cd2+ | 0.12–7.2 | 0.018 | This work |
| Pb2+ | 0.08–7.2 | 0.00324 |
| Interferences | Contribution (%) (Ip Cd2+ = 100%) | Contribution (%) (Ip Pb2+ = 100%) |
|---|---|---|
| Na | +3.15% | +9.46% |
| K | −8.77% | +2.85% |
| Ca | −8.81% | +3.92% |
| Mg | +6.11% | −2.64% |
| Al | −8.36% | 1.31% |
| Fe | 5.32% | 0.5% |
| Co | −0.75% | −0.7% |
| Ni | 4.94% | 8.8% |
| Cu | −13.86% | −5.38% |
| Zn | −4.19% | 5.9% |
| Original | Added (μM) | Found (μM) | Recovery (%) | |||
|---|---|---|---|---|---|---|
| (μM) | Cd2+ | Pb2+ | Cd2+ | Pb2+ | Cd2+ | Pb2+ |
| N.D | 1 | 1 | 1.0396 | 1.1315 | 103.96 | 113.15 |
| N.D | 2 | 2 | 2.1347 | 2.0334 | 106.74 | 101.67 |
| N.D | 3 | 3 | 3.0925 | 2.9944 | 103.08 | 99.81 |
| N.D | 4 | 4 | 3.9455 | 4.0102 | 98.64 | 100.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Ali, A.; Jamal, R.; Xiang, L.; Zhong, Z.; Abdiryim, T. An Electrochemical Sensor of Poly(EDOT-pyridine-EDOT)/Graphitic Carbon Nitride Composite for Simultaneous Detection of Cd2+ and Pb2+. Materials 2018, 11, 702. https://doi.org/10.3390/ma11050702
Ding S, Ali A, Jamal R, Xiang L, Zhong Z, Abdiryim T. An Electrochemical Sensor of Poly(EDOT-pyridine-EDOT)/Graphitic Carbon Nitride Composite for Simultaneous Detection of Cd2+ and Pb2+. Materials. 2018; 11(5):702. https://doi.org/10.3390/ma11050702
Chicago/Turabian StyleDing, Shuai, Ahmat Ali, Ruxangul Jamal, Ling Xiang, Ziping Zhong, and Tursun Abdiryim. 2018. "An Electrochemical Sensor of Poly(EDOT-pyridine-EDOT)/Graphitic Carbon Nitride Composite for Simultaneous Detection of Cd2+ and Pb2+" Materials 11, no. 5: 702. https://doi.org/10.3390/ma11050702
APA StyleDing, S., Ali, A., Jamal, R., Xiang, L., Zhong, Z., & Abdiryim, T. (2018). An Electrochemical Sensor of Poly(EDOT-pyridine-EDOT)/Graphitic Carbon Nitride Composite for Simultaneous Detection of Cd2+ and Pb2+. Materials, 11(5), 702. https://doi.org/10.3390/ma11050702