Fabrication of Functional Carbon/Magnetic Nanocomposites as A Promising Model of Utilization of Used Crosslinked Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
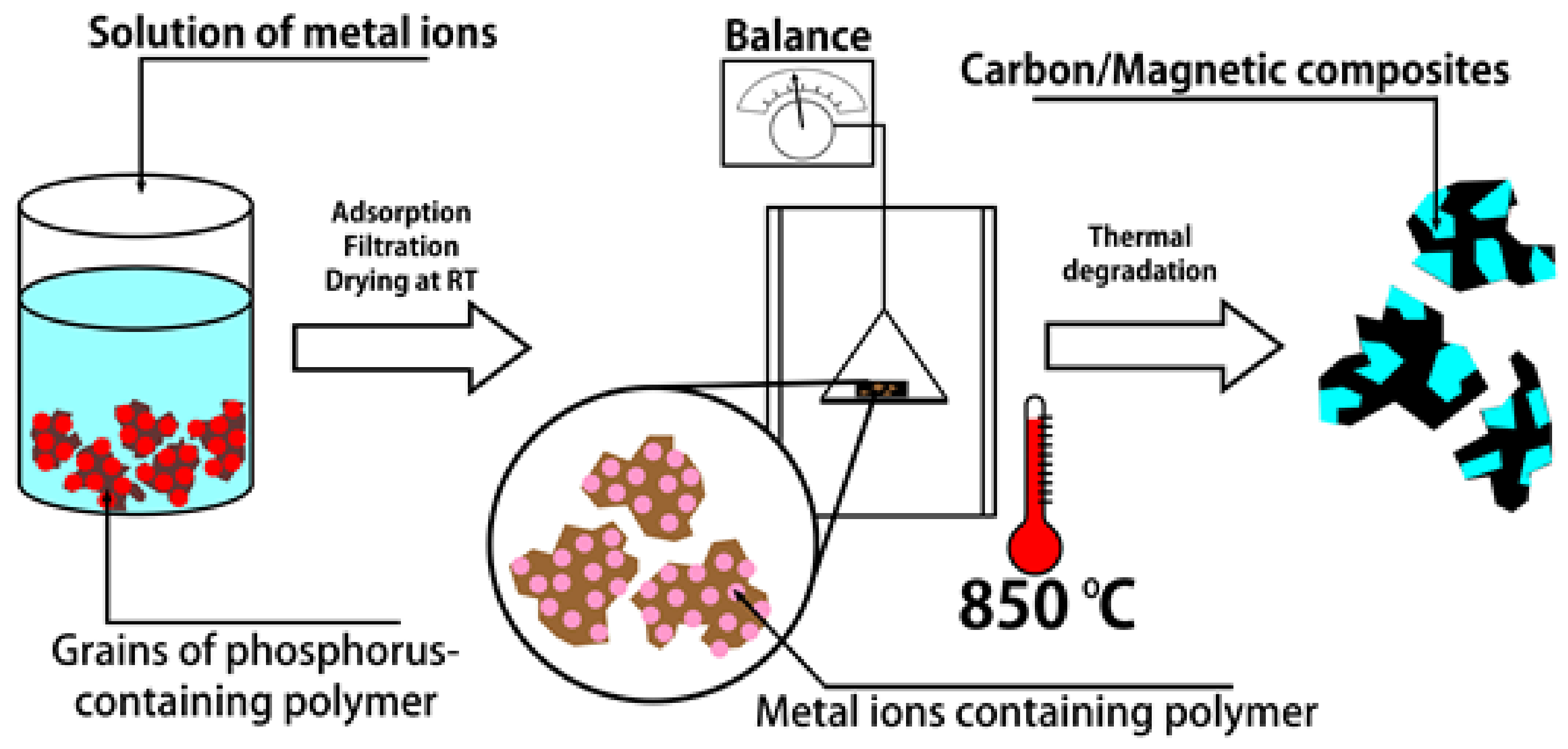
2.2. Preparation of PhCP Metal Forms
2.3. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.4. Thermal Degradation (TD)—Preparation of Carbon/Magnetic Composites
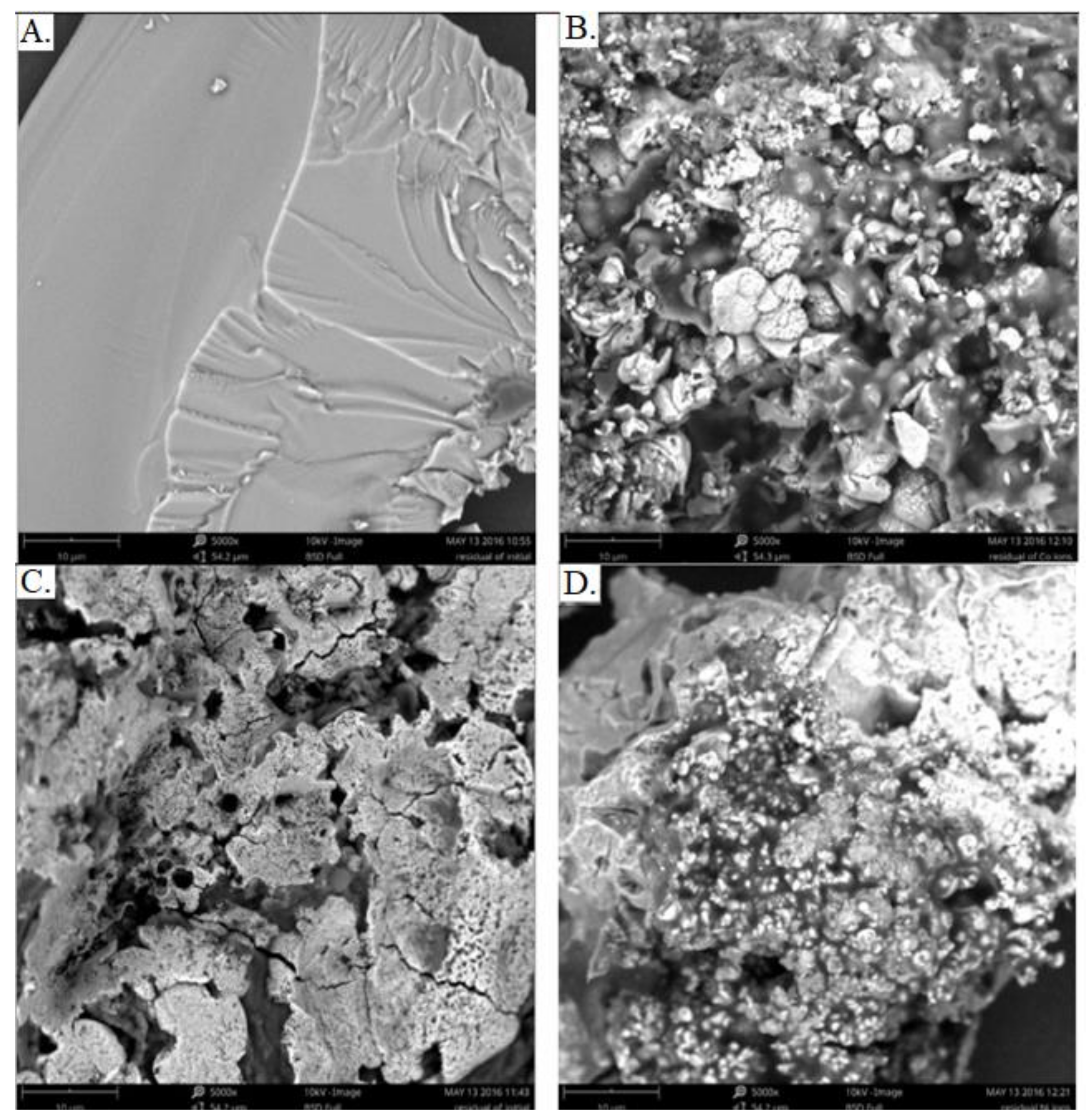
2.5. SEM Measurements
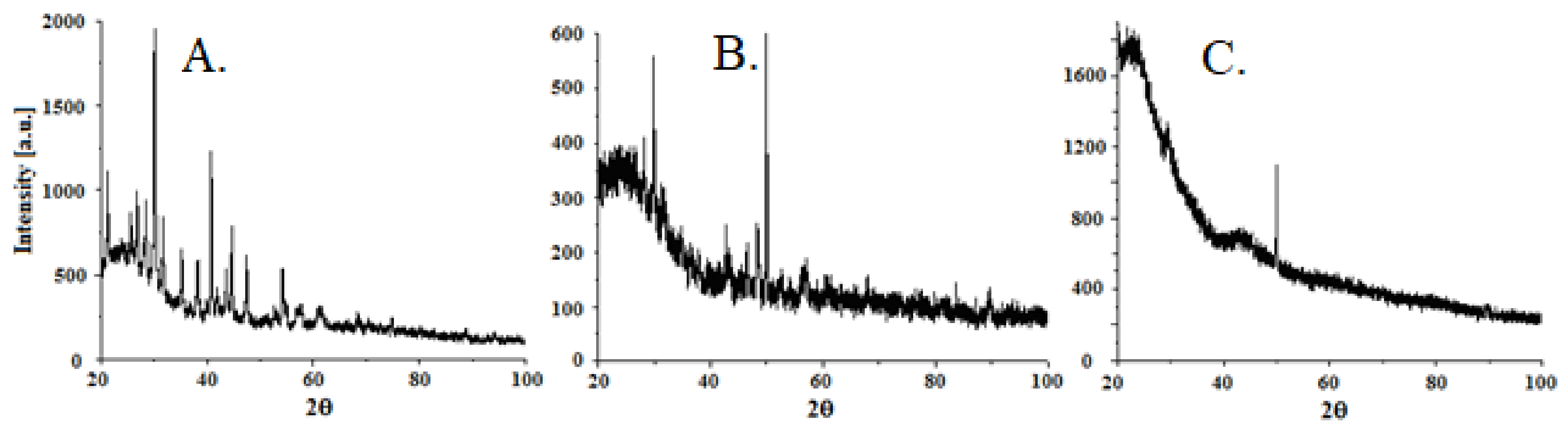
2.6. X-ray Diffraction (XRD) Study
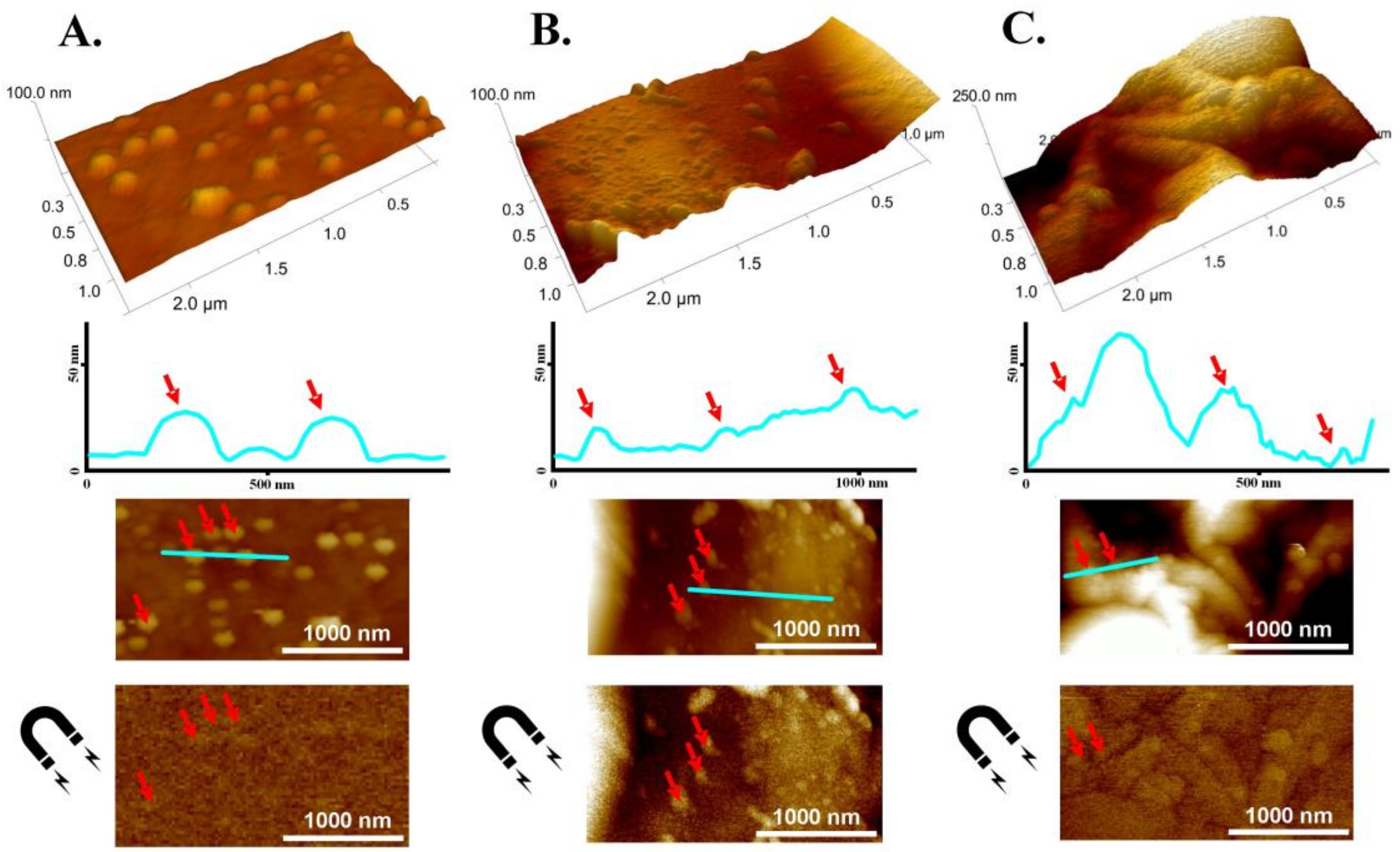
2.7. Atomic Force Microscopy (AFM) and Magnetic Force Microscopy (MFM) Measurements
3. Results
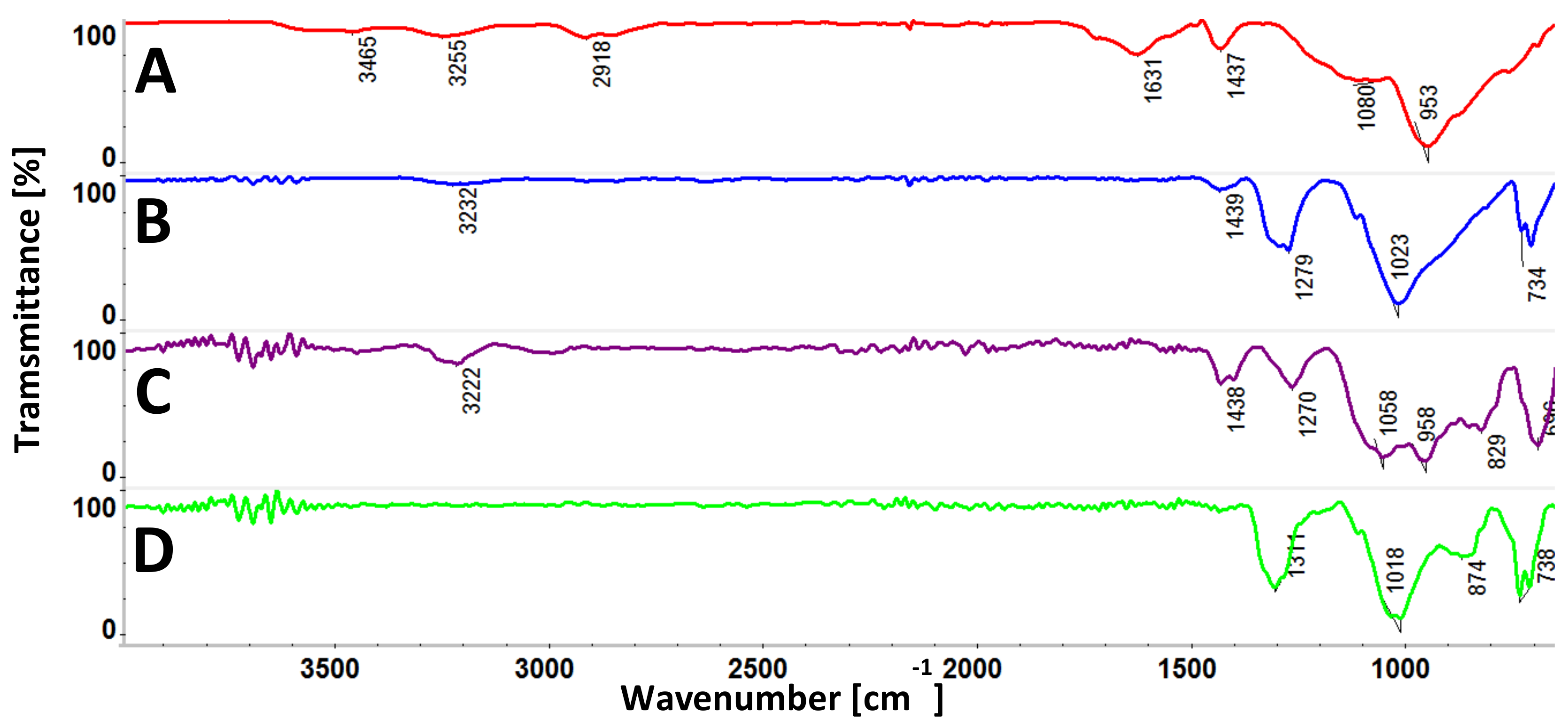
3.1. FTIR Spectra of PhCP and Its Metal Derivatives
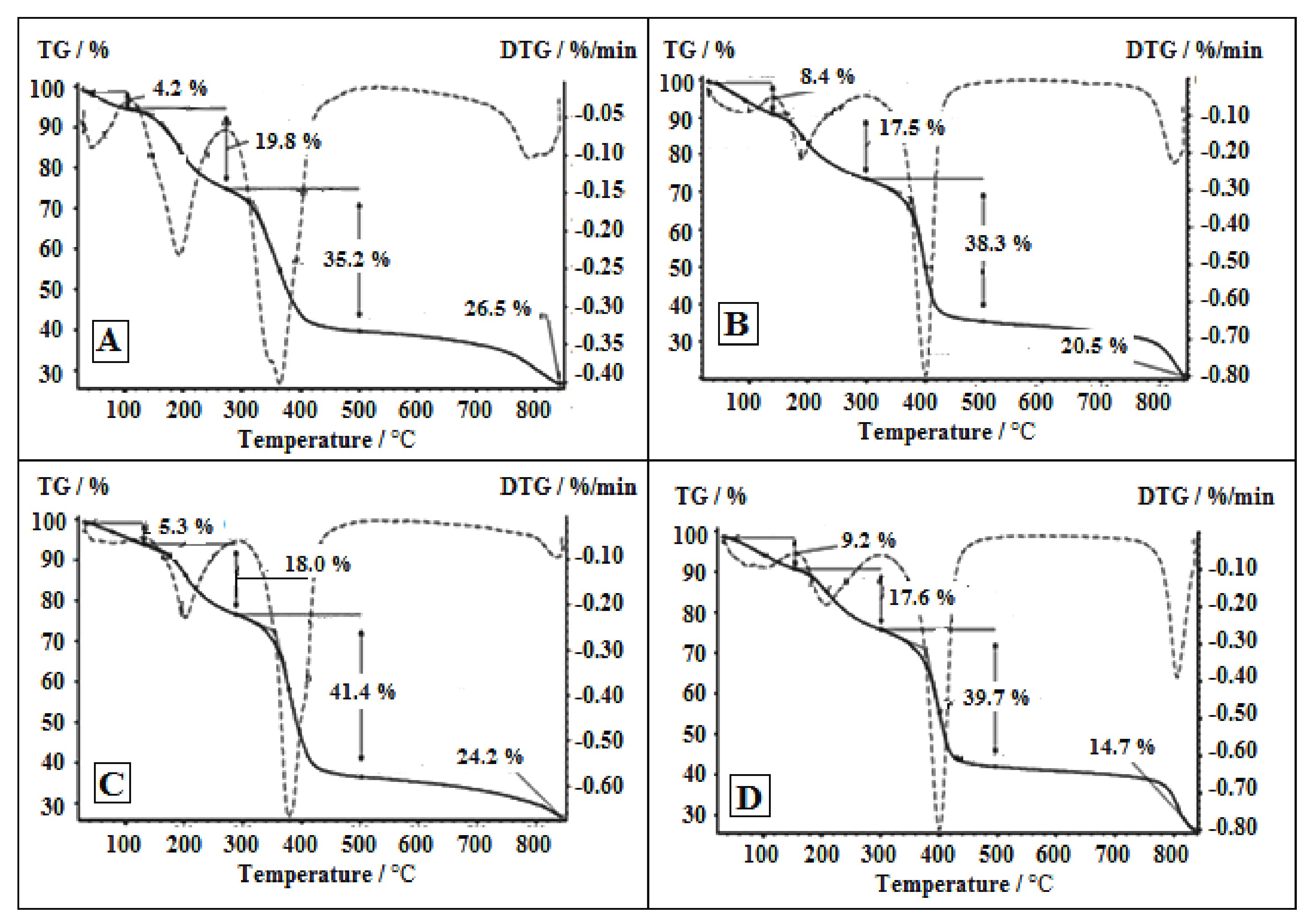
3.2. TD of the PhCP and Its Metal Forms (Co-PhCP, Fe-PhCP and Ni-PhCP)
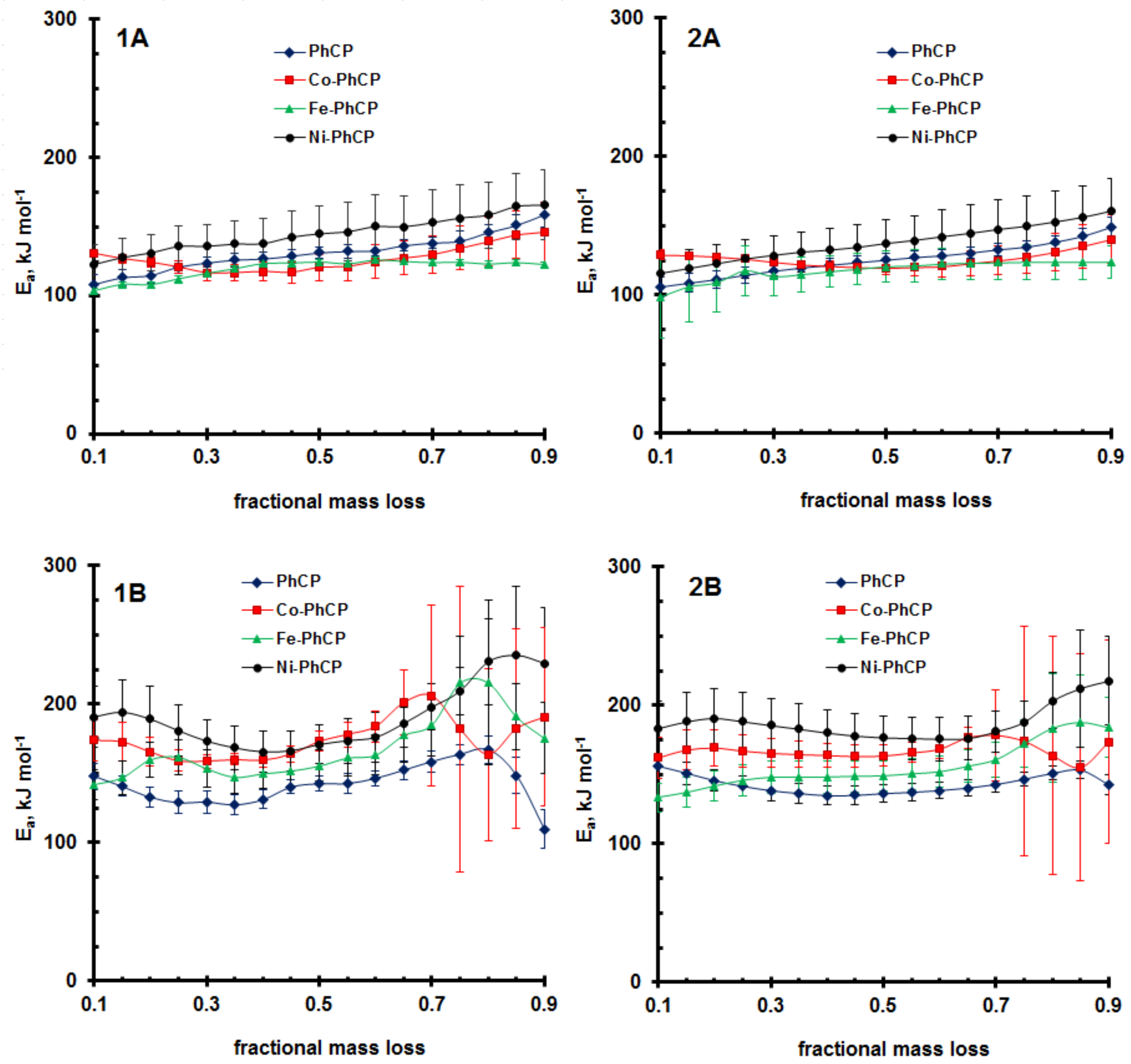
3.3. TD Kinetics of the PhCP and Its Metal Derivatives
3.4. Structural Characteristics of the Carbon Material and Carbon/Magnetic Composites
3.5. AFM-MFM Characteristics of the CM and CMCs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexandratos, S. Ion exchange resins: A retrospective from industrial and engineering chemistry research. Ind. Eng. Chem. Res. 2009, 48, 388–398. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Funabashi, K.; Kawamura, F.; Uchida, S.; Ohsumi, K. Application of Carboxylic Acid Cation Exchange Resin to Water Purification in Nuclear Power Plants. Nucl. Technol. 2017, 78, 62–68. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef]
- Van Deventer, J. Selected Ion Exchange Applications in the Hydrometallurgical Industry. Solvent Extr. Ion Exch. 2011, 29, 695–718. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, S.; Li, Y. A new regeneration approach to cation resins with aluminum salts: Application of desalination by its mixed bed. Front. Environ. Sci. Eng. 2012, 6, 45–50. [Google Scholar] [CrossRef]
- Chandrasekara, N.; Pashley, R. Study of a new process for the efficient regeneration of ion exchange resins. Desalination 2015, 357, 131–139. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Wang, C.; Ji, M. Cr (VI) Removal by a New Type of Anion Exchange Resin DEX-Cr: Adsorption Affecting Factors, Isotherms, Kinetics, and Desorption Regeneration. Environ. Prog. Sustain. 2015, 34, 387–393. [Google Scholar] [CrossRef]
- Zagarodni, A.; Kotova, D.L.; Selemenev, V.F. Infrared spectroscopy of ion exchange resins: Chemical deterioration of the resins. React. Funct. Polym. 2002, 53, 157–171. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Z. Treatment and disposal of spent radioactive ion-exchange resins produced in the nuclear industry. Prog. Nucl. Energy 2015, 78, 47–55. [Google Scholar] [CrossRef]
- Dubois, M.A.; Dozol, J.F.; Nicotra, C.; Serose, J.; Massiani, C. Pyrolysis and incineration of cationic and anionic ion-exchange resins—Identification of volatile degradation compounds. J. Anal. Appl. Pyrolysis. 1995, 31, 129–140. [Google Scholar] [CrossRef]
- Kinoshita, K.; Hirata, M.; Yahata, T. Treatment of ion-exchange resins by fluidized bed incinerator equipped with copper oxide catalyst. J. Nucl. Sci. Technol. 1991, 28, 228–238. [Google Scholar] [CrossRef]
- Juang, R.S.; Lee, T.S. Oxidative pyrolysis of organic ion exchange resins in the presence of metal oxide catalysts. J. Hazard. Mater. 2002, 92, 301–314. [Google Scholar] [CrossRef]
- Chun, U.K.; Choi, K.; Yang, K.H.; Park, J.K.; Song, M.J. Waste minimization pretreatment via pyrolysis and oxidative pyrolysis of organic ion exchange resin. Waste Manag. 1998, 18, 183–196. [Google Scholar] [CrossRef]
- Zhou, G.; Zhong, W.; Zhao, H.; Jin, B.; Wang, T.; Liu, F. Heat transfer of spent ion exchange resin in iron ore sintering process. Appl. Therm. Eng. 2015, 88, 258–264. [Google Scholar] [CrossRef]
- Wasielewski, R.; Sobolewski, A. Industrial Utilization of Spent Ion Exchange Resin in the Coke Battery. Coke Chem. 2011, 54, 66–71. [Google Scholar] [CrossRef]
- Soykan, C.; Coskun, R.; Delibas, A. Thermodegradation of poly(4-vinylpyridine-co-crotonic acid-co-divinylbenzene) and N-oxide derivatives. Therm Acta. 2007, 456, 152–157. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, H. Thermal behavior of typical weak basic ion exchange resin. J. Therm. Anal. Cal. 2014, 115, 875–880. [Google Scholar] [CrossRef]
- Alosmanov, R.; Wolski, K.; Matuschek, G.; Magerramov, A.; Azizov, A.; Zimmermann, R.; Aliyev, E.; Zapotoczny, S. Effect of functional groups on the thermal degradation of phosphorus- and phosphorus/nitrogen-containing functional polymers. J. Therm. Anal. Calorim. 2017, 130, 799–812. [Google Scholar] [CrossRef]
- Alosmanov, R.; Szuwarzyński, M.; Schnelle-Kreis, J.; Matuschek, G.; Magerramov, A.; Azizov, A.; Zimmermann, R.; Zapotoczny, S. Magnetic nanocomposites based on phosphorus-containing polymers—structural characterization and thermal analysis. Nanotechnology 2018, 29, 135708. [Google Scholar] [CrossRef] [PubMed]
- Gunko, V.M.; Leboda, R.; Skubiszewska-Zieba, J.; Charmas, B.; Oleszczuk, P. Carbon adsorbents from waste ion-exchange resin. Carbon 2005, 43, 1143–1150. [Google Scholar] [CrossRef]
- Bratek, W.; Bratek, K.; Kułazynski, M. The utilization of waste ion exchange resin in environmental protection. Fuel Process Technol 2002, 77–78, 431–436. [Google Scholar] [CrossRef]
- Bratek, W.; Bratek, K.; Kułazynski, M. Carbon adsorbents from waste ion-exchange resin. Carbon 2002, 40, 2213–2220. [Google Scholar] [CrossRef]
- Skubiszewska-Zieba, J.; Leboda, R.; Charmas, B.; Grzegorczyk, W.; Szmigielski, R. On the preparation of synthetic carbon adsorbents using the sulfonated ion exchange resin Duolite C-20. J. Therm. Anal. Calorim. 2006, 86, 187–194. [Google Scholar] [CrossRef]
- Long, C.; Lu, J.D.; Li, A.M.; Hu, D.B.; Liu, F.Q.; Zhang, Q.X. Adsorption of naphthalene onto the carbon adsorbent from waste ion exchange resin: Equilibrium and kinetic characteristics. J. Hazard. Mater. 2008, 150, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Li, A.; Zhou, Q.; Shuang, C.; Li, Y.; Ma, Y. Utilization of waste cation exchange resin to prepare carbon/iron composites for the adsorption of contaminants in water. J. Ind. Eng. Chem. 2014, 20, 4256–4260. [Google Scholar] [CrossRef]
- Kalia, S.; Kango, S.; Kumar, A.; Haldorai, Y.; Kumari, B.; Kumar, R. Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid Polym. Sci. 2014, 292, 2025–2052. [Google Scholar] [CrossRef]
- Behrens, S.; Appel, I. Magnetic nanocomposites. Curr. Opin. Biotechnol. 2016, 39, 89–96. [Google Scholar] [CrossRef]
- Sumaraj, L.; Padhye, P. Influence of surface chemistry of carbon materials on their interactions with inorganic nitrogen contaminants in soil and water. Chemosphere 2017, 184, 532–547. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I. The Properties Of Synthetic Carbon Derived From Nitrogen- and Phosphorus-Containing Polymer. Carbon 1998, 36, 45–50. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Martınez-Alonso, A.; Suarez-Garcıa, F.; Tascon, J.M.D. Synthetic carbons activated with phosphoric acid. I. Surface chemistry and ion binding properties. Carbon 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Akhmedov, V.M.; Alfadul, S.; Maharramov, A.M.; Azizov, A.A.; Alosmanov, R.M.; Buniyad-Zadeh, I.A. Modification of industrial divinyl rubber by oxidative chlorophosphorylation and assessment of metal ion removal efficiency of obtained polymer sorbent. Pol. J. Chem. Tech. 2015, 17, 112–118. [Google Scholar] [CrossRef]
- Alosmanov, R.M.; Azizov, A.A.; Magerramov, A.M. NMR Spectroscopic Study of Phosphorus-Containing Polymer Sorbent. Russ. J. Gen. Chem. 2011, 81, 1477–1479. [Google Scholar] [CrossRef]
- Alosmanov, R.M.; Azizov, A.A.; Maharramov, A.M.; Buniyadzadeh, I.A. Acid base and sorption properties of phosphorus containing polymeric sorbent. Mater. Res. Innov. 2010, 14, 414–418. [Google Scholar] [CrossRef]
- Marcelo, A.V.; de Santa Maria, L.C.; Costa, A.S.; Hui, W.S.; Costa, L.C.; Filho, H.C.; Amico, S.C. Synthesis, characterization and evaluation of phosphorylated resins in the removal of Pb2+ from aqueous solutions. Polym. Bull. 2011, 67, 237–249. [Google Scholar]
- Davidescu, C.M.; Ciopec, M.; Negrea, A.; Popa, A.; Lupa, L.; Dragan, S.; Ardelean, R.; Ilia, G.; Iliescu, S. Synthesis, characterization, and Ni(II) ion sorption properties of poly(styrene-co-divinylbenzene) functionalized with aminophosphonic acid groups. Polym. Bull. 2013, 70, 277–291. [Google Scholar] [CrossRef]
- Popa, A.; Muntean, S.G.; Paska, O.M.; Iliescu, S.; Ilia, G.; Zhang, Z. Resins containing a hydroxyphosphonic acid groups used for adsorption of dyes from wastewater. Polym. Bull. 2011, 66, 419–432. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. C Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Popescu, C. Integral method to analyse the kinetics of heterogeneous reactions under non-isothermal conditions. A variant on the Ozawa–Flynn–Wall method. Thermochim. Acta. 1996, 285, 309–323. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastics. J. Polym. Sci. C 1965, 6, 183–195. [Google Scholar] [CrossRef]
- NETZSCH-Thermokinetics 3.1 Software Help.
- Phadnis, A.; Deshpande, V. Determination of the kinetics and mechanism of a solid state reaction. A simple approach. Thermochim. Acta. 1983, 62, 361–367. [Google Scholar] [CrossRef]
- Zawadzki, J. Infrared spectroscopy in surface chemistry of carbons. Chem. Phys. Carbon 1989, 21, 147–386. [Google Scholar]
- Jagtoyen, M.; Thwaites, M.; Stencel, J.; McEnaney, B.; Derbyshire, F. Adsorbent carbon synthesis from coals by phosphoric acid activation. Carbon 1992, 30, 1089–1096. [Google Scholar] [CrossRef]
- Toles, C.; Rimmer, S.; Hower, J.C. Production of activated carbons from a Washington lignite using phosphoric acid activation. Carbon 1996, 34, 1419–1426. [Google Scholar] [CrossRef]
- Trojan, M.; Brandova, D. Study of thermal dehydration of Co12Mg12(H2PO4)2·3H2O Thermochim. Acta. 1990, 59, 1–12. [Google Scholar]
- Brandova, D.; Trojan, M.; Arnold, M.; Paulik, F. Thermal study of decomposition of Cu1/2Mg1/2(H2PO4)2.0.5 H2O. J. Therm. Anal. Calorim. 1990, 36, 677–684. [Google Scholar]

| Characteristic | Type |
|---|---|
| Polymer matrix structure | Cross-linked butadiene rubber |
| The main functional groups | Phosphonate (−OP(O)(OH)2); Phosphate (−P(O)(OH)2) |
| Other functional groups | >CH–Cl; >CH–OH |
| Ionic form | H+ |
| Total exchange, equiv/kg of dry polymer | 9.3 |
| Ionization constant: pKa1, pKa2 | 4.4; 8.6 |
| Size of particles, mm | 0.30−0.40 |
| Surface morphology | Porous and rough structure |
| Samples | Step I | Step II | Step III | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ea, kJ·mol−1 | lgA, s−1 | Ea, kJ·mol−1 | lgA, s−1 | Ea, kJ·mol−1 | lgA, s−1 | |||||||
| Friedman | OFW | Friedman | OFW | Friedman | OFW | Friedman | OFW | Friedman | OFW | Friedman | OFW | |
| PhCP | 62.4 ± 1.6 | 63.5 ± 2.2 | 7.14 ± 0.40 | 6.82 ± 0.96 | 131.4 ± 4.3 | 125.4 ± 4.6 | 11.89 ± 2.37 | 10.36 ± 3.06 | 141.8 ± 5.1 | 142.9 ± 5.6 | 9.04 ± 1.60 | 9.41 ± 1.48 |
| Co-PhCP | 71.7 ± 8.4 | 75.4 ± 7.6 | 7.56 ± 3.09 | 8.06 ± 3.59 | 127.3 ± 12.2 | 126.0 ± 12.0 | 11.11 ± 1.63 | 10.72 ± 0.79 | 175.0 ± 9.0 | 167.4 ± 6.7 | 10.18 ± 2.81 | 10.00 ± 1.56 |
| Fe-PhCP | 66.5 ± 21.9 | 70.1 ± 22.5 | 5.91 ± 1.66 | 6.14 ± 1.75 | 119.7 ± 8.7 | 117.8 ± 11.5 | 9.47 ± 0.83 | 9.22 ± 0.98 | 167.9 ± 14.4 | 155.7 ± 11.62 | 10.89 ± 2.85 | 9.55 ± 2.17 |
| Ni-PhCP | 73.0 ± 4.0 | 75.6 ± 3.7 | 7.67 ± 2.25 | 7.51 ± 2.54 | 145.0 ± 20.5 | 137.7 ± 17.06 | 11.53 ± 1.20 | 11.18 ± 1.62 | 190.5 ± 17.0 | 187.4 ± 15.1 | 12.37 ± 2.66 | 12.14 ± 1.61 |
| Samples | Step I | Step II | Step III |
|---|---|---|---|
| PhCP | 0.68 | 2.91 | 1.86 |
| Co-PhCP | 1.00 | 3.28 | 0.50 |
| Fe-PhCP | 1.30 | 2.88 | 1.69 |
| Ni-PhCP | 0.50 | 3.20 | 0.83 |
| Compound | The Lattice Parameters (nm) | Crystal System | Space Group | Space Group Number | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| Ni(PO3)2 | 1.11 | 0.82 | 0.98 | Monoclinic | C2/c | 15 |
| Ni2P | 0.86 | 0.86 | 0.34 | Hexagonal | P-62m | 189 |
| Ni2(P4O12) | 11.61 | 8.22 | 9.83 | Monoclinic | C2/c | 15 |
| Co(PO3)2 | 1.12 | 0.83 | 0.99 | Monoclinic | C2/c | 15 |
| CoP | 0.51 | 0.33 | 0.56 | Orthorhombic | Pnma | 62 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alosmanov, R.; Imanova, J.; Wolski, K.; Ziemmermann, R.; Fiejdasz, S.; Przewoźnik, J.; Goc, K.; Kapusta, C.; Zapotoczny, S.; Szuwarzyński, M. Fabrication of Functional Carbon/Magnetic Nanocomposites as A Promising Model of Utilization of Used Crosslinked Polymers. Materials 2018, 11, 2595. https://doi.org/10.3390/ma11122595
Alosmanov R, Imanova J, Wolski K, Ziemmermann R, Fiejdasz S, Przewoźnik J, Goc K, Kapusta C, Zapotoczny S, Szuwarzyński M. Fabrication of Functional Carbon/Magnetic Nanocomposites as A Promising Model of Utilization of Used Crosslinked Polymers. Materials. 2018; 11(12):2595. https://doi.org/10.3390/ma11122595
Chicago/Turabian StyleAlosmanov, Rasim, Jennet Imanova, Karol Wolski, Ralf Ziemmermann, Sylwia Fiejdasz, Janusz Przewoźnik, Kamil Goc, Czesław Kapusta, Szczepan Zapotoczny, and Michał Szuwarzyński. 2018. "Fabrication of Functional Carbon/Magnetic Nanocomposites as A Promising Model of Utilization of Used Crosslinked Polymers" Materials 11, no. 12: 2595. https://doi.org/10.3390/ma11122595
APA StyleAlosmanov, R., Imanova, J., Wolski, K., Ziemmermann, R., Fiejdasz, S., Przewoźnik, J., Goc, K., Kapusta, C., Zapotoczny, S., & Szuwarzyński, M. (2018). Fabrication of Functional Carbon/Magnetic Nanocomposites as A Promising Model of Utilization of Used Crosslinked Polymers. Materials, 11(12), 2595. https://doi.org/10.3390/ma11122595






