2.2. Separator Evaluation in the FPMFCs
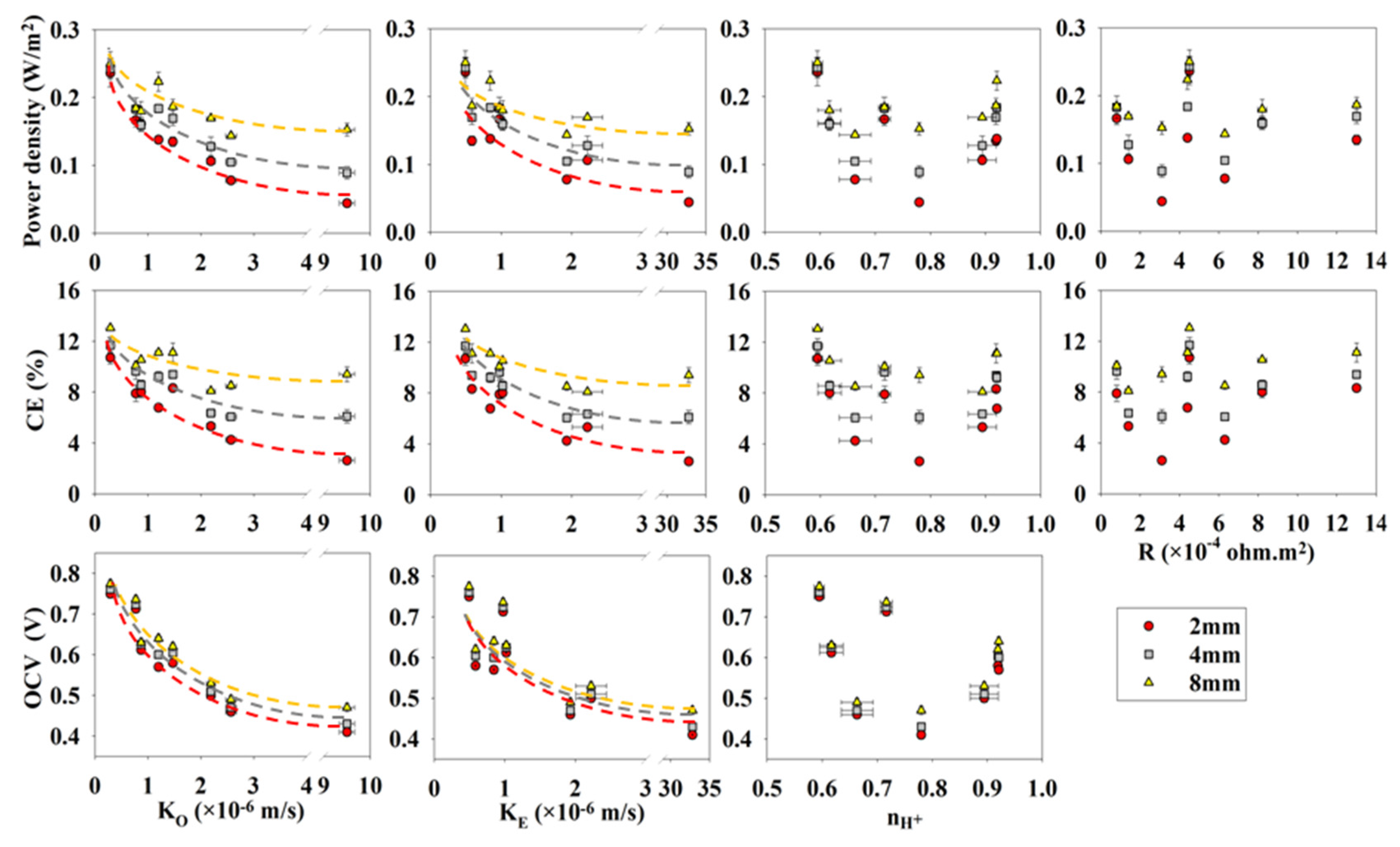
The performance of the passive air-breathing FPMFCs was greatly affected by the characteristics of the separators placed next to the air-cathode. Power density decreased from
ca. 0.25 W·m
−2 to
ca. 0.05 W·m
−2 as the mass transfer coefficient of oxygen increased from
ca. 0.3 × 10
−6 m·s
−1 to over 9 × 10
−6 m·s
−1 (
Figure 1). The mass transfer coefficient of oxygen through the air-cathode could be one order of magnitude higher than that in the separators [
22,
23]. That is why the separator played a crucial role in blocking the oxygen diffusion through the air-breathing cathode. The peak power density and coulombic efficiency (CE), therefore, increased significantly as the electrode spacing increased when separators with high oxygen crossover were used. The rate of decrease for superficial peak power density was 0.2 W·m
−2 per mm of the electrode spacing in the 2 mm FPMFC and it decreased to 0.02 W·m
−2 per mm of the electrode spacing in the 8 mm FPMFC. This indicated the high sensitivity of the peak power density and CE to the crossover in the 2 mm FPMFC. This sensitivity decreased as the electrode spacing increased.
Figure 1.
Performance of the 2, 4 and 8 mm FPMFCs versus the separator characteristics.
Figure 1.
Performance of the 2, 4 and 8 mm FPMFCs versus the separator characteristics.
The open circuit voltage was sensitive to the crossover in all three FPMFCs. It decreased from ca. 0.76 V to ca. 0.4 V as the mass transfer coefficients of oxygen and ethanol increased from 0.3 × 10−6 m·s−1 and 0.49 × 10−6 m·s−1 in Nafion®117 to 9.5 × 10−6 m·s−1 and 33 × 10−6 m·s−1 in J-cloth, respectively. The high sensitivity of the open circuit voltage (OCV) to crossover in the 8 mm FPMFC, as opposed to the relatively lower sensitivity of power density, indicated that although the presence of oxygen at the anode altered the anode potential, it did not inhibit the microbial culture significantly.
Power generation using the biofilm is dependent on the population of the microbial cells contributing to the current generation as well as the rate of the fuel uptake and the electron transfer to the anode [
24]. All MFC analysis tests were replicated at least three times and a maximum standard error of
ca. 0.02 W·m
−2 was recorded. The fairly reproducible performance data leads to the conclusion that the morphology of the microbial communities (and their characteristics) in the FPMFCs likely did not change significantly within the period of operation. Therefore, the presence of oxygen in the anode chamber likely reduced the number of the cells contributing to the current generation. As the electrode spacing increased, the concentration of oxygen decreased within the biofilm resulting in a less significant effect of oxygen crossover in the 4 and 8 mm FPMFCs, and in more cells contributing to the current generation. This explains why a larger variation in the peak power density and CE was observed as the electrode spacing decreased.
Table 3 presents the OCV, the superficial and volumetric peak power densities, and the CE of the 2, 4 and 8 mm FPMFCs operated with the selected separators. The highest peak power density in the 2 mm FPMFC was produced using Nafion
®117 (0.24 ± 0.02 W·m
−2). This was due to the low mass transfer coefficient of oxygen, ethanol, and acetate, which provided better condition for the anaerobic bacteria and also decreased the mixed potentials at the electrodes. Aquivion
® and glass fiber filter produced similar peak power densities after Nafion
®117, likely due to the similar crossover of oxygen and fuel, and proton transport number. Also, using glass fiber filter and Aquivion
® similar start-up times were observed, most likely resulting from their similar anode performance [
17]. The generated peak power density further decreased when Nylon mesh, SciMat
®, and Celgard
® were utilized. This was followed by a large decrease with the use of J-cloth which has much higher oxygen and fuel crossover. As the variation in the proton transport number of the separators indicated a maximum potential variation of
ca. 40 mV, the cathode performance was assumed to be similar using different separators in short-term. The anode performance, on the other hand, was significantly affected by the crossover of oxygen and therefore, the anode performance was likely responsible for the performance variation using different separators.
The contribution of oxygen crossover to the performance decreased when the electrode spacing increased. That is why diaphragms performed better in the 4 mm FPMFC compared to the 2 mm FPMFC. While the peak power density did not vary significantly using Nafion®117, Aquivion®, and glass fiber filter, it increased using diaphragms with higher permeability to oxygen. Zirfon® and Celgard® showed a greater extent of increase in the peak power density by increasing the electrode spacing, while the increase was less significant using Nylon mesh, SciMat®, and J-cloth. Although further increase of the electrode spacing resulted in an increased Ohmic overpotential, the 8 mm FPMFC showed an improved performance compared to the 4 and the 2 mm FPMFCs. This indicated that oxygen crossover was the main factor affecting the performance of the FPMFCs and Ohmic overpotential played a less significant role. Celgard® produced a peak power density of ca. 0.22 W·m−2 in the 8 mm FPMFC, close to that produced using Nafion®117, as a result of the decreased oxygen concentration at the anode, and a slightly improved cathode performance due to improved transfer of protons.
Table 3.
Performance characteristics of the 2 mm, 4 mm, and 8 mm FPMFCs with the selected separators.
Table 3.
Performance characteristics of the 2 mm, 4 mm, and 8 mm FPMFCs with the selected separators.
| Separator | OCV(V) ± 0.01 | Superficial Peak Power Density (W·m−2) | Volumetric Peak Power Density (W·m−3) | Coulombic Efficiency (%) |
|---|
| 2 mm | 4 mm | 8 mm | 2 mm | 4 mm | 8 mm | 2 mm | 4 mm | 8 mm | 2 mm | 4 mm | 8 mm |
|---|
| Nafion®117 | 0.75 | 0.76 | 0.77 | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.25 ± 0.02 | 110 ± 8 | 58 ± 4 | 30 ± 2 | 10.7 ± 0.5 | 11.7 ± 0.6 | 13.1 ± 0.2 |
| Aquivion®E79-03 | 0.71 | 0.72 | 0.73 | 0.17 ± 0.01 | 0.18 ± 0.02 | 0.18 ± 0.01 | 79 ± 5 | 43 ± 4 | 22 ± 1 | 7.9 ± 0.6 | 9.6 ± 0.6 | 10.1 ± 0.3 |
| Celgard®5511 | 0.57 | 0.60 | 0.64 | 0.14 ± 0.01 | 0.18 ± 0.01 | 0.22 ± 0.01 | 65 ± 3 | 44 ± 1 | 27 ± 1 | 6.8 ± 0.3 | 9.2 ± 0.4 | 11.1 ± 0.2 |
| Zirfon®Perl | 0.58 | 0.60 | 0.62 | 0.14 ± 0.01 | 0.17 ± 0.01 | 0.19 ± 0.01 | 64 ± 3 | 40 ± 2 | 22 ± 1 | 8.3 ± 0.2 | 9.4 ± 0.3 | 11.1 ± 0.8 |
| Nylon mesh | 0.50 | 0.51 | 0.53 | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.17 ± 0.01 | 50 ± 2 | 30 ± 3 | 20 ± 1 | 5.3 ± 0.2 | 6.4 ± 0.1 | 8.1 ± 0.2 |
| Glass fiber filter | 0.61 | 0.62 | 0.63 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.18 ± 0.01 | 77 ± 4 | 38 ± 2 | 21 ± 2 | 8.0 ± 0.5 | 8.6 ± 0.4 | 10.5 ± 0.1 |
| SciMat®700/20 | 0.46 | 0.47 | 0.49 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.14 ± 0.01 | 37 ± 1 | 25 ± 1 | 17 ± 1 | 4.3 ± 0.1 | 6.1 ± 0.1 | 8.5 ± 0.3 |
| J-cloth | 0.41 | 0.43 | 0.47 | 0.04 ± 0.0 | 0.09 ± 0.01 | 0.15 ± 0.01 | 18 ± 2 | 21 ± 2 | 18 ± 1 | 2.6 ± 0.1 | 6.1 ± 0.5 | 9.4 ± 0.6 |
Wastewater treatment efficiency decreased slightly as the electrode spacing increased (data not shown). This was due to a greater portion of the wastewater bypassing the 3D anode with larger electrode spacing. Also, the treatment efficiency increased as separators with larger pores were used, due to the high crossover of oxygen which contributed to the degradation of ethanol by the communities using oxygen as the final electron acceptor. The higher crossover of ethanol, on the other hand, resulted in direct ethanol oxidation at the cathode, and hence improved the overall treatment efficiency.
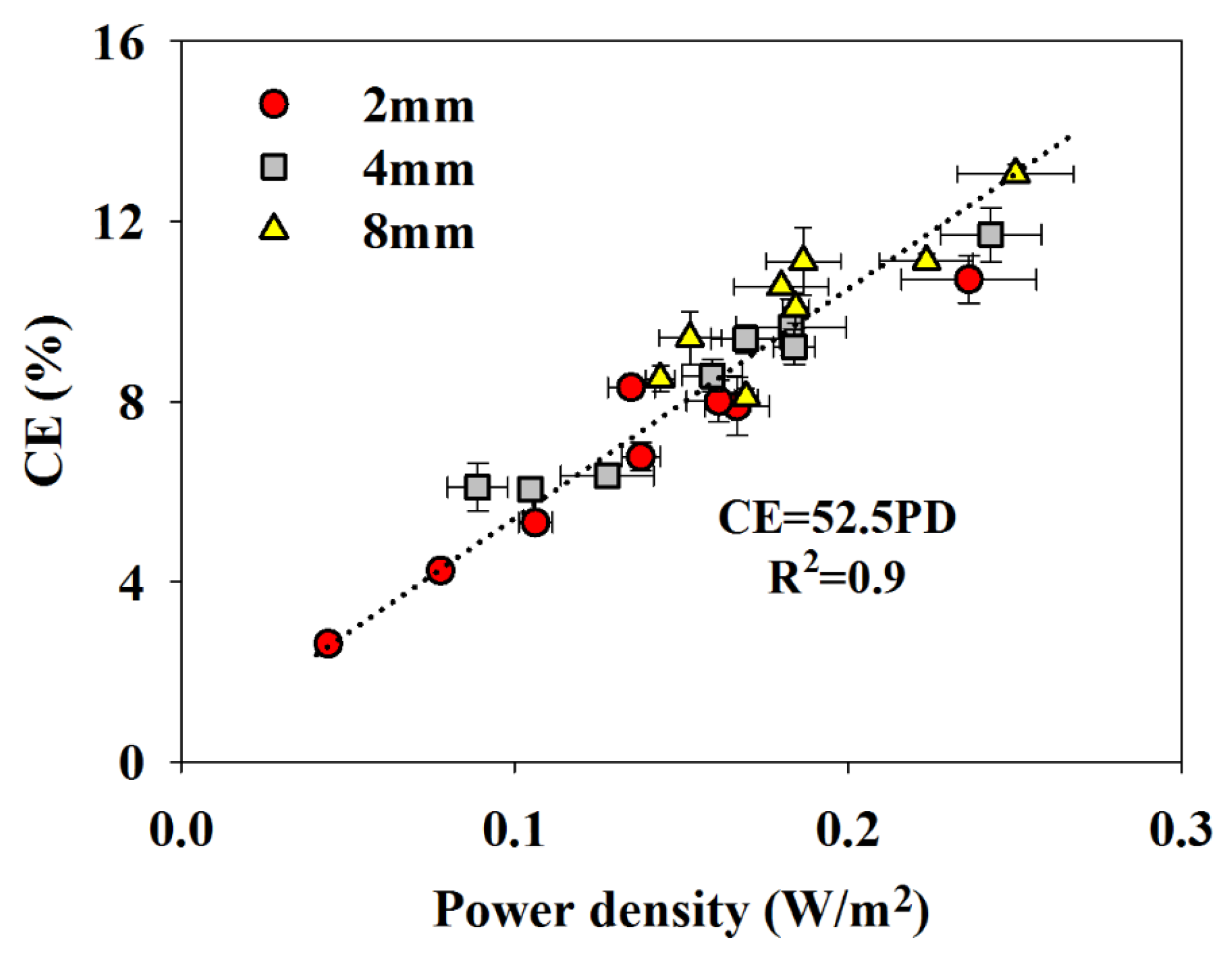
When the separators were examined in terms of the peak power density and CE, it was observed that those two parameters were linearly correlated (
Figure 2), showing that the power output was mainly affected by oxygen and fuel crossover. This was in contrast with the trend reported by Zhang
et al. [
12], who observed a decrease in the CE (from 70% to 40%) as the power density increased (from 1 W·m
−2 to 0.5 W·m
−2) in a passive air-breathing MFC with the cathode placed 2 cm apart from the anode. The authors indicated that the power output increased as separators with larger pores were used, which was due to the decreased Ohmic overpotential and the improved cathode performance. The CE, on the other hand, decreased as separators with larger pores were used due to the increased crossover.
Figure 2.
Coulombic efficiency versus peak power density (PD) with different separators in the 2 mm, 4 mm, and 8 mm FPMFC.
Figure 2.
Coulombic efficiency versus peak power density (PD) with different separators in the 2 mm, 4 mm, and 8 mm FPMFC.
It was observed that the peak power density of the FPMFCs decreased as separators with larger pores were used, indicating that the effect of proton transport number was less significant than the oxygen crossover. Variation of the proton transport number using the selected separators could result in only 0.04 V difference in the cathode potential, which could not significantly affect the performance. Presence of the phosphate buffer is suspected to play a role in balancing the pH of the anode.
The much smaller electrode spacing applied here increased the concentration of oxygen at the anode, resulting in a significant decrease in the peak power density and the CE. The extent of the fuel crossover likely did not vary significantly with electrode spacing in the FPMFCs while the CE and the peak power density increased as the electrode spacing increased. Therefore, it could be concluded that the oxygen crossover was playing a more important role in the FPMFC performance than the fuel crossover and the proton transport number of the separator.
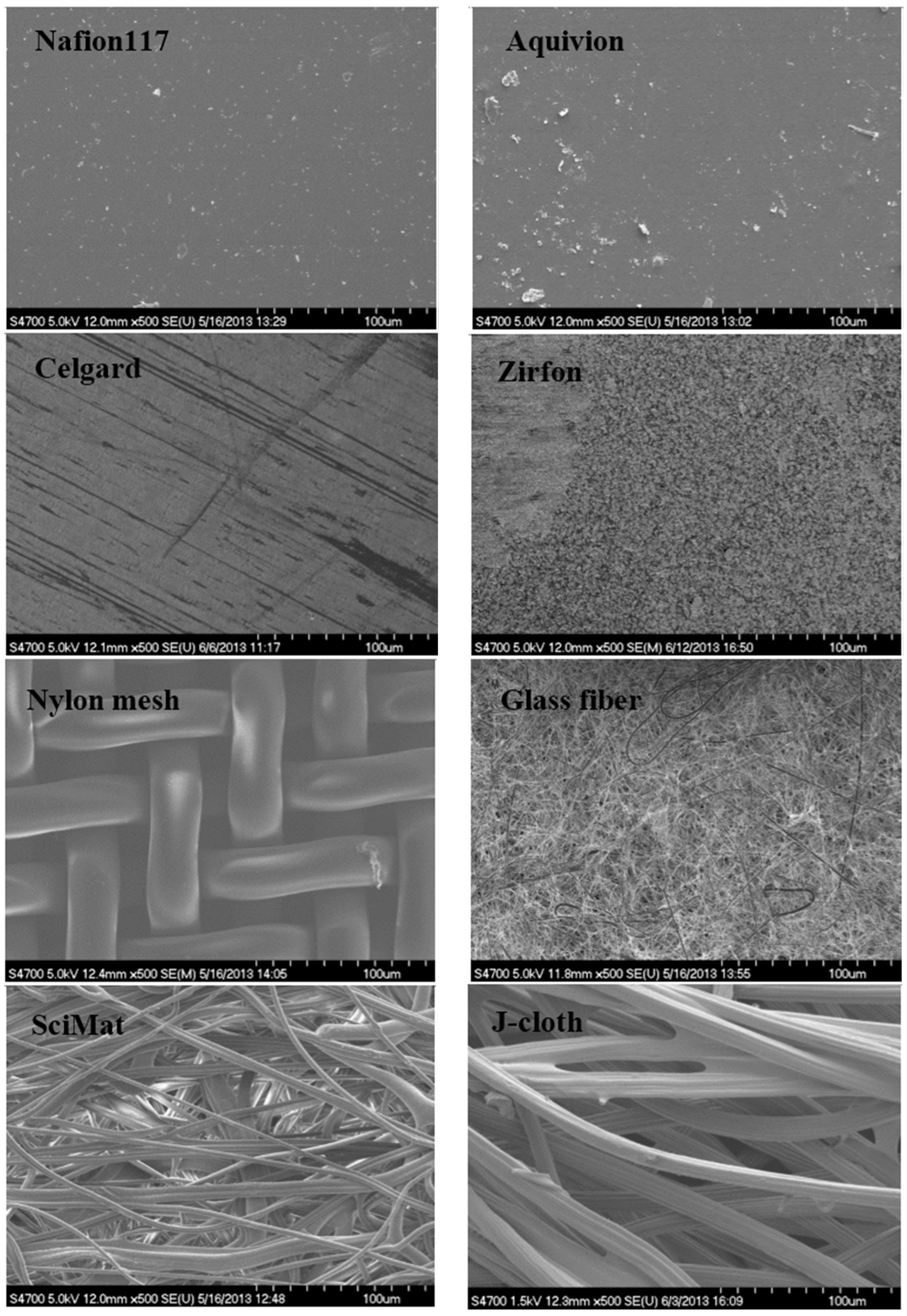
Scanning electron microscopy images from the anode-facing side of the separators from all three FPMFCs indicated significant biofilm growth on the surface of the separators, after 4 weeks of operation, except for the Nylon mesh filter (
Figure 3 and
Figure 4, comparison between virgin and (bio)fouled samples). In the diaphragms with pore sizes smaller than 1 µm, the pores seemed to be clogged, while in the coarse-pore diaphragms, more open areas were observed. Biofilm growth was also observed on the cathode surface with naked eye, when using diaphragms. Nylon mesh showed significant biofilm growth on the cathode, developing through the pores of Nylon.
The FPMFCs performance was characterized 2 weeks after starting to use each separator, with no significant variation in the power output with replications. This indicated that the fouling or biofilm formed on the separators did not hinder cations transfer or oxygen/ethanol crossover significantly in that period. Long-term operation of the FPMFCs using different separators and characterization of fouling or biofilm on the cathode and the separators was beyond the scope of this work and hence, not presented. Overall, the biofilm growth on the separator matrix could likely be in favor of the performance of the passive air-breathing FPMFC, especially when using micro-porous hydrophilic diaphragms. The biofilm development on the separator matrix will most likely cover the open areas shortly after the operation starts, and block the oxygen diffusing through the air cathode. The separator material should therefore be non-biodegradable or cheap to be replaced more frequently.
Figure 3.
SEM images from the surface of the virgin (unused) separators.
Figure 3.
SEM images from the surface of the virgin (unused) separators.
Figure 4.
SEM images from the surface of the (bio)fouled (used) separators.
Figure 4.
SEM images from the surface of the (bio)fouled (used) separators.
2.3. Economic Considerations
To be able to implement the MFCs into the current wastewater systems, it is critical to reduce the capital cost while increasing the power output so that this technology can be considered a viable alternative. Currently, the PEMs and the Pt-based cathodes contribute to up to 90% of the capital cost of the MFCs in lab-scale setups (resulting in a capital cost of
ca. $15 kg
−1 COD [
25]). At this stage, the capital cost of the MFCs can hardly compete with the activated sludge (
ca. $0.2 kg
−1 COD) and the anaerobic digestion (
ca. $0.02 kg
−1 COD) processes [
26,
27]. A major step in commercializing MFCs, therefore, seems to be the replacement of the currently used expensive PEMs as well as the Pt-based cathodes with less expensive alternatives.
A simple cost analysis was performed to evaluate the separators based on the cost they impose to the construction of the FPMFC. For this purpose, the cost of the separators as well as the cost of the other MFC components such as the Pt-based cathode, graphite felt anode, end plates, and current collectors were estimated based on the material used in the laboratory scale setup used in this research as well as some literature data. The costs of the separators were collected in some cases from the suppliers and in some other cases, from the literature, hence are rough estimates.
A lifetime of 5 years was assumed for the MFC components such as the separator (except for J-cloth with a lifetime of 1 year due to its biodegradability [
19]), anode, cathode, and current collectors, with no significant performance degradation, whereas a longer lifetime (25 years) was considered for the MFC end plates. The annual costs of the MFC components were calculated based on the lifetime of the different components and an annual interest rate of 6% in a 25-year period.
The configuration of the MFC clearly determines the output power, and thus, the cost of the separator per unit power (
Table 4). Because of the decreased oxygen concentration at the anode, increasing the anode chamber depth (from 2 mm to 8 mm) increases the power output and consequently decreases the separator cost per unit power. The removal efficiency, on the other hand, decreases as the anode chamber depth increases, since the portion of wastewater bypassing the anode increases.
Table 4.
Annual cost of the separator per watt of power output (estimated based on a lifetime of 1 year for J-cloth and 5 years for the other separators with an interest rate of 6% in a 25-year period).
Table 4.
Annual cost of the separator per watt of power output (estimated based on a lifetime of 1 year for J-cloth and 5 years for the other separators with an interest rate of 6% in a 25-year period).
| Separator | Cost ($·m−2) | Annual Cost ($·m−2) | Annual Cost ($·W−1) |
|---|
| 2 mm | 4 mm | 8 mm |
|---|
| Nafion®117 | 500 | 430 | 1800 | 1800 | 1700 |
| Aquivion®E79-03 | 500 | 430 | 2500 | 2400 | 2400 |
| Celgard®5511 | 20 | 20 | 120 | 100 | 80 |
| Zirfon®Perl | 200 | 170 | 1200 | 1000 | 900 |
| Nylon mesh | 5 | 5 | 40 | 30 | 20 |
| Glass fiber filter | 5 | 5 | 30 | 30 | 20 |
| SciMat®700/20 | 20 | 20 | 210 | 170 | 120 |
| J-cloth | 5 | 20 | 540 | 240 | 140 |
The cost imposed by the separator per kWh of the energy produced and the energy density per unit area of the separator within 5 years of continuous FPMFC operation is presented in
Table 5.
Table 5.
Cost of the separator and energy density over 5 years (estimated based on: interest rate of 6% in a 25-year period, $200 m−2 of graphite felt, $500 m−2 of Pt-based cathode, $50 m−2 of current collector, $7,000 m−3 of end plates, and $2,000 m−3 of other costs).
Table 5.
Cost of the separator and energy density over 5 years (estimated based on: interest rate of 6% in a 25-year period, $200 m−2 of graphite felt, $500 m−2 of Pt-based cathode, $50 m−2 of current collector, $7,000 m−3 of end plates, and $2,000 m−3 of other costs).
| Separator | Cost ($·m−2) | Energy Density (kWh·m−2) | Separator Cost ($·kWh−1) |
|---|
| 2 mm | 4 mm | 8 mm | 2 mm | 4 mm | 8 mm |
|---|
| Nafion®117 | 2,200 | 10 | 10 | 11 | 200 | 200 | 200 |
| Aquivion®E79-03 | 2,200 | 7 | 8 | 8 | 290 | 270 | 270 |
| Celgard®5511 | 100 | 6 | 8 | 10 | 10 | 10 | 10 |
| Zirfon®Perl | 850 | 6 | 7 | 8 | 140 | 110 | 100 |
| Nylon mesh | 30 | 5 | 6 | 7 | 5 | 5 | 5 |
| Glass fiber filter | 30 | 7 | 7 | 8 | 5 | 5 | 5 |
| SciMat®700/20 | 100 | 4 | 4 | 6 | 20 | 20 | 10 |
| J-cloth | 30 | 2 | 4 | 7 | 60 | 30 | 20 |
It was assumed that the costs of the other MFC components do not vary when different separators are used, so the costs of the separators were compared only. This may not be the case in an actual case where using diaphragms could lower the cathode lifetime. The analysis was also performed based on the assumption that the superficial power density would remain the same when scaling up the FPMFC, which in reality is not necessarily the case.
As can be seen in
Table 5, energy densities in the 2 mm FPMFC varied significantly using different separators, with Nafion
®117, Aquivion
®, and glass fiber filter resulting in the highest. The sensitivity of energy density to separator type decreased as the electrode spacing increased, as a result of reduced oxygen flux towards the anaerobic anode. Overall, Nafion
®117, Aquivion
®, and Zirfon
® (PEMs) impose the highest costs to the FPMFC construction per kWh of the energy produced. The significantly lower cost of porous diaphragms, on the other hand, indicates that there is a higher possibility for a positive offset using these separators, especially when the electrode spacing increases. Glass fiber filter and Nylon mesh provide the lowest cost per kWh, followed by Celgard
® in all of the FPMFCs. While application of Nylon mesh results in significant bacterial growth on the cathode, application of Celgard
® and glass fiber filter, which yield higher power output than the coarse-pore diaphragms, especially at large electrode spacings, is likely a necessary step for this technology to become practically viable.