Sensitive Five-Fold Local Symmetry to Kinetic Energy of Depositing Atoms in Cu-Zr Thin Film Growth
Abstract
:1. Introduction
2. Models and Methods
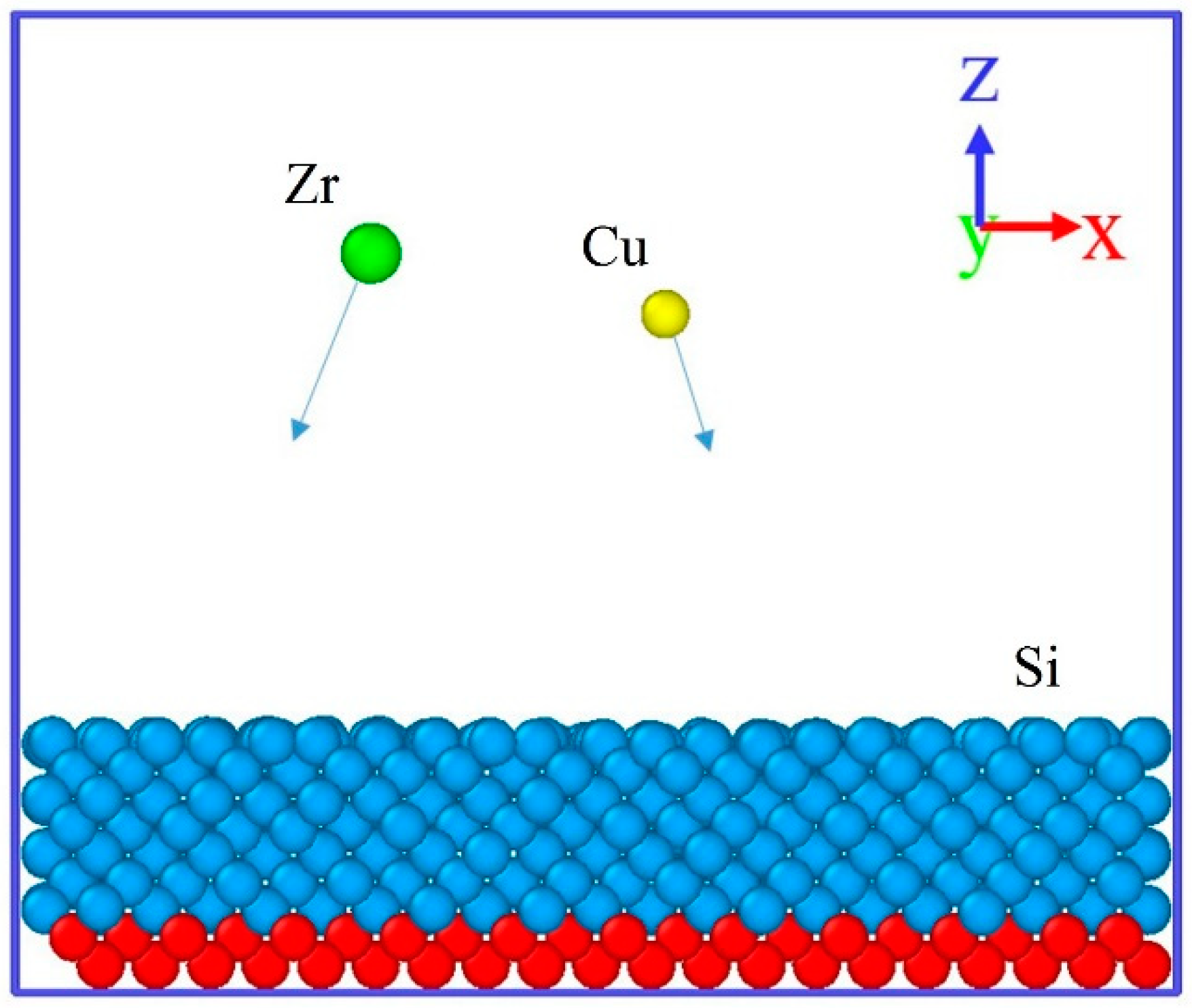
2.1. Modeling
2.2. Simulation Process
3. Results and Discussion
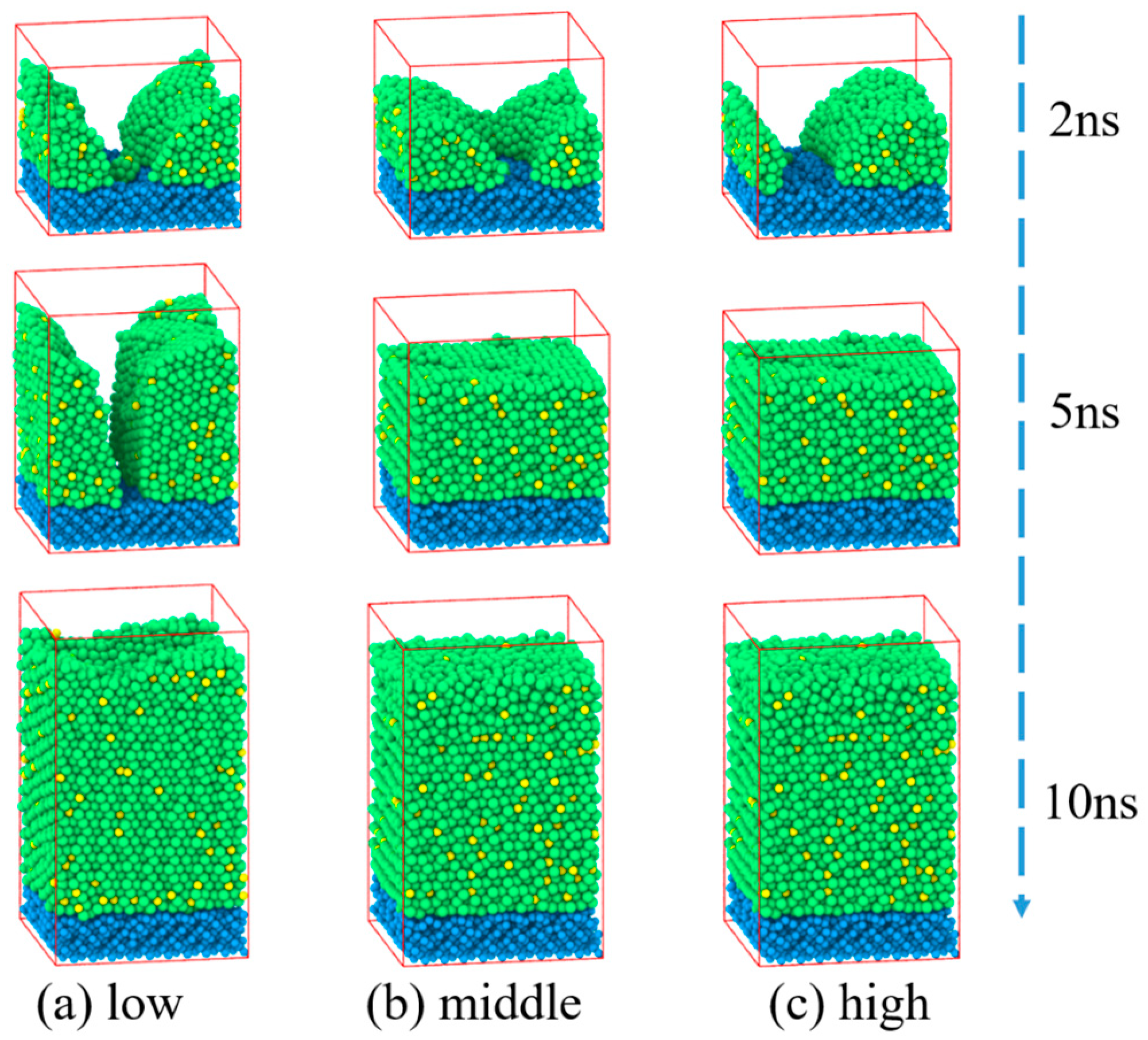
3.1. Zr50Cu50
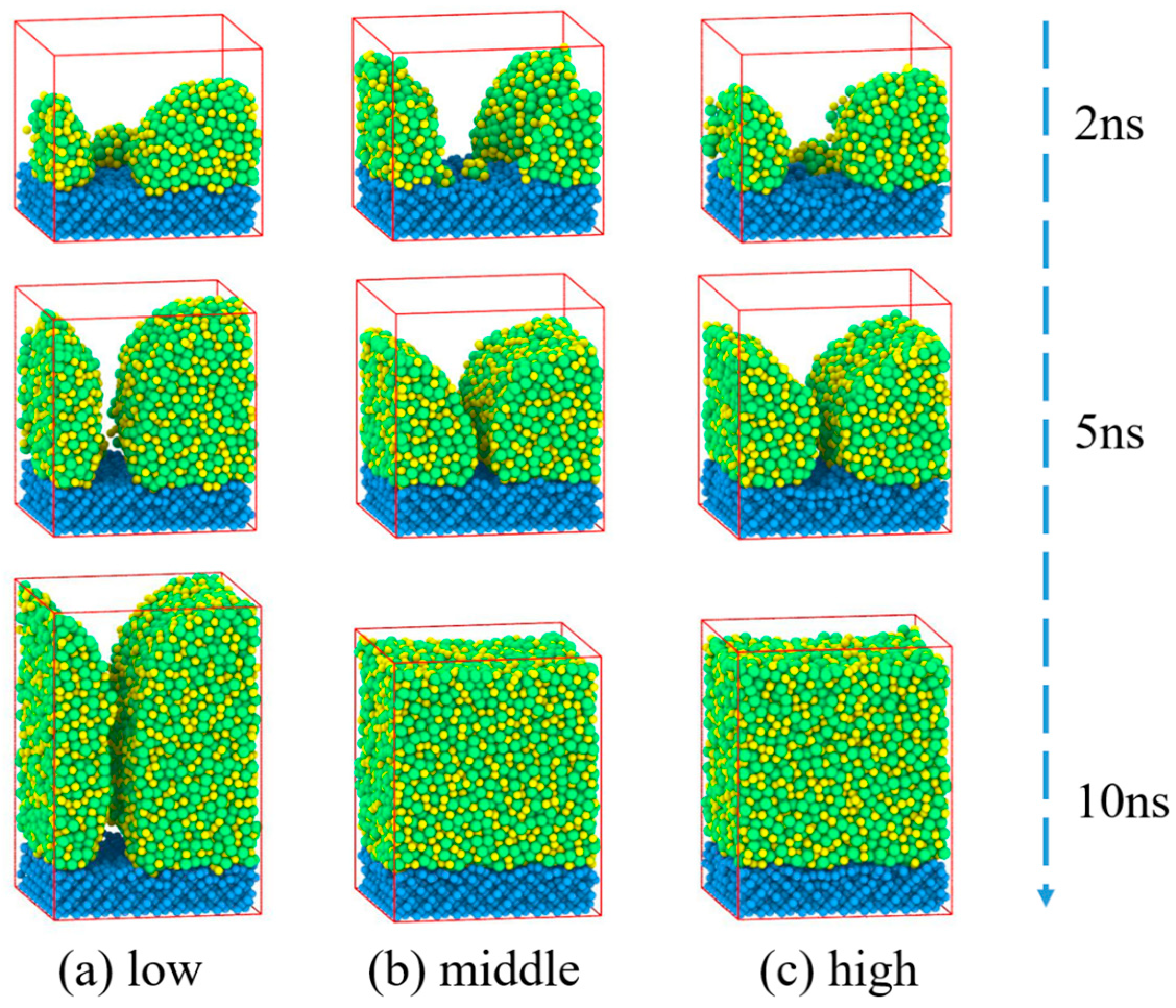
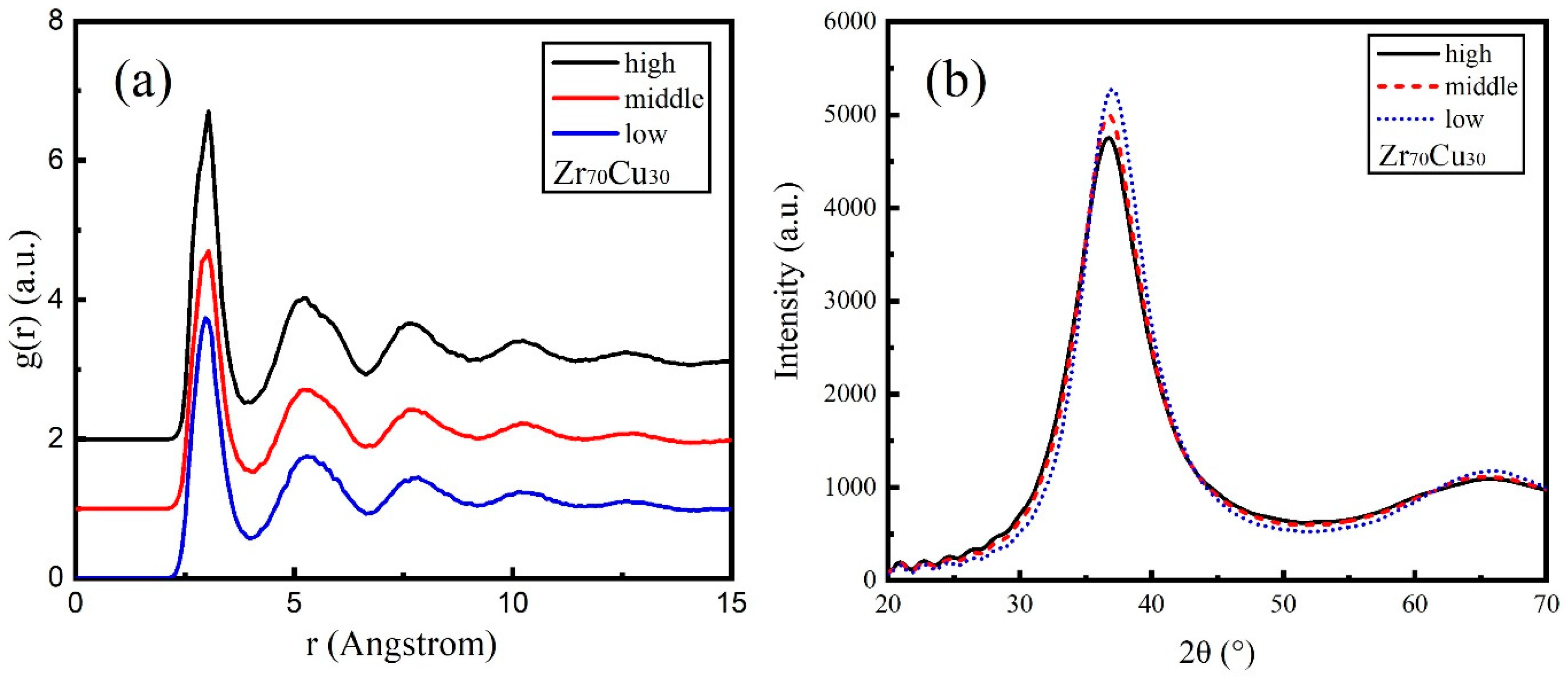
3.2. Zr70Cu30
3.3. Zr90Cu10
3.4. Glass Forming Ability
3.5. Five-Fold Local Symmetry Analysis.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.H. The elastic properties, elastic models and elastic perspectives of metallic glasses. Prog. Mater. Sci. 2012, 57, 487–656. [Google Scholar] [CrossRef]
- Si, J.; Mei, J.; Wang, R.; Chen, X.; Hui, X. Fe-B-Si-Zr bulk metallic glasses with ultrahigh compressive strength and excellent soft magnetic properties. Mater. Lett. 2016, 181, 282–284. [Google Scholar] [CrossRef]
- Niu, B.; Sun, M.H.; Wang, D.; Wang, L.N. Thermoplasticity of Cu45Zr42.55Y3.45Al9 metallic glass. Mater. Heat. Treat 2010, 8, 6–9. [Google Scholar]
- Liao, G.; Long, Z.; Zhao, M.; Zhong, M.; Liu, W.; Chai, W. Serrated flow behavior in a Pd-based bulk metallic glass under nanoindentation. J. Non-Cryst. Solids 2017, 460, 47–53. [Google Scholar] [CrossRef]
- Mattern, N. Structure Formation in Metallic Glasses. Available online: http://www.ww.tu-freiberg.de/mk/bht/Abstracts/mattern.pdf (accessed on 13 December 2018).
- Masumoto, T. Recent progress of amorphous metallic materials. Mater. Sci. Eng. A 1994, 179–180, 8–16. [Google Scholar] [CrossRef]
- Laws, K.J.; Miracle, D.B.; Ferry, M. A predictive structural model for bulk metallic glasses. Nat. Commun. 2015, 6, 8123. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.Q.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Zeman, P.; Zítek, M.; Zuzjaková, Š.; Čerstvý, R. Amorphous Zr-Cu thin-film alloys with metallic glass behavior. J. Alloys Compd. 2017, 696, 1298–1306. [Google Scholar] [CrossRef]
- Musil, J.; Daniel, R. Structure and mechanical properties of magnetron sputtered Zr–Ti–Cu–N films. Surf. Coat. Technol. 2003, 166, 243–253. [Google Scholar] [CrossRef]
- Eckert, J.; Das, J.; Kim, K.B.; Baier, F.; Tang, M.B.; Wang, W.H.; Zhang, Z.F. High strength ductile Cu-base metallic glass. Intermetallics 2006, 14, 876–881. [Google Scholar] [CrossRef]
- Xu, D.; Lohwongwatana, B.; Duan, G.; Johnson, W.; Garland, C. Bulk metallic glass formation in binary Cu-rich alloy series—Cu100−xZrx (x= 34, 36, 38.2, 40 at %) and mechanical properties of bulk Cu64Zr36 glass. Acta Mater. 2004, 52, 2621–2624. [Google Scholar] [CrossRef]
- Karpe, N.; Bøttiger, J.; Krog, J.P.; Nordström, A.; Rapp, Ö. Influence of deposition conditions and ion irradiation on thin films of amorphous Cu-Zr superconductors. Thin Solid Films 1996, 275, 82–86. [Google Scholar] [CrossRef]
- Dudonis, J.; Bručas, R.; Miniotas, A. Synthesis of amorphous Zr-Cu alloys by magnetron co-sputtering. Thin Solid Films 1996, 275, 164–167. [Google Scholar] [CrossRef]
- Apreutesei, M.; Djemia, P.; Belliard, L.; Abadias, G.; Esnouf, C.; Billard, A.; Steyer, P. Structural-elastic relationships of Zr-TL (TL = Cu, Co, Ni) thin films metallic glasses. J. Alloys Compd. 2017, 707, 126–131. [Google Scholar] [CrossRef]
- Aji, D.P.B.; Hirata, A.; Zhu, F.; Pan, L.; Reddy, K.M.; Song, S.; Liu, Y.; Fujita, T.; Kohara, S.; Chen, M. Ultrastrong and Ultrastable Metallic Glass. Available online: https://arxiv.org/ftp/arxiv/papers/1306/1306.1575.pdf (accessed on 13 December 2018).
- Xu, B.; Falk, M.L.; Li, J.F.; Kong, L.T. Predicting Shear Transformation Events in Metallic Glasses. Phys. Rev. Lett. 2018, 120, 125503. [Google Scholar] [CrossRef]
- Peng, Q.; Meng, F.; Yang, Y.; Lu, C.; Deng, H.; Wang, L.; De, S.; Gao, F. Shockwave generates <100> dislocation loops in bcc iron. Nat. Commun. 2018, 9, 4880. [Google Scholar] [CrossRef] [PubMed]
- Utz, M.; Peng, Q.; Nandagopal, M. Athermal simulation of plastic deformation in amorphous solids at constant pressure. J. Polym. Sci. B Polym. Phys. 2004, 42, 2057–2065. [Google Scholar]
- Sha, Z.D.; Zhang, Y.W.; Feng, Y.P.; Li, Y. Molecular dynamics studies of short to medium range order in Cu64Zr36 metallic glass. J. Alloys Compd. 2011, 509, 8319–8322. [Google Scholar] [CrossRef]
- Wu, T.W.; Feng, S.D.; Qi, L.; Gao, W.; Ma, M.Z.; Zhang, X.Y.; Li, G.; Jing, Q.; Liu, R.P. The compressive behaviour after crystallisation in Zr 85 Cu 15 metallic glasses studied by molecular dynamics simulations. J. Non-Cryst. Solids 2017, 468, 41–45. [Google Scholar] [CrossRef]
- Yang, G.J.; Xu, B.; Kong, L.T.; Li, J.F.; Zhao, S. Size effects in Cu 50 Zr 50 metallic glass films revealed by molecular dynamics simulations. J. Alloys Compd. 2016, 688, 88–95. [Google Scholar] [CrossRef]
- Xie, L.; Brault, P.; Bauchire, J.-M.; Thomann, A.-L.; Bedra, L. Molecular dynamics simulations of clusters and thin film growth in the context of plasma sputtering deposition. J. Phys. D Appl. Phys. 2014, 47, 224004. [Google Scholar] [CrossRef]
- Sha, Z.; Feng, Y.; Li, Y. Statistical composition-structure-property correlation and glass-forming ability based on the full icosahedra in Cu–Zr metallic glasses. Appl. Phys. Lett. 2010, 96, 061903. [Google Scholar] [CrossRef]
- Hajlaoui, K.; Alsaleh, N.; Alrasheedi, N.H.; Yavari, A.R. Coalescence and subsequent twinning of nanocrystals during deformation of CuZr-based metallic glasses: The grain size effect. J. Non-Cryst. Solids 2017, 464, 39–43. [Google Scholar] [CrossRef]
- Almyras, G.A.; Lekka, C.E.; Mattern, N.; Evangelakis, G.A. On the microstructure of the Cu65Zr35 and Cu35Zr65 metallic glasses. Scr. Mater. 2010, 62, 33–36. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Chattopadhyay, P.P.; Ganguly, S. A predictable glass forming ability expression by statistical learning and evolutionary intelligence. Intermetallics 2017, 90, 9–15. [Google Scholar] [CrossRef]
- Turchanin, A.A.; Tomilin, I.A.; Turchanin, M.A.; Belokonenko, I.V.; Agraval, P.G. Enthalpies of formation of liquid and amorphous Cu–Zr alloys. J. Non-Cryst. Solids 1999, 250–252, 582–585. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Hu, Y.C.; Li, F.X.; Li, M.Z.; Bai, H.Y.; Wang, W.H. Five-fold symmetry as indicator of dynamic arrest in metallic glass-forming liquids. Nat. Commun. 2015, 6, 8310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lü, Y.J.; Bi, Q.L.; Huang, H.S.; Pang, H.H. Role of fivefold symmetry in the dynamical slowing down of metallic glass-forming liquids. Phys. Rev. B 2017, 96, 064301. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Daw, M.S.; Baskes, M.I. Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals. Phys. Rev. B 1984, 29, 6443–6453. [Google Scholar] [CrossRef]
- Graves, D.B.; Brault, P. Molecular dynamics for low temperature plasma–surface interaction studies. J. Phys. D Appl. Phys. 2009, 42, 194011. [Google Scholar] [CrossRef] [Green Version]
- Zhen, S.; Davies, G.J. Calculation of the Lennard-Jones n–m potential energy parameters for metals. Phys. Status Solid 1983, 78, 595–605. [Google Scholar] [CrossRef]
- Xie, L.; Brault, P.; Thomann, A.-L.; Bedra, L. Molecular dynamic simulation of binary ZrxCu100−x metallic glass thin film growth. Appl. Surf. Sci. 2013, 274, 164–170. [Google Scholar] [CrossRef]
- Finney, J.L. Modelling the structures of amorphous metals and alloys. Nature 1977, 266, 309–314. [Google Scholar] [CrossRef]
- Wu, Z.W.; Li, M.Z.; Wang, W.H.; Liu, K.X. Hidden topological order and its correlation with glass-forming ability in metallic glasses. Nat. Commun. 2015, 6, 6035. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Xu, Y.; Lu, Z.P.; Hui, X.; Chen, G.L.; Zheng, G.P.; Liu, C.T. Atomic packing symmetry in the metallic liquid and glass states. Acta Mater. 2011, 59, 6480–6488. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Li, M.Z.; Peng, H.L.; Hu, Y.C.; Li, F.X.; Zhang, H.P.; Wang, W.H. Five-fold local symmetry in metallic liquids and glasses. Chin. Phys. B 2017, 26, 016104. [Google Scholar] [CrossRef]
- Spaepen, F. Condensed-matter science: Five-fold symmetry in liquids. Nature 2000, 408, 781–782. [Google Scholar] [CrossRef] [PubMed]


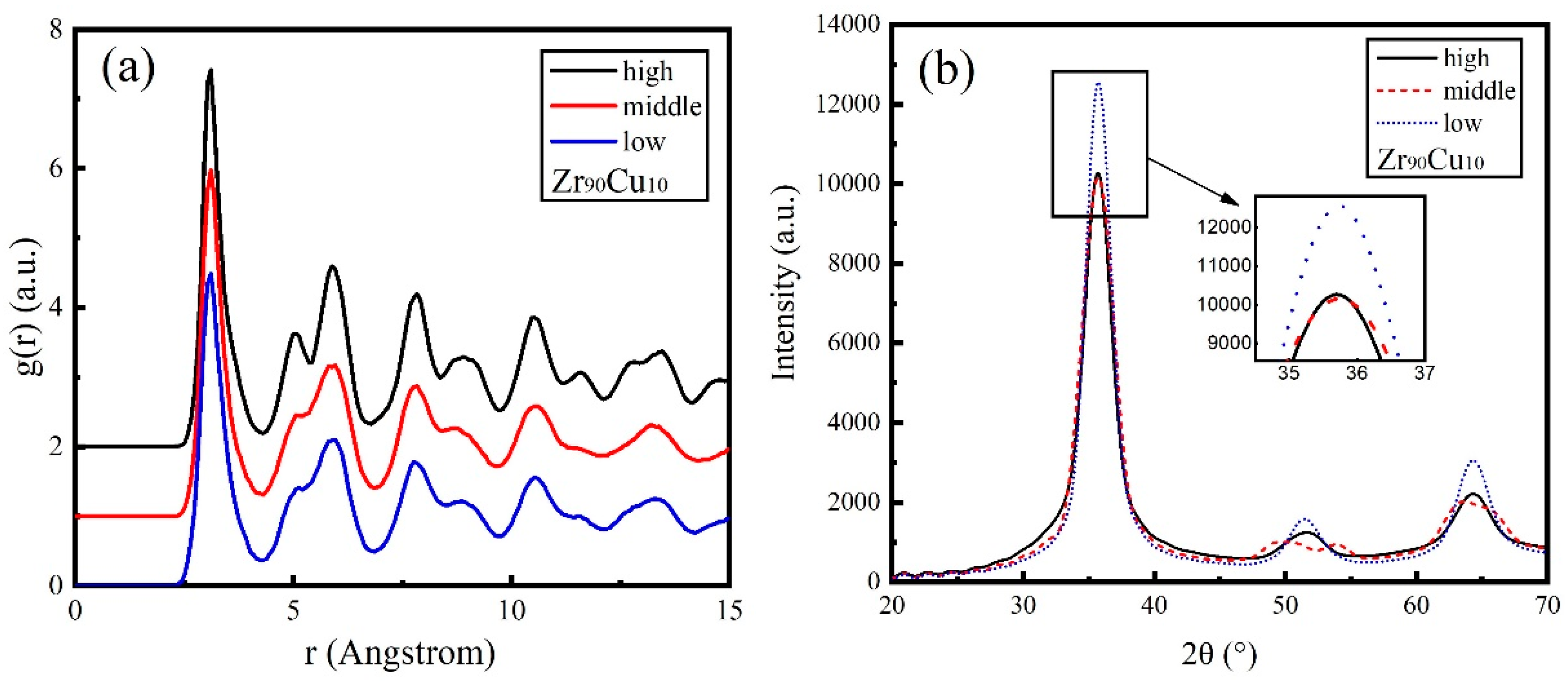
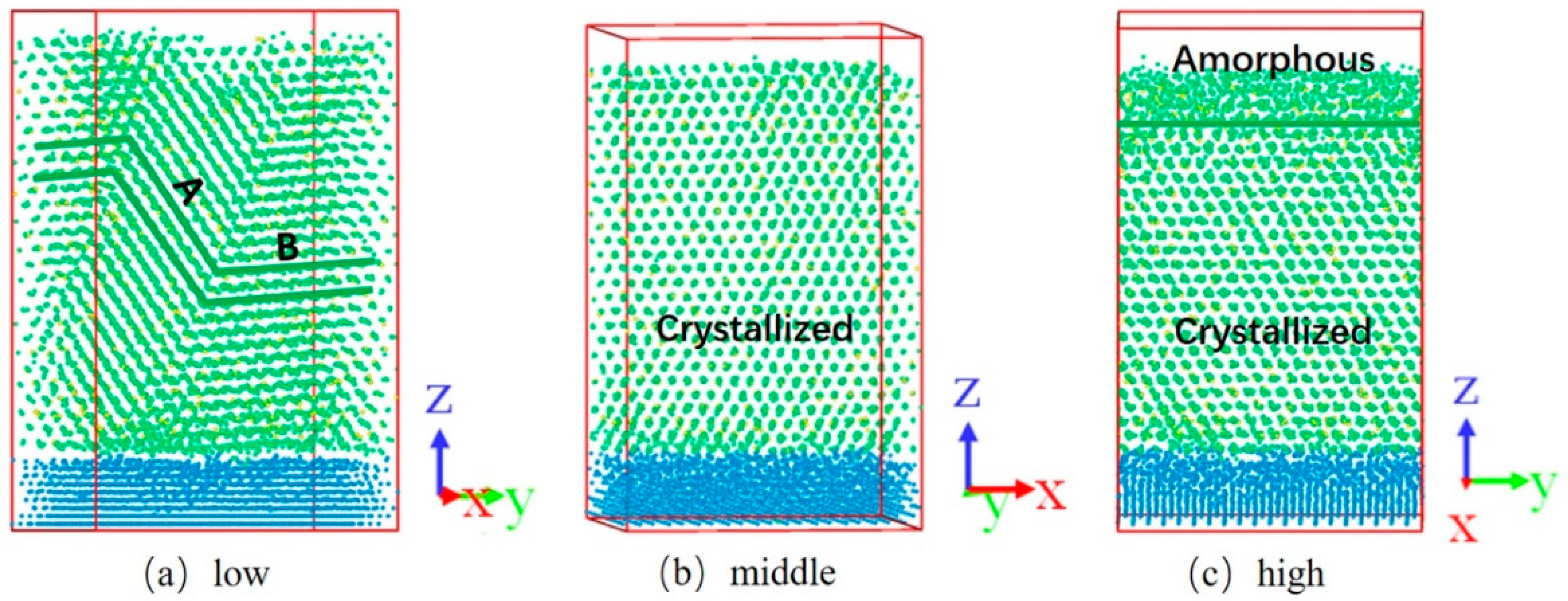
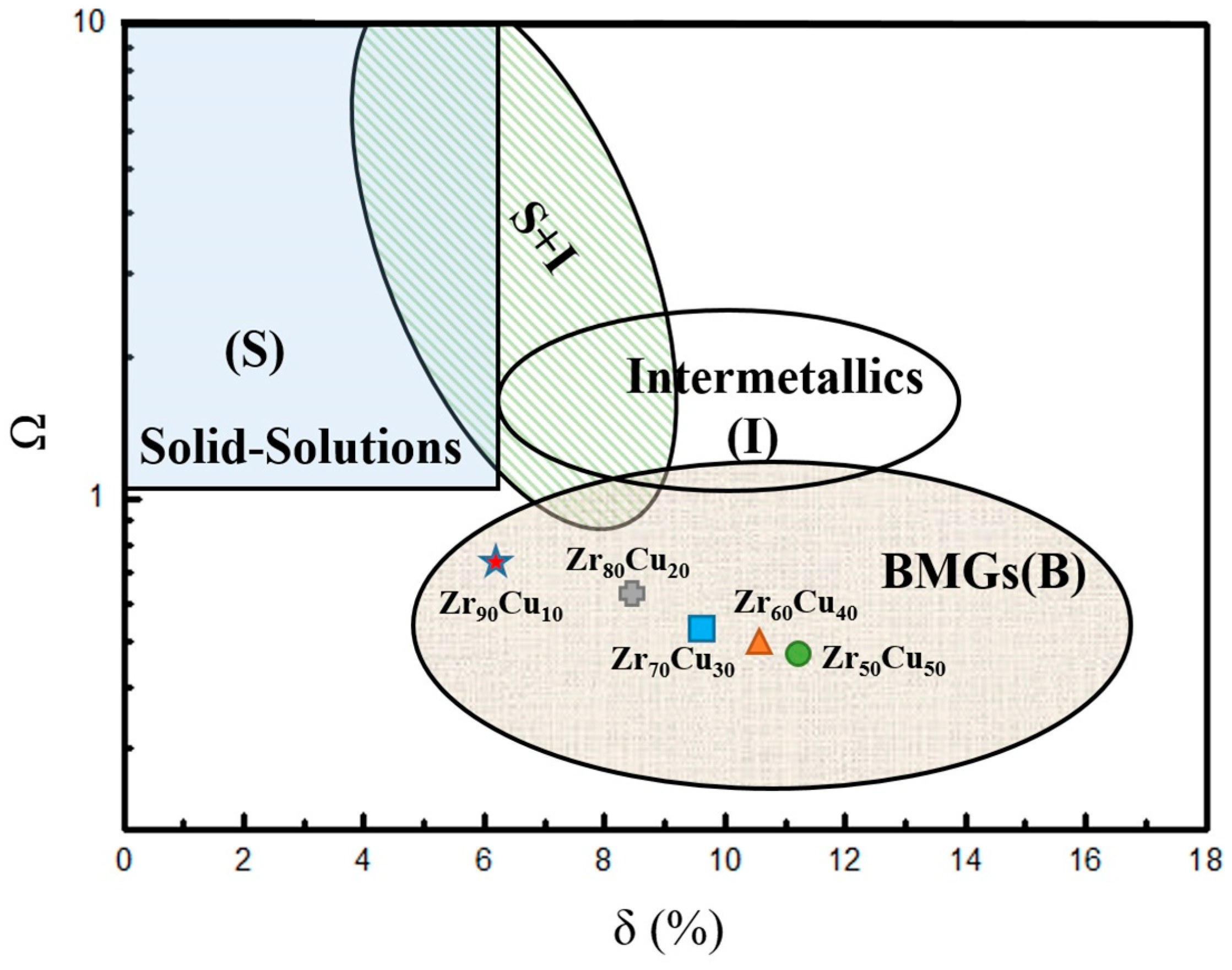
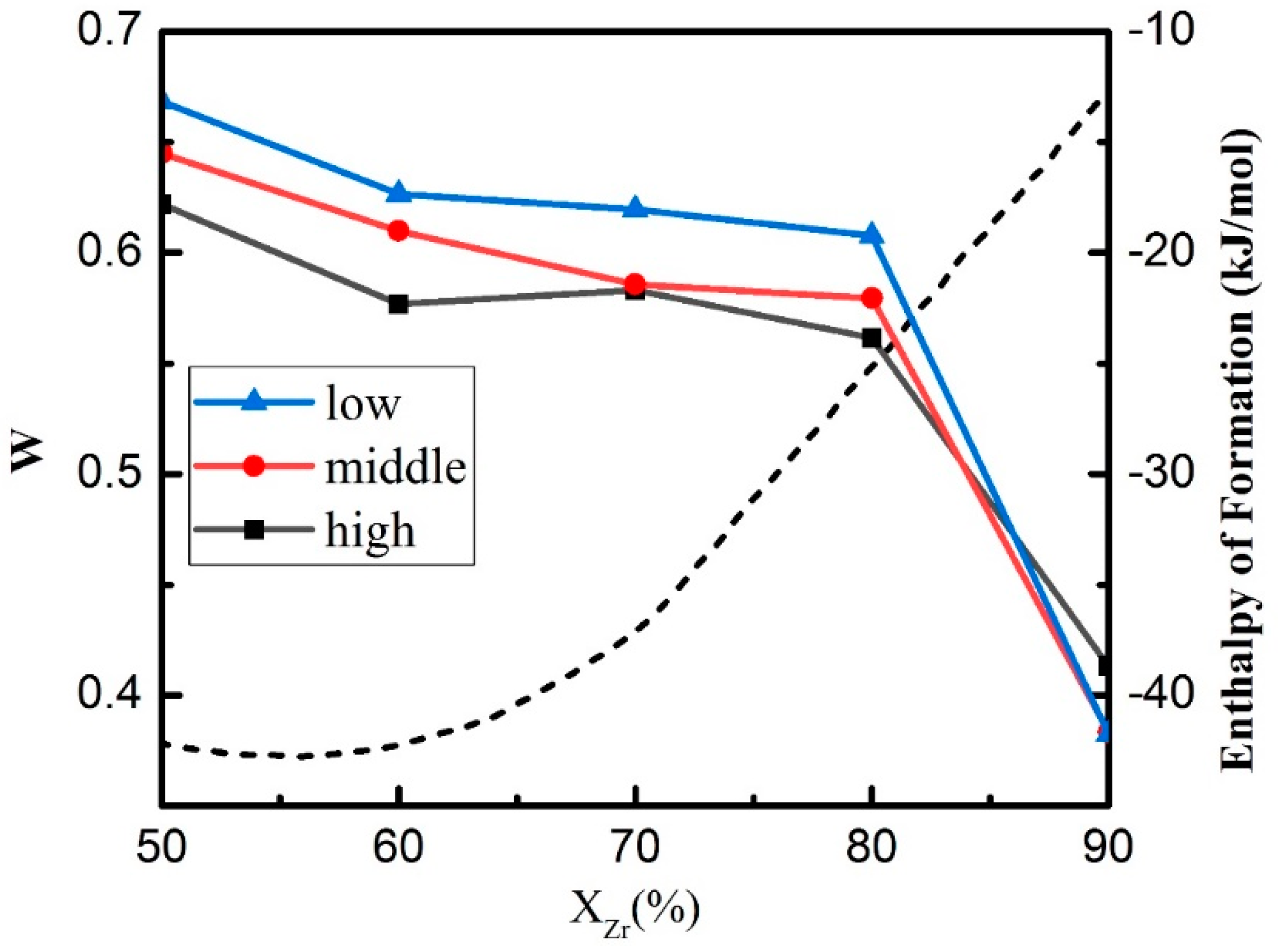
| Sample of Mean Kinetic Energy | Zr (eV) | Cu (eV) |
|---|---|---|
| (a) Low energy group | 0.13 | 0.34 |
| (b) Middle energy group | 7.65 | 6.67 |
| (c) High energy group | 12.6 | 9.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; An, H.; Peng, Q.; Qin, Q.; Zhang, Y. Sensitive Five-Fold Local Symmetry to Kinetic Energy of Depositing Atoms in Cu-Zr Thin Film Growth. Materials 2018, 11, 2548. https://doi.org/10.3390/ma11122548
Xie L, An H, Peng Q, Qin Q, Zhang Y. Sensitive Five-Fold Local Symmetry to Kinetic Energy of Depositing Atoms in Cu-Zr Thin Film Growth. Materials. 2018; 11(12):2548. https://doi.org/10.3390/ma11122548
Chicago/Turabian StyleXie, Lu, Haojie An, Qing Peng, Qin Qin, and Yong Zhang. 2018. "Sensitive Five-Fold Local Symmetry to Kinetic Energy of Depositing Atoms in Cu-Zr Thin Film Growth" Materials 11, no. 12: 2548. https://doi.org/10.3390/ma11122548
APA StyleXie, L., An, H., Peng, Q., Qin, Q., & Zhang, Y. (2018). Sensitive Five-Fold Local Symmetry to Kinetic Energy of Depositing Atoms in Cu-Zr Thin Film Growth. Materials, 11(12), 2548. https://doi.org/10.3390/ma11122548







