Fabrication of Silver Nanoparticles Using a Gas Phase Nanocluster Device and Preliminary Biological Uses
Abstract
:1. Introduction
2. Materials and Methods
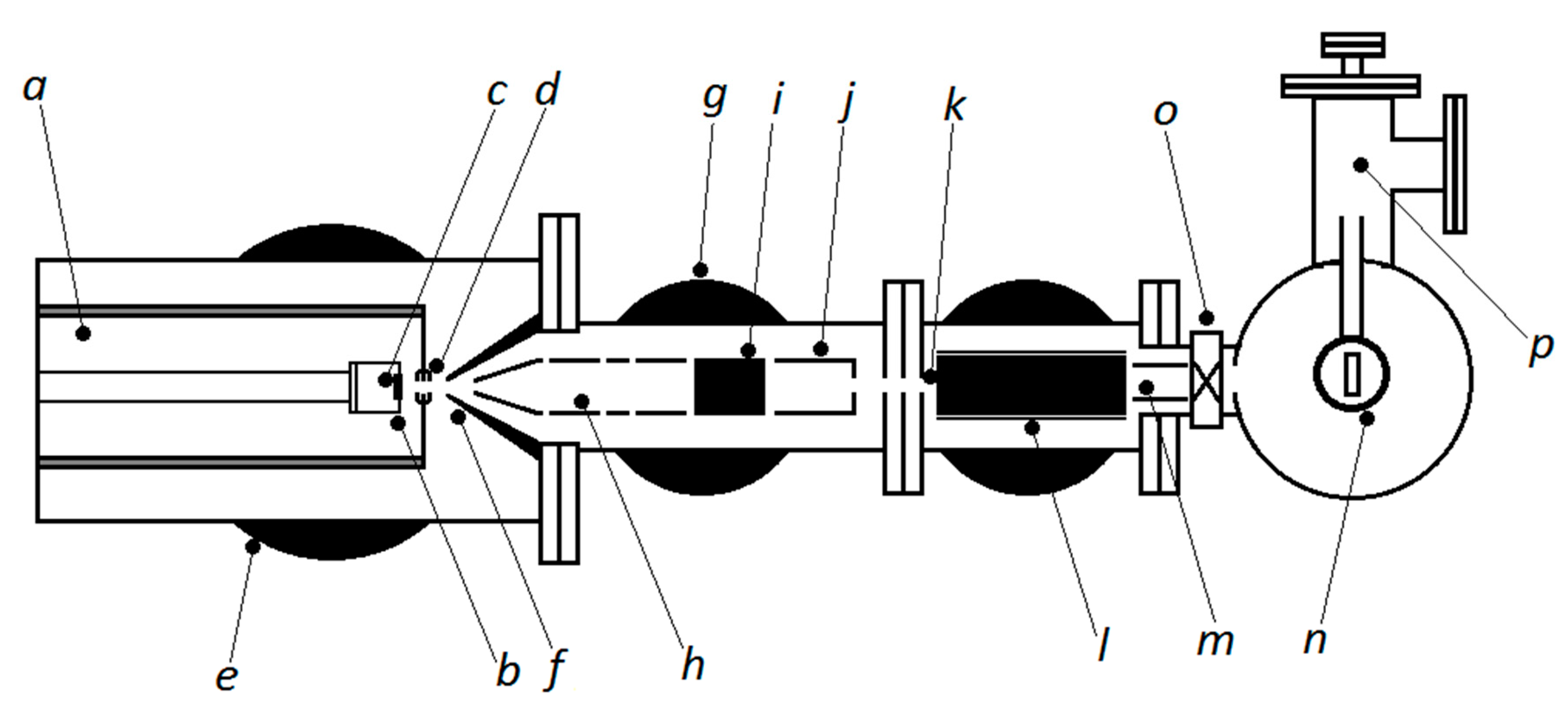
2.1. Cluster Equipment, Working Principles of the Nanoparticle System for Sample Production
2.2. Sample Fabrication: Ag Nanoparticles
2.3. Sample Characterization
2.4. Cell Culture
2.5. Cell Spreading and Morphological Assay
2.6. Statistical Analysis
3. Results and Discussion
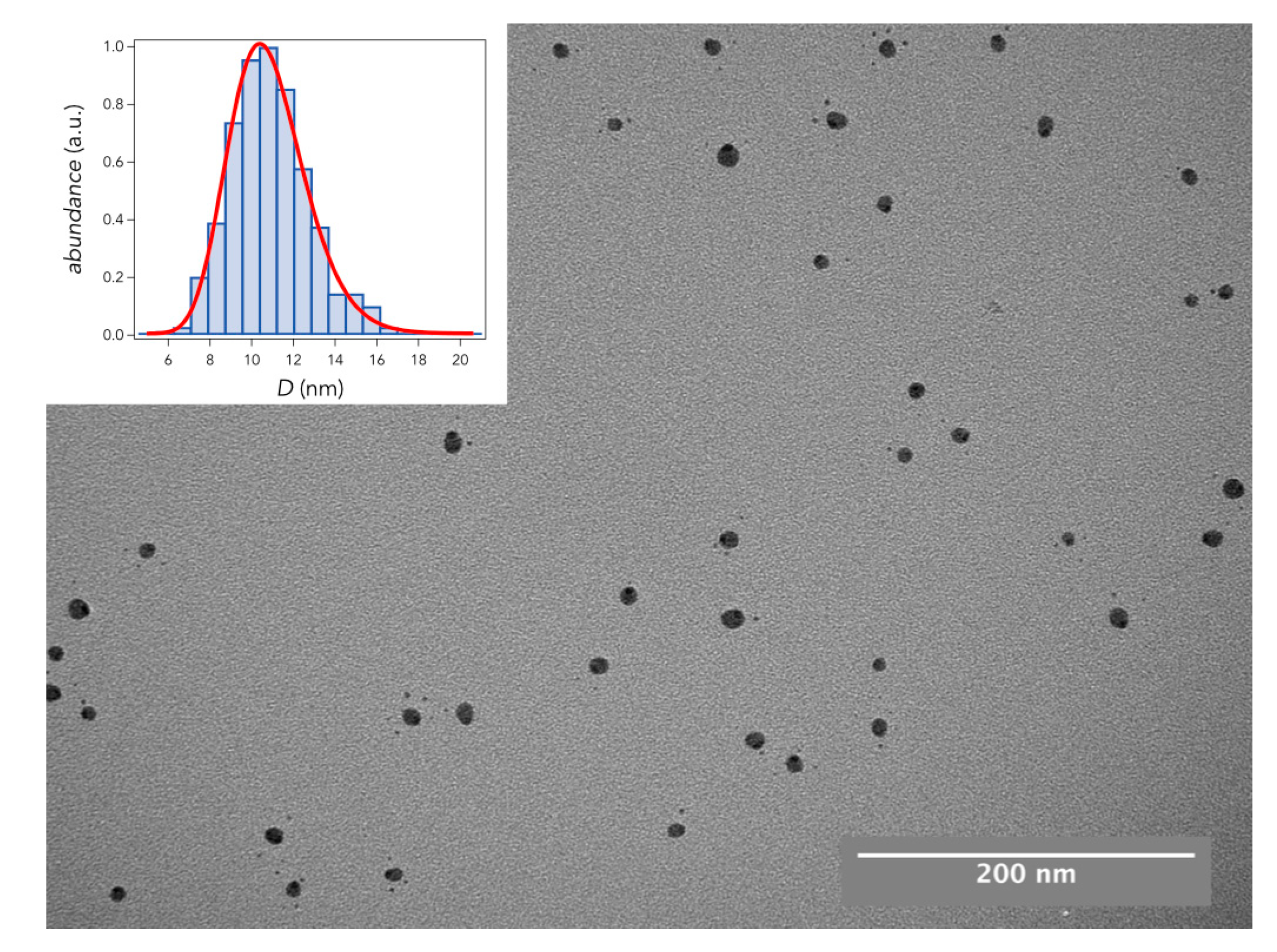
3.1. Nanoparticle Sample Fabrication
3.2. Biological Response of Myoblast Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References and Note
- Canali, L. Novel Scanning Probes Applied to the Study of Nanostructures; Delft University of Technology: Delft, The Netherlands, 2000; ISBN 90-6464-78-79. [Google Scholar]
- Reinhard, P.G.; Suraud, E. Introduction to Cluster Dynamics; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2004; p. 40. ISBN 3-527-40345-0. [Google Scholar]
- Suzhou SinoRaybo Nanotechnology Co., Ltd., Zone D, No. 99, Ren’ai Road, Suzhou Industrial Park.
- Morris, J.E. Nanopackaging: Nanotechnologies and Electronics Packaging. In Nanopackaging; Morris, J., Ed.; Springer: Boston, MA, USA, 2008. [Google Scholar]
- Solairajan, S.A.; Alexraj, S.; Kumar, G.P.; Rajan, V.P. Review on nano fabrication and application. Adv. Mater. Res. 2014, 984–985, 508–513. [Google Scholar] [CrossRef]
- Wang, H.-H.; Lin, C.-A.J.; Lee, C.-H.; Lin, Y.-C.; Tseng, Y.-M.; Hsieh, C.-L.; Chen, C.-H.; Tsai, C.-H.; Hsieh, C.-T.; Shen, J.-L.; et al. Fluorescent Gold Nanoclusters as a Biocompatible Marker for In Vitro and In Vivo Tracking of Endothelial Cells. ACS Nano 2011, 5, 4337–4344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Chen, J.; Luo, Z.; Wu, D.; Shen, X.; Song, S.S.; Sun, Y.M.; Liu, P.X.; Zhao, J.; Huo, S.; et al. Enhanced Tumor Accumulation of Sub-2 nm Gold Nanoclusters for Cancer Radiation Therapy. Adv. Healthc. Mater. 2014, 3, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.; Thapa, A.; Haberstroh, K.M.; Webster, T.J. Nanostructured polymer/nanophase ceramic composites enhance osteoblast and chondrocyte adhesion. Tissue Eng. 2002, 8, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.; Vindigni, V.; Azzena, B.; Barbante, C.; et al. Active silver NPs for wound healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Gao, W.J.; Gu, H.Y. Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns 2008, 34, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative toxicity of 24 manufactured NPs in human alveolar epithelial and macrophage cell lines. Part. Fibre Toxicol. 2009, 6, 14. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metalbased nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef]
- Available online: https://www.nanosilver.eu/T-shirts/ (accessed on November 2018).
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]
- Romero, C.P.; Volodin, A.; Paddubrouskaya, H.; Van Bael, M.J.; Van Haesendonck, C.; Lievens, P. Tailoring magnetic properties of Co nanocluster assembled films using hydrogen. J. Magn. Magn. Mater. 2018, 458, 261–267. [Google Scholar] [CrossRef]
- Cuppens, J.; Romero, C.P.; Lievens, P.; Van Bael, M.J. Superconductivity in Pb cluster assembled systems with different degrees of coagulation. Phys. Rev. B 2010, 81, 064517. [Google Scholar] [CrossRef]
- Romero, C.P.; Volodin, A.; Di Vece, M.; Paddubrouskaya, H.; Huan Wang Vantomme, A.; Van Haesendonck, C.; Lievens, P. Passivation of cobalt nanocluster assembled thin films with hydrogen. Thin Solid Films 2012, 520, 5584–5588. [Google Scholar] [CrossRef] [Green Version]
- Goldby, I.M.; von Issendorff, B.; Kuipers, L.; Palmer, R.E. Gas condensation source for production and deposition of size-selected metal clusters. Rev. Sci. Instrum. 1997, 68, 3327. [Google Scholar] [CrossRef]
- von Issendorff, B.; Palmer, R.E. A new high transmission infinite range mass selector for cluster and nanoparticle beams. Rev. Sci. Instrum. 1999, 70, 4497. [Google Scholar] [CrossRef]
- Mendoza, P.; Ortiz, R.; Díaz, J.; Quest, A.F.; Leyton, L.; Stupack, D.; Torres, V.A. Rab5 activation promotes focal adhesion disassembly, migration and invasiveness in tumor cells. J. Cell Sci. 2013, 126, 3835–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, V.; Fourel, L.; Destaing, O.; Gilde, F.; Albigès-Rizo, C.; Picart, C.; Boudou, T. Signal mingle: Micropatterns of BMP-2 and fibronectin on soft biopolymeric films regulate myoblast shape and SMAD signaling. Sci. Rep. 2017, 7, 41479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bereiter-Hahn, J.; Lück, M.; Miebach, T.; Stelzer, H.K.; Vöth, M. Spreading of trypsinized cells: Cytoskeletal dynamics and energy requirements. J. Cell Sci. 1990, 96, 171–188. [Google Scholar]
- Burattini, S.; Ferri, P.; Battistelli, M.; Curci, R.; Luchetti, F.; Falcieri, E. C2C12 murine myoblasts as a model of skeletal muscle development: Morpho-functional characterization. Eur. J. Histochem. 2004, 48, 223–233. [Google Scholar]
- Grabowska, I.; Szeliga, A.; Moraczewski, J.; Czaplicka, I.; Brzóska, E. Comparison of satellite cell-derived myoblasts and C2C12 differentiation in two- and three-dimensional cultures: Changes in adhesion protein expression. Cell Biol. Int. 2011, 35, 125–133. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells In Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, J.; Ruan, H.; Wang, W.; Wu, T.; Cui, W.; Fan, C. Antibacterial and anti-adhesion effects of the silver nanoparticles-loaded poly(L-lactide) fibrous membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1176–1182. [Google Scholar] [CrossRef] [PubMed]


| Sample | Deposition Time (s) | Observed Average Coverage (ng/cm2) |
|---|---|---|
| 1 | 2389 | 620 |
| 2 | 2160 | 400 |
| 3 | 1237 | 490 |
| 4 | 750 | 190 |
| 5 | 316 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mery, M.; Orellana, N.; Acevedo, C.A.; Oyarzún, S.; Araneda, F.; Herrera, G.; Aliaga, D.; Creixell, W.; Corrales, T.P.; Romero, C.P. Fabrication of Silver Nanoparticles Using a Gas Phase Nanocluster Device and Preliminary Biological Uses. Materials 2018, 11, 2574. https://doi.org/10.3390/ma11122574
Mery M, Orellana N, Acevedo CA, Oyarzún S, Araneda F, Herrera G, Aliaga D, Creixell W, Corrales TP, Romero CP. Fabrication of Silver Nanoparticles Using a Gas Phase Nanocluster Device and Preliminary Biological Uses. Materials. 2018; 11(12):2574. https://doi.org/10.3390/ma11122574
Chicago/Turabian StyleMery, M., N. Orellana, C. A. Acevedo, S. Oyarzún, F. Araneda, G. Herrera, D. Aliaga, W. Creixell, T. P. Corrales, and C. P. Romero. 2018. "Fabrication of Silver Nanoparticles Using a Gas Phase Nanocluster Device and Preliminary Biological Uses" Materials 11, no. 12: 2574. https://doi.org/10.3390/ma11122574
APA StyleMery, M., Orellana, N., Acevedo, C. A., Oyarzún, S., Araneda, F., Herrera, G., Aliaga, D., Creixell, W., Corrales, T. P., & Romero, C. P. (2018). Fabrication of Silver Nanoparticles Using a Gas Phase Nanocluster Device and Preliminary Biological Uses. Materials, 11(12), 2574. https://doi.org/10.3390/ma11122574





