Modeling of Production and Quality of Bioethanol Obtained from Sugarcane Fermentation Using Direct Dissolved Sugars Measurements
Abstract
:1. Introduction
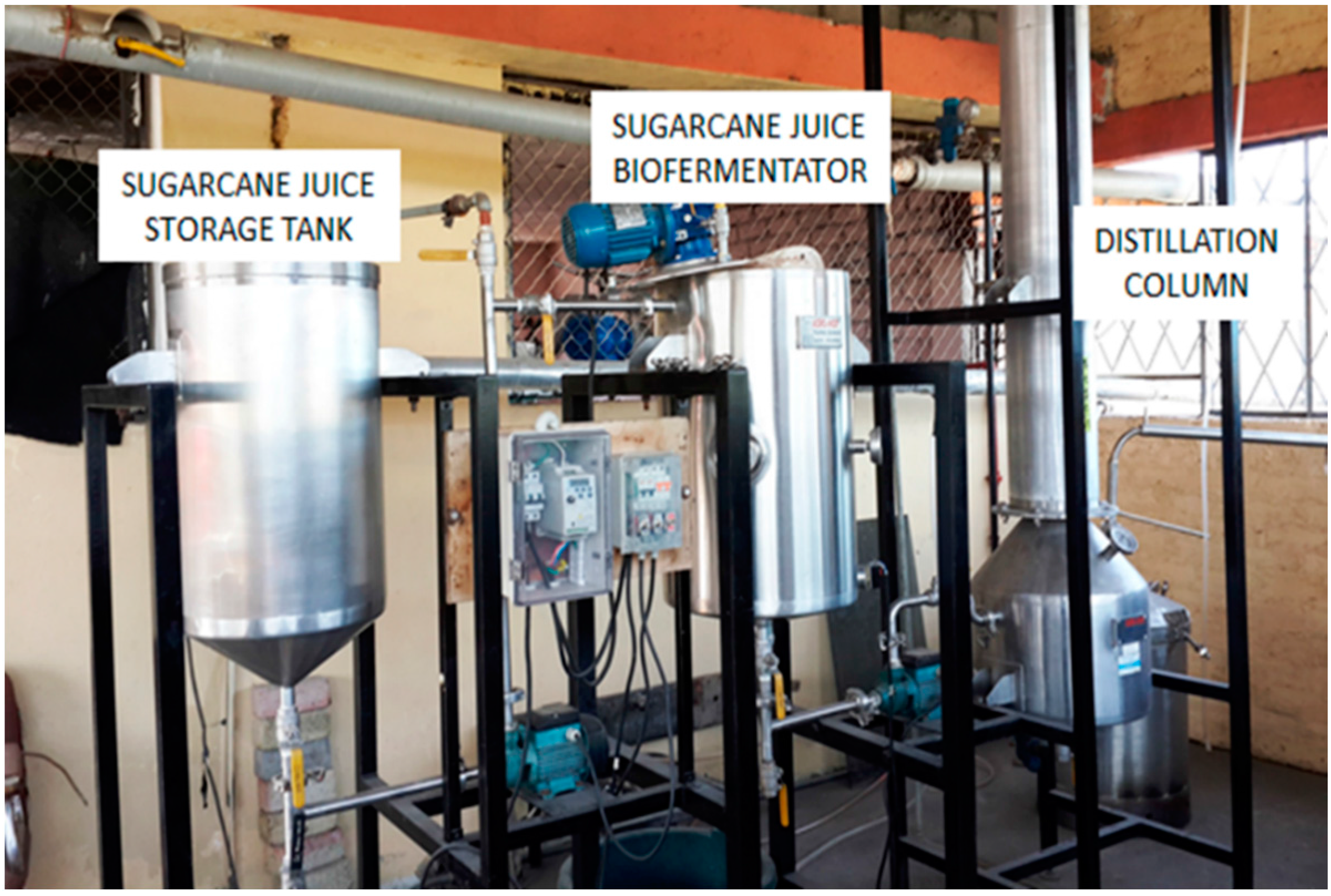
2. Material and Methods
2.1. Sample Preparation
2.2. Mathematical Modeling to Predict Performance and Product Quality
3. Results and Discussion
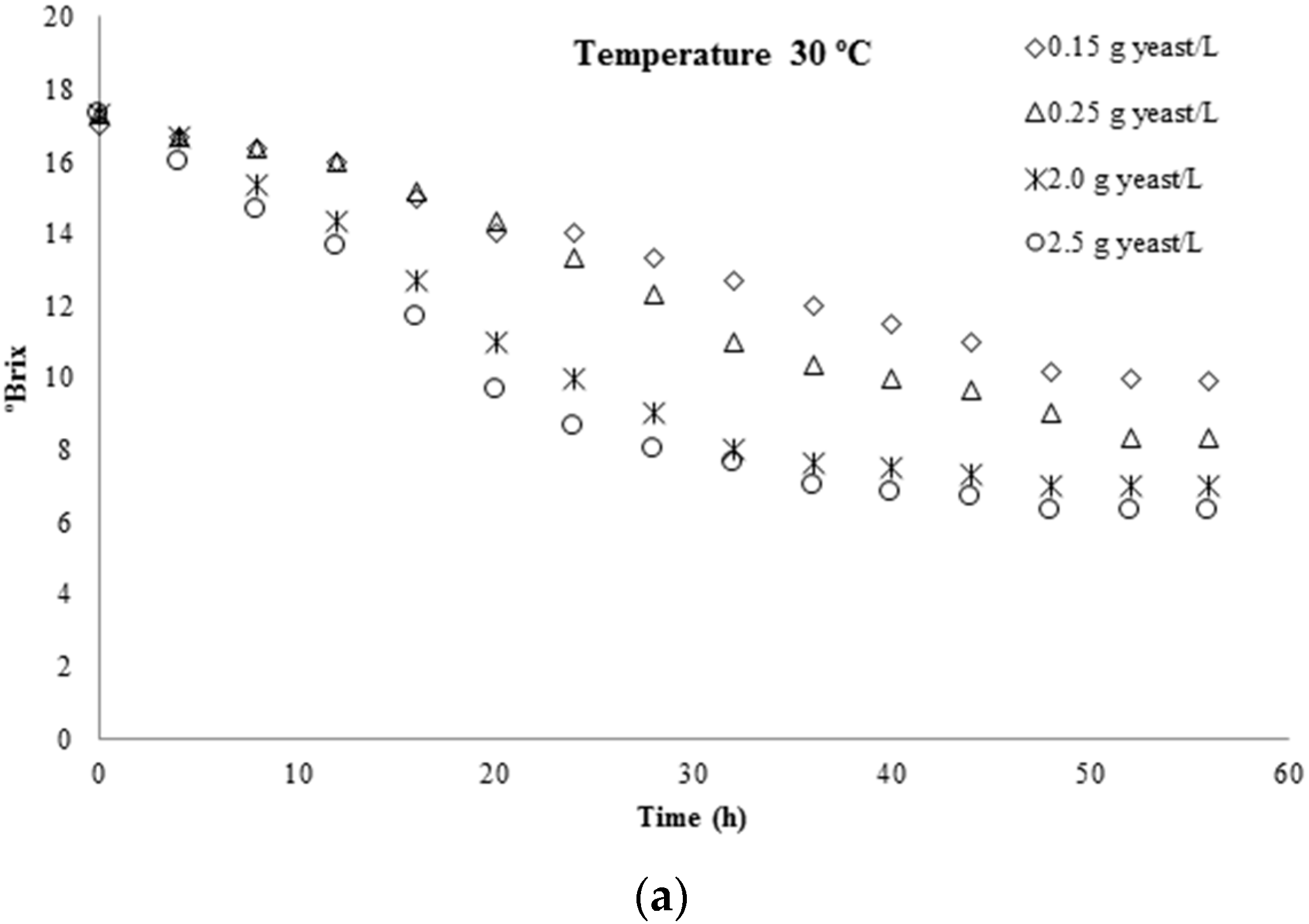
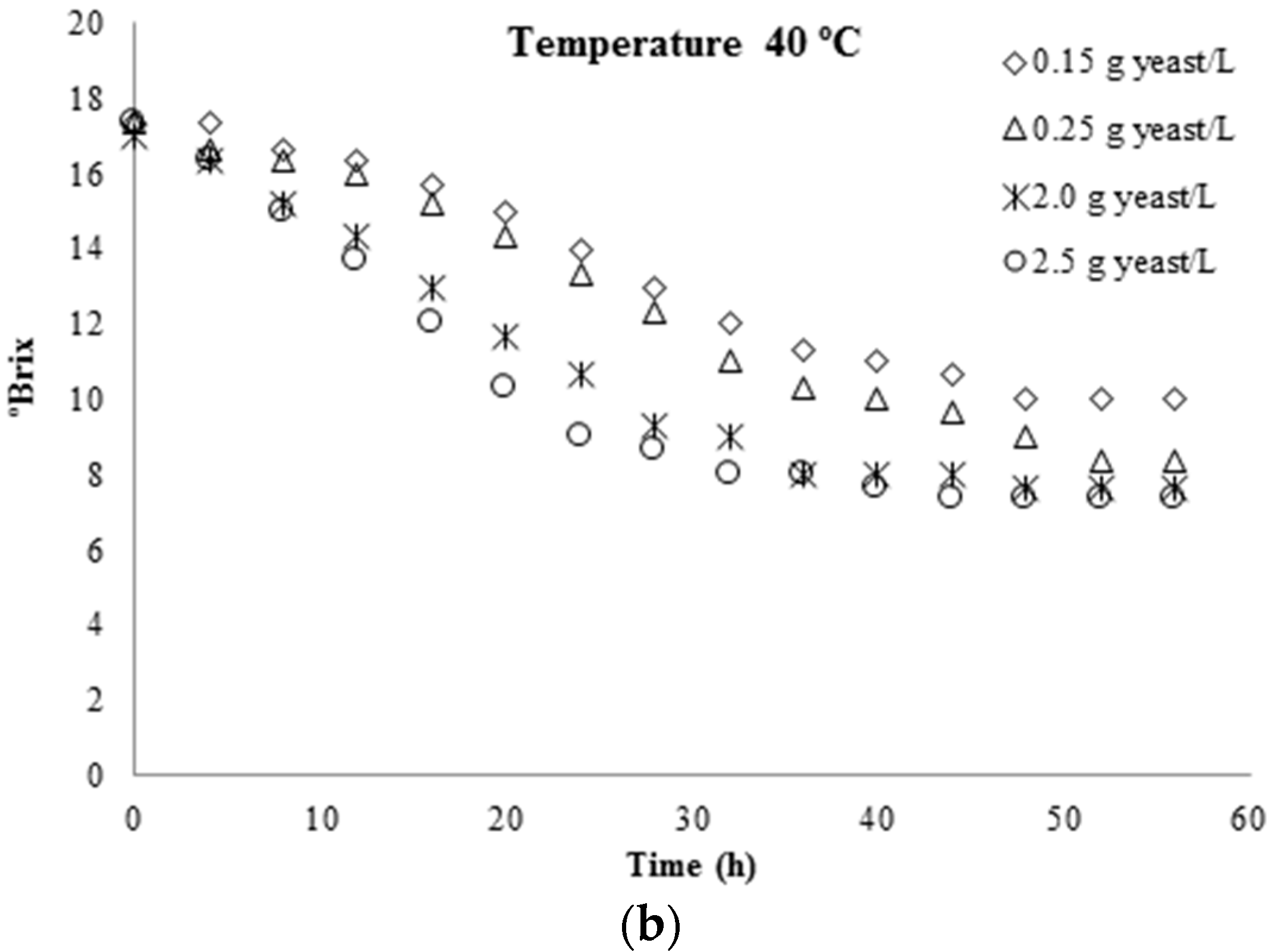
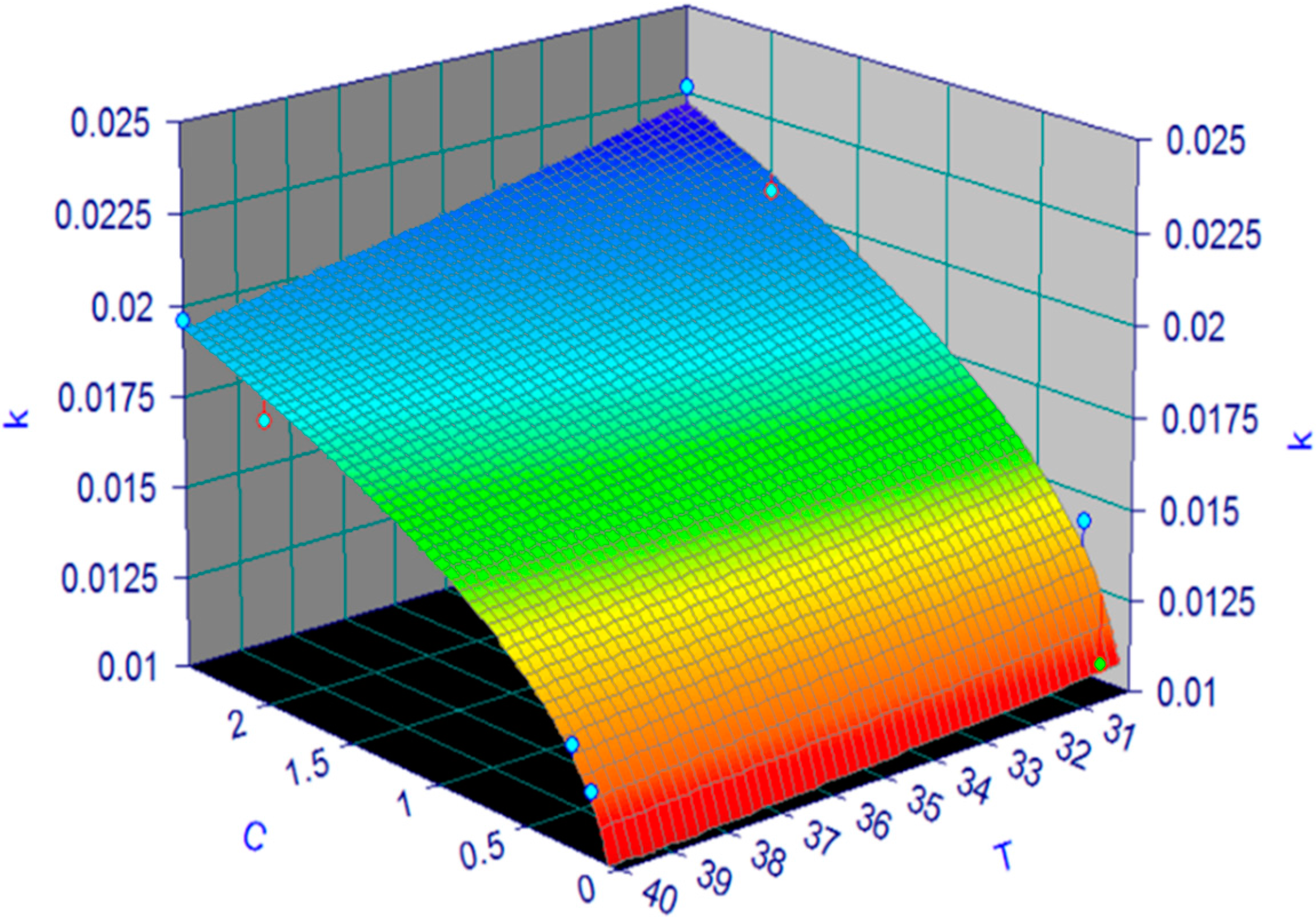
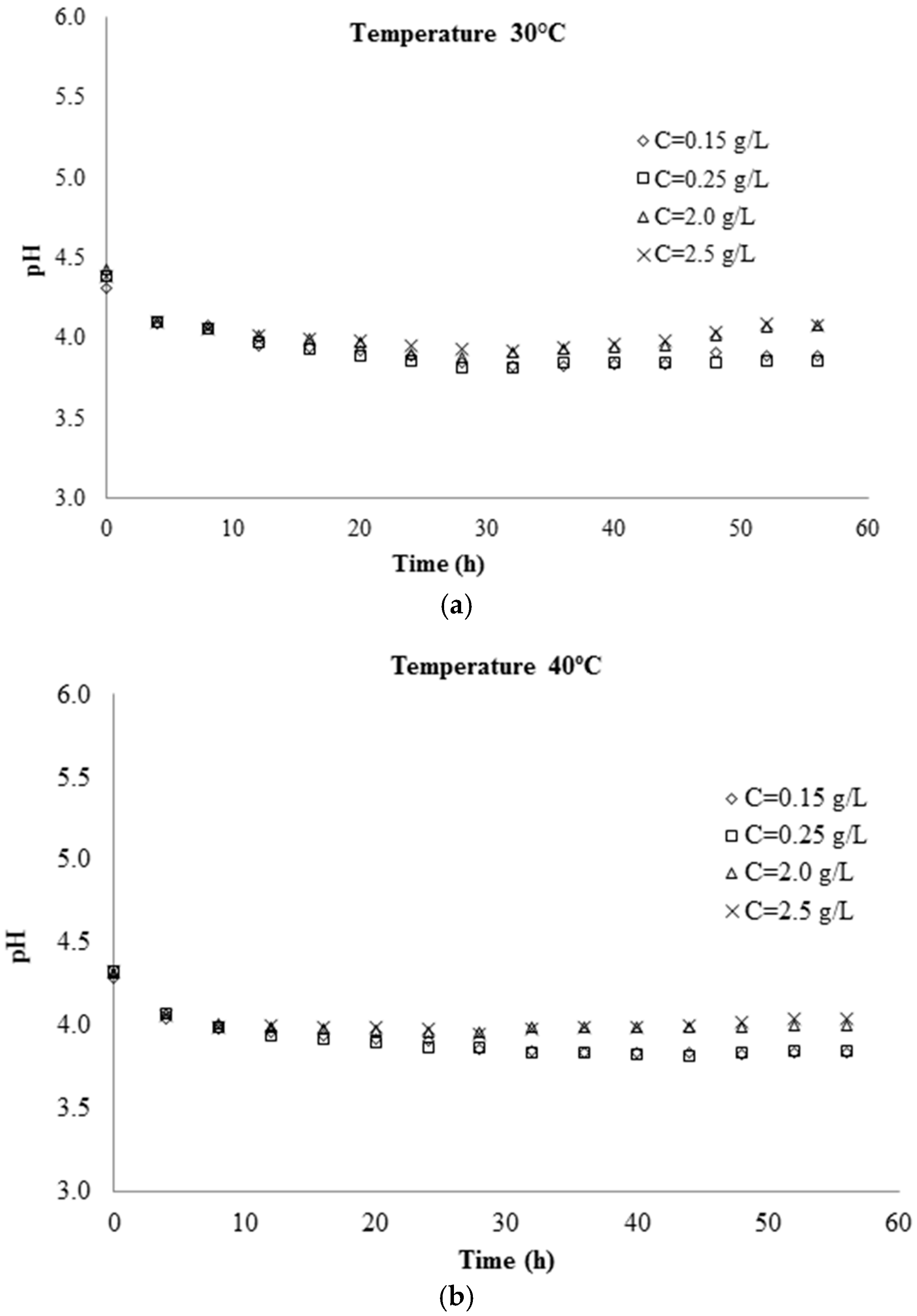
3.1. Kinetic Parameters Determination
3.2. Prediction of Alcohol Volume
3.3. Prediction of Alcoholic Degree (AD)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Acronyms and Symbols
| °Bo | Initial Brix degree |
| °B(t) | |
| C | Concentration of yeast |
| (°Brix−1) | Proportionality constant |
| Volumetric fraction of alcohol | |
| INEN | Instituto Ecuatoriano de Normalización |
| k (h−1) | Kinetic rate constant |
| MCPEC | Ministerio Coordinator de Produccion, Empleo y Competividad |
| NIR | Near infrared |
| Standard deviation | |
| t (s) | Time |
| T (°C) | Temperature |
| Va (mL) | Alcohol volume |
| Vo (mL) | Initial sugar juice volume |
| UTM | Universal Transverse Mercator |
References
- Xavier, M.R. The Brazilian sugarcane ethanol experience. Issue Anal. 2007. Available online: https://cei.org/search/node/ethanol?f[0]=created%3A2007&f[1]=field_experts%3A48291 (accessed on 2 February 2014).
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemicalpathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Kim, S.; Dale, E.B. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- James, S.A.; Carvajal Barriga, E.J.; Barahona, P.P.; Nueno-Palop, C.; Cross, K.; Bond, C.J.; Roberts, I.N. Kazachstania yasuniensis sp. nov., an ascomycetous yeast species found in mainland Ecuador and on the Galápagos. Int. J. Syst. Evol. Microbiol. 2015, 65, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, T. Chemical treatments of crop residues. J. Anim. Sci. 1978, 46, 841–848. [Google Scholar]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2009, 101, 4754–4766. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agroindustrial residues. I: Sugarcane baggase. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Pandey, A. Handbook of Plant-Based Biofuels; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Luttmann, R.; Bracewell, D.G.; Cornelissen, G.; Gernaey, K.V.; Glassey, J.; Hass, V.C.; Kaiser, C.; Lidnstöm, I.M.; Preusee, C.; Striedner, G.; et al. Soft sensors in bioprocessing: A status report and recommendation. Biotechnol. J. 2012, 7, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Faassen, S.M.; Hitzmann, B. Fluorescence Spectroscopy and Chemometric Modeling for Bioprocess Monitoring. Sensors 2015, 15, 10271–10291. [Google Scholar] [CrossRef] [PubMed]
- Alatraktchi, F.A.; Bakmand, T.; Dimaki, M.; Svendsen, W.E. Novel Membrane-Based Electrochemical Sensor for Real-Time Bio-Applications. Sensors 2014, 14, 22128–22139. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.S.A.; Inoue, H.; Endo, T.; Yano, S.; Bon, E.P.S. Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 2010, 101, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Klinke, H.B.; Thomsen, A.B. Wet oxidation as a pretreatment method for enhancing the enzymatic convertibility of sugarcane bagasse. Enzyme Microb. Technol. 2007, 40, 426–432. [Google Scholar] [CrossRef]
- Singh, P.; Suman, A.; Tiwari, P.; Arya, N.; Gaur, A.; Shrivastava, A.K. Biological pretreatment of sugarcane trash for its conversion to fermentable sugars. World J. Microbiol. Biotechnol. 2008, 2, 4667–4673. [Google Scholar] [CrossRef]
- Rao, R.S.; Jyothi, C.P.; Prakasham, R.S.; Sarma, P.N.; Rao, L.V. Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresour. Technol. 2006, 97, 1974–1978. [Google Scholar] [CrossRef] [PubMed]
- Sendelius, J. Steam Pretreatment Optimisation for Sugarcane Bagasse in Bioethanol Production. Master’s Thesis, Lund University, Lund, Sweden, 2005. [Google Scholar]
- Arroyo-Lopez, F.N.; Querol, A.; Barrio, E. Application of a substrate inhibition model to estimate the effect of fructose concentration on the growth of diverse Saccharomyces cerevisiae strains. J. Ind. Microbiol. Biotechnol. 2009, 36, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.; Arango, R. Evaluación de la producción de etanol utilizando cepas recombinantes de Saccharomyces cerevisiae a partir de melaza de caña de azúcar. Dyna 2009, 76, 153–161. [Google Scholar]
- Amores, M.J.; Mele, F.D.; Jiménez, L.; Castells, F. Life cycle assessment of fuel ethanol from sugarcane in Argentina. Int. J. Life Cycle Assess. 2013, 18, 1344–1357. [Google Scholar] [CrossRef]
- Balakumar, S.; Arasaratnam, V. Enhanced production of ethanol by high gravity glucose fermentation at temperatures above 40 °C by Saccharomyces cerevisiae S1 using a soya flour supplemented medium. J. Natl. Sci. Sri Lanka 2014, 42, 111–117. [Google Scholar] [CrossRef]
- Fruit Juice, Purees, Concentrates, Nectar and Beverage, 1st ed.; NTE INEN 2337:2008; Instituto Ecuatoriano de Normalización, Norma Técnica Ecuatoriana: Quito, Ecuador, 2008.
- Bebidas Alcohólicas. Determinación del Grado Alcohólico; NTE INEN 0340:1994; Instituto Ecuatoriano de Normalización, Norma Técnica Ecuatoriana: Quito, Ecuador, 1994.

| Sector | UTM | ||
|---|---|---|---|
| X | Y | Z | |
| Allaga Paredes Zoila Rosa | 714117 | 9869856 | 1664 |
| Caiza Yanchaliquín Luis Ernesto | 713832 | 9867747 | 1870 |
| Falcón Mera Dolores Narcisa | 705624 | 9871465 | 631 |
| Flores Ruiz Felix | 715428 | 9866975 | 1718 |
| Guerrero Meneses Angel Froilan | 710081 | 9870847 | 976 |
| Jaramillo Pacha Bertha Lucía | 708897 | 9871408 | 813 |
| Merino Ayme Rosa Adela | 715358 | 9866797 | 1658 |
| Ruiz Guerrero Wilsón Rodrigo | 709556 | 9872138 | 814 |
| Zapata Guerrero Arcadio Heremitis | 709677 | 9870184 | 1075 |
| Temperature (°C) | 30 | 40 | ||||||
|---|---|---|---|---|---|---|---|---|
| Yeast Concentration (g·L−1) | 0.15 | 0.25 | 2.0 | 2.5 | 0.15 | 0.25 | 2.0 | 2.5 |
| °Bo (Brix) | 17.544 | 18.145 | 17.493 | 17.042 | 18.171 | 17.844 | 17.074 | 16.878 |
| k (h−1) | 0.0104 | 0.0142 | 0.0204 | 0.0227 | 0.0117 | 0.0127 | 0.0178 | 0.0196 |
| R2 | 0.987 | 0.977 | 0.962 | 0.954 | 0.973 | 0.977 | 0.961 | 0.932 |
| Temperature (°C) | 30 | 40 | ||||||
|---|---|---|---|---|---|---|---|---|
| Yeast Concentration (g·L−1) | 0.15 | 0.20 | 2.0 | 2.5 | 0.15 | 0.20 | 2.0 | 2.5 |
| °Bo (Brix) | 17.54 | 18.14 | 17.49 | 17.04 | 18.17 | 17.84 | 17.07 | 16.88 |
| k (h−1) | 0.0104 | 0.0142 | 0.0204 | 0.0227 | 0.0117 | 0.0127 | 0.0178 | 0.0196 |
| Va (mL) | 33.67 | 34.67 | 47.67 | 40.33 | 33.33 | 33.33 | 36.33 | 40.00 |
| Vo (mL) | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| t (h) | 56 | 56 | 56 | 56 | 56 | 56 | 56 | 56 |
| (adim.) | 0.13 | 0.14 | 0.19 | 0.16 | 0.13 | 0.13 | 0.15 | 0.13 |
| (Brix−1) | 0.017 | 0.014 | 0.016 | 0.013 | 0.015 | 0.015 | 0.014 | 0.014 |
| Temperature (°C) | 30 | 40 | |||
|---|---|---|---|---|---|
| Yeast Concentration (g·L−1) | 0.2 | 1.5 | 0.2 | 1.5 | |
| °Bo (Brix) | 17.33 | 17.00 | 17.33 | 17.00 | |
| Sugar juice volume V0 (mL) | 250 | 250 | 250 | 250 | |
| k (h−1) Equation (6) | 0.0130 | 0.0180 | 0.0115 | 0.0165 | |
| t (h) | 56 | 56 | 56 | 56 | |
| average (Brix−1) | 0.0148 | 0.0148 | 0.0148 | 0.0148 | |
| Va (mL): experiment | 32.33 | 40.33 | 32.33 | 39.00 | |
| Va (mL): predicted Equation (5) | 32.98 | 39.81 | 30.31 | 37.84 | |
| Error (%) | 2.01 | 1.29 | 6.24 | 2.97 | |
| Temperature | T = 30 °C | T = 40 °C | ||||
|---|---|---|---|---|---|---|
| Yeast Concentration (g·L−1) | 0.15 | 0.20 | 0.15 | 0.15 | 0.20 | 0.15 |
| oBo (Brix): Exp. | 17.00 | 17.331 | 17.33 | 17.00 | 17.33 | 17.33 |
| Vo (mL): Exp. | 15000 | 15000 | 15000 | 15000 | 15000 | 15000 |
| k (h−1): Equation (6) | 0.0128 | 0.0130 | 0.0131 | 0.0113 | 0.0115 | 0.0117 |
| t (h): Exp. | 56 | 56 | 56 | 56 | 56 | 56 |
| average (Brix−1) | 0.0148 | 0.0148 | 0.0148 | 0.0148 | 0.0148 | 0.0148 |
| Va (mL): Exp. | 2375 | 2475 | 2490 | 2290 | 2520 | 1375 |
| Va (mL): model Equation (5) | 2342.1 | 2412.8 | 2437.2 | 2149.3 | 2217.8 | 2244.4 |
| Error (%) | 1.39 | 2.51 | 2.12 | 6.14 | 7.20 | 5.50 |
| AD (Experimental) | Yeast Concentration C (g·L−1) | Alcohol Volume
Va (mL) | Yeast Concentration/Alcohol Volume (C/Va) |
|---|---|---|---|
| 32.5 | 0.15 | 33.67 | 0.0045 |
| 32.6 | 0.2 | 32.33 | 0.0062 |
| 31 | 0.25 | 33.33 | 0.0075 |
| 33.7 | 1.5 | 39.00 | 0.0385 |
| 36.2 | 2 | 36.33 | 0.0550 |
| 40.1 | 2.5 | 40.33 | 0.0620 |
| 38.5 | 2.5 | 40.00 | 0.0625 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velazquez-Marti, B.; Pérez-Pacheco, S.; Gaibor-Chávez, J.; Wilcaso, P. Modeling of Production and Quality of Bioethanol Obtained from Sugarcane Fermentation Using Direct Dissolved Sugars Measurements. Energies 2016, 9, 319. https://doi.org/10.3390/en9050319
Velazquez-Marti B, Pérez-Pacheco S, Gaibor-Chávez J, Wilcaso P. Modeling of Production and Quality of Bioethanol Obtained from Sugarcane Fermentation Using Direct Dissolved Sugars Measurements. Energies. 2016; 9(5):319. https://doi.org/10.3390/en9050319
Chicago/Turabian StyleVelazquez-Marti, Borja, Sergio Pérez-Pacheco, Juan Gaibor-Chávez, and Paola Wilcaso. 2016. "Modeling of Production and Quality of Bioethanol Obtained from Sugarcane Fermentation Using Direct Dissolved Sugars Measurements" Energies 9, no. 5: 319. https://doi.org/10.3390/en9050319
APA StyleVelazquez-Marti, B., Pérez-Pacheco, S., Gaibor-Chávez, J., & Wilcaso, P. (2016). Modeling of Production and Quality of Bioethanol Obtained from Sugarcane Fermentation Using Direct Dissolved Sugars Measurements. Energies, 9(5), 319. https://doi.org/10.3390/en9050319







