Single Crystalline Iron Silicide and Beta-Iron Disilicide Nanowires Formed through Chemical Vapor Deposition
Abstract
1. Introduction
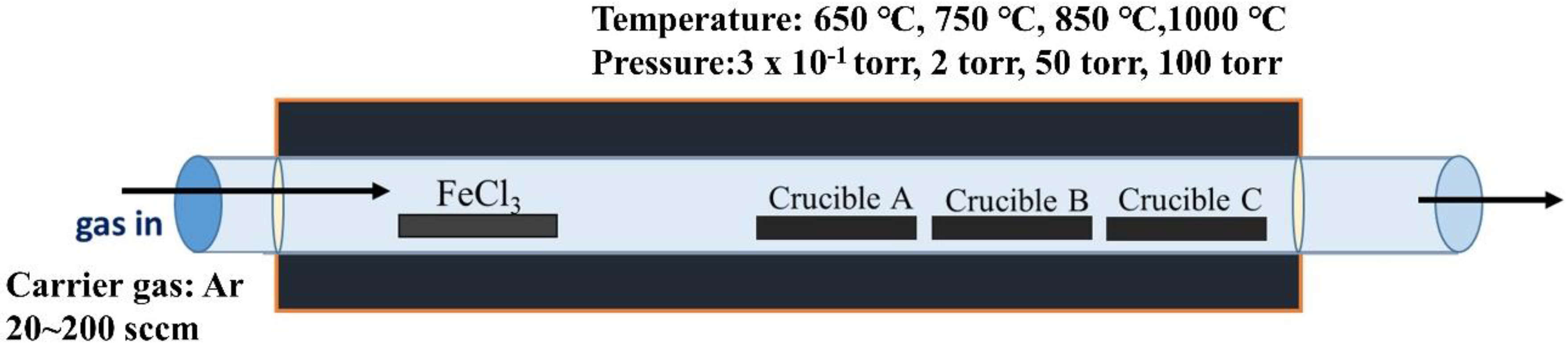
2. Materials and Methods
3. Results and Discussion
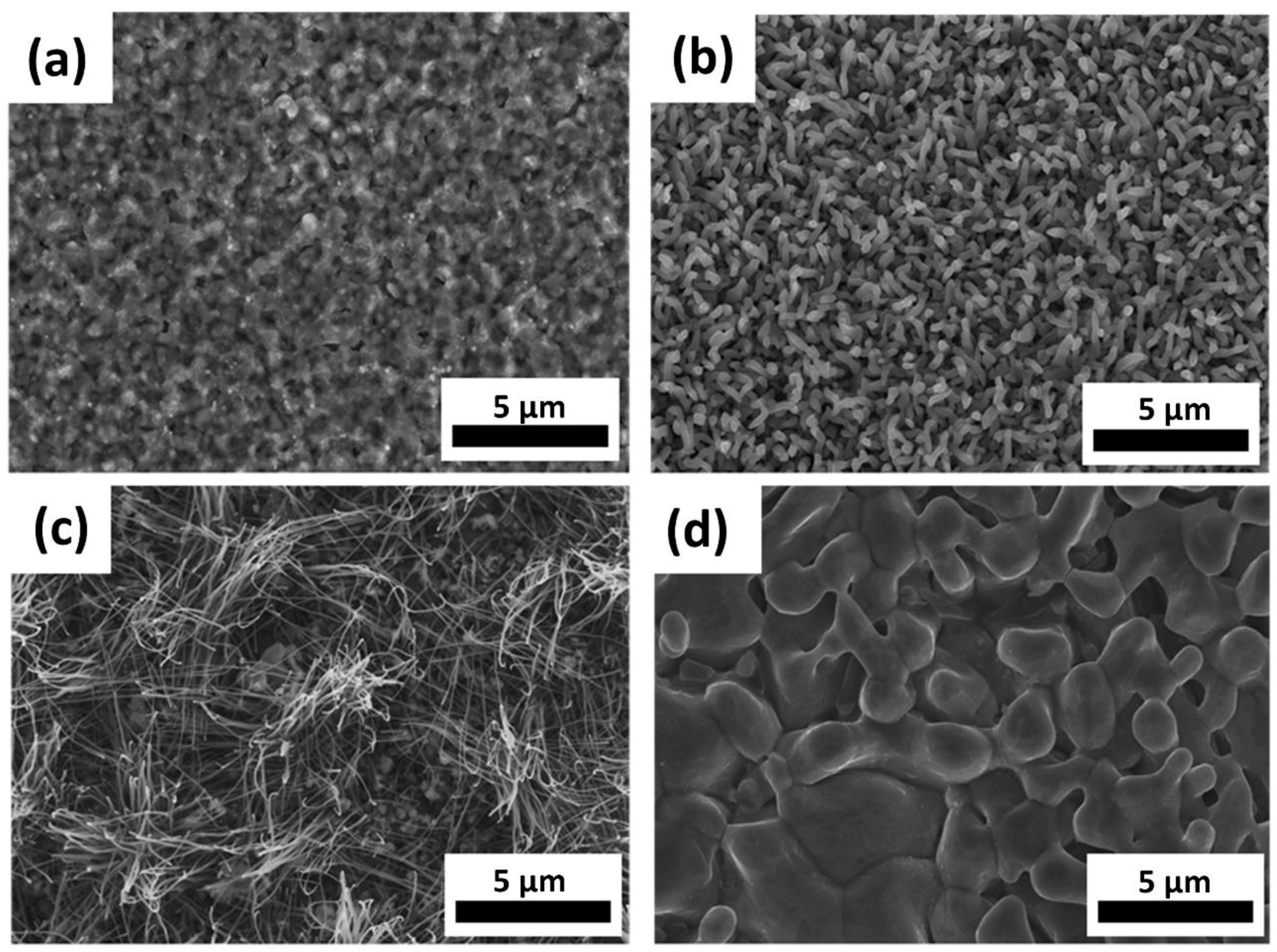
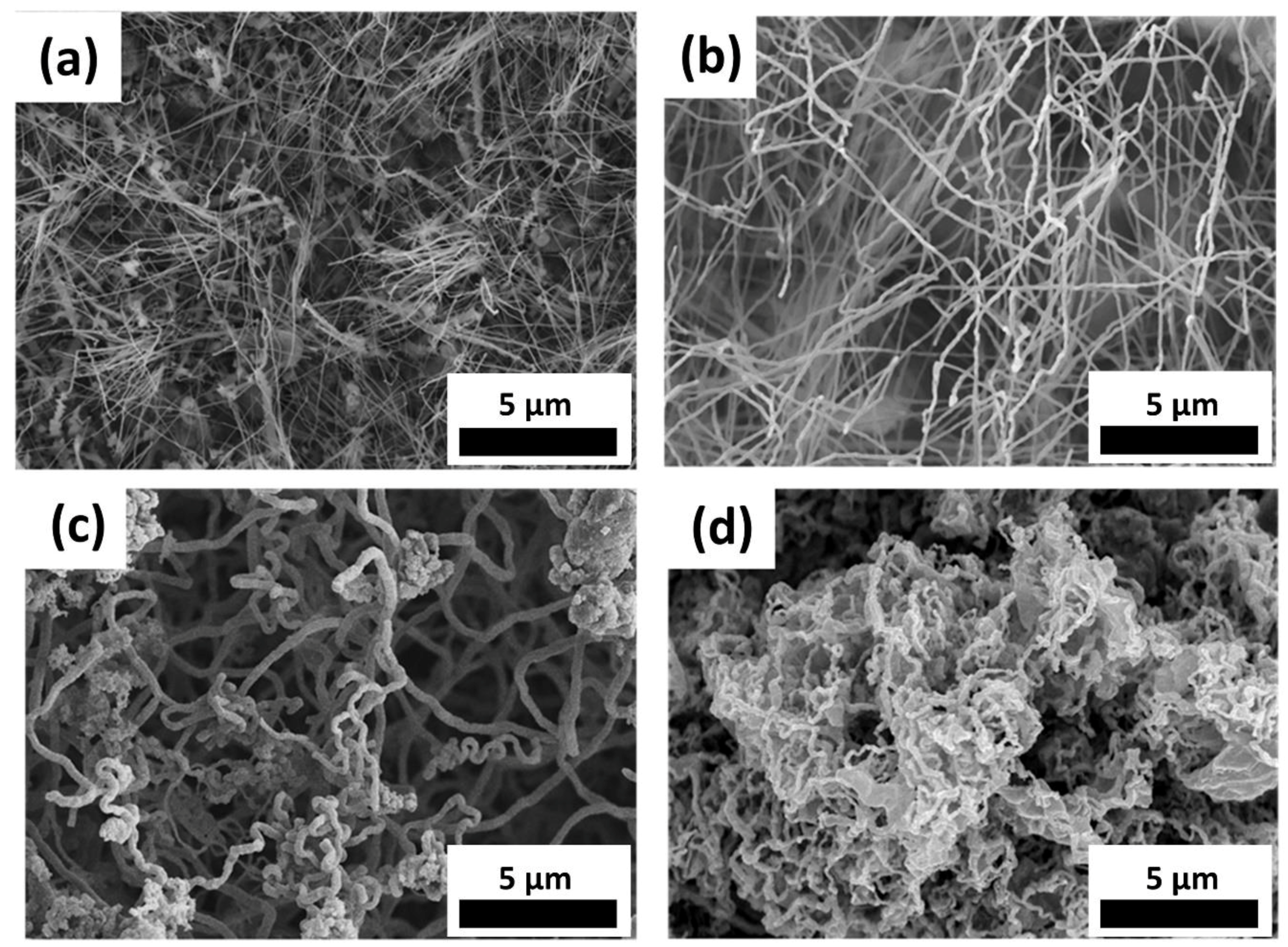
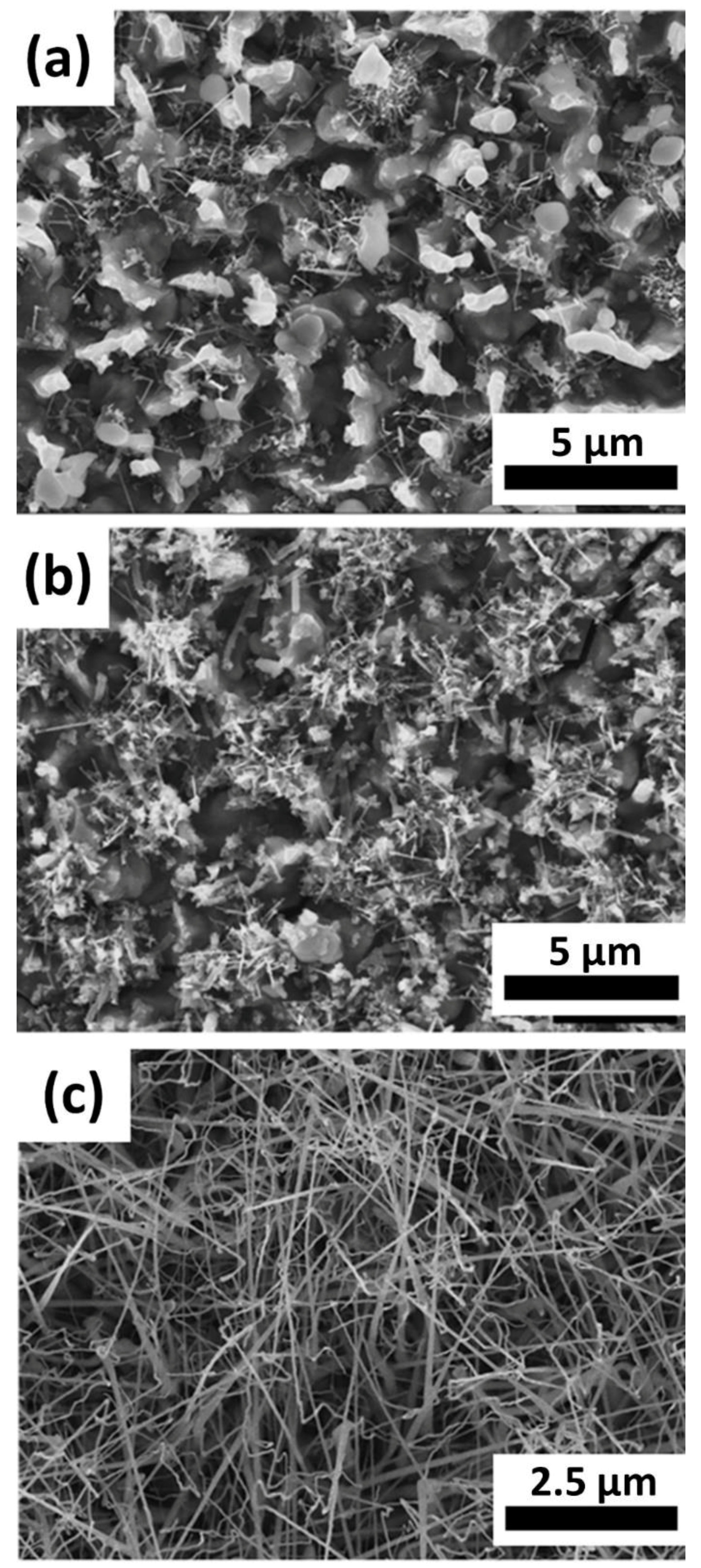
3.1. Morphology
3.1.1. Temperature
3.1.2. Pressure and Flow Rate
3.2. Structure Analysis
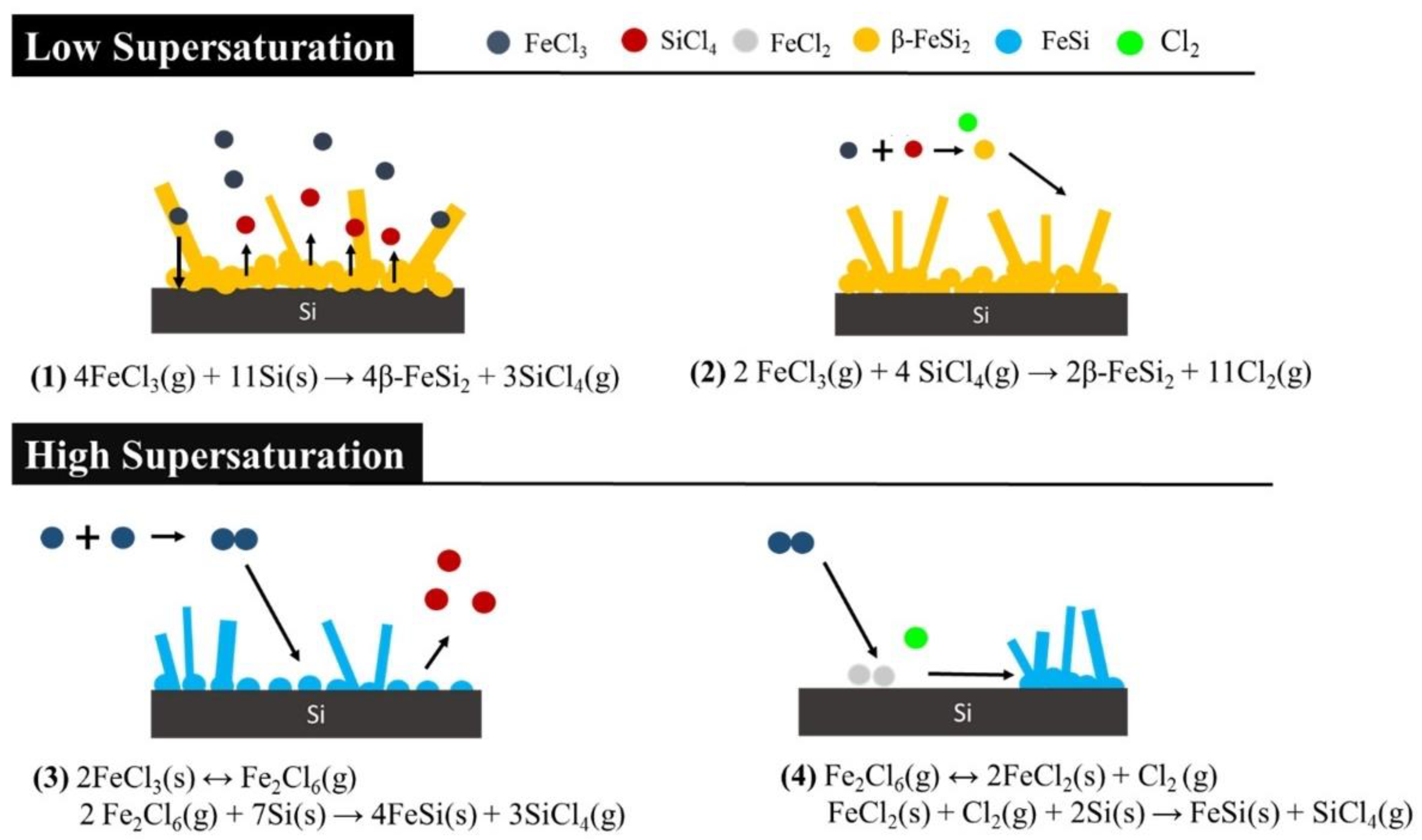
3.3. Growth Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pham, C.V.; Repp, S.; Thomann, R.; Krueger, M.; Weber, S.; Erdem, E. Charge transfer and surface defect healing within ZnO nanoparticle decorated graphene hybrid materials. Nanoscale 2016, 8, 9682–9687. [Google Scholar] [CrossRef] [PubMed]
- Erdem, E.; Kiraz, K.; Somer, M.; Eichel, R.A. Size effects in Fe3+-doped PbTiO3 nanocrystals—Formation and orientation of (FeTi′VO) defect-dipoles. J. Eur. Ceram. Soc. 2010, 30, 289–293. [Google Scholar] [CrossRef]
- Park, S.; Lim, S.; Choi, H. Chemical Vapor Deposition of Iron and Iron Oxide Thin Films from Fe(II) Dihydride Complexes. Chem. Mater. 2006, 18, 5150–5152. [Google Scholar] [CrossRef]
- Lu, C.M.; Hsu, H.F.; Lu, K.C. Growth of single-crystalline cobalt silicide nanowires and their field emission property. Nanoscale Res. Lett. 2013, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.L.; Chiu, C.H.; Chen, J.Y.; Huang, C.W.; Huang, Y.T.; Lu, K.C.; Hsin, C.L.; Yeh, P.H.; Wu, W.W. Single-crystalline delta-Ni2Si nanowires with excellent physical properties. Nanoscale Res. Lett. 2013, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Hsin, C.L.; Huang, C.W.; Yu, S.Y.; Wang, C.W.; Lu, C.M.; Lu, K.C.; Wu, W.W. Single-crystalline Ge nanowires and Cu3Ge/Ge nano-heterostructures. CrystEngComm 2012, 14, 4570–4574. [Google Scholar] [CrossRef]
- Lu, K.C.; Wu, W.W.; Ouyang, H.; Lin, Y.C.; Huang, Y.; Wang, C.W.; Wu, Z.W.; Huang, C.W.; Chen, L.J.; Tu, K.N. The influence of surface oxide on the growth of metal/semiconductor nanowires. Nano Lett. 2011, 11, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.W.; Lu, K.C.; Chen, K.N.; Yeh, P.H.; Wang, C.W.; Lin, Y.C.; Huang, Y. Controlled large strain of Ni silicide/Si/Ni silicide nanowire heterostructures and their electron transport properties. Appl. Phys. Lett. 2010, 97, 203110. [Google Scholar] [CrossRef]
- Wu, W.W.; Lu, K.C.; Wang, C.W.; Hsieh, H.Y.; Chen, S.Y.; Chou, Y.C.; Yu, S.Y.; Chen, L.J.; Tu, K.N. Growth of multiple metal/semiconductor nanoheterostructures through point and line contact reactions. Nano Lett. 2010, 10, 3984–3989. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Lu, K.C.; Tu, K.N. Nucleation and growth of epitaxial silicide in silicon nanowires. Mater. Sci. Eng. R Rep. 2010, 70, 112–125. [Google Scholar] [CrossRef]
- Riffel, M.; Gross, E.; Stohrer, U. Electrical contacts for FeSi2 and higher manganese silicide thermoelectric elements. J. Mater. Sci. Mater. Electron. 1995, 6, 182–185. [Google Scholar] [CrossRef]
- Rowe, D.M. CRC Handbook of Thermoelectrics; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Jin, S.; Schmitt, A.L.; Zhu, L.; Song, Y.; Szcech, J. Synthesis, characterization, and physical properties of transition metal silicide nanowires. In Proceedings of the Nanomaterial Synthesis and Integration for Sensors, Electronics, Photonics, and Electro-Optics, Boston, MA, USA, 1–4 October 2006. [Google Scholar]
- Preinesberger, C.; Vandre, S.; Kalka, T.; Dahne-Prietsch, M. Formation of dysprosium silicide wires on Si(001). J. Phys. D 1998, 31, L43–L45. [Google Scholar] [CrossRef]
- Chen, L.J.; Wu, W.W. Metal silicide nanowires. Jpn. J. Appl. Phys. 2015, 54, 07JA04. [Google Scholar] [CrossRef]
- Chen, Y.; Ohlberg, D.A.A.; Medeiros-Ribeiro, G.; Chang, Y.A.; Williams, R.S. Self-assembled growth of epitaxial erbium disilicide nanowires on silicon (001). Appl. Phys. Lett. 2000, 76, 4004–4006. [Google Scholar] [CrossRef]
- Chen, Y.; Ohlberg, D.A.A.; Williams, R.S. Nanowires of four epitaxial hexagonal silicides grown on Si(001). J. Appl. Phys. 2002, 91, 3213–3218. [Google Scholar] [CrossRef]
- Preinesberger, C.; Becker, S.K.; Vandre, S.; Kalka, T.; Dahne, M. Structure of DySi2 nanowires on Si(001). J. Appl. Phys. 2002, 91, 1695–1697. [Google Scholar] [CrossRef]
- Stevens, M.; He, Z.; Smith, D.J.; Bennett, P.A. Structure and orientation of epitaxial titanium silicide nanowires determined by electron microdiffraction. J. Appl. Phys. 2003, 93, 5670–5674. [Google Scholar] [CrossRef]
- Nogami, J.; Liu, B.Z.; Katkov, M.V.; Ohbuchi, C.; Birge, N.O. Self-assembled rare-earth silicide nanowires on Si(001). Phys. Rev. B 2001, 63, 233305. [Google Scholar] [CrossRef]
- Lian, O.Y.; Thrall, E.S.; Deshmukh, M.M.; Park, H. Vapor-phase synthesis and characterization of epsilon-FeSi nanowires. Adv. Mater. 2006, 18, 1437–1440. [Google Scholar]
- Schmitt, A.L.; Bierman, M.J.; Schmeisser, D.; Himpsel, F.J.; Jin, S. Synthesis and properties of single-crystal FeSi nanowires. Nano Lett. 2006, 6, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Borisenko, V.E. Semiconducting Silicides; Springer: Berlin, Germany, 2000. [Google Scholar]
- Vonkanel, H.; Onda, N.; Sirringhaus, H.; Mullergubler, E.; Goncalvesconto, S.; Schwarz, C. Epitaxial phase transitions in the iron/silicon system. Appl. Surf. Sci. 1993, 70, 559–563. [Google Scholar] [CrossRef]
- Desimoni, J.; Bernas, H.; Behar, M.; Lin, X.W.; Washburn, J.; Lilientalweber, Z. Ion beam synthesis of cubic FeSi2. Appl. Phys. Lett. 1993, 62, 306–308. [Google Scholar] [CrossRef]
- Alvarez, J.; Hinarejos, J.J.; Michel, E.G.; Miranda, R. Determination of the Fe/Si(111) phase diagram by means of photoelectron spectroscopies. Surf. Sci. 1993, 287, 490–494. [Google Scholar] [CrossRef]
- Christensen, N.E. Electronic structure of beta-FeSi2. Phys. Rev. B 1990, 42, 7148–7153. [Google Scholar] [CrossRef]
- Leong, D.; Harry, M.; Reeson, K.J.; Homewood, K.P. A silicon/iron-disilicide light-emitting diode operating at a wavelength of 1.5 μm. Nature 1997, 387, 686. [Google Scholar] [CrossRef]
- Manyala, N.; Sidis, Y.; DiTusa, J.F.; Aeppli, G.; Young, D.P.; Fisk, Z. Magnetoresistance from quantum interference effects in ferromagnets. Nature 2000, 404, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Goldberg, R.; Burshtein, G.; Lumelsky, V.; Subannajui, K.; Zacharias, M.; Lifshitz, Y. Role of carrier gas flow and species diffusion in nanowire growth from thermal CVD. J. Phys. Chem. C 2012, 116, 5524–5530. [Google Scholar] [CrossRef]
- Hung, S.W.; Yeh, P.H.; Chu, L.W.; Chen, C.D.; Chou, L.J.; Wu, Y.J.; Chen, L.J. Direct growth of beta-FeSi2 nanowires with infrared emission, ferromagnetism at room temperature and high magnetoresistance via a spontaneous chemical reaction method. J. Mater. Chem. 2011, 21, 5704–5709. [Google Scholar] [CrossRef]
- Chang, C.M.; Chang, Y.C.; Chung, Y.A.; Lee, C.Y.; Chen, L.J. Synthesis and properties of the low resistivity tisi2 nanowires grown with TiF4 precursor. J. Phys. Chem. C 2009, 113, 17720–17723. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-J.; Chen, Y.-Y.; Hsu, H.-M.; Lu, K.-C. Single Crystalline Iron Silicide and Beta-Iron Disilicide Nanowires Formed through Chemical Vapor Deposition. Materials 2018, 11, 2384. https://doi.org/10.3390/ma11122384
Huang W-J, Chen Y-Y, Hsu H-M, Lu K-C. Single Crystalline Iron Silicide and Beta-Iron Disilicide Nanowires Formed through Chemical Vapor Deposition. Materials. 2018; 11(12):2384. https://doi.org/10.3390/ma11122384
Chicago/Turabian StyleHuang, Wei-Jie, Yu-Yang Chen, Hsiu-Ming Hsu, and Kuo-Chang Lu. 2018. "Single Crystalline Iron Silicide and Beta-Iron Disilicide Nanowires Formed through Chemical Vapor Deposition" Materials 11, no. 12: 2384. https://doi.org/10.3390/ma11122384
APA StyleHuang, W.-J., Chen, Y.-Y., Hsu, H.-M., & Lu, K.-C. (2018). Single Crystalline Iron Silicide and Beta-Iron Disilicide Nanowires Formed through Chemical Vapor Deposition. Materials, 11(12), 2384. https://doi.org/10.3390/ma11122384



