Effects of Zr Addition on Strengthening Mechanisms of Al-Alloyed High-Cr ODS Steels
Abstract
1. Introduction
2. Experimental
2.1. Material
2.2. Microstructure Characterization
2.3. Tensile Test
3. Results and Discussion
3.1. Microstructure/Substructure Examination
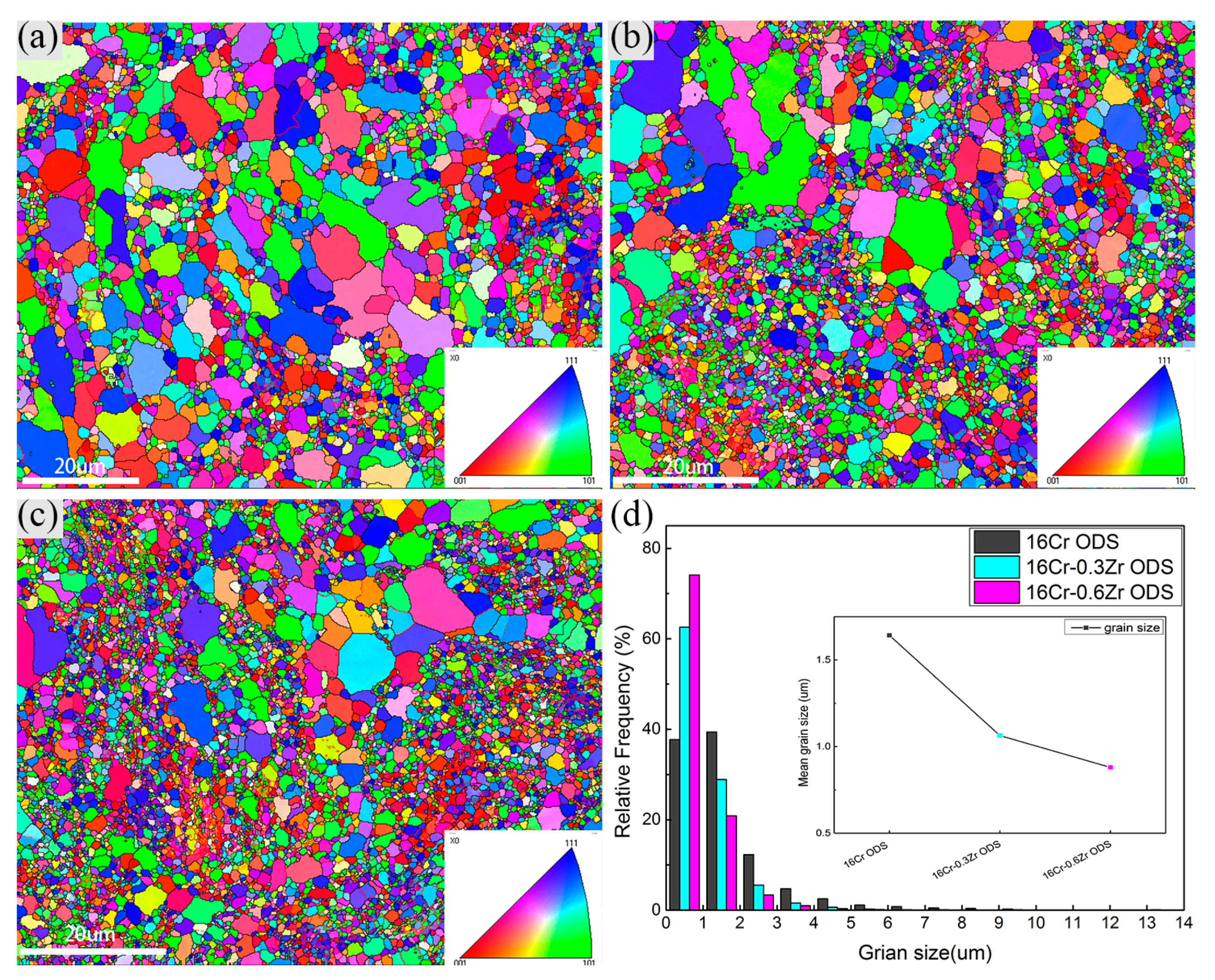
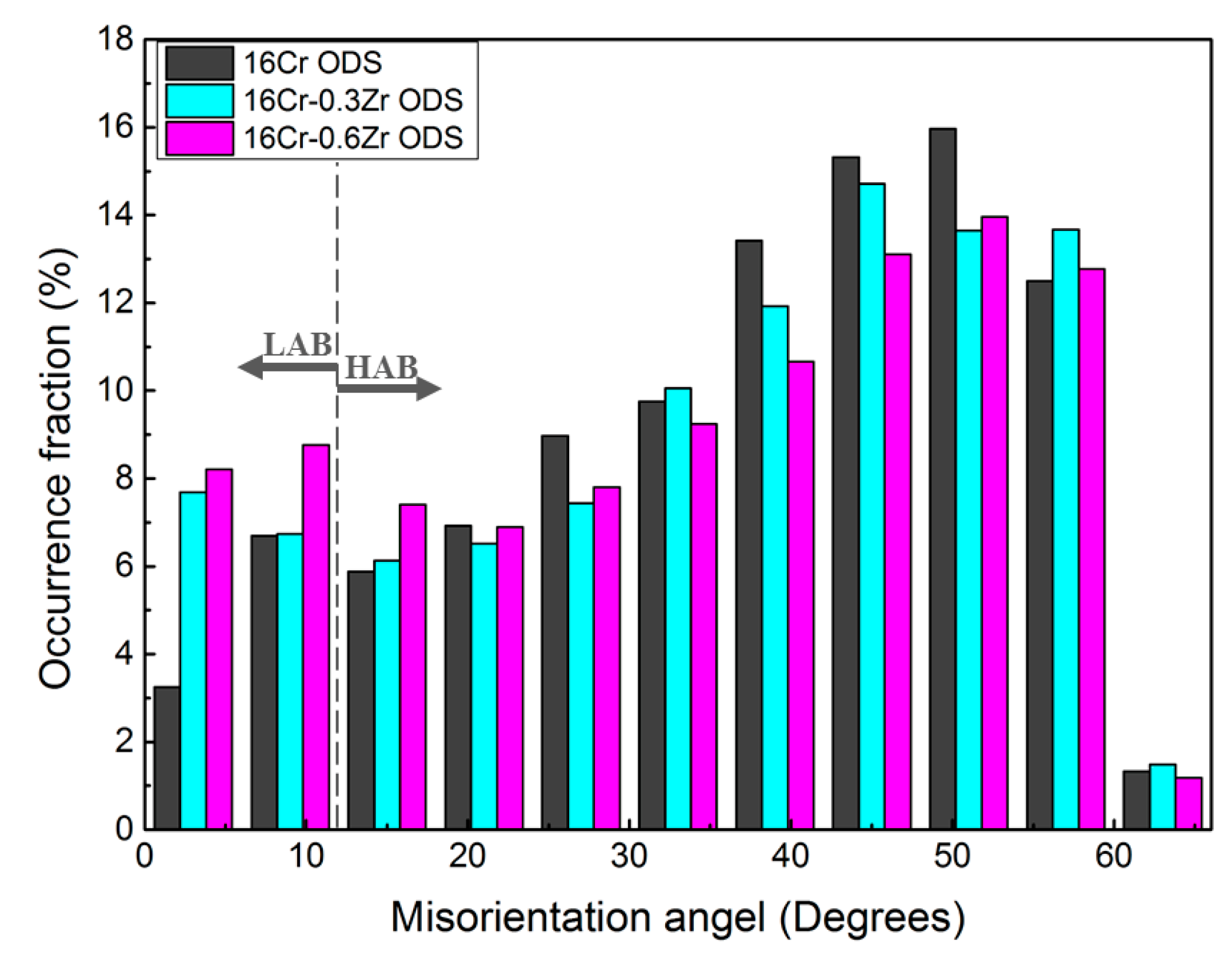
3.1.1. Grain Morphologies
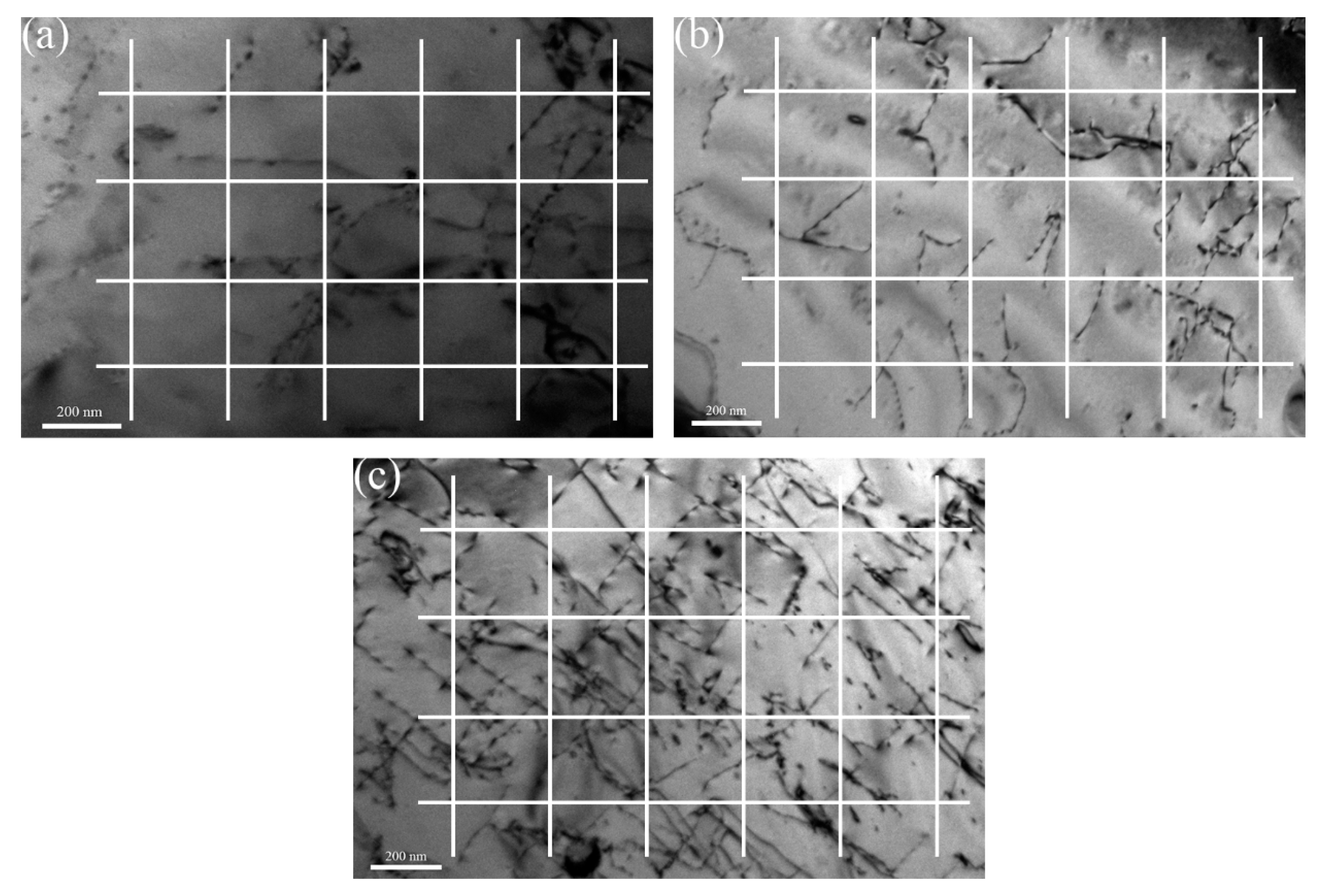
3.1.2. Dislocation Density
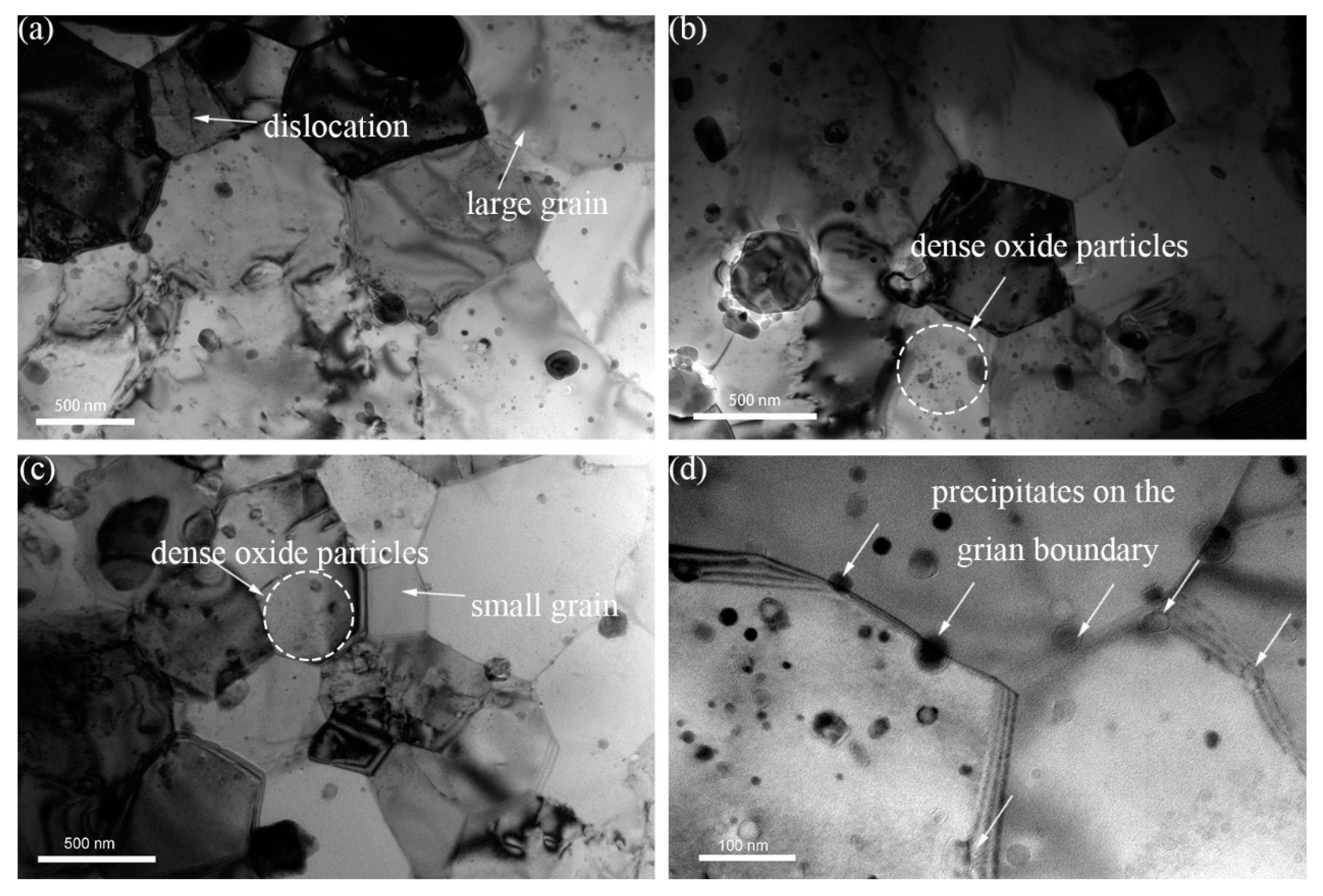
3.1.3. Spatial and Size Distributions of Oxide Particles
3.1.4. Crystal Structures of Oxide Particles Studied by HRTEM
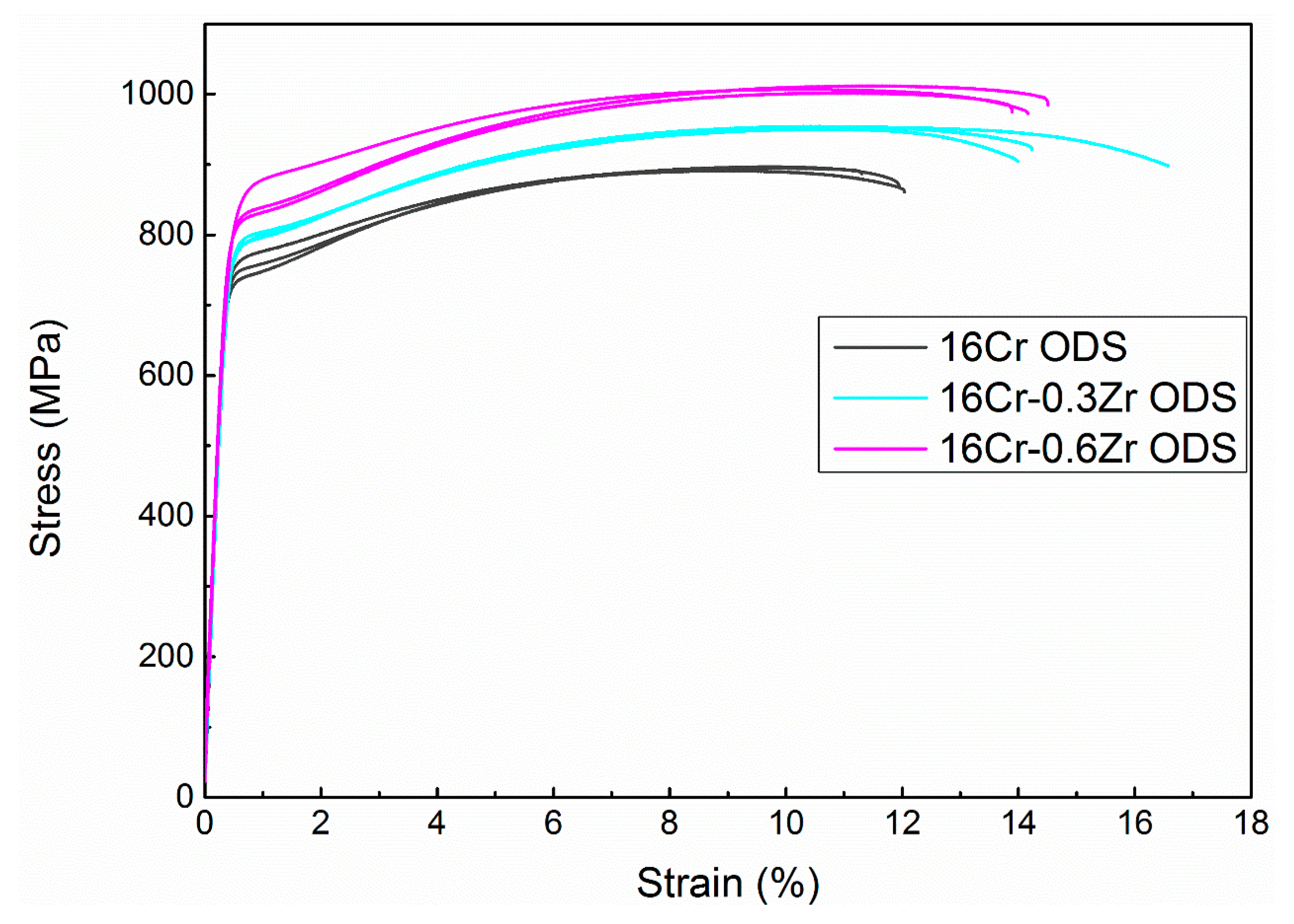
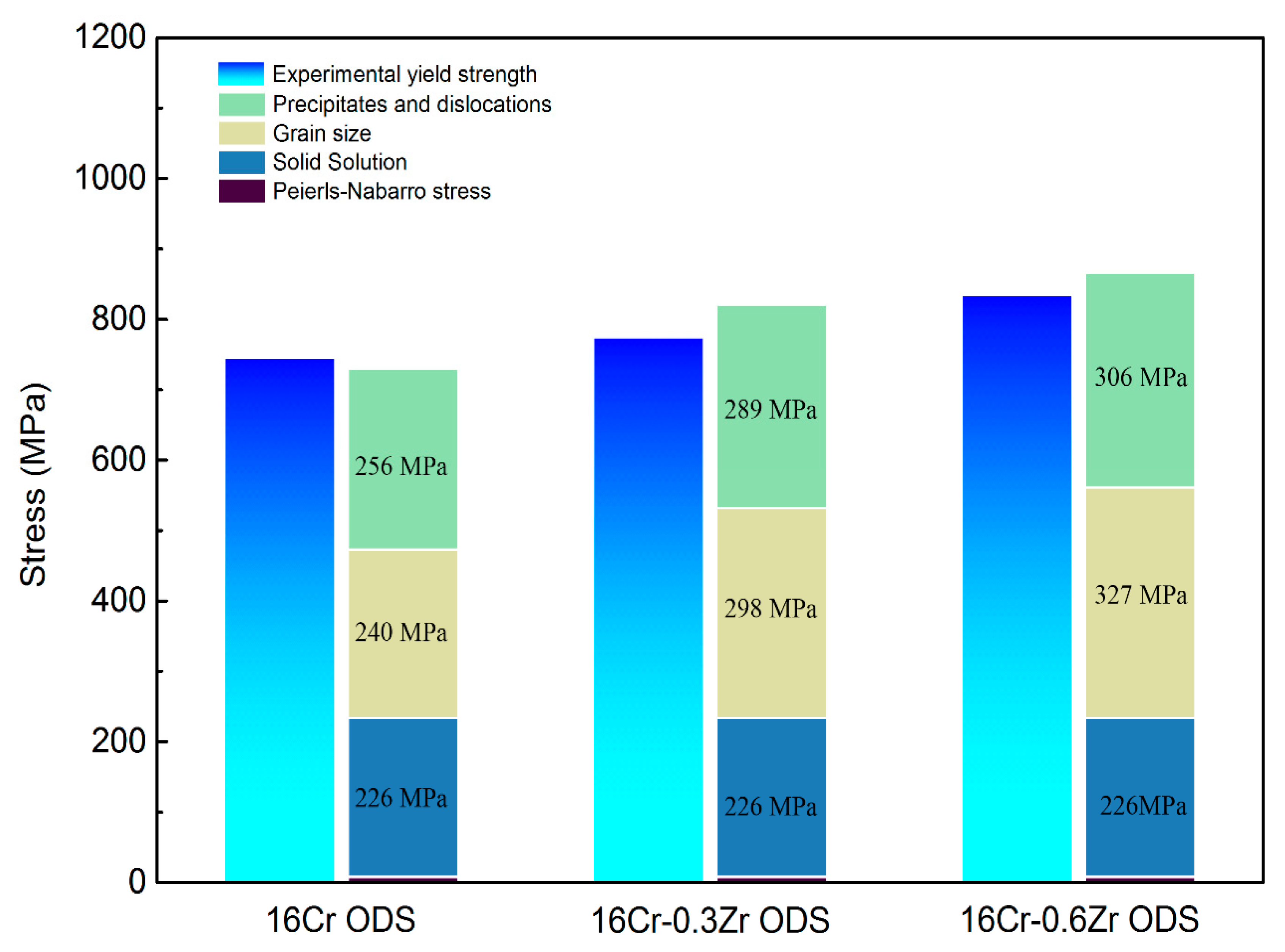
3.2. Mechanical Properties
4. Conclusions
- (1)
- The oxides in 16Cr ODS steel are mainly Y-Al-O nanoparticles; however, with Zr addition in 16Cr-0.3Zr ODS and 16Cr-0.6Zr ODS steels, some precipitates are identified as Y-Zr-O nanoparticles. Y-Zr-O nanoparticles exhibit a smaller size compared to Y-Al-O particles.
- (2)
- Smaller Y-Zr-O oxide nanoparticles in Zr-contained ODS steels can lead to the formation of more homogenous and dispersive oxides. The oxides in Zr-contained ODS steels exhibit a smaller size, higher number density and more homogeneous distribution compared to those in 16Cr ODS. These refined oxides inhabit the grain growth during HIP and lead to a more refined grain size. Therefore, the Zr addition could decrease the grain size and increase the LAB of Al-alloyed 16Cr ODS steels.
- (3)
- Decrease in grain size and refinement of oxide particle increase the yield strength of ODS steels with Zr addition. The major strengthening mechanisms change from dispersion strengthening and dislocation hardening to grain boundary strengthening in 16Cr-0.3Zr and 16Cr-0.6Zr ODS steels when compared to 16Cr ODS steel.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Odette, G.R. Recent Progress in Developing and Qualifying Nanostructured Ferritic Alloys for Advanced Fission and Fusion Applications. JOM 2014, 66, 2427–2441. [Google Scholar] [CrossRef]
- Lu, C.; Lu, Z.; Wang, X.; Xie, R.; Li, Z.; Higgins, M.; Liu, C.; Gao, F.; Wang, L. Enhanced Radiation-tolerant Oxide Dispersion Strengthened Steel and its Microstructure Evolution under Helium-implantation and Heavy-ion Irradiation. Sci. Rep. 2017, 7, 40343. [Google Scholar] [CrossRef] [PubMed]
- Mo, K.; Yun, D.; Miao, Y.; Liu, X.; Pellin, M.; Almer, J.; Park, J.-S.; Stubbins, J.F.; Zhu, S.; Yacout, A.M. Investigation of High-Energy Ion-Irradiated MA957 Using Synchrotron Radiation under In-Situ Tension. Materials 2016, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Ukai, S.; Ohtsuka, S.; Kaito, T.; Sakasegawa, H.; Chikata, N.; Hayashi, S.; Ohnuki, S. High-temperature strength characterization of advanced 9Cr-ODS ferritic steels. Mater. Sci. Eng. A 2009, 510–511, 115–120. [Google Scholar] [CrossRef]
- Ukai, S.; Mizuta, S.; Yoshitake, T.; Okuda, T.; Fujiwara, M.; Hagi, S.; Kobayashi, T. Tube manufacturing and characterization of oxide dispersion strengthened ferritic steels. J. Nucl. Mater. 2000, 283, 702–706. [Google Scholar] [CrossRef]
- Alinger, M.J.; Odette, G.R.; Hoelzer, D.T. The development and stability of Y–Ti–O nanoclusters in mechanically alloyed Fe–Cr based ferritic alloys. J. Nucl. Mater. 2004, 329–333, 382–386. [Google Scholar] [CrossRef]
- McClintock, D.A.; Sokolov, M.A.; Hoelzer, D.T.; Nanstad, R.K. Mechanical properties of irradiated ODS-EUROFER and nanocluster strengthened 14YWT. J. Nucl. Mater. 2009, 392, 353–359. [Google Scholar] [CrossRef]
- Klueh, R.L.; Shingledecker, J.P.; Swindeman, R.W.; Hoelzer, D.T. Oxide dispersion-strengthened steels: A comparison of some commercial and experimental alloys. J. Nucl. Mater. 2005, 341, 103–114. [Google Scholar] [CrossRef]
- Yu, H.; Ukai, S.; Hayashi, S.; Oono, N. Effect of Al content on the high-temperature oxidation of Co-20Cr-(5, 10)Al oxide dispersion strengthened superalloys. Corros. Sci. 2017, 118, 49–59. [Google Scholar] [CrossRef]
- Gao, R.; Xia, L.L.; Zhang, T.; Wang, X.P.; Fang, Q.F.; Liu, C.S. Oxidation resistance in LBE and air and tensile properties of ODS ferritic steels containing Al/Zr elements. J. Nucl. Mater. 2014, 455, 407–411. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Shen, H.; Chou, W.; Iwata, N.Y.; Kimura, A. The effects of Cr and Al concentrations on the oxidation behavior of oxide dispersion strengthened ferritic alloys. Corros. Sci. 2013, 76, 310–316. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takasugi, T. Mapping of 475 °C embrittlement in ferritic Fe–Cr–Al alloys. Scr. Mater. 2010, 63, 1104–1107. [Google Scholar] [CrossRef]
- Airiskallio, E.; Nurmi, E.; Heinonen, M.H.; Väyrynen, I.J.; Kokko, K.; Ropo, M.; Punkkinen, M.P.J.; Pitkänen, H.; Alatalo, M.; Kollár, J.; et al. High temperature oxidation of Fe–Al and Fe–Cr–Al alloys: The role of Cr as a chemically active element. Corros. Sci. 2010, 52, 3394–3404. [Google Scholar] [CrossRef]
- Nana, S.; Cortie, M.B. Retardation of intermetallic phase formation in experimental superferritic stainless steels. Metall. Mater. Trans. A 1996, 27, 2436–2444. [Google Scholar] [CrossRef]
- Dong, H.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Effect of hafnium addition on the microstructure and tensile properties of aluminum added high-Cr ODS steels. J. Alloys Compd. 2017, 702, 538–545. [Google Scholar] [CrossRef]
- Dou, P.; Kimura, A.; Kasada, R.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F.; Jiang, S.; et al. TEM and HRTEM study of oxide particles in an Al-alloyed high-Cr oxide dispersion strengthened ferritic steel with Hf addition. J. Nucl. Mater. 2017, 485, 189–201. [Google Scholar] [CrossRef]
- Dou, P.; Kimura, A.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F. Polymorphic and coherency transition of Y–Al complex oxide particles with extrusion temperature in an Al-alloyed high-Cr oxide dispersion strengthened ferritic steel. Acta Mater. 2011, 59, 992–1002. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, T.; Wang, X.P.; Fang, Q.F.; Liu, C.S. Effect of zirconium addition on the microstructure and mechanical properties of ODS ferritic steels containing aluminum. J. Nucl. Mater. 2014, 444, 462–468. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, Z.; Hu, H.; Zhang, G.; Li, S.; Wang, M. Effects of aluminum on microstructure and mechanical behavior of 14Cr–ODS steels. J. Nucl. Mater. 2015, 462, 502–507. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Z.; Mo, K.; Miao, Y.; Li, S.; Liu, X.; Wang, M.; Park, J.-S.; Almer, J.; Stubbins, J.F. The comparison of microstructures and mechanical properties between 14Cr-Al and 14Cr-Ti ferritic ODS alloys. Mater. Des. 2016, 98, 61–67. [Google Scholar] [CrossRef]
- Isselin, J.; Kasada, R.; Kimura, A.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F. Effects of Zr Addition on the Microstructure of 14%Cr 4%Al ODS Ferritic Steels. Mater. Trans. 2010, 51, 1011–1015. [Google Scholar] [CrossRef]
- Rahmanifard, R.; Farhangi, H.; Novinrooz, A.J. Effect of zirconium and tantalum on the microstructural characteristics of 12YWT ODS steel nanocomposite. J. Alloys Compd. 2015, 622, 948–952. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Z.; Wang, D.; Liu, C. Effect of zirconium addition on the microstructure and mechanical properties of 15Cr-ODS ferritic Steels consolidated by hot isostatic pressing. Fusion Eng. Des. 2017, 114, 33–39. [Google Scholar] [CrossRef]
- Kotan, H.; Darling, K.A.; Scattergood, R.O.; Koch, C.C. Influence of Zr and nano-Y2O3 additions on thermal stability and improved hardness in mechanically alloyed Fe base ferritic alloys. J. Alloys Compd. 2014, 615, 1013–1018. [Google Scholar] [CrossRef]
- Pešička, J.; Kužel, R.; Dronhofer, A.; Eggeler, G. The evolution of dislocation density during heat treatment and creep of tempered martensite ferritic steels. Acta Mater. 2003, 51, 4847–4862. [Google Scholar] [CrossRef]
- Loretto, M.H. Electron Beam Analysis of Materials; Chapman and Hall: London, UK, 1984; pp. 97–100. [Google Scholar]
- He, P.; Klimenkov, M.; Möslang, A.; Lindau, R.; Seifert, H.J. Correlation of microstructure and low cycle fatigue properties for 13.5Cr1.1W0.3Ti ODS steel. J. Nucl. Mater. 2014, 455, 167–173. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Yu, L.; Ma, Z.; Guo, Q.; Huang, Y.; Li, H. Microstructure characteristic and mechanical property of transformable 9Cr-ODS steel fabricated by spark plasma sintering. Mater. Des. 2017, 132, 158–169. [Google Scholar] [CrossRef]
- Dadé, M.; Malaplate, J.; Garnier, J.; De Geuser, F.; Barcelo, F.; Wident, P.; Deschamps, A. Influence of microstructural parameters on the mechanical properties of oxide dispersion strengthened Fe-14Cr steels. Acta Mater. 2017, 127, 165–177. [Google Scholar] [CrossRef]
- Auger, M.A.; de Castro, V.; Leguey, T.; Monge, M.A.; Muñoz, A.; Pareja, R. Microstructure and tensile properties of oxide dispersion strengthened Fe–14Cr–0.3Y2O3 and Fe–14Cr–2W–0.3Ti–0.3Y2O3. J. Nucl. Mater. 2013, 442, S142–S147. [Google Scholar] [CrossRef]
- Ramar, A.; Schäublin, R. Analysis of hardening limits of oxide dispersion strengthened steel. J. Nucl. Mater. 2013, 432, 323–333. [Google Scholar] [CrossRef]
- Li, Q. Modeling the microstructure–mechanical property relationship for a 12Cr–2W–V–Mo–Ni power plant steel. Mater. Sci. Eng. A 2003, 361, 385–391. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Li, F.; Yang, H.; Zhao, Z.; Kano, S.; Matsukawa, Y.; Satoh, Y.; Abe, H. Microstructural characterization and strengthening mechanisms of a 12Cr-ODS steel. Mater. Sci. Eng. A 2016, 673, 624–632. [Google Scholar] [CrossRef]
- Praud, M.; Mompiou, F.; Malaplate, J.; Caillard, D.; Garnier, J.; Steckmeyer, A.; Fournier, B. Study of the deformation mechanisms in a Fe–14% Cr ODS alloy. J. Nucl. Mater. 2012, 428, 90–97. [Google Scholar] [CrossRef]
- Martin, J.W. Micromechanisms in Particle-Hardened Alloys; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Nishizawa, T.; Ohnuma, I.; Ishida, K. Examination of the Zener Relationship between Grain Size and Particle Dispersion. Mater. Trans. 1997, 38, 950–956. [Google Scholar] [CrossRef]
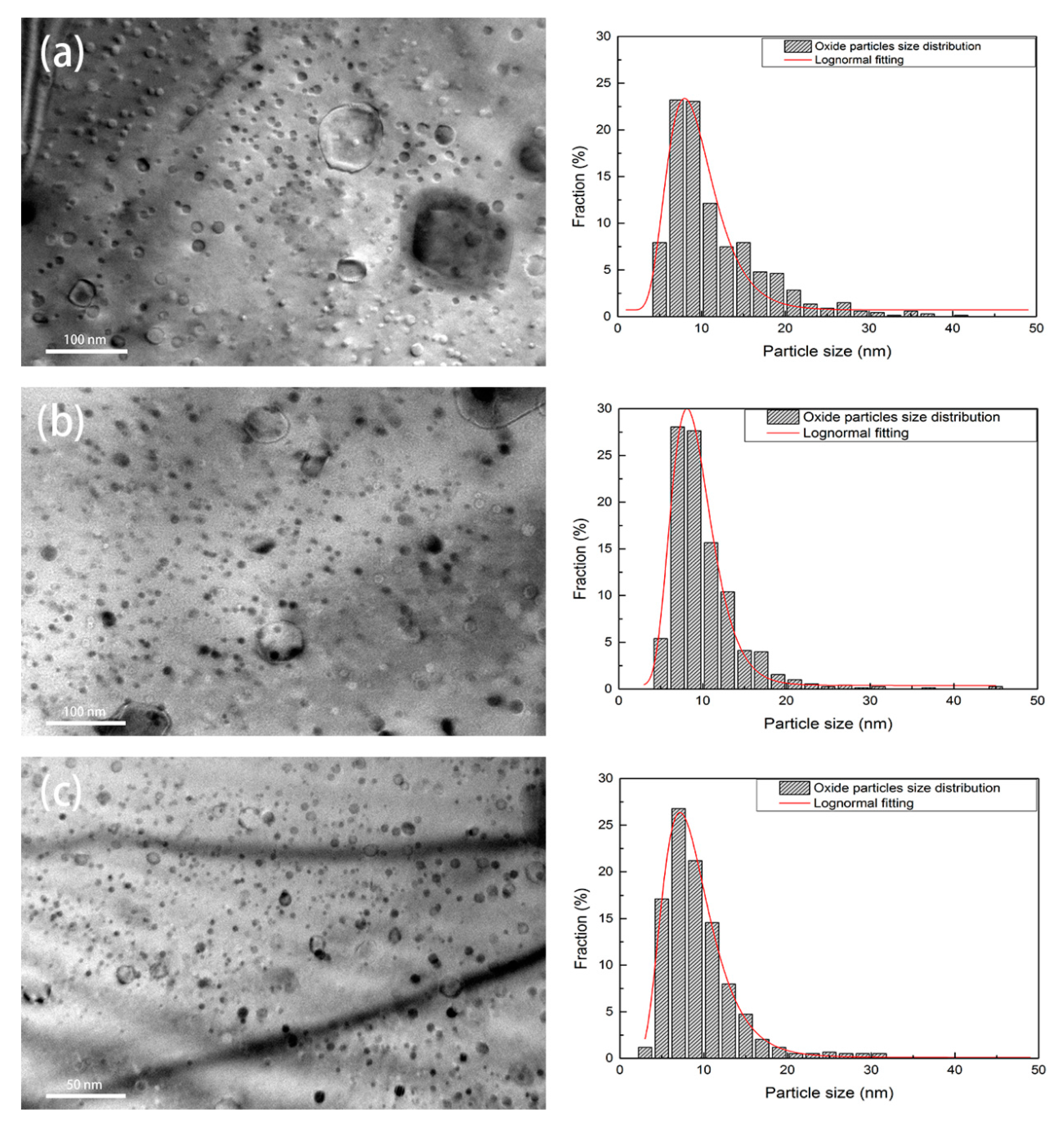
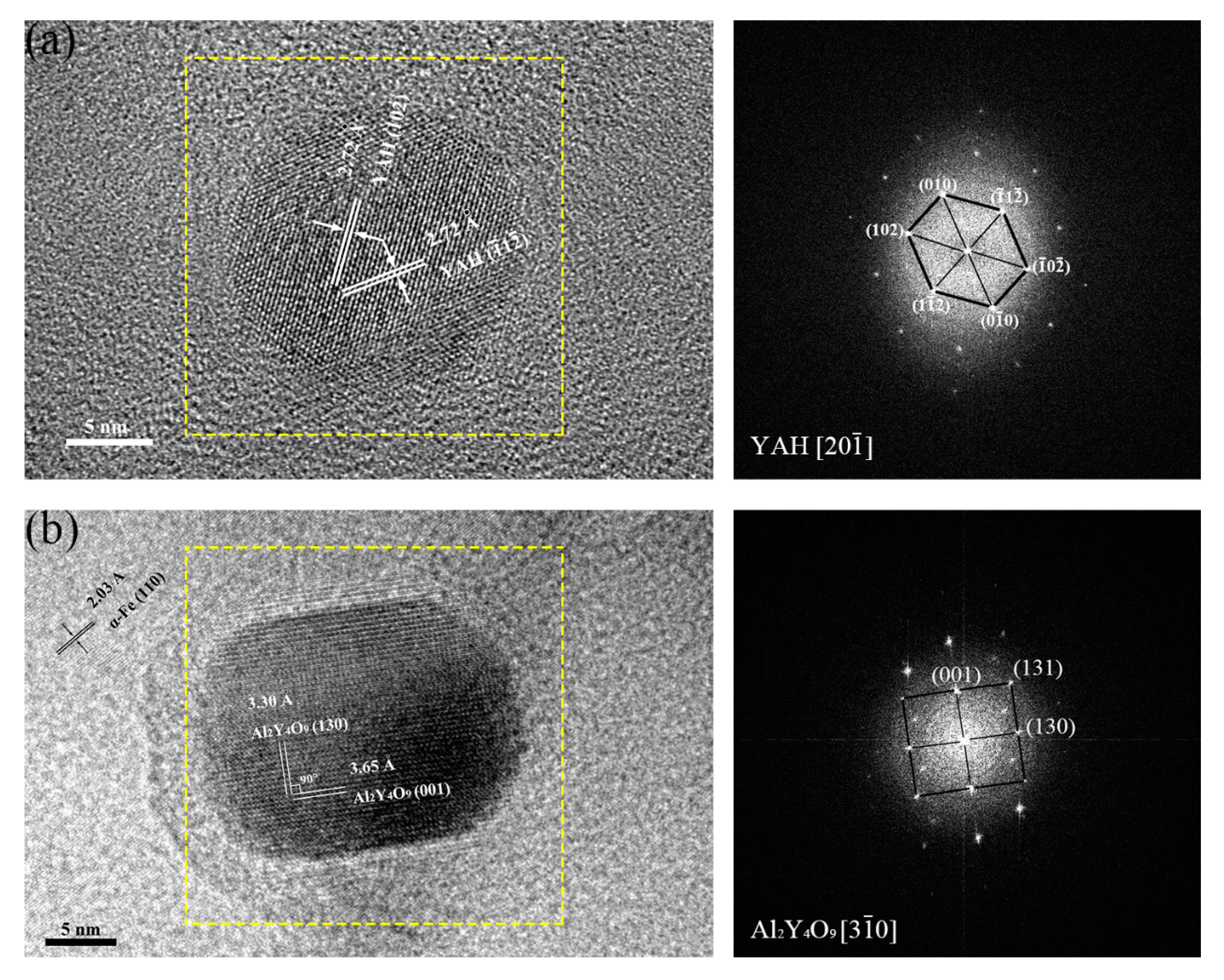
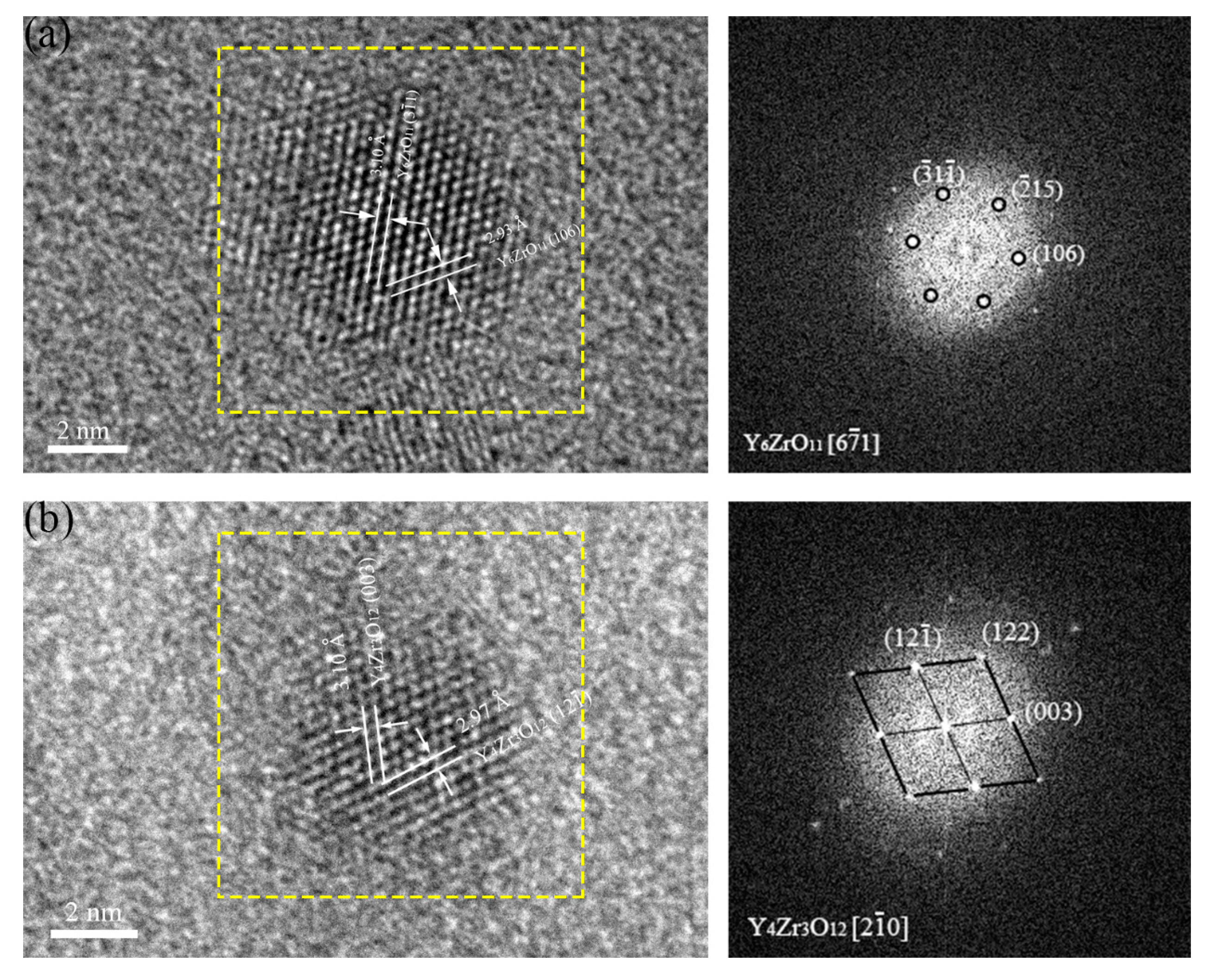
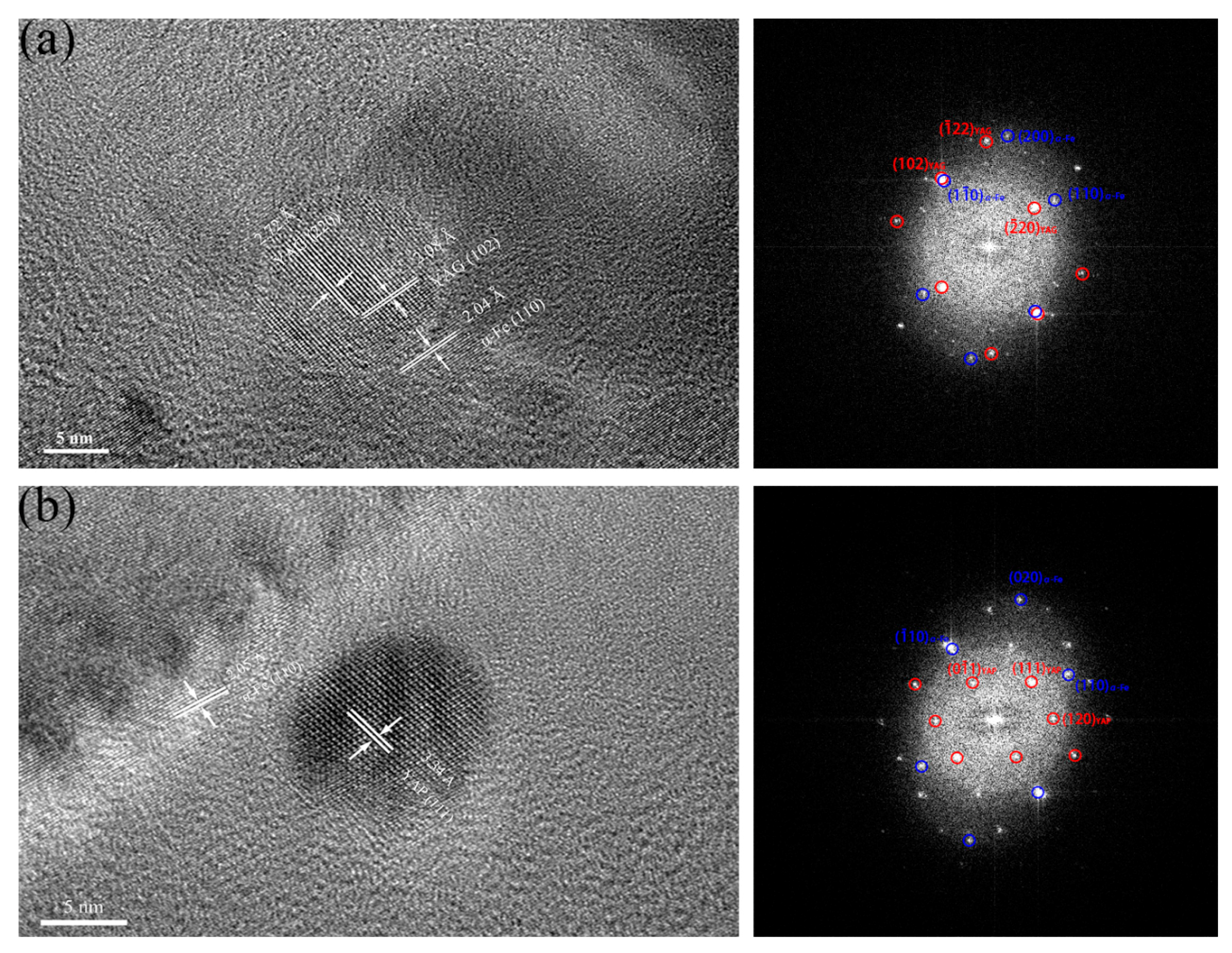
| ODS Samples | Fe | Cr | Al | W | Zr | Y2O3 |
|---|---|---|---|---|---|---|
| 16Cr-ODS | Bal. | 16 | 3 | 1.5 | - | 0.35 |
| 16Cr-0.3Zr-ODS | Bal. | 16 | 3 | 1.5 | 0.3 | 0.35 |
| 16Cr-0.6Zr-ODS | Bal. | 16 | 3 | 1.5 | 0.6 | 0.35 |
| ODS Samples | (nm) | (nm) | (m−3) |
|---|---|---|---|
| 16Cr ODS | 11.59 ± 0.60 | 30.03 ± 1.50 | 1.33 × 2023 ± 0.5 × 2023 |
| 16Cr-0.3Zr ODS | 10.21 ± 0.45 | 25.56 ± 1.75 | 1.81 × 2023 ± 0.8 × 2023 |
| 16Cr-0.6Zr ODS | 9.5 ± 0.50 | 23.33 ± 2.0 | 2.04 × 2023 ± 0.6 × 2023 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Effects of Zr Addition on Strengthening Mechanisms of Al-Alloyed High-Cr ODS Steels. Materials 2018, 11, 118. https://doi.org/10.3390/ma11010118
Ren J, Yu L, Liu Y, Liu C, Li H, Wu J. Effects of Zr Addition on Strengthening Mechanisms of Al-Alloyed High-Cr ODS Steels. Materials. 2018; 11(1):118. https://doi.org/10.3390/ma11010118
Chicago/Turabian StyleRen, Jian, Liming Yu, Yongchang Liu, Chenxi Liu, Huijun Li, and Jiefeng Wu. 2018. "Effects of Zr Addition on Strengthening Mechanisms of Al-Alloyed High-Cr ODS Steels" Materials 11, no. 1: 118. https://doi.org/10.3390/ma11010118
APA StyleRen, J., Yu, L., Liu, Y., Liu, C., Li, H., & Wu, J. (2018). Effects of Zr Addition on Strengthening Mechanisms of Al-Alloyed High-Cr ODS Steels. Materials, 11(1), 118. https://doi.org/10.3390/ma11010118






