Polymer Electrode Materials for Sodium-ion Batteries
Abstract
1. Introduction
1.1. The Importance of Energy Storage
1.2. Sodium-Ion Batteries
1.3. Electrode Materials for Sodium-Ion Batteries
1.4. Polymer Electrode Materials for Sodium-Ion Batteries
2. Polymer Electrode Materials for NIBs
2.1. Polymers with Carbonyl Functional Groups
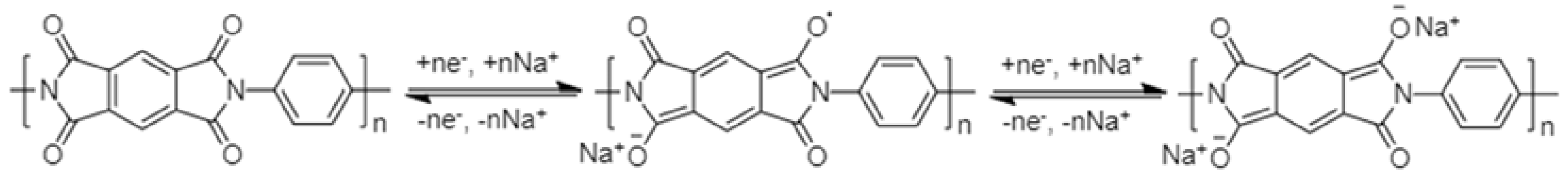
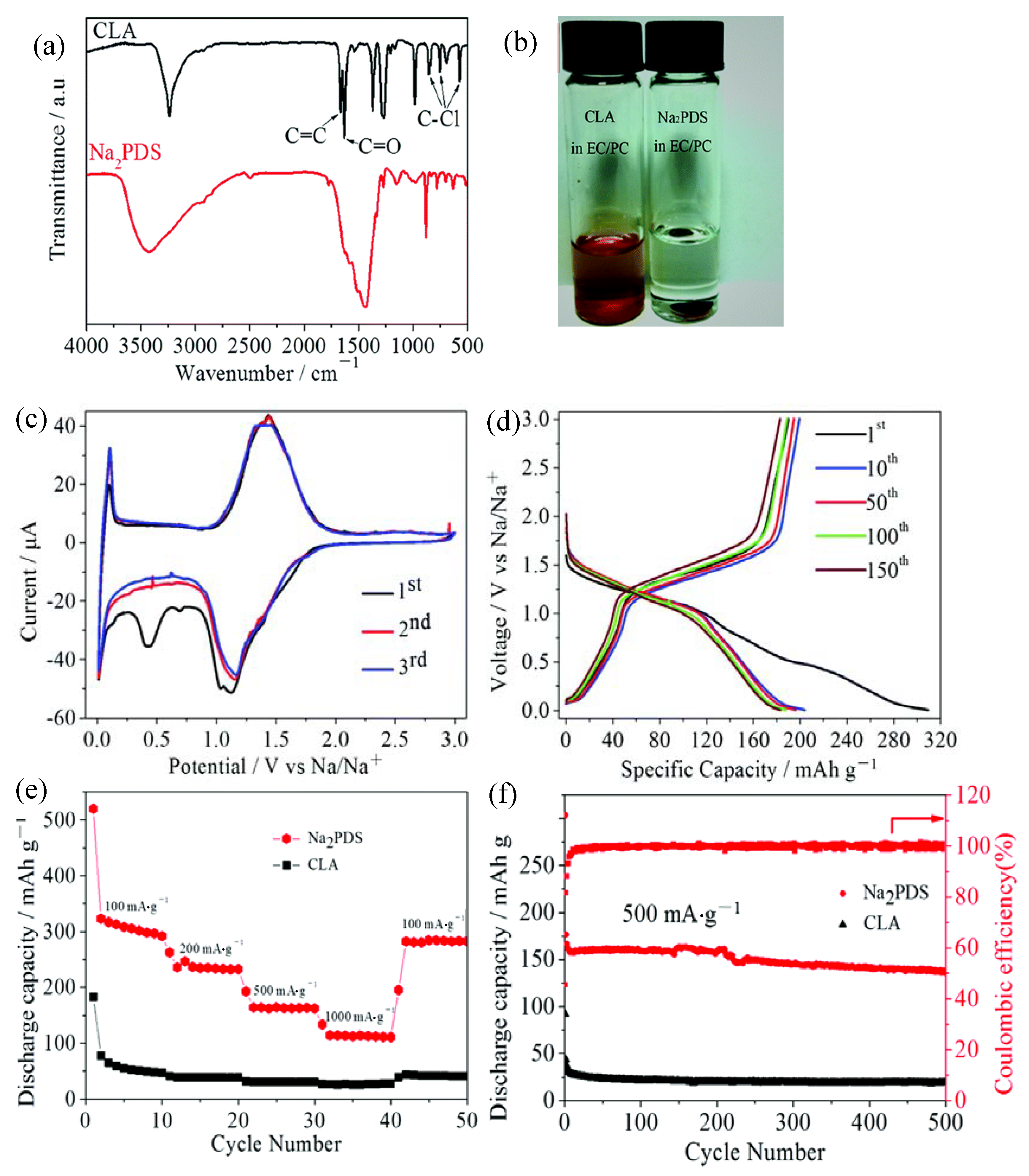
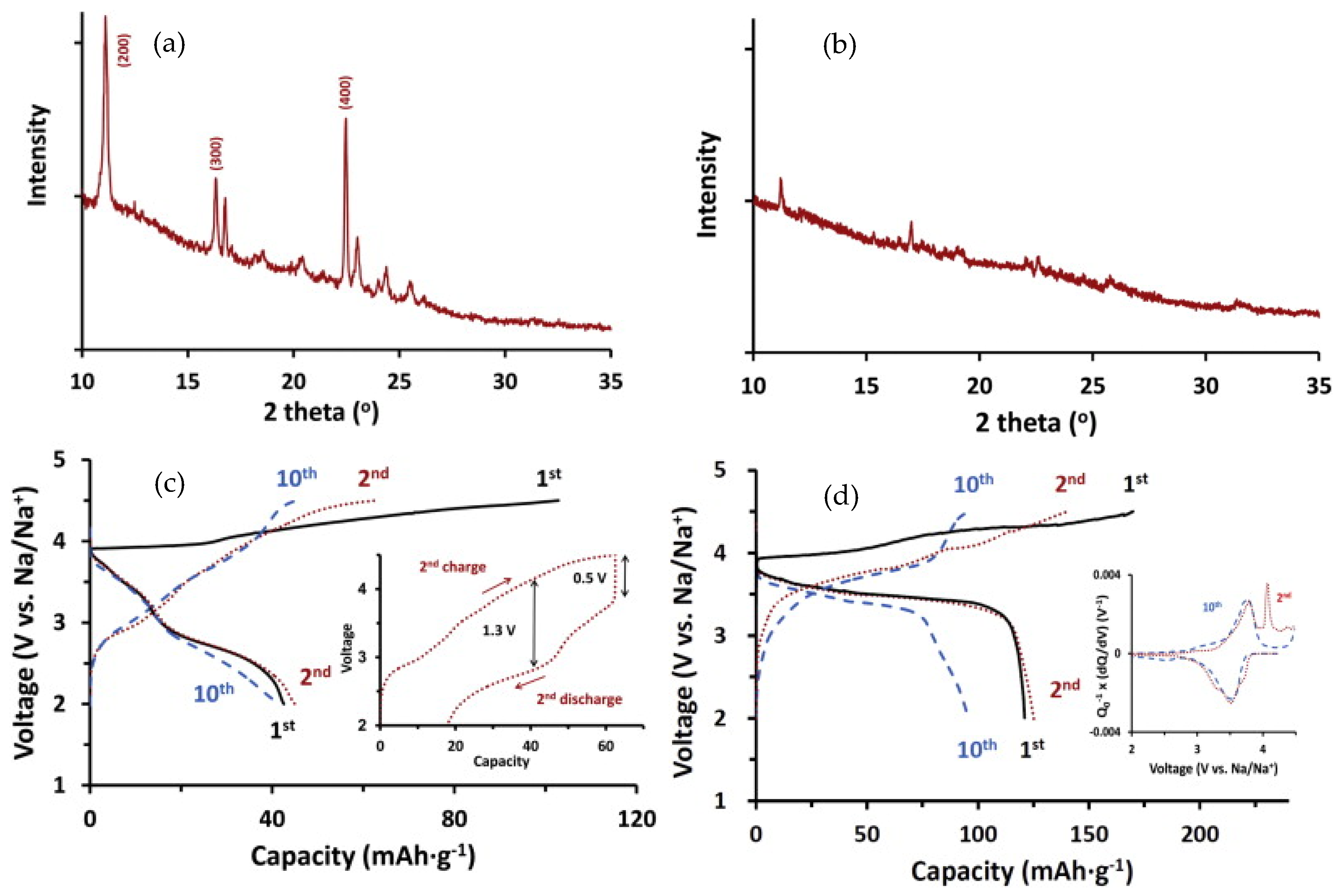
2.1.1. Polyimides
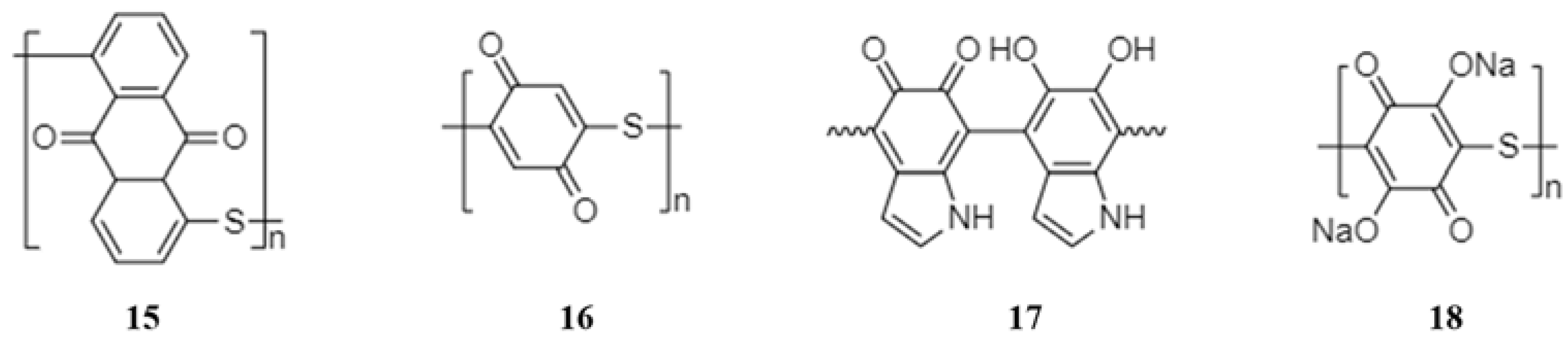
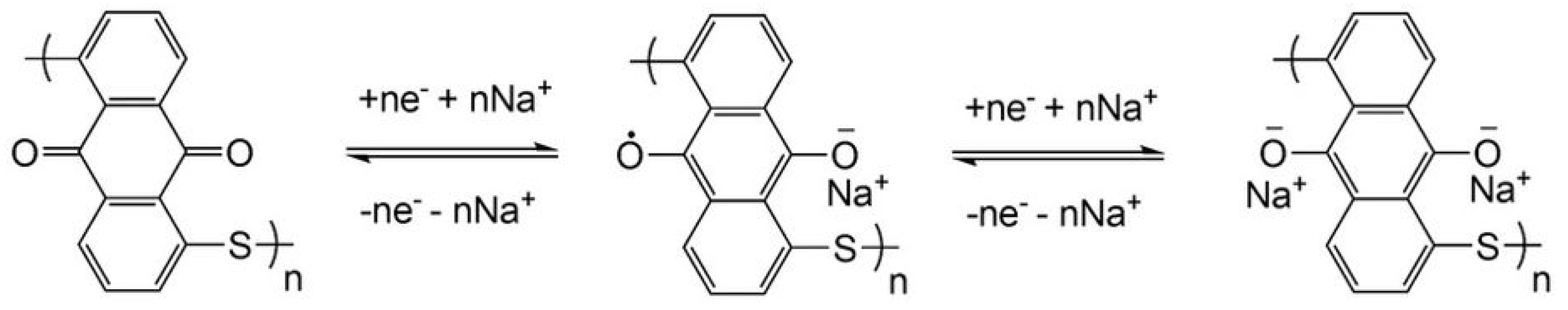
2.1.2. Polyquinones
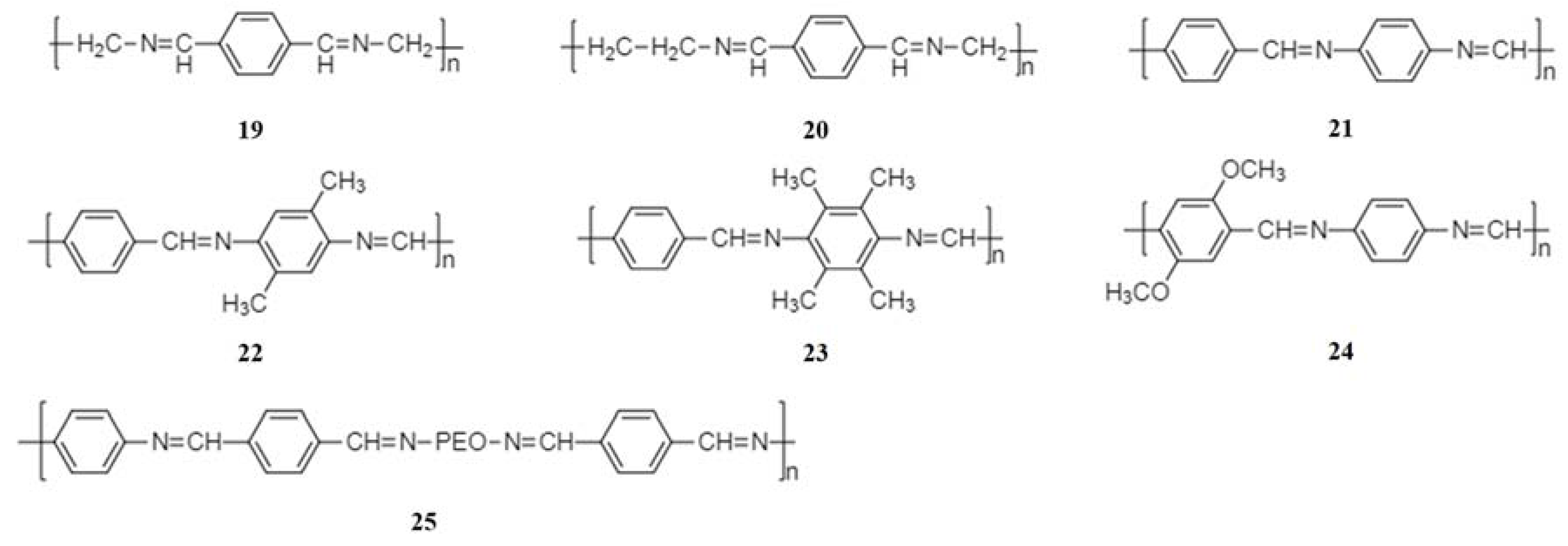
2.2. Schiff Base Polymer Electrode Materials
2.2.1. Synthesis and Structural Diversity
2.2.2. Charge Storage Mechanism in Schiff Base Polymers
2.2.3. Recent Development of Schiff Base Polymer Electrode Materials for NIBs
2.2.4. Challenges and Opportunities in Developing Schiff Base Polymer Electrode Materials
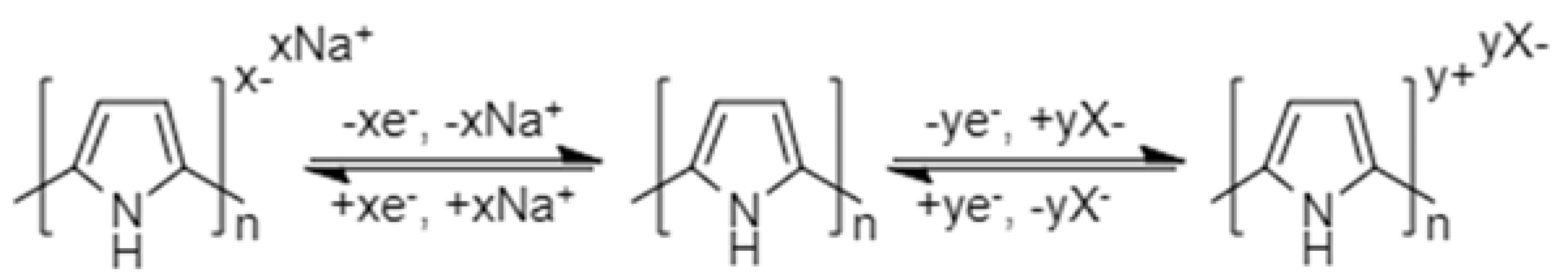
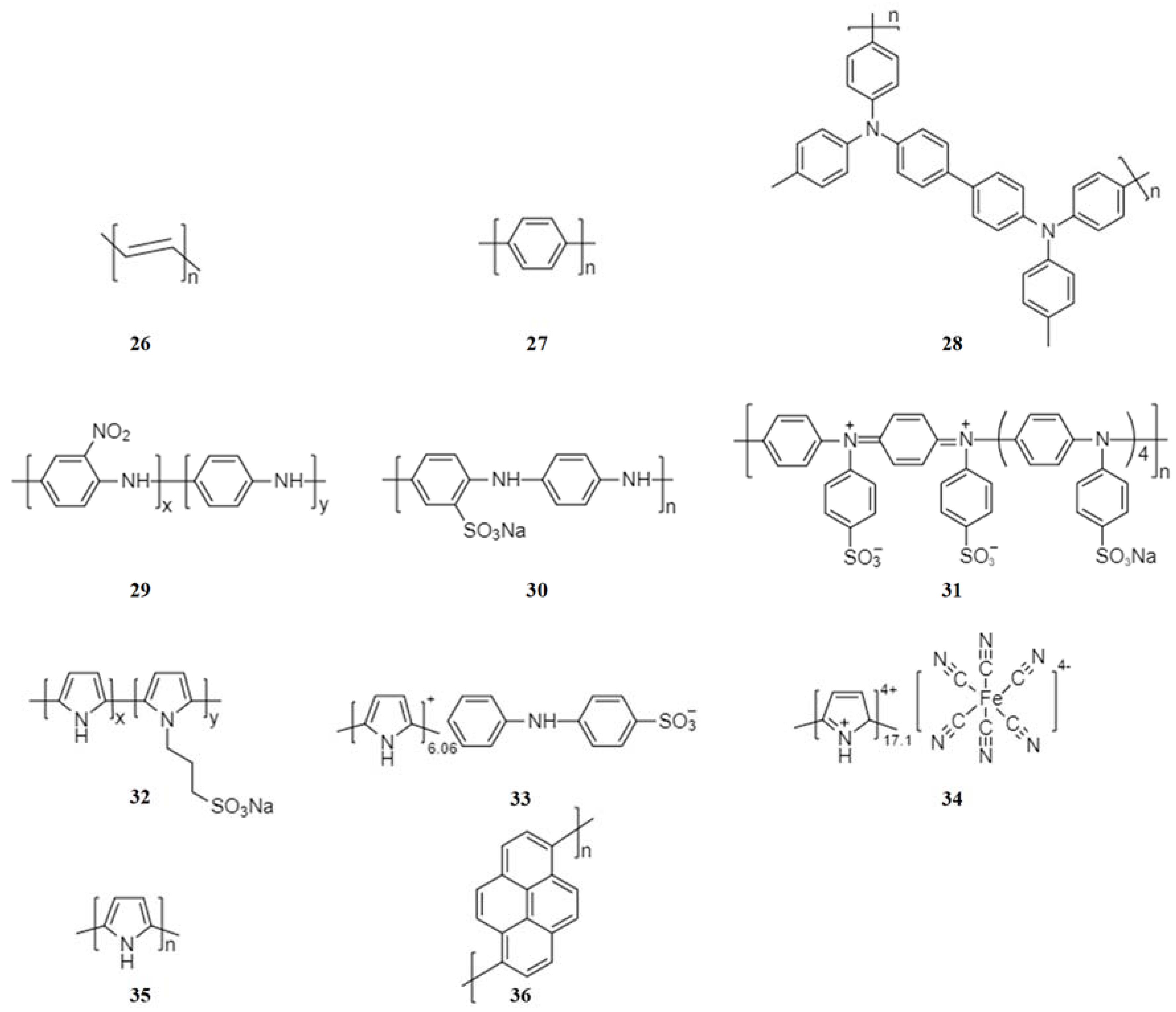
2.3. Conducting Polymer Electrode Materials
2.3.1. Conjugated Conducting Polymers
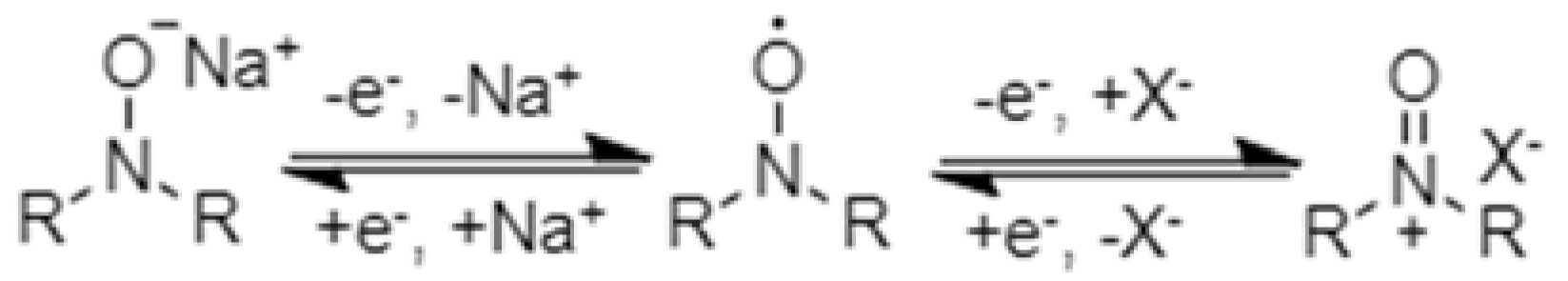
2.3.2. Non-Conjugated Conductive Radical Polymers
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Häupler, B.; Wild, A.; Schubert, U.S. Carbonyls: Powerful Organic Materials for Secondary Batteries. Adv. Energy Mater. 2015, 5, 1402034. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.S.; Chen, L. Room-Temperature Stationary Sodium-Ion Batteries for Large-Scale Electric Energy Storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-Gonzάlez, J.; Rojo, T. Na-Ion Batteries, Recent Advances and Present Challenges to Become Low Cost Energy Storage Systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Grosjean, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of World Lithium Resources and Consequences of Their Geographic Distribution on the Expected Development of the Electric Vehicle Industry. Renew. Sustain. Energy Rev. 2012, 16, 1735–1744. [Google Scholar] [CrossRef]
- Yaksic, A.; Tilton, J.E. Using the Cumulative Availability Curve to Assess the Threat of Mineral Depletion: The Case of Lithium. Resour. Policy 2009, 34, 185–194. [Google Scholar] [CrossRef]
- Tarascon, J.M. Is Lithium the New Gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, K.H.; Oh, S.M.; Lee, K.T. High-Capacity Anode Materials for Sodium-Ion Batteries. Chem.-Eur. J. 2014, 20, 11980–11992. [Google Scholar] [CrossRef]
- Nagelberg, A.S.; Worrell, W.L. A Thermodynamic Study of Sodium-Intercalated TaS2 and TiS2. J. Solid State Chem. 1979, 29, 345–354. [Google Scholar] [CrossRef]
- Braconnier, J.J.; Delmas, C.; Fouassier, C.; Hagenmuller, P. Comportement Electrochimique Des Phases NaxCoO2. Mater. Res. Bull. 1980, 15, 1797–1804. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x < −1): A New Cathode Material for Batteries of High Energy Density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded Graphite as Superior Anode for Sodium-Ion Batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271–1273. [Google Scholar] [CrossRef]
- Balogun, M.S.; Luo, Y.; Qiu, W.; Liu, P.; Tong, Y. A Review of Carbon Materials and Their Composites with Alloy Metals for Sodium Ion Battery Anodes. Carbon 2016, 98, 162–178. [Google Scholar] [CrossRef]
- Górka, J.; Vix-Guterl, C.; Ghimbeu, C.M. Recent Progress in Design of Biomass-Derived Hard Carbons for Sodium Ion Batteries. C 2016, 2, 24. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Wang, L.P.; Yu, L.; Wang, X.; Srinivasan, M.; Xu, Z.J. Recent Developments in Electrode Materials for Sodium-Ion Batteries. J. Mater. Chem. A 2015, 3, 9353–9378. [Google Scholar] [CrossRef]
- Han, M.H.; Gonzalo, E.; Singh, G.; Rojo, T. A Comprehensive Review of Sodium Layered Oxides: Powerful Cathodes for Na-Ion Batteries. Energy Environ. Sci. 2015, 8, 81–102. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent Progress in Electrode Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Wang, P.F.; You, Y.; Yin, Y.X.; Guo, Y.G. Layered Oxide Cathodes for Sodium-Ion Batteries: Phase Transition, Air Stability, and Performance. Adv. Energy Mater. 2018, 8, 1701912. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, Y.; Chen, J. Advanced Organic Electrode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1601792. [Google Scholar] [CrossRef]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L. Na-Ion Battery Anodes: Materials and Electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, X.; Yang, S. Partially Singl-Crystalline Mesoporous Nb2O5 Nanosheets in between Graphene for Ultrafast Sodium Storage. Adv. Mater. 2016, 28, 7672–7679. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, H. Towards Sustainable and Versatile Energy Storage Devices: An Overview of Organic Electrode Materials. Energy Environ. Sci. 2013, 6, 2280–2301. [Google Scholar] [CrossRef]
- Abouimrane, A.; Weng, W.; Eltaye, H.; Cui, Y.; Niklas, J.; Poluektov, O.; Amine, K. Sodium Insertion in Carboxylate based Materials and Their Application in 3.6 V Full Sodium Cells. Energy Environ. Sci. 2012, 5, 9632–9638. [Google Scholar] [CrossRef]
- Emanuelsson, R.; Sterby, M.; Strømme, M.; Sjödin, M. An All-Organic Proton Battery. J. Am. Chem. Soc. 2017, 139, 4828–4834. [Google Scholar] [CrossRef]
- Tarascon, J.M. Key Challenges in Future Li-Battery Research. Phil. Trans. R. Soc. A 2010, 368, 3227–3241. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zhang, F.; Jin, J. Interface Chemistry Guided Long-Cycle-Life Li-S Battery. Nano Lett. 2013, 13, 4206–4211. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Meng, Q.; Yang, Q.; Zhang, M.; Lu, K.; Wei, Z. Large-Area Polyimide/SWCNT Nanocable Cathode for Flexible Lithium-Ion Batteries. Adv. Mater. 2015, 27, 6504–6510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Riduan, S.N. Strategies Toward Improving the Performance of Organic Electrodes in Rechargeable Lithium (Sodium) Batteries. J. Mater. Chem. A 2016, 4, 14902–14914. [Google Scholar] [CrossRef]
- Wilson, D.; Stenzenberger, H.D.; Hergenrother, P.M. Polyimides, 1st ed.; Blackie and Son: Glasgow, Scotland, 1990; pp. 1–2. [Google Scholar]
- Baumgartner, B.; Bojdys, M.J.; Unterlass, M.M. Geomimetics for Green Polymer Synthesis: Highly Ordered Polyimides via Hydrothermal Techniques. Polym. Chem. 2014, 5, 3771–3776. [Google Scholar] [CrossRef]
- Song, Z.; Zhan, H.; Zhou, Y. Polyimides: Promising Energy-Storage Materials. Angew. Chem. 2010, 122, 8622–8626. [Google Scholar] [CrossRef]
- Wang, H.G.; Yuan, S.; Ma, D.L.; Huang, X.L.; Meng, F.L.; Zhang, X.B. Tailored Aromatic Carbonyl Derivative Polyimides for High-Power and Long-Cycle Sodium-Organic Batteries. Adv. Energy Mater. 2014, 4, 1301651. [Google Scholar] [CrossRef]
- Banda, H.; Damien, D.; Nagarajan, K.; Hariharan, M.; Shaijumon, M.M. A Polyimide based All-Organic Sodium Ion Battery. J. Mater. Chem. A 2015, 3, 10453–10458. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Wang, Y.; Wang, C.; Xia, Y. Polyimide as Anode Electrode Material for Rechargeable Sodium Batteries. RSC Adv. 2014, 4, 25369–25373. [Google Scholar] [CrossRef]
- Qin, H.; Song, Z.P.; Zhan, H.; Zhou, Y.H. Aqueous Rechargeable Alkali-Ion Batteries with Polyimide Anode. J. Power Sources 2014, 249, 367–372. [Google Scholar] [CrossRef]
- Zhao, Q.; Gaddam, R.R.; Yang, D.; Strounina, E.; Whittaker, A.K.; Zhao, X.S. Pyromellitic Dianhydride-Based Polyimide Anodes for Sodium-Ion Batteries. Electrochim. Acta 2018, 265, 702–708. [Google Scholar] [CrossRef]
- Xu, F.; Xia, J.; Shi, W.; Cao, S. Sulfonyl-Based Polyimide Cathode for Lithium and Sodium Secondary Batteries: Enhancing the Cycling Performance by the Electrolyte. Mater. Chem. Phys. 2016, 169, 192–197. [Google Scholar] [CrossRef]
- Xu, F.; Xia, J.; Shi, W. Anthraquinone-Based Polyimide Cathodes for Sodium Secondary Batteries. Electrochem. Commun. 2015, 60, 117–120. [Google Scholar] [CrossRef]
- Xu, F.; Wang, H.; Lin, J.; Luo, X.; Cao, S.; Yang, H. Poly(anthraquinonyl imide) as a High Capacity Organic Cathode Material for Na-Ion Batteries. J. Mater. Chem. A 2016, 4, 11491–11497. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Xu, R.; Liu, S.; Wang, Y.; Li, P.; Wu, W.; Wu, M. Synthesis of Three Dimensional Extended Conjugated Polyimide and Application as Sodium-Ion Battery Anode. Chem. Eng. J. 2016, 287, 516–522. [Google Scholar] [CrossRef]
- Deng, W.; Liang, X.; Wu, X.; Qian, J.; Cao, Y.; Ai, X.; Feng, J.; Yang, H. A Low Cost, all-Organic Na-Ion Battery Based on Polymeric Cathode and Anode. Sci. Rep. 2013, 3, 2671. [Google Scholar] [CrossRef]
- Song, Z.; Qian, Y.; Zhang, T.; Otani, M.; Zhou, H. Poly(benzoquinonyl sulfide) as a High-Energy Organic Cathode for Rechargeable Li and Na Batteries. Adv. Sci. 2015, 2, 1500124. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, Z.J.; Wang, H.G.; Bao, D.; Meng, F.L.; Zhang, X.B. A Biodegradable Polydopamine-Derived Electrode Material for High-Capacity and Long-Life Lithium-Ion and Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 10662–10666. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, R.; Nan, J.; Shu, D.; Qiu, Y.; Chou, S.L. Quinone Electrode Materials for Rechargeable Lithium/Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1700278. [Google Scholar] [CrossRef]
- Wu, D.; Huang, Y.; Hu, X. A Sulfurization-Based Oligomeric Sodium Salt as a High-Performance Organic Anode for Sodium Ion Batteries. Chem. Commun. 2016, 52, 11207–11210. [Google Scholar] [CrossRef]
- Tunҫel, M.; Ӧzbülbül, A.; Serı, S. Synthesis and Characterization of Thermally Stable Schiff base Polymers and Their Copper(II), Cobalt(II) and Nickel(II) Complexes. React. Funct. Polym. 2008, 68, 292–306. [Google Scholar] [CrossRef]
- Castillo-Martínez, E.; Carretero-González, J.; Armand, M. Polymeric Schiff Bases as Low-Voltage Redox Centers for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2014, 53, 5341–5345. [Google Scholar] [CrossRef]
- Fernάndez, N.; Sάnchez-Fontecoba, P.; Castillo-Martínez, E.; Carretero-Gonzάlez, J.; Rojo, T.; Armand, M. Polymeric Redox-Active Electrodes for Sodium-Ion Batteries. ChemSusChem 2018, 11, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Gutch, P.K.; Banerjee, S.; Gupta, D.C.; Jaiswal, D.K. Poly-Schiff Bases. V. Synthesis and Characterization of Novel Soluble Fluorine-Containing Polyether Azomethines. J. Polym. Sci. Poly. Chem. 2001, 39, 383–388. [Google Scholar] [CrossRef]
- Li, X.; Jiao, Y.; Li, S. The Syntheses, Properties and Application of New Conducting Polymers. Eur. Polym. J. 1991, 27, 1345–1351. [Google Scholar] [CrossRef]
- Shacklette, L.W.; Toth, T.E.; Murthy, N.S.; Baughman, R.H. Polyacetylene and Polyphenylene as Anode Materials for Nonaqueous Secondary Batteries. J. Electrochem. Soc. 1985, 132, 1529–1535. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, R.; Deng, W.; Ai, X.; Yang, H.; Cao, Y. An All-Solid-State and All-Organic Sodium-Ion Battery based on Redox-Active Polymers and Plastic Crystal Electrolyte. Electrochim. Acta 2015, 178, 55–59. [Google Scholar] [CrossRef]
- Zhou, M.; Li, W.; Gu, T.; Wang, K.; Cheng, S.; Jiang, K. A Sulfonated Polyaniline with High Density and High Rate Na-Storage Performances as a Flexible Organic Cathode for Sodium Ion Batteries. Chem. Commun. 2015, 51, 14354–14356. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.F.; Yuan, D.D.; Ai, X.P.; Yang, H.X.; Zhou, M. Poly(diphenylaminesulfonic acid sodium) as a Cation-Exchanging Organic Cathode for Sodium Batteries. Electrochem. Commun. 2014, 49, 5–8. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, Y.; Sun, M.; Qian, J.; Cao, Y.; Ai, X.; Yang, H. Self-Doped Polypyrrole with Ionizable Sodium Sulfonate as a Renewable Cathode Material for Sodium Ion Batteries. Chem. Commun. 2013, 49, 11370–11372. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Zhao, R.; Zhu, L.; Cao, Y.; Ai, X.; Yang, H.X. An Aniline-Nitroaniline Copolymer as a High Capacity Cathode for Na-Ion Batteries. Electrochem. Commun. 2012, 21, 36–38. [Google Scholar] [CrossRef]
- Zhou, M.; Xiong, Y.; Cao, Y.; Ai, X.; Yang, H. Electroactive Organic Anion-Doped Polypyrrole as a Low Cost and Renewable Cathode for Sodium-Ion Batteries. J. Polym. Sci. Poly. Phys. 2013, 51, 114–118. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, L.; Cao, Y.; Zhao, R.; Qian, J.; Ai, X.; Yang, H. Fe(CN)64−-Doped Polypyrrole: A High-Capacity and High-Rate Cathode Material for Sodium-Ion Batteries. RSC Adv. 2012, 2, 5495–5498. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yan, Z.; Huang, Z.; Zhou, Q.; Guo, G.; Wang, X. The Excellent Cycling Stability and Superior Rate Capability of Polypyrrole as the Anode Material for Rechargeable Sodium Ion Batteries. RSC Adv. 2016, 6, 2345–2351. [Google Scholar] [CrossRef]
- Su, D.; Zhang, J.; Dou, S.; Wang, G. Polypyrrole Hollow Nanospheres: Stable Cathode Materials for Sodium-Ion Batteries. Chem. Commun. 2015, 51, 16092–16095. [Google Scholar] [CrossRef]
- Liu, S.; Wang, F.; Dong, R.; Zhang, T.; Zhang, J.; Zhuang, X.; Mai, Y.; Feng, X. Dual-Template Synthesis of 2D Mesoporous Polypyrrole Nanosheets with Controlled Pore Size. Adv. Mater. 2016, 28, 8365–8370. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Bae, E.G.; Lim, H.; Pyo, M. Non-Crystalline Oligopyrene as a Cathode Material with a High-Voltage Plateau for Sodium Ion Batteries. J. Power Sources 2014, 254, 73–79. [Google Scholar] [CrossRef]
- Nakahara, K.; Iwasa, S.; Satoh, M.; Morioka, Y.; Iriyama, J.; Suguro, M.; Hasegawa, E. Rechargeable Batteries with Organic Radical Cathodes. Chem. Phys. Lett. 2002, 359, 351–354. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, Y.; Gao, L.; Xu, G.; Xie, J. A Sodium Ion Based Organic Radical Battery. Electrochem. Solid State Lett. 2010, 13, A22–A24. [Google Scholar] [CrossRef]
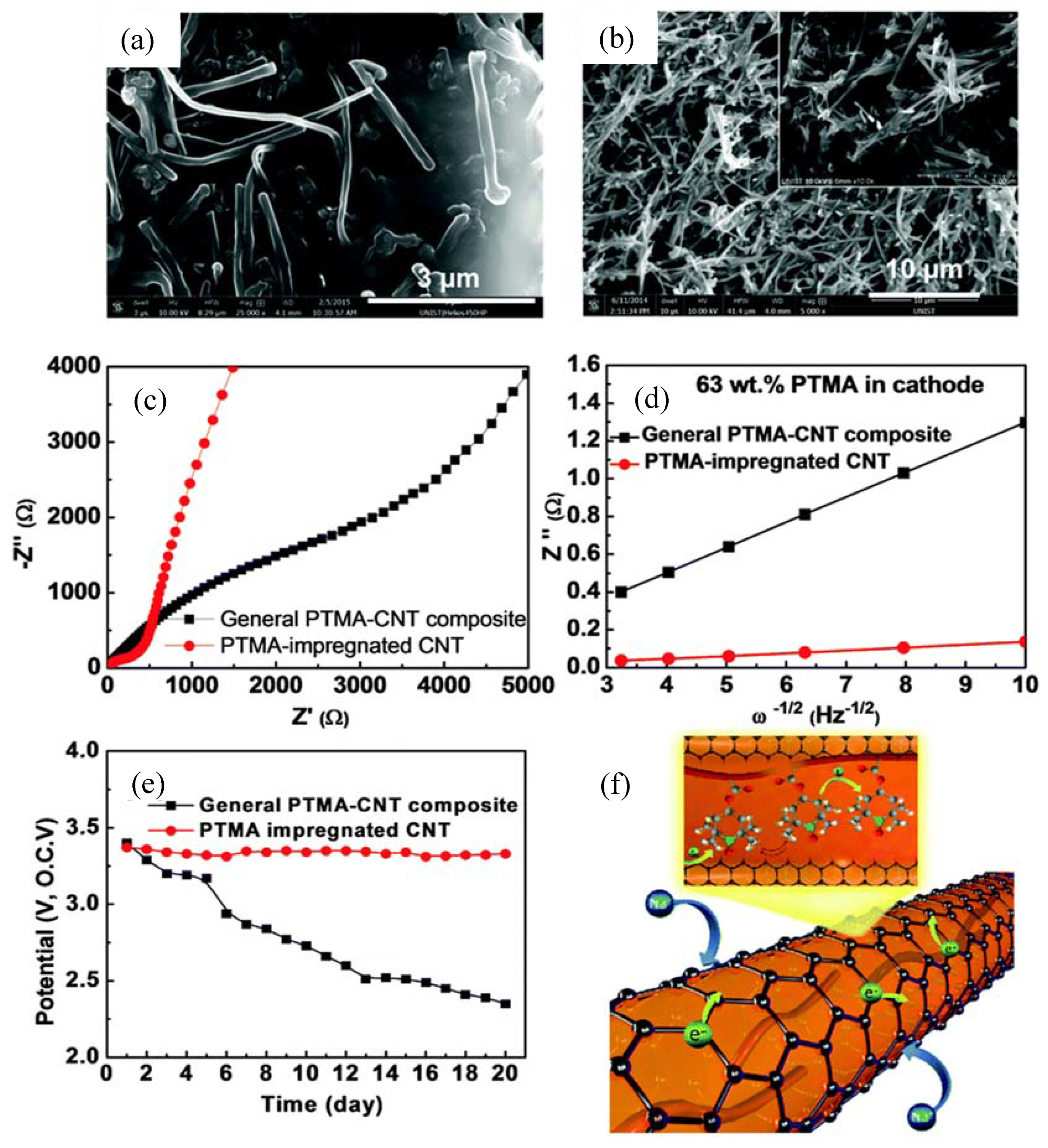
- Kim, J.K.; Kim, Y.; Park, S.; Ko, H.; Kim, Y. Encapsulation of Organic Active Materials in Carbon Nanotubes for Application to High-Electrochemical-Performance Sodium Batteries. Energy Eviron. Sci. 2016, 9, 1264–1269. [Google Scholar] [CrossRef]
- Janoschka, T.; Hager, M.D.; Schubert, U.S. Powering up the Future: Radical Polymers for Battery Applications. Adv. Mater. 2012, 24, 6397–6409. [Google Scholar] [CrossRef] [PubMed]
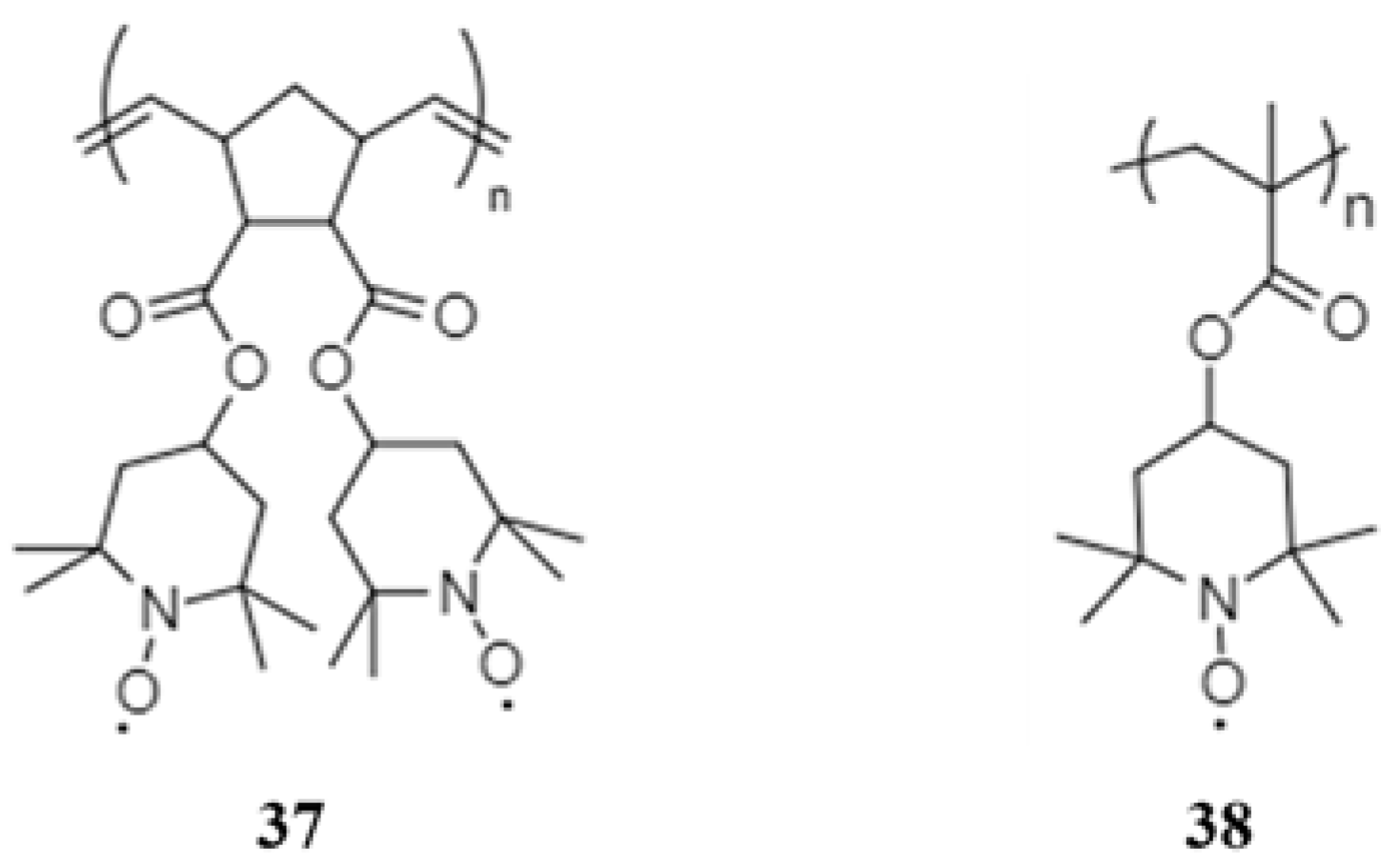
- Bugnon, L.; Morton, C.J.H.; Novak, P.; Vetter, J.; Nesvadba, P. Synthesis of Poly(4-methacryloyloxy-TEMPO) via Group-Transfer Polymerization and Its Evaluation in Organic Radical Battery. Chem. Mater. 2007, 19, 2910–2914. [Google Scholar] [CrossRef]


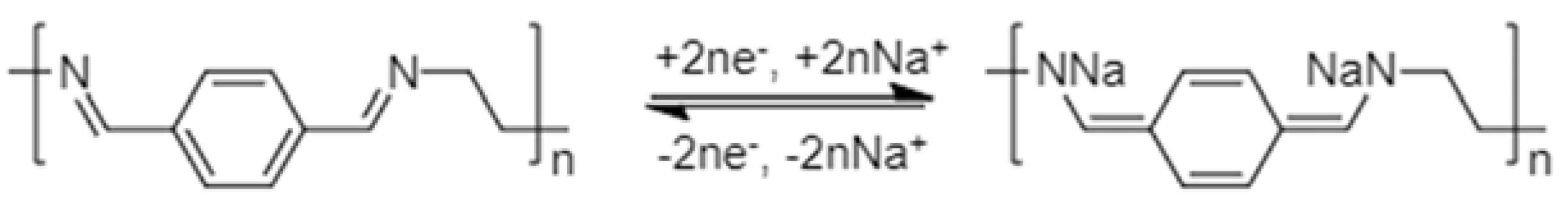
| Electrode Materials | Advantages | Disadvantages | |
|---|---|---|---|
| Inorganic materials | Good conductivity | Potential environmental contamination; Large volume changes; Irreversible phase transitions. | |
| Organic materials | Small organic molecules | Environmental friendliness; Low cost; Structural diversity; Tenability; Flexibility. | High solubility in electrolyte; Poor conductivity. |
| Polymers | The same advantages of small organic molecules with lower solubility. | Potential solubility in electrolyte; Poor conductivity except conducting polymers. | |
| No. | Cathodic/Anodic Peak Potential (V vs. Na/Na+) | The 1st and 2nd Discharge Capacity(mAh·g−1) | Cycling Performance: Discharge Capacity Retention/Cycle/Current Density (mA·g−1) | Rate Capability: Discharge Capacity (mAh·g−1)/Current Density (mA·g−1) | Conductive Additive (wt. %) | Electrolyte * |
|---|---|---|---|---|---|---|
| 1 [37] | 1.90/2.20 | 112, NA | 89%/600/25 | 137.1/50, 38.9/20000 | 30 | 1 M NaPF6 in EC/DMC (1/1) |
| 2 [38] | 1.86/1.97, 2.45/2.75 | 126, 121 | 90%/50/100 | 120/25, 90/800 | 30 | 1 M NaPF6 in PC |
| 3 [39] | 1.80/2.10, 2.25/2.4 | 140, NA | 90%/500/140 | 140/140, 84/2520 | 50 | 1 M NaClO4 in EC/DEC (1/1) |
| 4 [40] | 2.07/2.22, 2.27/2.47 | 184, 170 | 74%/20/50 | NA | 30 | 5 M NaNO3 in H2O |
| 5 [41] | 0.75/1~2, 1.25/1~2 | NA | NA | 125/25, 43/2000 | 30 | 1 M NaClO4 in EC/PC (1/1) plus 0.3 wt% FEC |
| 6 [42] | 1.90/2.30, 2.10/2.80 | 122, NA | 70%/100/50 | NA | 30 | Saturated NaPF6 in DME/DOL (1/1) |
| 7 [43] | 1.45/1.70, 1.80/2.10, 2.10/2.45 | 202, NA | 95%/150/50 | NA | 40 | Saturated NaPF6 in DME/DOL (1/1) |
| 8 [43] | 1.30/1.75, 1.75/2.25 | 179, NA | 92%/150/50 | NA | 40 | Saturated NaPF6 in DME/DOL (1/1) |
| 9 [44] | 1.42/1.78, 1.65/2.0, 2.00/2.25, 2.12/2.40 | 211, NA | 57%/150/50 | NA | 40 | Saturated NaPF6 in DME/DOL (1/1) |
| 10 [44] | 1.52/1.68, 1.85/2.0, 2.15/2.30 | 234, NA | 82%/150/50 | 200/50, 65/1000 | 40 | Saturated NaPF6 in DME/DOL (1/1) |
| 11 [44] | 1.35/1.65, 1.80/2.0, 1.90/2.25 | 242, NA | 62%/150/50 | NA | 40 | Saturated NaPF6 in DME/DOL (1/1) |
| 12 [44] | 1.42/1.58, 1.85/2.15 | 248, NA | 75%/150/50 | NA | 40 | Saturated NaPF6 in DME/DOL (1/1) |
| 13 [45] | 1.00/1.30, 1.76/2.20 | 1161.2, NA | 15%/100/500 | 330.8/100, 102.3/5000 | 30 | 1 M NaClO4 in EC/DEC (1/1) |
| 14 [45] | 0.90/1.30, 1.40/1.90 | NA | NA | 137.0/100, 69.4/5000 | 30 | 1 M NaClO4 in EC/DEC (1/1) |
| 15 [46] | 1.46/1.8, 1.85/2.18 | 185, NA | 100%/200/1600 | 210/400, 160/6000 | 50 | Saturated NaPF6 in DME/DOL (1/1) |
| 16 [47] | 2.08 | 247, NA | 68%/100/50 | NA | 30 | 1 M NaN(CF3SO2)2 in DME/DOL (1/1) |
| 17 [48] | NA | 1081, NA | 47%/1024/50 | 433/100, 122/3200 | 20 | 1 M NaPF6 in EC/DEC (1/1) |
| 18 [50] | 1.02/1.11, 1.38/1.44 | 309, 224.9 | 45%/500/500 | 225/100, 131/1000 | 30 | 1 M NaClO4 in EC/PC (1/1) |
| 19 [52] | 0.47/0.85 | 150, NA | NA | NA | 20 | 1M NaN(SO2F)2 in Me-THF |
| 20 [52] | 0.37/0.79 | NA | NA | NA | 20 | 1 M NaN(SO2F)2 in Me-THF |
| 21 [52] | 0.75/0.95 | 360, NA | 53%/75/26 | NA | 50 | 1 M NaN(SO2F)2 in Me-THF |
| 22 [52] | 0.65/0.79 | NA | NA | NA | 20 | 1 M NaN(SO2F)2 in Me-THF |
| 23 [52] | 0.34/0.87 | NA | NA | NA | 20 | 1 M NaN(SO2F)2 in Me-THF |
| 24 [52] | 0.60/0.87 | NA | NA | NA | 20 | 1 M NaN(SO2F)2 in Me-THF |
| 25 [53] | 0.66, 1.04 | 206, NA | 89%/25/19.7 | NA | 20 | 1 M NaN(SO2F)2 in Me-THF |
| 28 [46] | 3.4/3.7 | 91, NA | 97%/200/500 | 96/200, 88/2000 | 50 | Saturated NaPF6 in DME/DOL (1/1) |
| 29 | NA | 120, NA | 94%/20/50 | NA | 20 | 5 mol% NaClO4 in SCN [57] |
| 2.65/3.15, 3.5/3.75 | 181, NA | 96%/50/50 | 180/50, 165/200 | 20 | 1M NaPF6 in EC/DMC/DEC (1/1/1) [62] | |
| 30 [58] | 2.8/3.0, 3.4/3.6 | 122, NA | 97%/200/100 | 123/100, 76/800 | 10 | 1 M NaPF6 in EC/DEC (1/1) |
| 31 [59] | 3.3/3.5, 3.6/3.7 | 103, NA | 70%/100/50 | 92/100, 43/400 | 40 | 1M NaPF6 in EC/ DEC (1/1) |
| 32 [60] | 3.0/3.3 | 75, NA | 100%/100/40 | NA | 30 | 1M NaPF6 in EC/ DEC (1/1) |
| 33 [63] | 2.5, 3.7 | 115, NA | 82%/50/50 | 105/100, 85/800 | 10 | 1M NaPF6 in EC/ DEC (1/1) |
| 34 [64] | 2.35/2.75 | 120, NA | 92%/100/50 | 135/50, 75/1600 | 10 | 1M NaPF6 in EC/ DEC (1/1) |
| 35 | NA | 471.2, NA | 40%/100/200 | 159.2/1800, 137.7/7200 | 20 | 1 M NaClO4 in PC [65] |
| 2.5/2.8 | 70, NA | 79%/1000/400 | 100/20, 69/320 | 10 | 1 M NaClO4 in EC/PC (1/1) [66] | |
| 2.5/2.7 | 83, NA | 100%/120/300 | 107/100, 75/300 | 15 | 1 M NaPF6 in DEGDME [67] | |
| 36 [68] | 3.54/3.78 | 121, NA | 79%/10/20 | 60/100, 10/500 | 10 | 1 M NaClO4 in PC |
| 37 [70] | 3.38/3.42 | 75, 73 | 65%/50/50 | NA | 60 | 1M NaClO4 in EC/DMC (1/1) |
| 38 [71] | 2.1/2.51, 3.36/3.51 | 217, NA | 92%/100/112.5 | 222/22.5, 190/1125 | 30 | 1M NaClO4 in EC/DEC (1/1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Whittaker, A.K.; Zhao, X.S. Polymer Electrode Materials for Sodium-ion Batteries. Materials 2018, 11, 2567. https://doi.org/10.3390/ma11122567
Zhao Q, Whittaker AK, Zhao XS. Polymer Electrode Materials for Sodium-ion Batteries. Materials. 2018; 11(12):2567. https://doi.org/10.3390/ma11122567
Chicago/Turabian StyleZhao, Qinglan, Andrew K. Whittaker, and X. S. Zhao. 2018. "Polymer Electrode Materials for Sodium-ion Batteries" Materials 11, no. 12: 2567. https://doi.org/10.3390/ma11122567
APA StyleZhao, Q., Whittaker, A. K., & Zhao, X. S. (2018). Polymer Electrode Materials for Sodium-ion Batteries. Materials, 11(12), 2567. https://doi.org/10.3390/ma11122567





