Study of the Thermal Properties and the Fire Performance of Flame Retardant-Organic PCM in Bulk Form
Abstract
:1. Introduction
2. Materials and Methods
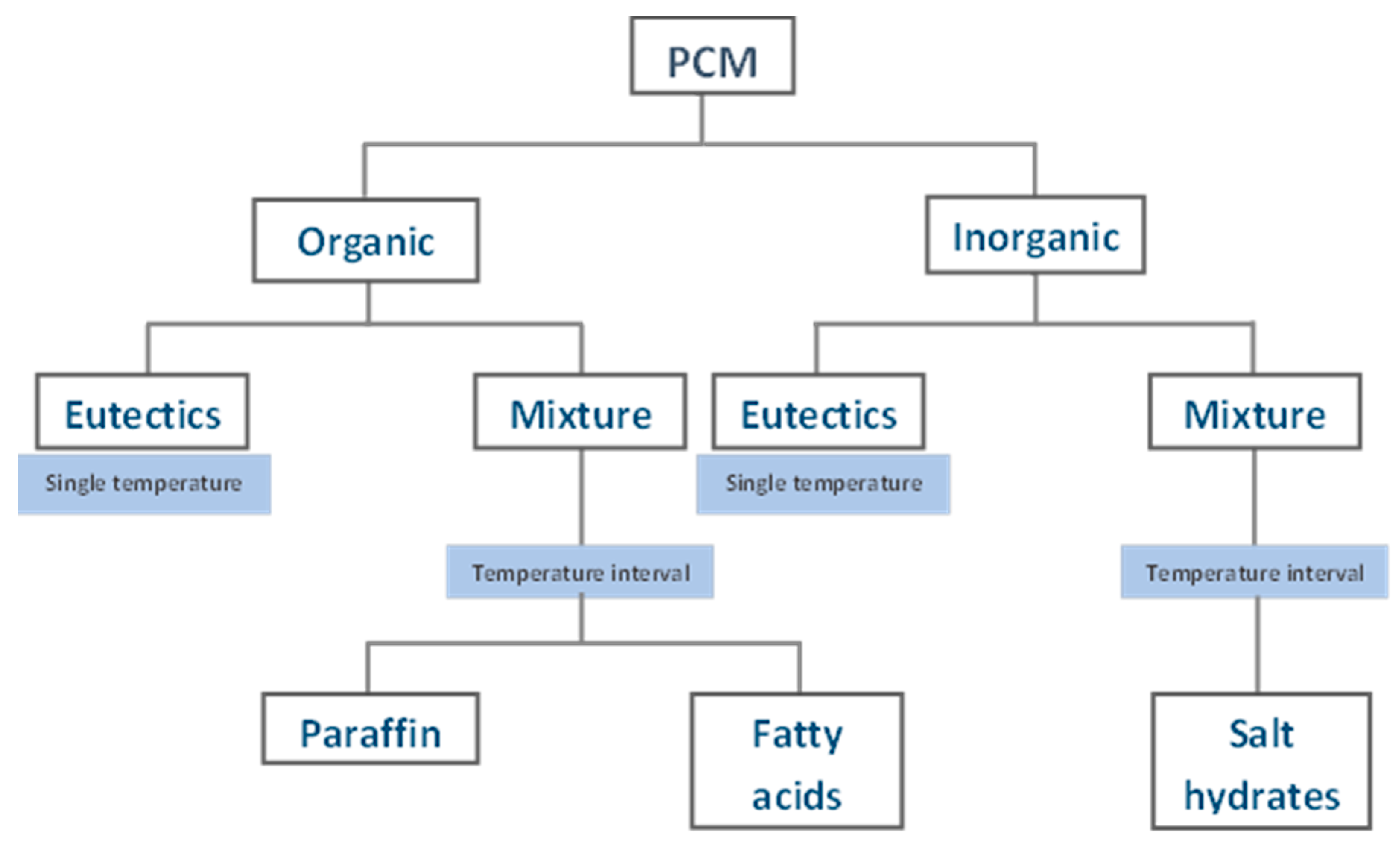
2.1. Phase Change Materials
2.2. Flame Retardants
- Gas phase flame retardants: The flame retardant interferes with the free radical combustion reaction. Halogenated flame retardants are an example of this category.
- Endothermic flame retardants undergo an endothermic decomposition in the range of temperatures at which combustion takes place. This endothermic reaction helps to withdraw heat from the substrate. Furthermore, these compounds evolve non-flammable gases such as water or CO2 that have a dilution effect. The metal oxide formed during the decomposition of a metal hydroxide form an insulating protective coating on the condensed phase.
- Char-forming flame retardants: In this case, the flame retardant promotes the formation of a protective coating on the flammable material that hinders the heat and oxygen transfer. Polyphosphates and intumescent flame retardants (IFRs) are among this category. IFRs consist of a combination of a carbon source, an acid source and a foaming agent. Usually, ammonium polyphosphate, pentaerythritol and melamine are the main ingredients of an IFR.
2.3. Formulations
3. Experimental Methods
3.1. Thermal Stability
3.2. Pyrolysis Combustion Flow Calorimeter (PCFC)
3.3. Dripping Test
3.4. DSC
4. Results and Discussion
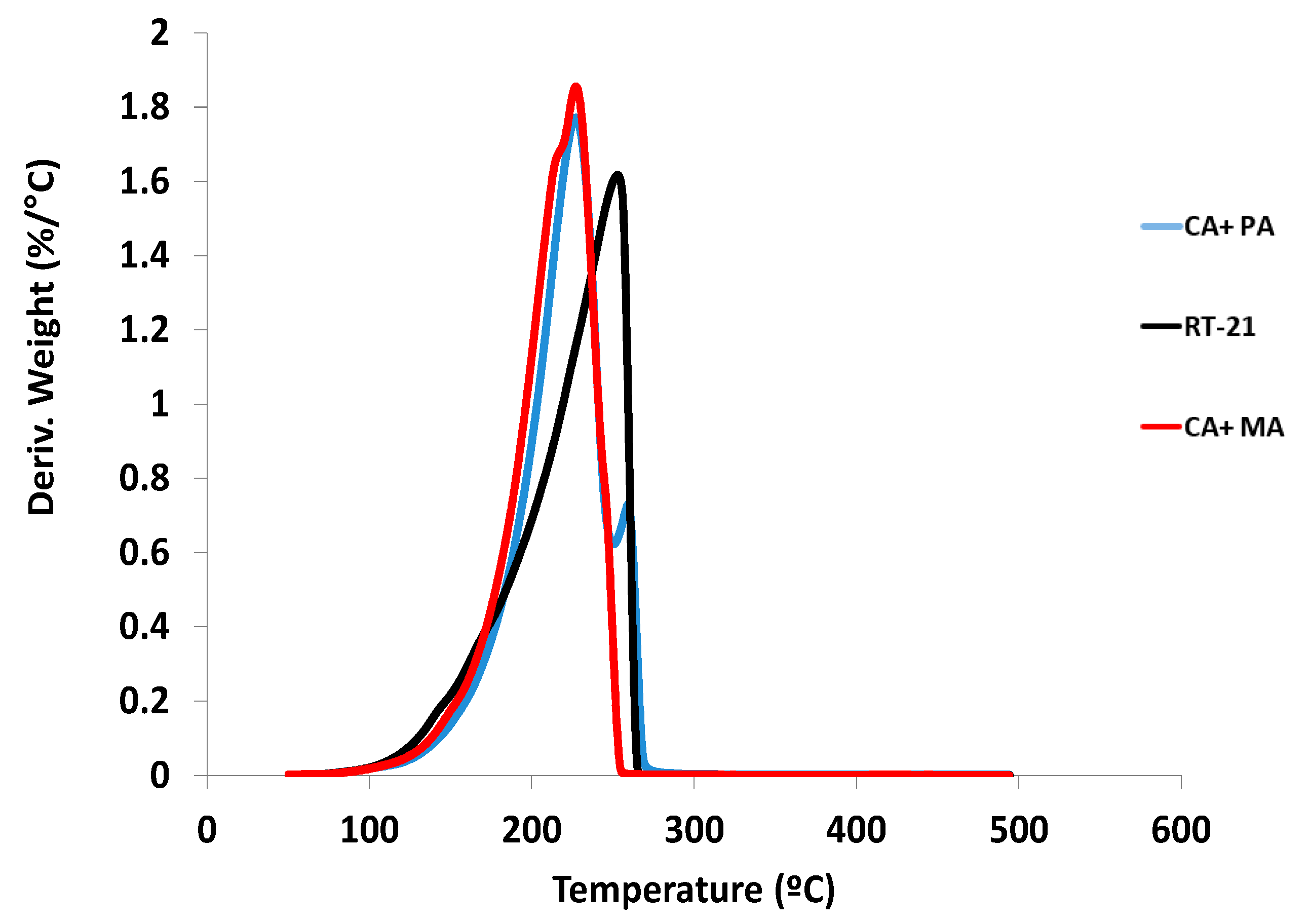
4.1. Thermal Stability
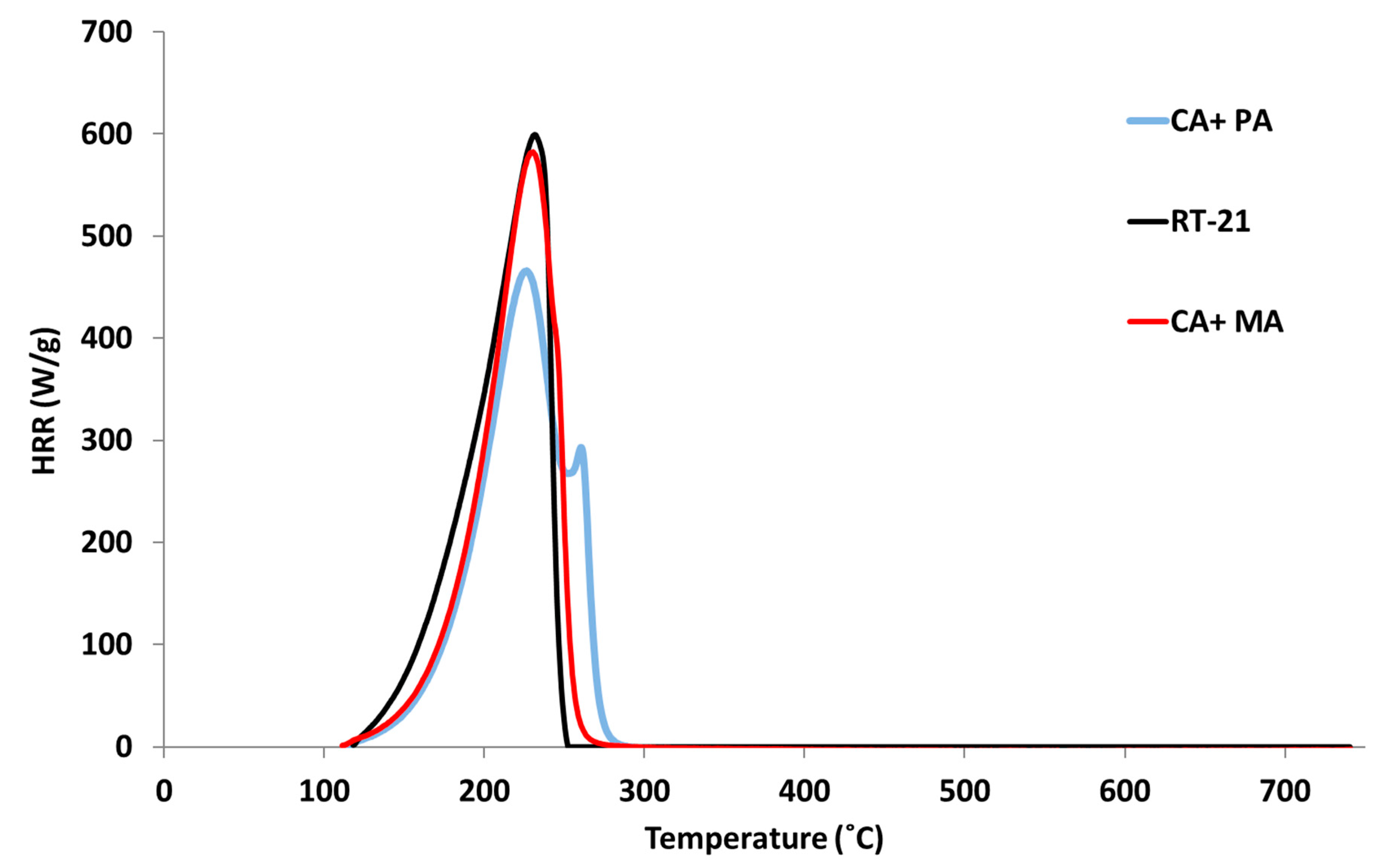
4.2. PCFC
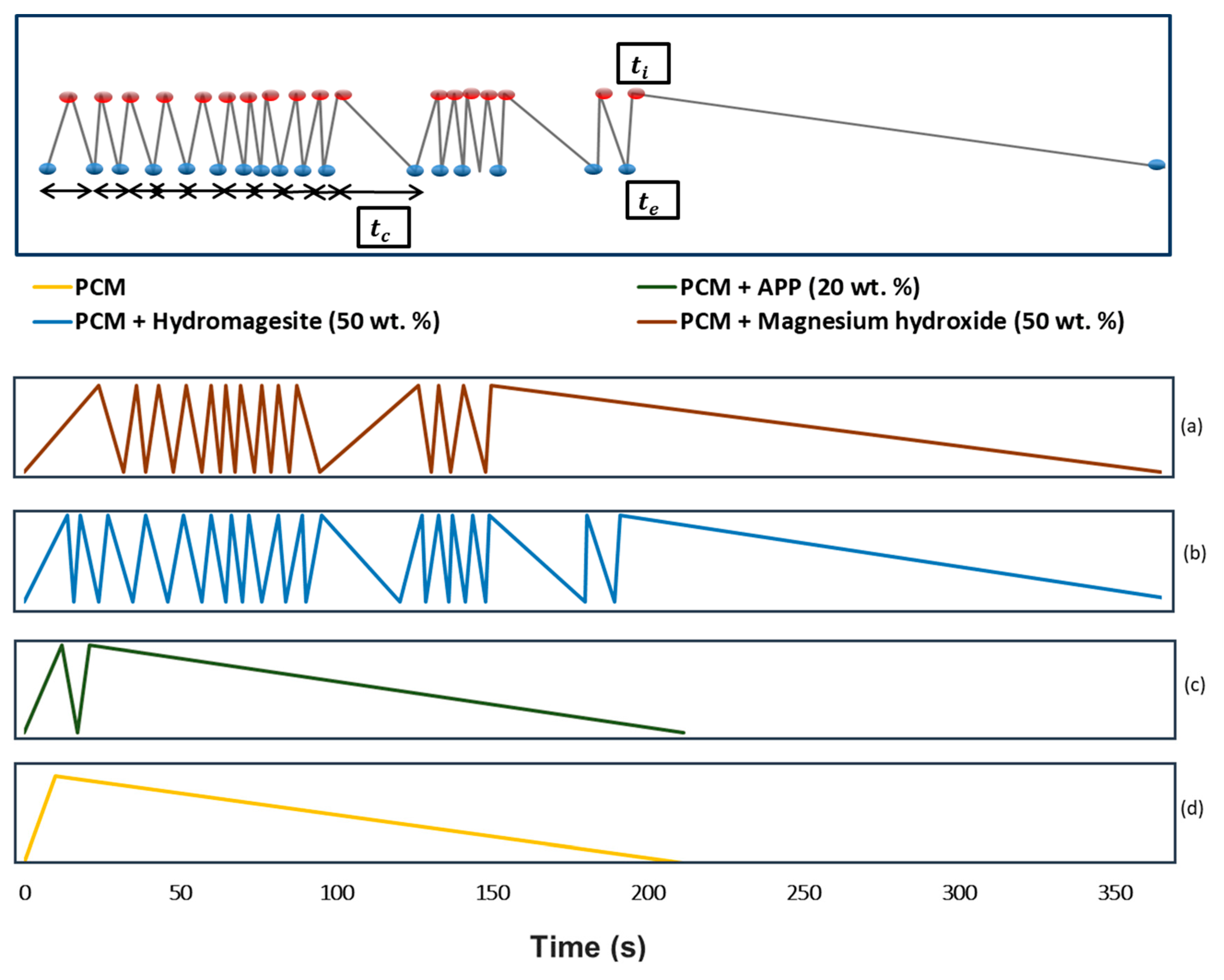
4.3. Dripping Test
4.4. Thermal Characterization
4.5. Further Work
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Energy Agency (IEA). Energy Technology Perspectives 2012 Pathways to a Clean Energy System. Energy Technol. Perspect. 2012, 1–12. [Google Scholar] [CrossRef]
- Kundakçı, K.B.; Yılmaz, Z. The performance comparison of fan-assisted Trombe wall system. ITU J. Fac. Arch. 2013, 10, 198–211. [Google Scholar]
- Özbalta, T.G.; Kartal, S. Heat gain through Trombe wall using solar energy in a cold region of Turkey. Sci. Res. Essays 2010, 5, 2768–2778. [Google Scholar]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- De Gracia, A.; Navarro, L.; Castell, A.; Ruiz-Pardo, Á.; Alvárez, S.; Cabeza, L.F. Experimental study of a ventilated facade with PCM during winter period. Energy Build. 2013, 58, 324–332. [Google Scholar] [CrossRef]
- De Gracia, A.; Oró, E.; Farid, M.M.; Cabeza, L.F. Thermal analysis of including phase change material in a domestic hot water cylinder. Appl. Therm. Eng. 2011, 31, 3938–3945. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellón, C.; Nogués, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F.; Lin, S.; Chen, F.; Gao, W. Thermal stability, latent heat and flame retardant properties of the thermal energy storage phase change materials based on paraffin/high density polyethylene composites. Renew. Energy 2009, 34, 2117–2123. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Chen, Z.; Liu, X. Preparation and properties of palmitic acid/SiO2 composites with flame retardant as thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2011, 95, 1875–1881. [Google Scholar] [CrossRef]
- Sittisart, P.; Farid, M.M. Fire retardants for phase change materials. Appl. Energy 2011, 88, 3140–3145. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, Y.; Song, L.; Ni, J.; Xing, W.; Wang, J. Effect of expanded graphite on properties of high-density polyethylene/paraffin composite with intumescent flame retardant as a shape-stabilized phase change material. Sol. Energy Mater. Sol. Cells 2010, 94, 360–365. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F.; Gao, W. Preparation and properties studies of halogen-free flame retardant form-stable phase change materials based on paraffin/high density polyethylene composites. Appl. Energy 2008, 85, 765–775. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, Y.; Song, L.; Tang, Y.; Yang, R.; Zhang, Y.; Chen, Z.; Fan, W. Flammability and thermal properties of high density polyethylene/paraffin hybrid as a form-stable phase change material. J. Appl. Polym. Sci. 2006, 99, 1320–1327. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Morikawa, J.; Hashimoto, T. Simultaneous measurement of heat capacity and thermal diffusivity in solid–solid and solid–liquid phase transitions of n-alkane. Thermochim. Acta 2000, 353, 291–296. [Google Scholar] [CrossRef]
- Inaba, H.; Tu, P. Evaluation of thermophysical characteristics on shape-stabilized paraffin as a solid-liquid phase change material. Heat Mass Transf. 1997, 32, 307–312. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Kurajica, S.; Šipušiæ, J. Thermophysical Comparison of Five Commercial Paraffin Waxes as Latent Heat Storage Materials. Chem. Biochem. Eng. Q. 2010, 24, 129–137. [Google Scholar]
- Código Técnico de la Edificación (Spain). Available online: https://www.codigotecnico.org/images/stories/pdf/ahorroEnergia/DcmHE.pdf (accessed on 12 November 2017).
- RT-21 Paraffin Datasheet. Available online: https://www.rubitherm.eu/media/products/datasheets/Techdata_-RT21_EN_05112015.PDF (accessed on 12 November 2017).
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Nguyen, N.P.M.Q. A review on fire protection for phase change materials in building applications. In From Materials to Structures: Advancement through Innovation; Attard, M.M., Samali, B., Song, C., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 561–566. [Google Scholar]
- Haurie, L.; Fernández, A.I.; Velasco, J.I.; Chimenos, J.M.; Lopez Cuesta, J.-M.; Espiell, F. Synthetic hydromagnesite as flame retardant. Evaluation of the flame behaviour in a polyethylene matrix. Polym. Degrad. Stab. 2006, 91, 989–994. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gi, J.W. An overview of flame retardancy of polymeric materials: application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Flame retardancy of thermoplastic polyesters—A review of the recent literature. Polym. Int. 2005, 54, 11–35. [Google Scholar] [CrossRef]
- Hapuarachchi, T.D.; Peijs, T. Aluminium trihydroxide in combination with ammonium polyphosphate as flame retardants for unsaturated polyester resin. Express Polym. Lett. 2009, 3, 743–751. [Google Scholar] [CrossRef]
- Zhang, S.; Horrocks, A.R. A review of flame retardant polypropylene fibres. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Fan, Z.; Tsakiropoulos, P.; Miodownik, A.P. A generalized law of mixtures. J. Mater. Sci. 1994, 29, 141–150. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N. Pyrolysis combustion flow calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27–46. [Google Scholar] [CrossRef]
- ASTM D7309: Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry; ASTM International: West Conshohocken, PA, USA, 2013.
- UNE-23727:1990: Ensayos de Reacción al Fuego de los Materiales de Construcción. Clasificación de los Materiales Utilizados en la Construcción; Asociacion Espanola de Normalizacion: Madrid, Spain, 1990.
- Lazaro, A.; Peñalosa, C.; Solé, A.; Diarce, G.; Haussmann, T.; Fois, M.; Zalba, B.; Gshwander, S.; Cabeza, L.F. Intercomparative tests on phase change materials characterisation with differential scanning calorimeter. Appl. Energy 2013, 109, 415–420. [Google Scholar] [CrossRef]
- Barreneche, C.; Solé, A.; Miró, L.; Martorell, I.; Fernández, A.I.; Cabeza, L.F. Study on differential scanning calorimetry analysis with two operation modes and organic and inorganic phase change material (PCM). Thermochim. Acta 2013, 553, 23–26. [Google Scholar] [CrossRef]
- Hirschler, M.M. Flame retardants and heat release: review of data on individual polymers. Fire Mater. 2015, 39, 232–258. [Google Scholar] [CrossRef]
- Babrauskas, V.; Peacock, R.D. Heat Release Rate: The Single Most Important Variable in Fire Hazard. Fire Saf. J. 1992, 18, 255–272. [Google Scholar] [CrossRef]
- De Gracia, A.; Barreneche, C.; Farid, M.M.; Cabeza, L.F. New equipment for testing steady and transient thermal performance of multilayered building envelopes with PCM. Energy Build. 2011, 43, 3704–3709. [Google Scholar] [CrossRef]
- ASTM E 1321: Standard Test Method for Determining Material Ignition and Flame Spread Properties; ASTM International: West Conshohocken, PA, USA, 2009.
- Mowrer, F.W. Analysis of Effective Thermal Properties of Thermall Thick Materials; Grant/Contract Reports (NISTGCR) 03-855; The National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2003; p. 25. [Google Scholar]
| Compound | Melting Temperature (°C) | Heat of Fusion (kJ/kg) | Thermal Conductivity (W/m·°C) |
|---|---|---|---|
| Paraffin RT-21 | 21 [23] | 100 [23] | 0.2 [23] |
| 73.5% Capric acid + 26.5% Myristic acid | 24.1 [4] | 152 [4] | n.a |
| 75.2% Capric acid + 24.8% Palmitic acid | 22.1 [4] | 153 [4] | n.a |
| Compound | Method | Onset Decomposition Temperature (°C) | Enthalpy of Decomposition (kJ/kg) |
|---|---|---|---|
| Aluminum hydroxide | Endothermic decomposition | 180 | 1300 |
| Magnesium hydroxide | Endothermic decomposition | 340 | 1450 |
| Hydromagnesite | Endothermic decomposition | 200 | 800 |
| APP | Char forming | 190 | - |
| IFR | Char forming | 190 | - |
| Formulations |
|---|
| PCM + (15%-20%-25%-40%) APP |
| PCM + (40%-50%-60%) Hydromagnesite |
| PCM + (40%-50%-60%) Magnesium hydroxide |
| PCM + (40%-50%-60%) Aluminum hydroxide |
| PCM + (15%-20%-25%) IFR |
| Flame Retardant (wt %) | Ignition Time (s) | N° of Ignitions | Average Combustion Time (s) |
|---|---|---|---|
| Paraffin RT-21 | 26 | 1 | 300 |
| 60% Paraffin + 40% APP | 20 | 3 | 82 |
| 50% Paraffin + 50% HM | 26 | 1 | 293 |
| 60% Paraffin + 20% IFR | 50 | 2 | 239 |
| 50% Paraffin + 50% Al(OH)3 | 27 | 1 | 288 |
| 50% Paraffin + 50% Mg(OH)2 | 26 | 1 | 286 |
| CA + MA | 19 | 1 | 200 |
| 80% (CA + MA) + 20% APP | 12 | 2 | 109 |
| 50% (CA + MA) + 50% HM | 14 | 15 | 8 |
| 80% (CA + MA) + 20% IFR | 21 | 2 | 102 |
| 50% (CA + MA) + 50% Al(OH)3 | 19 | 2 | 117 |
| 50% (CA + MA) + 50% Mg(OH)2 | 24 | 17 | 4 |
| CA + PA | 12 | 1 | 224 |
| 80% (CA + PA) + 20% APP | 10 | 2 | 104 |
| 50% (CA + PA) + 50% HM | 9 | 17 | 7 |
| 80% (CA + PA) + 20% IFR | 16 | 3 | 106 |
| 50% (CA + PA) + 50% Al(OH)3 | 14 | 1 | 322 |
| 50% (CA + PA) + 50% Mg(OH)2 | 32 | 26 | 4 |
| Compositions | Melting Enthalpy (kJ/kg) | Peak Temperature (°C) | |
|---|---|---|---|
| PCMs | Paraffin RT-21 | 118 ± 3 | 22.3 ± 0.2 |
| 73.5% Capric acid + 26.5% Myristic acid | 143 ± 3 | 24.1 ± 0.2 | |
| 75.2% Capric acid + 24.8% Palmitic acid | 141 ± 3 | 23.3 ± 0.2 | |
| PCM + Flame Retardant | 60% Paraffin RT-21 + 40% APP | 111 ± 4 | 22.6 ± 0.2 |
| 50% CA + MA + 50% Hydromagnesite | 53 ± 3 | 22.0 ± 0.2 | |
| 50% CA + MA + 50% Magnesium hydroxide | 55 ± 3 | 24.4 ± 0.2 | |
| 50% CA+PA + 50% Hydromagnesite | 56 ± 1 | 19.0 ± 0.6 | |
| 50% CA+PA + 50% Magnesium hydroxide | 55 ± 2 | 23.0 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios, A.; De Gracia, A.; Haurie, L.; Cabeza, L.F.; Fernández, A.I.; Barreneche, C. Study of the Thermal Properties and the Fire Performance of Flame Retardant-Organic PCM in Bulk Form. Materials 2018, 11, 117. https://doi.org/10.3390/ma11010117
Palacios A, De Gracia A, Haurie L, Cabeza LF, Fernández AI, Barreneche C. Study of the Thermal Properties and the Fire Performance of Flame Retardant-Organic PCM in Bulk Form. Materials. 2018; 11(1):117. https://doi.org/10.3390/ma11010117
Chicago/Turabian StylePalacios, Anabel, Alvaro De Gracia, Laia Haurie, Luisa F. Cabeza, A. Inés Fernández, and Camila Barreneche. 2018. "Study of the Thermal Properties and the Fire Performance of Flame Retardant-Organic PCM in Bulk Form" Materials 11, no. 1: 117. https://doi.org/10.3390/ma11010117
APA StylePalacios, A., De Gracia, A., Haurie, L., Cabeza, L. F., Fernández, A. I., & Barreneche, C. (2018). Study of the Thermal Properties and the Fire Performance of Flame Retardant-Organic PCM in Bulk Form. Materials, 11(1), 117. https://doi.org/10.3390/ma11010117









