Spherical α-MnO2 Supported on N-KB as Efficient Electrocatalyst for Oxygen Reduction in Al–Air Battery
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of MnO2
2.2. Preparation of N-KB
2.3. Characterization
2.4. Electrochemical Measurements
2.5. Electrochemical Test of Al–Air Batteries
3. Results and Discussion
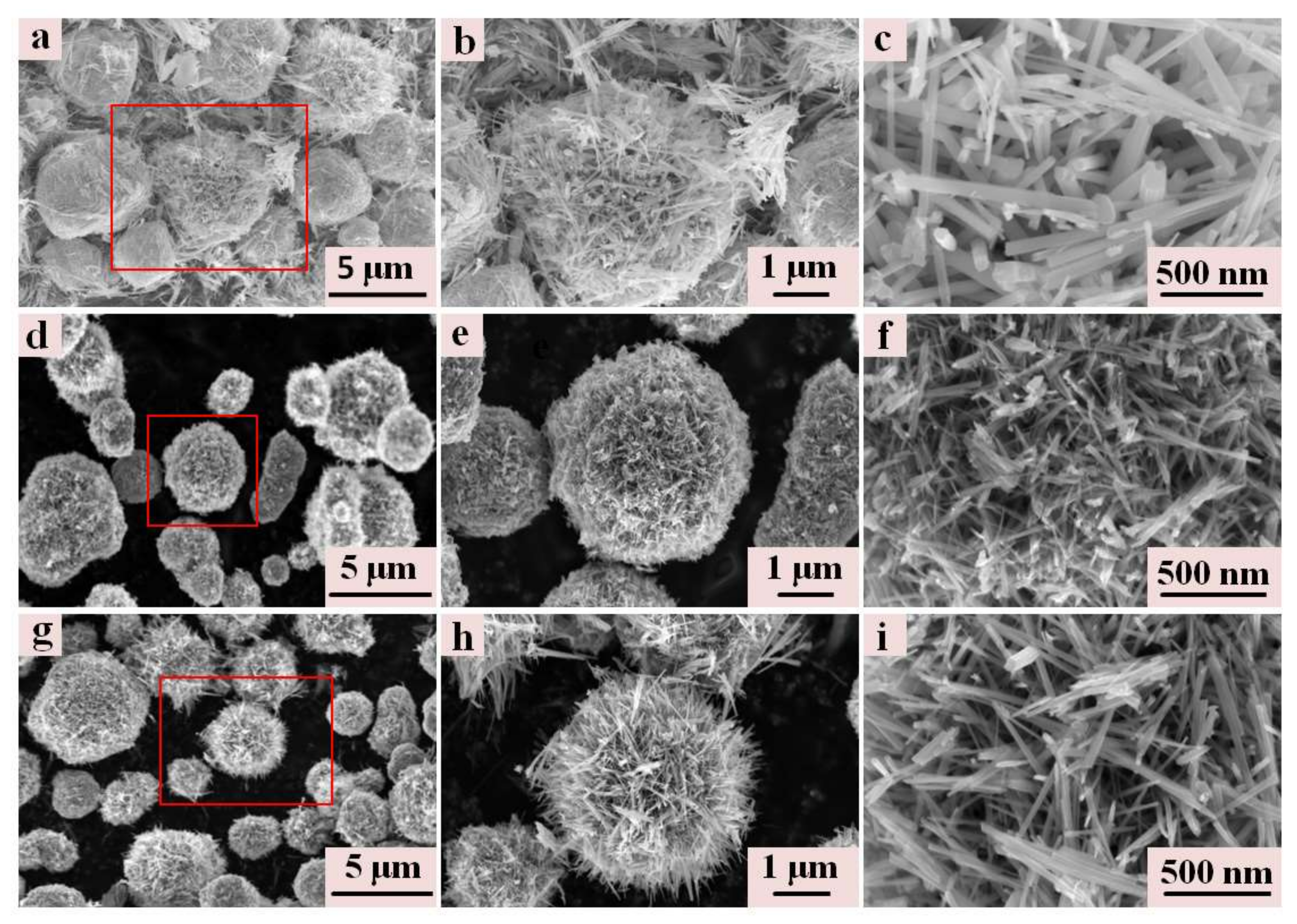
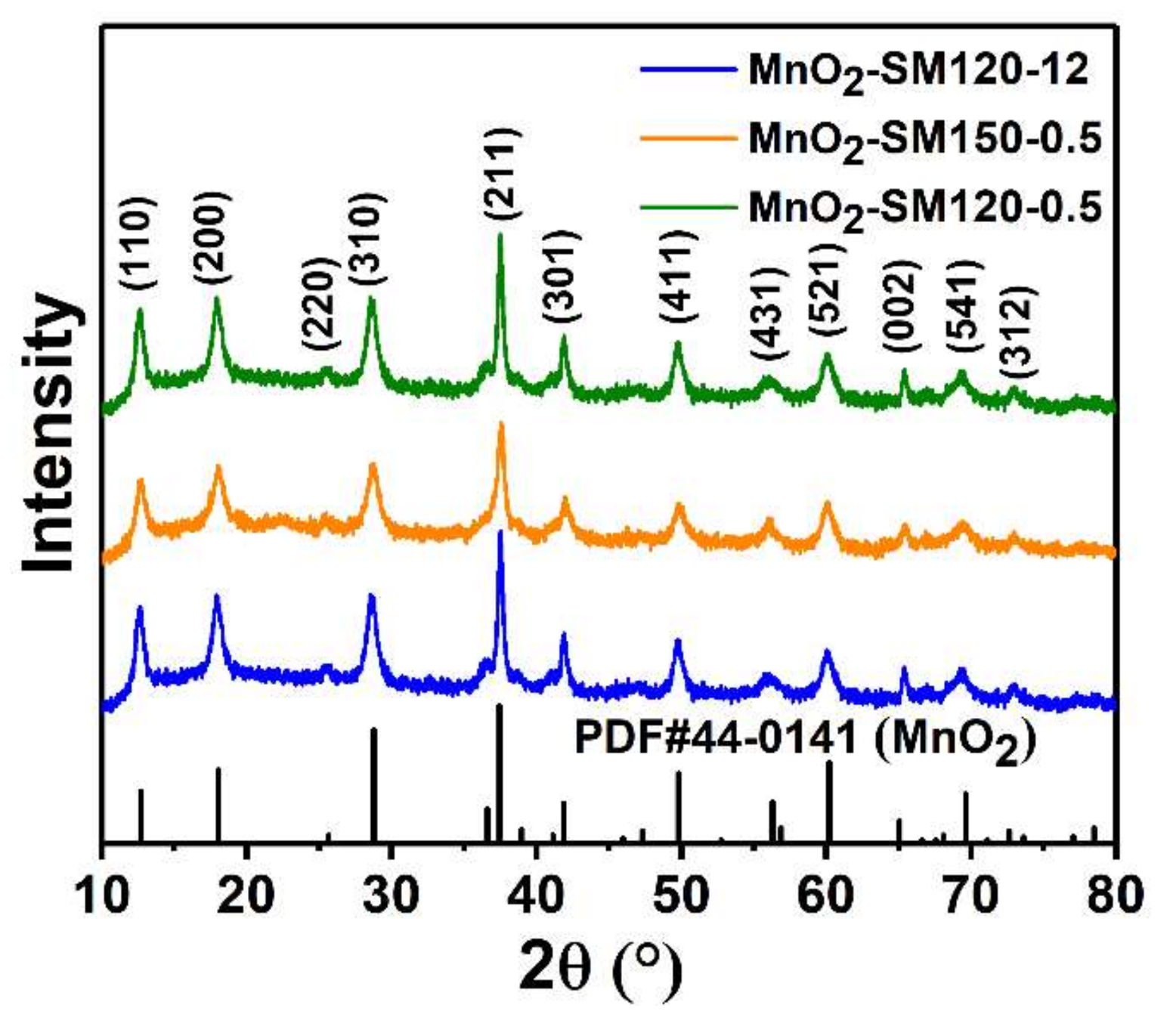
3.1. Micromorphological and Microstructural Properties
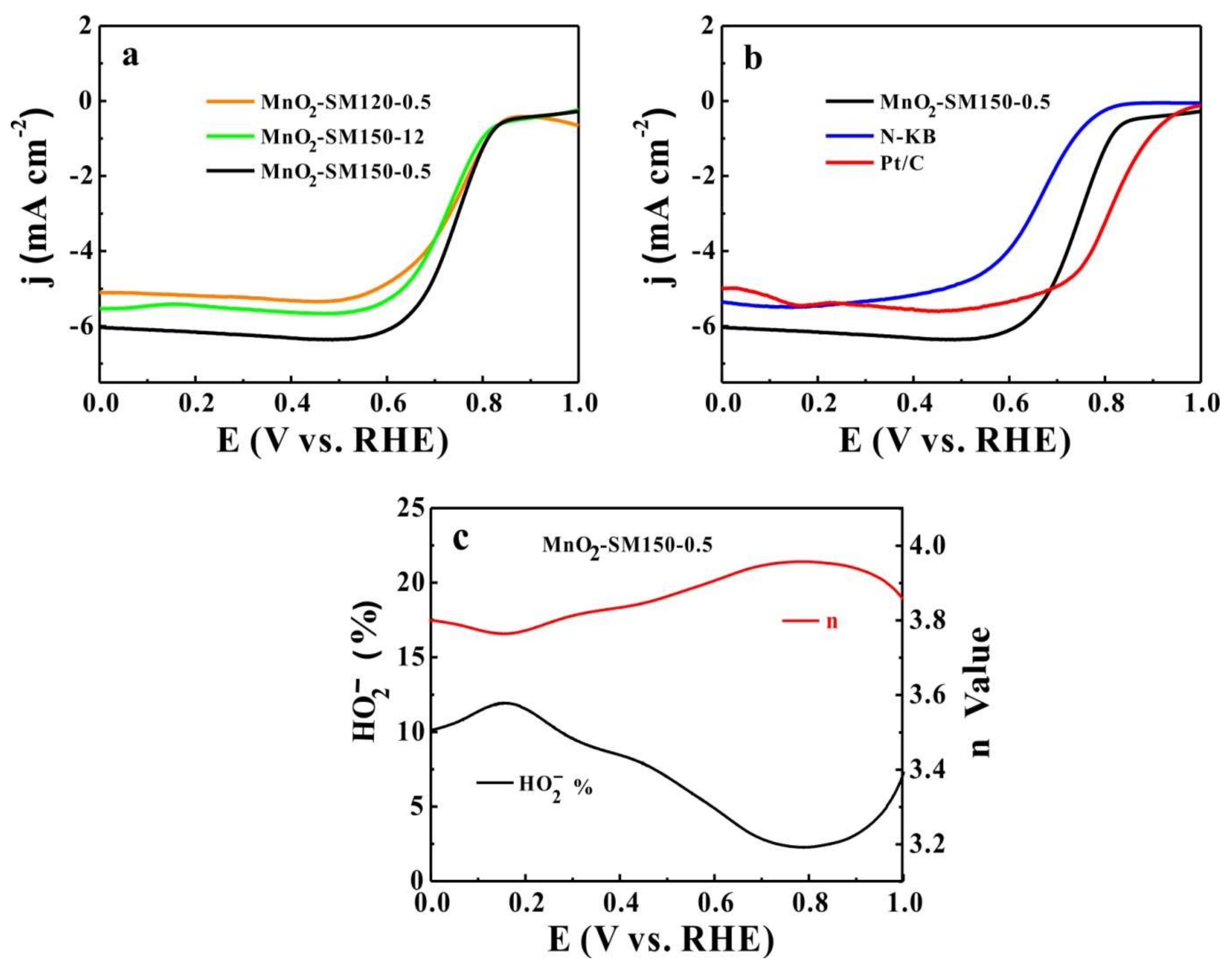
3.2. ORR Activity and Stability
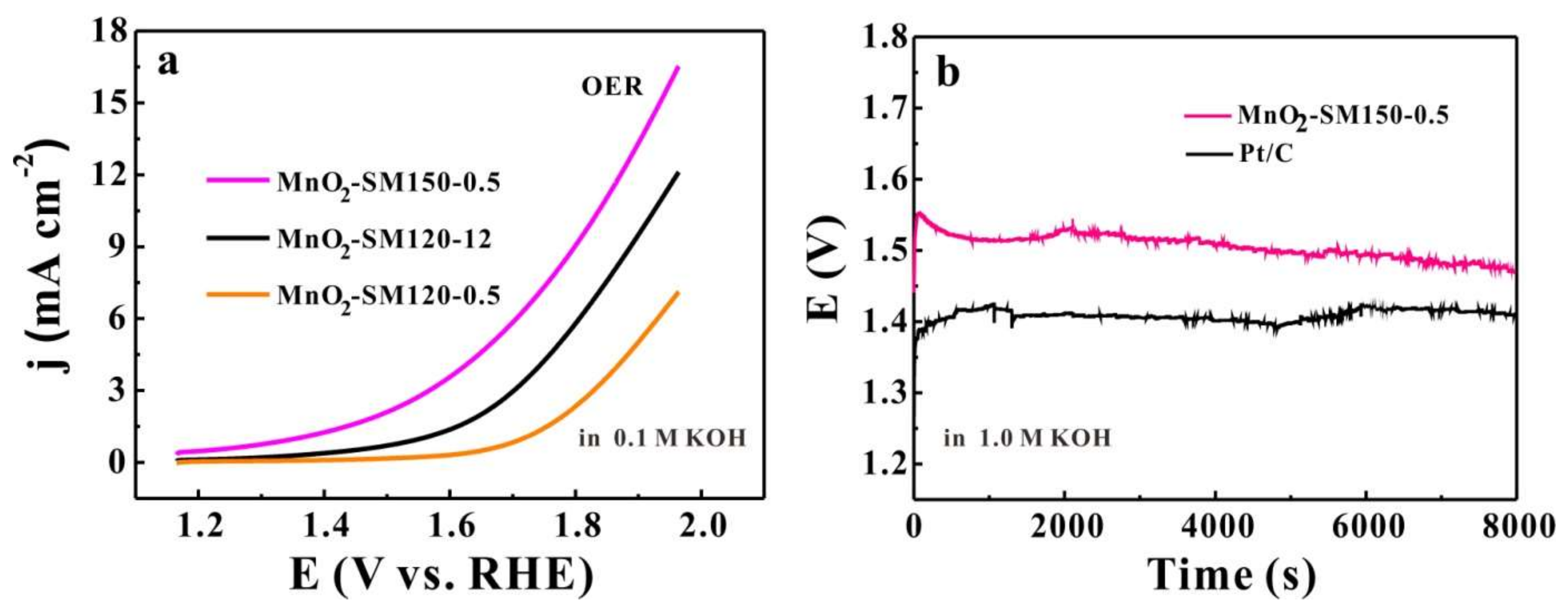
3.3. OER Activity and Stability
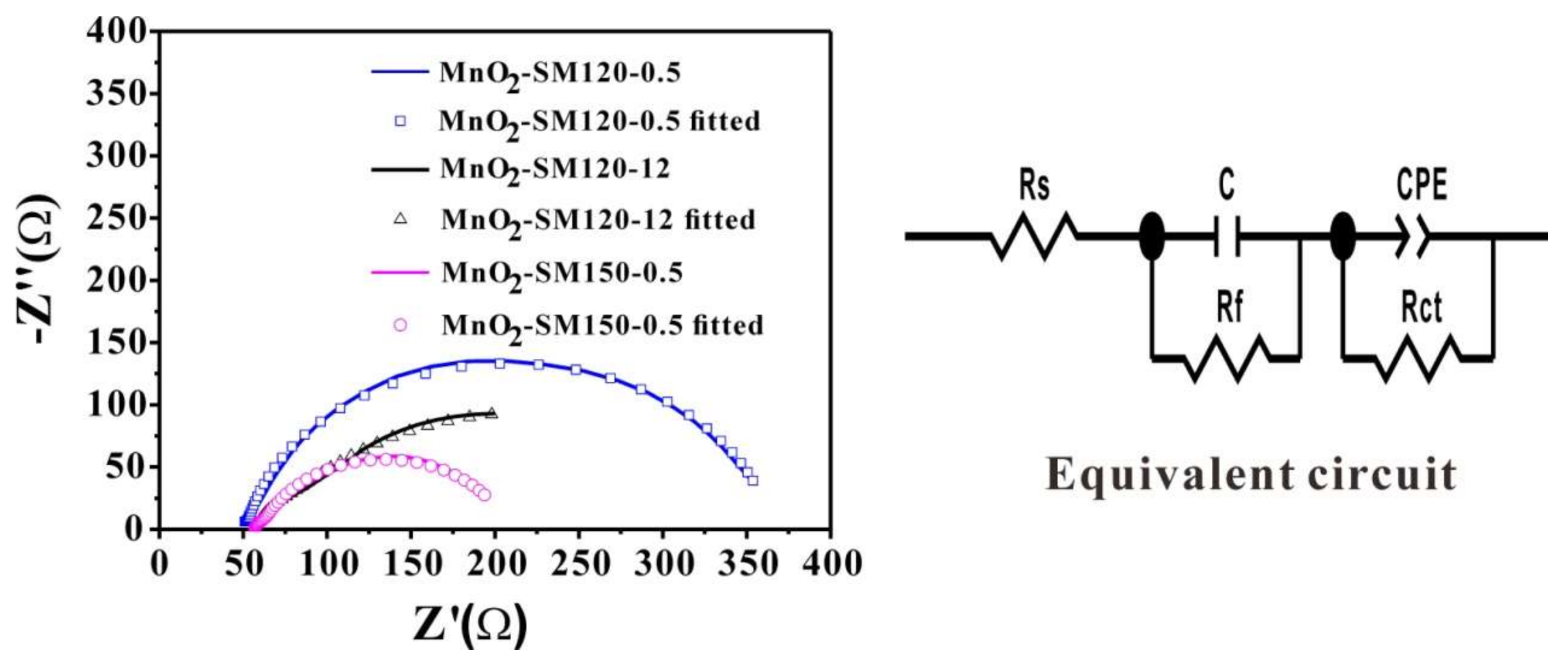
3.4. EIS Performance
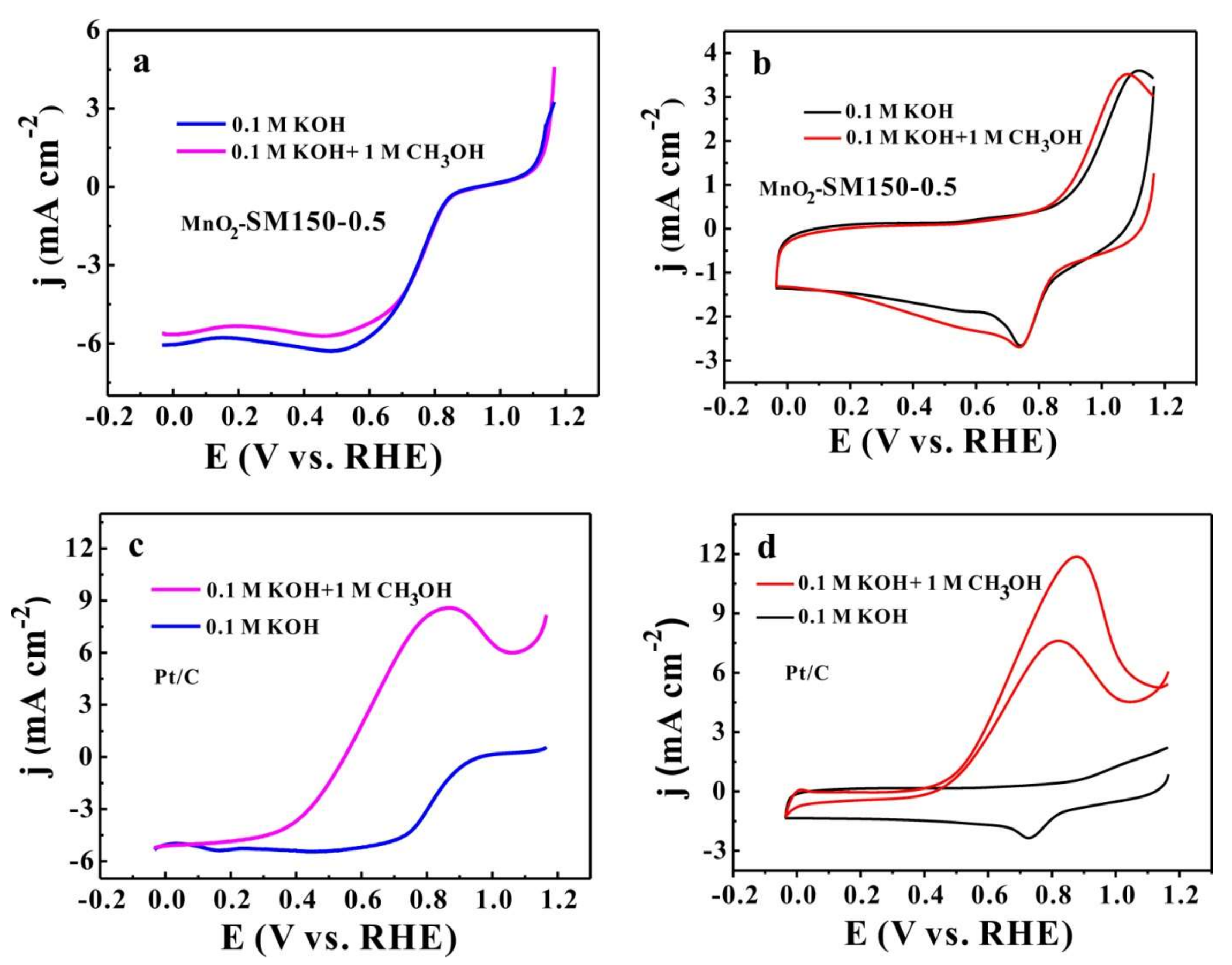
3.5. Methanol Tolerance Performance
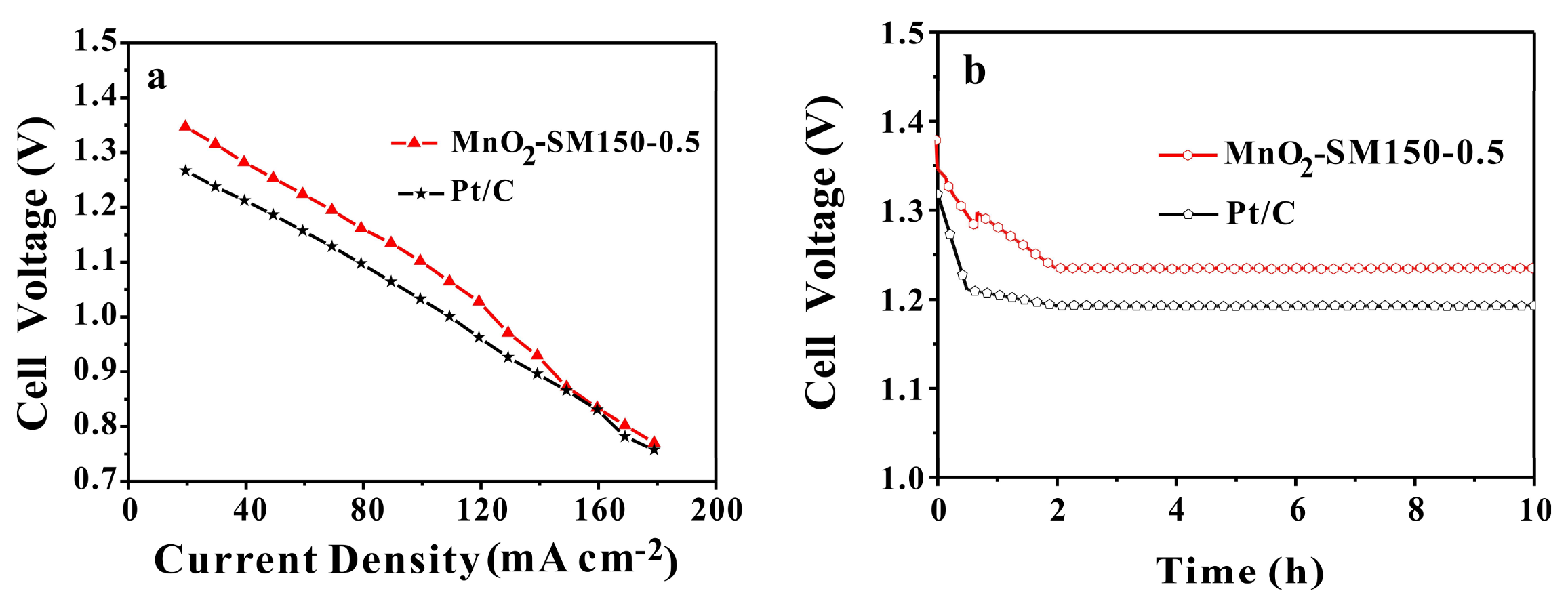
3.6. Application in Al-Air Battery
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef]
- Marković, N.M.; Schmidt, T.J.; Stamenković, V.; Ross, P.N. Oxygen reduction reaction on Pt and Pt bimetallic surfaces: A selective review. Fuel Cells 2001, 1, 105–116. [Google Scholar] [CrossRef]
- Li, J.; Xi, Z.; Pan, Y.-T.; Spendelow, J.S.; Duchesne, P.N.; Su, D.; Li, Q.; Yu, C.; Yin, Z.; Shen, B.; et al. Fe stabilization by intermetallic L10-FePt and Pt catalysis enhancement in L10-FePt/Pt nanoparticles for efficient oxygen reduction reaction in fuel cells. J. Am. Chem. Soc. 2018, 140, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Z.; Cao, Z.; Mao, X.; Shi, M.; Li, Y.; Zhang, R.; Yin, Y. Facile synthesis of well dispersed spinel cobalt manganese oxides microsphere as efficient bi-functional electrocatalysts for oxygen reduction reaction and oxygen evolution reaction. J. Alloys Compd. 2017, 721, 482–491. [Google Scholar] [CrossRef]
- Xue, Y.; Miao, H.; Sun, S.; Wang, Q.; Li, S.; Liu, Z. (La1−x Srx)0.98 MnO3 perovskite with A-site deficiencies toward oxygen reduction reaction in aluminum-air batteries. J. Power Sources 2017, 342, 192–201. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Hu, T.; Zhang, L.; Deng, Y. Carbon supported MnO2-CoFe2O4 with enhanced electrocatalytic activity for oxygen reduction and oxygen evolution. Appl. Surf. Sci. 2017, 403, 51–56. [Google Scholar] [CrossRef]
- Wang, B.; Pan, S.; Qin, Y.; Tan, W.; Tao, Y.; Li, K.; Kong, Y. Synthesis of efficient electrocatalyst for oxygen reduction reaction by using poly(m-phenylenediamine) as the interlayer spacer and the sources of N-doped carbon and MnO. Synth. Met. 2017, 224, 92–98. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, T.; Guo, W.; Chen, S.; Li, Y.; Song, J.; Chang, L.; Mu, S.; Zhao, Y.; Gao, F. Reduced graphene oxide supported MnS nanotubes hybrid as a novel non-precious metal electrocatalyst for oxygen reduction reaction with high performance. J. Power Sources 2017, 362, 1–9. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, L.; Lan, B.; Cheng, G.; Lin, T.; He, B.; Ye, W.; Sun, M.; Ye, F. Three-dimensional radial α-MnO2 synthesized from different redox potential for bifunctional oxygen electrocatalytic activities. J. Power Sources 2017, 362, 332–341. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, D.; Lou, X.W. Shape-Controlled Synthesis of MnO2 Nanostructures with enhanced electrocatalytic activity for oxygen reduction. J. Phys. Chem. C 2010, 114, 1430–1434. [Google Scholar] [CrossRef]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.Y.; Suib, S.L. Structure-property relationship of bifunctional MnO2 nanostructures: Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, R.; Wang, H.; Key, J.; Ji, S. Control of MnO2 nanocrystal shape from tremella to nanobelt for ehancement of the oxygen reduction reaction activity. J. Power Sources 2015, 280, 526–532. [Google Scholar] [CrossRef]
- Selvakumar, K.; Thangamuthu, R.; Ganesan, K.; Murugan, P.; Rajput, P.; Jha, S.N.; Bhattacharyya, D. Physiochemical investigation of shape-designed MnO2 nanostructures and their influence on oxygen reduction reaction activity in alkaline solution. J. Phys. Chem. C 2015, 119, 6604–6618. [Google Scholar] [CrossRef]
- Selvakumar, K.; Kumar, S.M.S.; Thangamuthu, R.; Kruthika, G.; Murugan, P. Development of shape-engineered α-MnO2 materials as bi-functional catalysts for oxygen evolution reaction and oxygen reduction reaction in alkaline medium. Int. J. Hydrogen Energy 2014, 39, 21024–21036. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Dong, J.; Ding, F.; Li, X.; Zhang, B.; Yang, S.; Zhang, K. Facile general strategy toward hierarchical mesoporous transition metal oxides arrays on three-dimensional macroporous foam with superior lithium storage properties. Nano Energy 2015, 13, 77–91. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, Y.; Wang, S.; Ren, Z.; Yu, J. Transition metal doped MnO2 nanosheets grown on internal surface of macroporous carbon for supercapacitors and oxygen reduction reaction electrocatalysts. Appl. Mater. Today 2016, 3, 63–72. [Google Scholar] [CrossRef]
- Wu, X.; Gao, X.; Xu, L.; Huang, T.; Yu, J.; Wen, C.; Chen, Z.; Han, J. Mn2O3 doping induced the improvement of catalytic performance for oxygen reduction of MnO. Int. J. Hydrogen Energy 2016, 41, 16087–16093. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, D.; Dou, S.; Ma, Z.; Wu, J.; Tao, L.; Shen, A.; Ouyang, C.; Liu, Q.; et al. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2014, 50, 4839–4842. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fu, L.; Li, J.; Yan, J.; Tang, Y.; Pan, Y.; Wang, H. Ag/Fe3O4-N-doped ketjenblack carbon composite as highly efficient oxygen reduction catalyst in al-air batteries. J. Electrochem. Soc. 2017, 164, A3595–A3601. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Wang, H.; Ren, Y.; Liu, K.; Tang, Y.; Shao, M. Fe/N co-doped carbon materials with controllable structure as highly efficient electrocatalysts for oxygen reduction reaction in Al-air batteries. Energy Storage Mater. 2017, 8, 49–58. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Risch, M.; Han, B.; Yang, S.H. Recent insights into manganese oxides in catalyzing oxygen reduction kinetics. ACS Catal. 2015, 5, 446–449. [Google Scholar] [CrossRef]
- Liu, K.; Peng, Z.; Wang, H.; Ren, Y.; Liu, D.; Li, J.; Tang, Y.; Zhang, N. Fe3C@Fe/N doped graphene-like carbon sheets as a highly efficient catalyst in Al-Air batteries. J. Electrochem. Soc. 2017, 164, F475–F483. [Google Scholar] [CrossRef]
- Pargoletti, E.; Cappelletti, G.; Minguzzi, A.; Rondinini, S.; Leoni, M.; Marelli, M.; Vertova, A. High-performance of bare and Ti-doped α-MnO2 nanoparticles in catalyzing the oxygen reduction reaction. J. Power Sources 2016, 325, 116–128. [Google Scholar] [CrossRef]
- Ozcan, S.; Tokur, M.; Cetinkaya, T.; Guler, A.; Uysal, M.; Guler, M.O.; Akbulut, H. Free standing flexible graphene oxide + α-MnO2 composite cathodes for Li–Air batteries. Solid State Ion. 2016, 286, 34–39. [Google Scholar] [CrossRef]
- Wei, Z.H.; Zhao, T.S.; Zhu, X.B.; Tan, P. MnO2−x nanosheets on stainless steel felt as a carbon- and binder-free cathode for non-aqueous lithium-oxygen batteries. J. Power Sources 2016, 306, 724–732. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, X.; Feng, X.; Qiu, G.; Tan, W.; Liu, F. Large-scale size-controlled synthesis of cryptomelane-type manganese oxide OMS-2 in lateral and longitudinal directions. J. Mater. Chem. A 2011, 21, 462–465. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Geng, D.; Chen, Y.; Li, R.; Cai, M.; Sun, X. A highly durable platinum nanocatalyst for proton exchange membrane fuel cells: Multiarmed starlike nanowire single crystal. Angew. Chem. 2011, 123, 442–446. [Google Scholar] [CrossRef]
- Jahan, M.; Liu, Z.; Loh, K.P. A Graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for HER, OER, and ORR. Adv. Funct. Mater. 2013, 23, 5363–5372. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, T.; Zhang, Y.; Du, J.; Han, X.; Chen, J. Enhancing electrocatalytic oxygen reduction on MnO2 with vacancies. Angew. Chem. Int. Ed. 2013, 52, 2474–2477. [Google Scholar] [CrossRef] [PubMed]
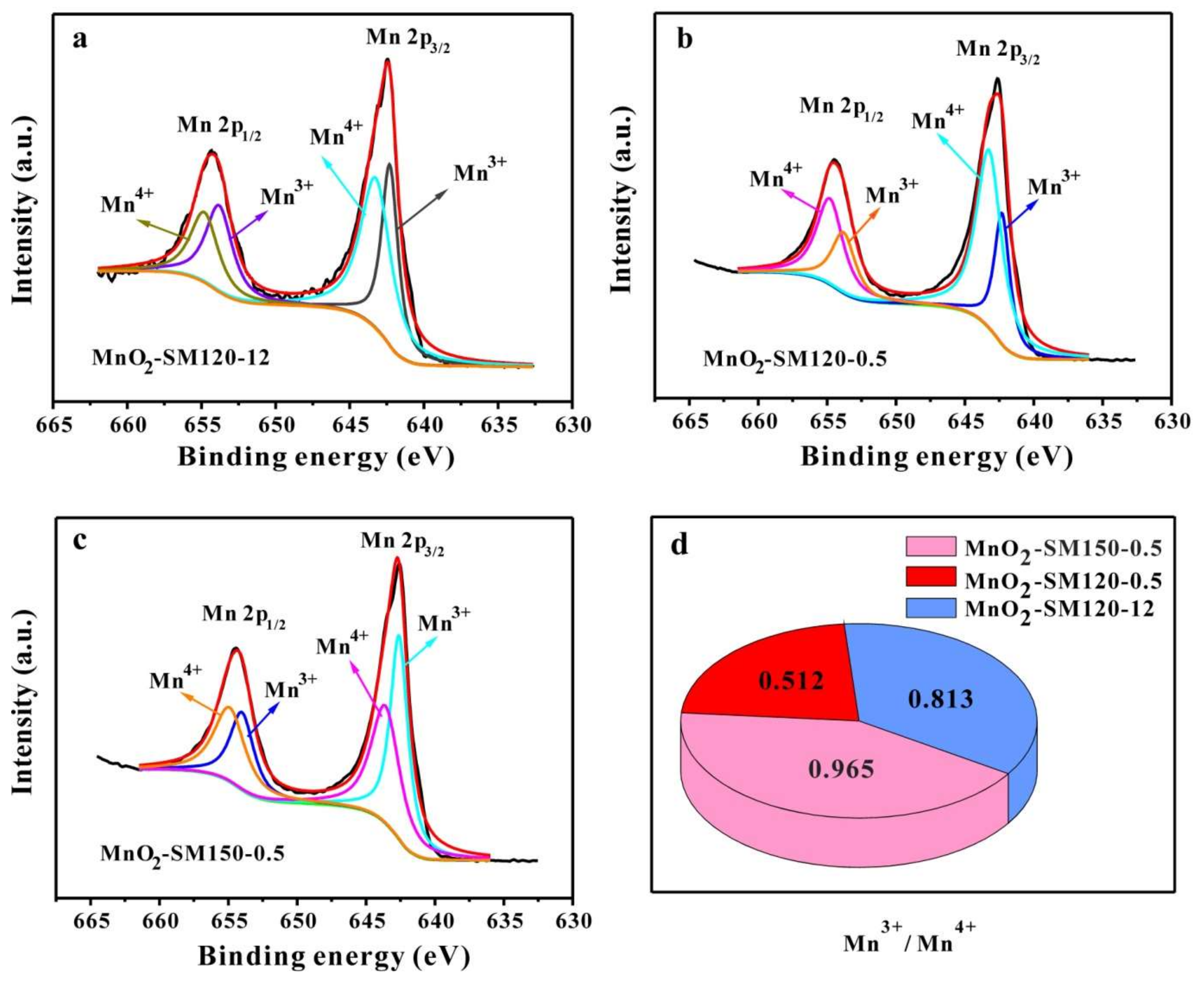

| Samples | Species | Peak Position (eV) | Peak Area | Mn3+/Mn4+ |
|---|---|---|---|---|
| MnO2-SM150-0.5 | 2p 3/2 Mn3+ | 642.60 | 36429.72 | 0.965 |
| 2p 3/2 Mn4+ | 643.60 | 33877.38 | ||
| 2p 1/2 Mn3+ | 654.00 | 18763.28 | ||
| 2p 1/2 Mn4+ | 654.90 | 23322.43 | ||
| MnO2-SM120-0.5 | 2p 3/2 Mn3+ | 642.30 | 20298.63 | 0.512 |
| 2p 3/2 Mn4+ | 643.25 | 44859.42 | ||
| 2p 1/2 Mn3+ | 653.80 | 14298.36 | ||
| 2p 1/2 Mn4+ | 654.80 | 22753.79 | ||
| MnO2-SM120-12 | 2p 3/2 Mn3+ | 642.30 | 29778.53 | 0.813 |
| 2p 3/2 Mn4+ | 643.25 | 45449.07 | ||
| 2p 1/2 Mn3+ | 653.80 | 23045.48 | ||
| 2p 1/2 Mn4+ | 654.80 | 19499.66 |
| Sample | Rs (Ω) | Rf (Ω) | Rct (Ω) | C (F) | CPE-T | CPE-P |
|---|---|---|---|---|---|---|
| MnO2-SM150-0.5 | 55.72 | 4.77 | 146.4 | 0.001010 | 0.002368 | 0.842 |
| MnO2-SM120-12 | 61.75 | 8.89 | 257.1 | 0.005600 | 0.003926 | 0.692 |
| MnO2-SM120-0.5 | 62.01 | 9.69 | 313.5 | 0.000031 | 0.002031 | 0.895 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Wang, M.; Li, G.; He, Q.; Liu, J.; Li, F. Spherical α-MnO2 Supported on N-KB as Efficient Electrocatalyst for Oxygen Reduction in Al–Air Battery. Materials 2018, 11, 601. https://doi.org/10.3390/ma11040601
Chen K, Wang M, Li G, He Q, Liu J, Li F. Spherical α-MnO2 Supported on N-KB as Efficient Electrocatalyst for Oxygen Reduction in Al–Air Battery. Materials. 2018; 11(4):601. https://doi.org/10.3390/ma11040601
Chicago/Turabian StyleChen, Kui, Mei Wang, Guangli Li, Quanguo He, Jun Liu, and Fuzhi Li. 2018. "Spherical α-MnO2 Supported on N-KB as Efficient Electrocatalyst for Oxygen Reduction in Al–Air Battery" Materials 11, no. 4: 601. https://doi.org/10.3390/ma11040601
APA StyleChen, K., Wang, M., Li, G., He, Q., Liu, J., & Li, F. (2018). Spherical α-MnO2 Supported on N-KB as Efficient Electrocatalyst for Oxygen Reduction in Al–Air Battery. Materials, 11(4), 601. https://doi.org/10.3390/ma11040601






