Evaluation of a New Dental Implant Cervical Design in Comparison with a Conventional Design in an Experimental American Foxhound Model
Abstract
:1. Introduction
2. Materials and Methods
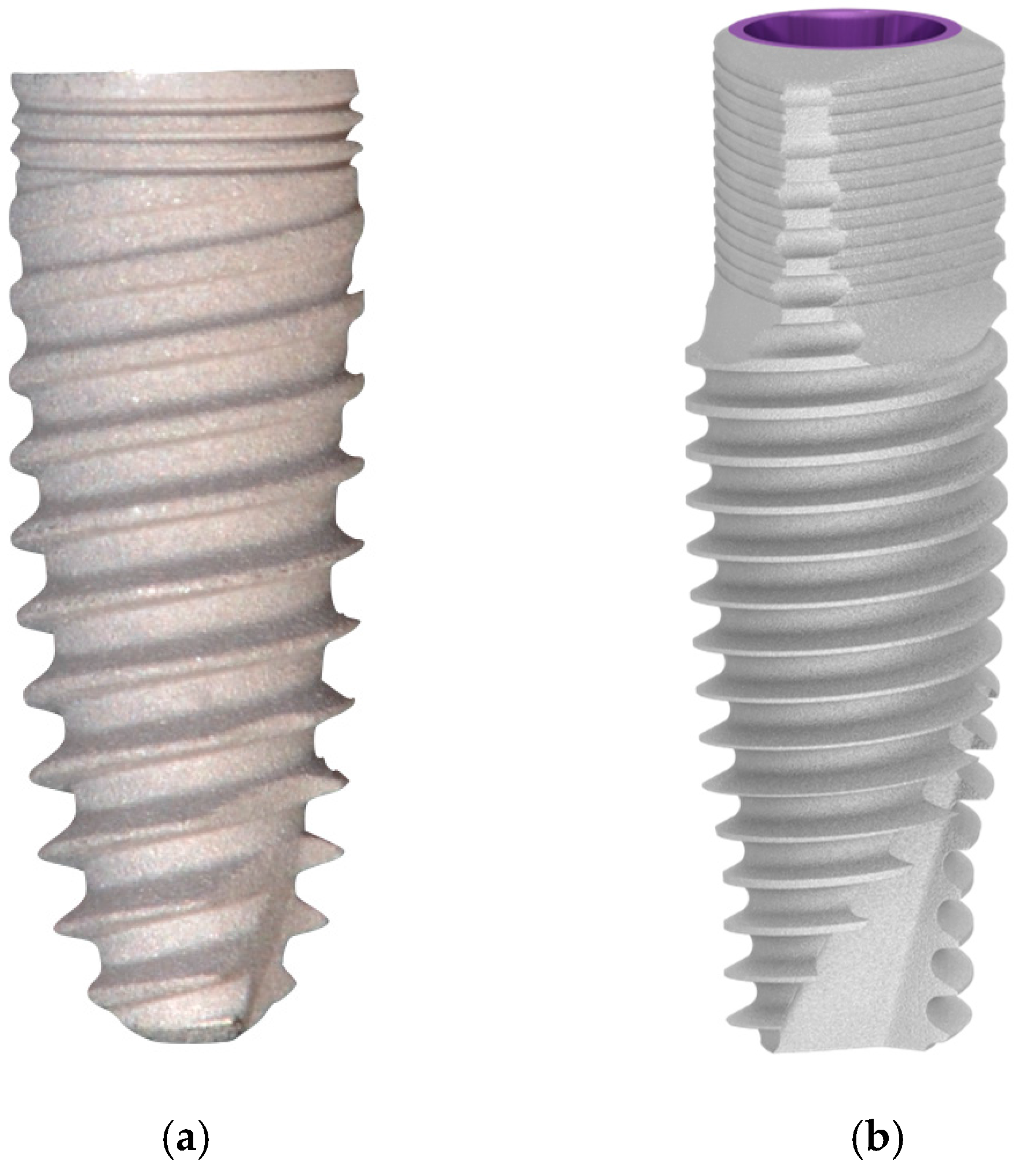
2.1. Implant Characteristics
2.2. Sample Characteristics
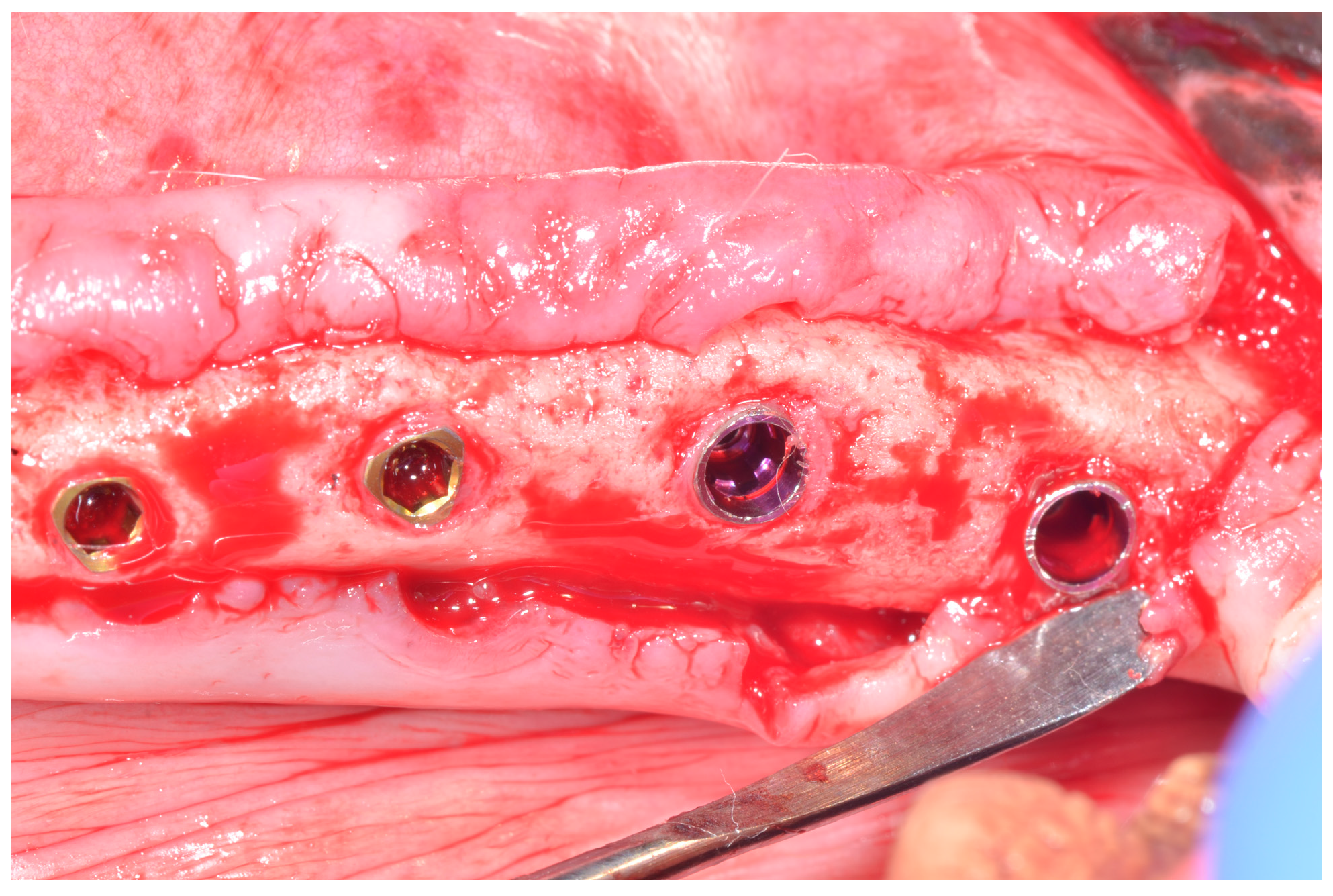
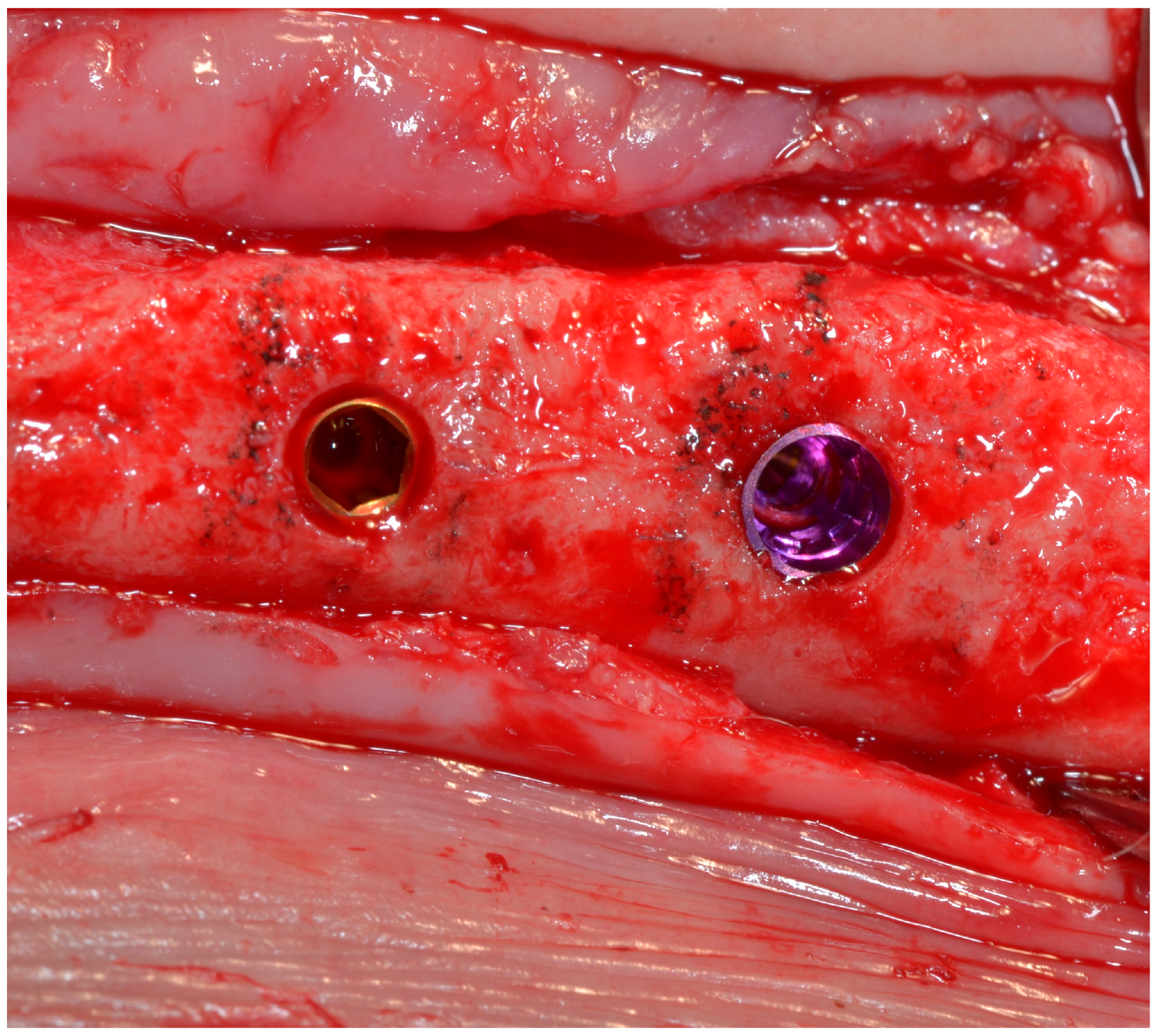
2.3. Surgical Procedure
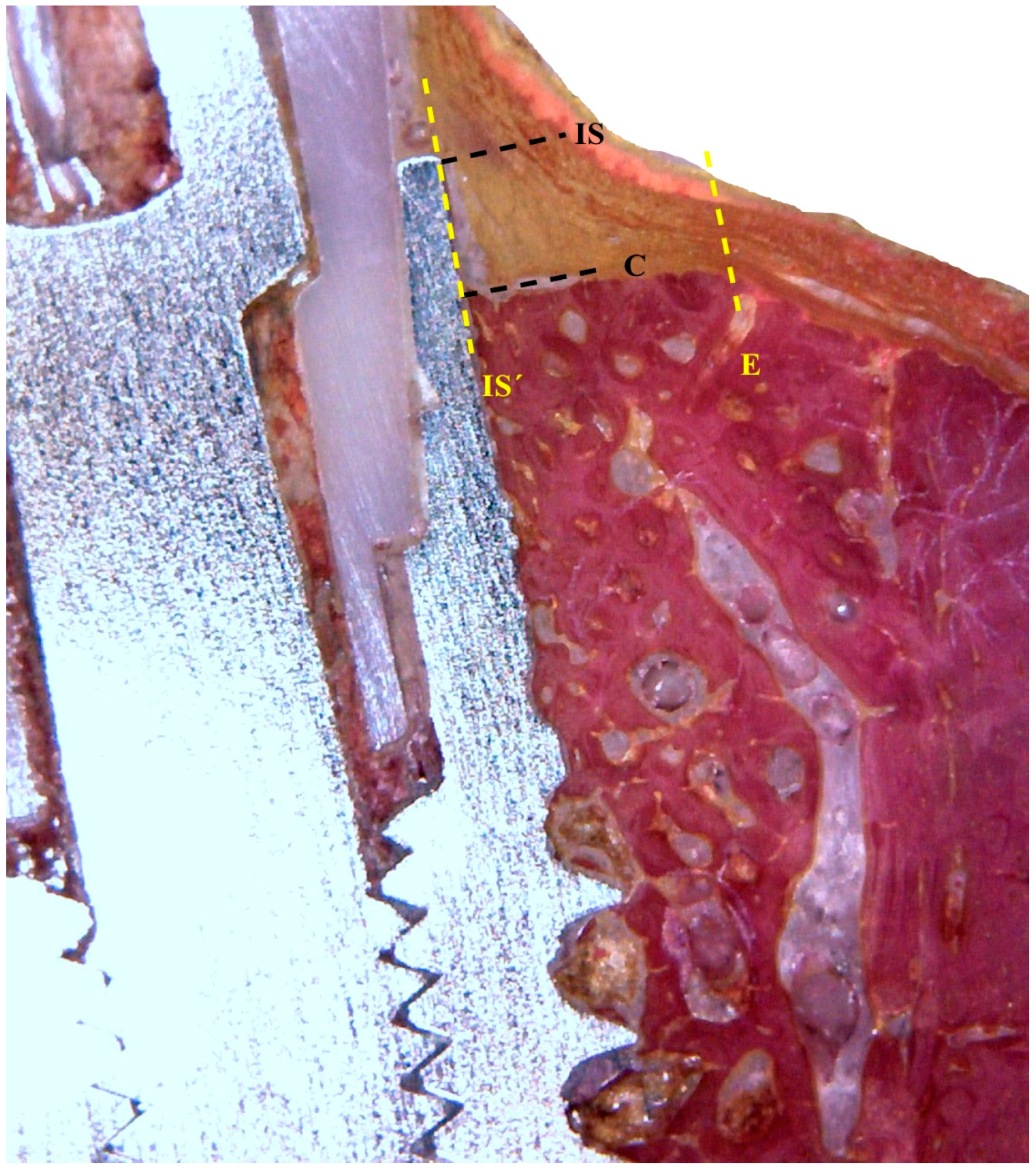
2.4. Histological Preparation
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Degidi, M.; Novaes, A.B., Jr.; Nardi, D.; Piattelli, A. Outcome analysis of immediately placed, immediately restored implants in the esthetic area: The clinical relevance of different interimplant distances. J. Periodontol. 2008, 79, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Grunder, U.; Polizzi, G.; Goené, R.; Hatano, N.; Henry, P.; Jackson, W.J.; Kawamura, K.; Köhler, S.; Renouard, F.; Rosenberg, R.; et al. A 3-year prospective multicenter follow-up report on the immediate and delayed-immediate placement of implants. Int. J. Oral Maxillofac. Implants 1999, 14, 210–216. [Google Scholar] [PubMed]
- Mangano, F.; Mangano, C.; Ricci, M.; Sammons, R.L.; Shibli, J.A.; Piattelli, A. Single-tooth Morse taper connection implants placed in fresh extraction sockets of the anterior maxilla: An aesthetic evaluation. Clin. Oral Implants Res. 2012, 23, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Buser, D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla—A systematic review. Int. J. Oral Maxillofac. Implants 2014, 29 (Suppl.), 186–215. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Gomez-Moreno, G.; Aguilar-Salvatierra, A.; Guardia, J.; Delgado-Ruiz, R.A.; Romanos, G.E. Marginal bone loss evaluation around immediate non-occlusal microthreaded implants placed in fresh extraction sockets in the maxilla: A 3-year study. Clin. Oral Implants Res. 2014, 26, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Javed, F. Platform switching minimises crestal bone loss around dental implants: Truth or myth? J. Oral Rehabil. 2014, 41, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Berglundh, T.; Lindhe, J. Hard-tissue alterations following immediate implant placement in extraction sites. J. Clin. Periodontol. 2004, 31, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Negri, B.; Calvo-Guirado, J.L.; Pardo Zamora, G.; Ramírez Fernández, M.P.; Delgado Ruiz, R.; Munoz Guzon, F. Peri-implant bone reactions to immediate implants placed at different levels in relation to crestal bone. Part I: A pilot study in dogs. Clin. Oral Implants Res. 2012, 23, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Palacci, P. Aesthetic treatment of the anterior maxilla: Soft and hard tissue considerations. Oral Maxillofac. Surg. Clin. N. Am. 2004, 16, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; López-López, P.J.; Pérez-Albacete Martínez, C.; Javed, F.; Granero-Marín, J.M.; Maté Sánchez de Val, J.E.; Ramírez Fernández, M.P. Peri-implant bone loss clinical and radiographic evaluation around rough neck and microthread implants: A 5-year study. Clin. Oral Implants Res. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Jones, A.A.; Bakaeen, L.G.; Buser, D.; Schoolfield, J.D.; Cochran, D.L. Influence of a machined collar on crestal bone changes around titanium implants: A histometric study in the canine mandible. J. Periodontol. 2011, 82, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Hegewald, A.; Becker, J. Impact of implant-abutment connection and positioning of the machined collar/microgap on crestal bone level changes: A systematic review. Clin. Oral Implants Res. 2014, 25, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.J.; Yoon, J.; Misch, C.E.; Wang, H.L. The causes of early implant bone loss: Myth or science? J. Periodontol. 2002, 73, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ciurana, X.; Vela-Nebot, X.; Segala-Torres, M.; Calvo-Guirado, J.L.; Cambra, J.; Mendez-Blanco, V.; Tarnow, D.P. The effect of interimplant distance on the height of the interimplant bone crest when using platform-switched implants. Int. J. Periodontics Restor. Dent. 2009, 29, 141–151. [Google Scholar]
- Calvo-Guirado, J.L.; Ortiz-Ruiz, A.J.; Negri, B.; López-Marí, L.; Rodríguez-Barba, C.; Schlottig, F. Histological and histomorphometric evaluation of immediate implant placement on a dog model with a new implant surface treatment. Clin. Oral Implants Res. 2010, 21, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-Y.; Han, D.-H. Influence of microgrooved collar design on soft and hard tissue healing of immediate implantation in fresh extraction sites in dogs. Clin. Oral Implants Res. 2009, 21, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Choi, Y.S.; Park, K.H.; Kim, C.S.; Moon, I.S. Effect of microthread on the maintenance of marginal bone level, a 3-year prospective study. Clin. Oral Implants Res. 2007, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Stanford, C.M. Surface modifications of dental implants. Aust. Dent. J. 2008, 53 (Suppl. 1), 26–33. [Google Scholar] [CrossRef] [PubMed]
- Alomrani, A.N.; Hermann, J.S.; Jones, A.A.; Buser, D.; Schoolfield, J.; Cochran, D.L. The effect of a machined collar on coronal hard tissue around titanium implants: A radiographic study in the canine mandible. Int. J. Oral Maxillofac. Implants 2005, 20, 677–686. [Google Scholar] [PubMed]
- Calvo-Guirado, J.L.; Pérez-Albacete, C.; Aguilar-Salvatierra, A.; de Val Maté-Sánchez, J.E.; Delgado-Ruiz, R.A.; Abboud, M.; Velasco, E.; Gómez-Moreno, G.; Romanos, G.E. Narrow-versus mini-implants at crestal and subcrestal bone levels. Experimental study in beagle dogs at three months. Clin. Oral Investig. 2015, 19, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Hermann, F.; Lerner, H.; Palti, A. Factors influencing the preservation of the periimplant marginal bone. Implant Dent. 2015, 16, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Tovar, N.; Marin, C.; Teixeira, H.S.; Anchieta, R.B.; Silveira, L.M.; Janal, M.N.; Shibli, J.A.; Coelho, P.G. The impact of a modified cutting flute implant design on osseointegration. Int. J. Oral Maxillofac. Surg. 2014, 43, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Lee, C.C.; Fu, P.Y.; Lin, S.C. The effects of flute shape and thread profile on the insertion torque and primary stability of dental implants. Med. Eng. Phys. 2012, 34, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Bratu, E.A.; Tandlich, M.; Shapira, L. A rough surface implant neck with microthreads reduces the amount of marginal bone loss: A prospective clinical study. Clin. Oral Implants Res. 2009, 20, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Berglundh, T. Tissue characteristics at microthreaded implants: An ex- perimental study in dogs. Clin. Implant Dent. Relat. Res. 2006, 8, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Han, C.H.; Heo, S.J.; Kim, S.; Chun, H.J. Radiographic evaluation of marginal bone level around implants with different neck designs after 1 year. Int. J. Oral Maxillofac. Implants 2006, 21, 789–794. [Google Scholar] [PubMed]
- Nickenig, H.-J.; Wichmann, M.; Schlegel, K.A.; Nkenke, E.; Eitner, S. Radiographic evaluation of marginal bone levels adjacent to parallel-screw cylinder machined-neck implants and rough-surfaced microthreaded implants using digitized panoramic radiographs. Clin. Oral Implants Res. 2009, 20, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Hürzeler, M.; Fickl, S.; Zuhr, O.; Wachtel, H.C. Peri-implant bone level around implants with platform-switched abutments: Preliminary data from a prospective study. J. Oral Maxillofac. Surg. 2007, 65 (Suppl. 1), 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, M.; Luongo, R.; Di Iorio, D.; Bugea, C.; Cocchetto, R.; Celletti, R. Evaluation of peri-implant bone loss around platform-switched implants. Int. J. Periodontics Restor. Dent. 2008, 28, 347–355. [Google Scholar]
- Fickl, S.; Zuhr, O.; Stein, J.M.; Hürzeler, M.B. Peri-implant bone level around implants with platform-switched abutments. Int. J. Oral Maxillofac. Implants 2010, 25, 577–581. [Google Scholar] [PubMed]
- Ferrus, J.; Cecchinato, D.; Pjetursson, E.B.; Lang, N.P.; Sanz, M.; Lindhe, J. Factors influencing ridge alterations following immediate implant placement into extraction sockets. Clin. Oral Implants Res. 2010, 21, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Sanz, M.; Cecchinato, D.; Pjetursson, B.; Ferrus, J.; Lang, N.P.; Lindhe, J. Bone dimensional variations at implants placed in fresh extraction sockets: A multilevel multivariate analysis. Clin. Oral Implants Res. 2010, 21, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Buser, D.; Schenk, R.K.; Cochran, D.L. Crestal bone changes around titanium implants. A histometric evaluation of unloaded non- submerged and submerged implants in the canine mandible. J. Periodontol. 2000, 71, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Vela-Nebot, X.; Rodríguez-Ciurana, X.; Rodado-Alonso, C.; Segalà-Torres, M. Benefits of an implant platform modification technique to reduce crestal bone resorption. Implant Dent. 2006, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Brägger, U.; Bürgin, W.; Lang, N.P. The effect of subcrestal placement of the polished surface of ITI implants on marginal soft and hard tissues. Clin. Oral Implants Res. 1996, 7, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.; Hitomi-Nagata, M.J.; Bell, M.; Nascimento de Melo, L.G.; Bosco, A.F. Influence of microgao location and configuration on peri-implant bone morphology in nonsubmerged implants: An experimental study in dogs. Int. J. Oral Maxillofac. Implants 2010, 25, 540–547. [Google Scholar] [PubMed]
- Weng, D.; Hitomi-Nagata, M.J.; Bosco, A.F.; Nascimento de Melo, L.G. Influence of microgap location and configuration on radiographic bone loss around submerged implants: An experimental study in dogs. Int. J. Oral Maxillofac. Implants 2011, 26, 941–946. [Google Scholar] [PubMed]
- Pontes, A.E.F.; Ribeiro, F.S.; Iezzi, G.; Piattelli, A.; Cirelli, J.A.; Marcantonio, E., Jr. Biologic width changes around loaded implants inserted in different levels in relation to crestal bone: Histometric evaluation in canine mandible. Clin. Oral Implants Res. 2008, 19, 483–490. [Google Scholar] [CrossRef] [PubMed]

| ISQ Values | Baseline | 12 Weeks | p Values Intergroup | ||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| Implants C1® (Control group) | 69.56 ± 3.17 | 69.91 | 70.35 ± 3.42 | 71.35 | 0.17 |
| Implants V3® (Test group) | 73.82 ± 2.78 | 70.79 | 74.02 ± 3.96 | 74.56 | 0.21 |
| p Value intergroup | 0.048 * | – | 0.041 * | – | – |
| 12 WEEKS | IS-C | |
|---|---|---|
| BUCCAL | LINGUAL | |
| Implants C1® (Control group) | 0.71 ± 0.28 mm | 0.42 ± 0.30 mm |
| Implants V3® (Test group) | 0.31 ± 0.24 mm | 0.35 ± 0.14 mm |
| p Value | 0.0019 * | 0.132 |
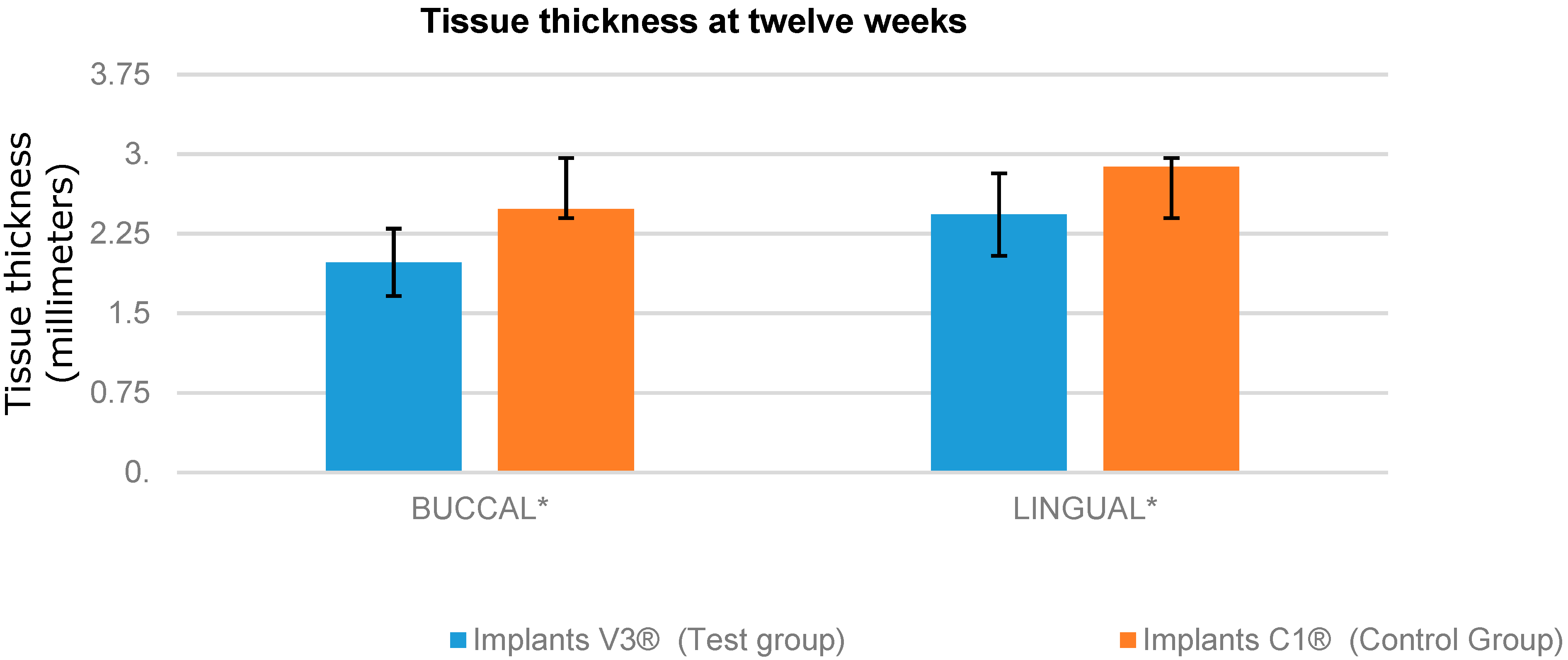
| 12 WEEKS | IS’-E | |
|---|---|---|
| BUCCAL | LINGUAL | |
| Implants V3® (Test group) | 1.98 ± 0.17 mm | 2.43 ± 0.93 mm |
| Implants C1® (Control group) | 2.48 ± 0.61 mm | 2.88 ± 0.14 mm |
| p Value | 0.0043 * | 0.0029 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Albacete Martínez, M.Á.; Pérez-Albacete Martínez, C.; Maté Sánchez De Val, J.E.; Ramos Oltra, M.L.; Fernández Domínguez, M.; Calvo Guirado, J.L. Evaluation of a New Dental Implant Cervical Design in Comparison with a Conventional Design in an Experimental American Foxhound Model. Materials 2018, 11, 462. https://doi.org/10.3390/ma11040462
Pérez-Albacete Martínez MÁ, Pérez-Albacete Martínez C, Maté Sánchez De Val JE, Ramos Oltra ML, Fernández Domínguez M, Calvo Guirado JL. Evaluation of a New Dental Implant Cervical Design in Comparison with a Conventional Design in an Experimental American Foxhound Model. Materials. 2018; 11(4):462. https://doi.org/10.3390/ma11040462
Chicago/Turabian StylePérez-Albacete Martínez, Maria Ángeles, Carlos Pérez-Albacete Martínez, José Eduardo Maté Sánchez De Val, María Luisa Ramos Oltra, Manuel Fernández Domínguez, and Jose Luis Calvo Guirado. 2018. "Evaluation of a New Dental Implant Cervical Design in Comparison with a Conventional Design in an Experimental American Foxhound Model" Materials 11, no. 4: 462. https://doi.org/10.3390/ma11040462
APA StylePérez-Albacete Martínez, M. Á., Pérez-Albacete Martínez, C., Maté Sánchez De Val, J. E., Ramos Oltra, M. L., Fernández Domínguez, M., & Calvo Guirado, J. L. (2018). Evaluation of a New Dental Implant Cervical Design in Comparison with a Conventional Design in an Experimental American Foxhound Model. Materials, 11(4), 462. https://doi.org/10.3390/ma11040462






