The BioSCWG Project: Understanding the Trade-Offs in the Process and Thermal Design of Hydrogen and Synthetic Natural Gas Production
Abstract
:1. Introduction
- (1)
- At lower temperatures—up to 250 °C: the product is primarily a carbon rich solid commonly known as hydro or bio-char and is reported to be as energetically dense as lignite [4].
- (2)
- At higher temperatures—up to 400 °C: a de-oxygenated liquid commonly known as bio-oil or biocrude is the main product, accompanied with an aqueous stream with organic soluble compounds, a carbon dioxide rich gas and solid char residue as coproducts [11,17]. The bio-oil, consisting mainly of hydrolyzed organics, with a carbon partitioning as high as 40–45 wt. % per carbon feed, has a heating value that could reach between 24 and 37 MJ/kg and offers a potential substitute for existing liquid fuels. However, it has been reported that significant upgrading is required to adjust the liquid viscosity levels for longer storage periods and match the lower oxygen and nitrogen content normally found in the corresponding petroleum crude products [15].
- (1)
- At lower temperatures—from 370 to 550 °C: under non-catalytic conditions, water soluble organics are the primary product. While with the introduction of either metallic or alkali based catalysts, a carbon rich syngas is released due to further de-polymerization, dehydration, dehydrogenation and decarboxylation reactions taking place. The product gas consists primarily of a carbon dioxide and methane mixture [5,7,11].
- (2)
- At higher temperatures—beyond 550 °C: catalytic and non-catalytic conditions yield a hydrogen rich syngas, as a result of kinetically driven gas reforming reactions [15]. Some literature has reported experimental results that show complete partitioning and conversion of carbon from model compounds or from catalyzed real biomass feedstocks into syngas at temperatures around 600 °C and beyond [16].
2. State of the Art
2.1. Supercritical Water Gasification Process Synthesis and Simulation
2.1.1. Supercritical Water Gasification Reactor Model
2.1.2. Conceptual Supercritical Water Gasification Bio-Refinery Design
2.2. Algal Biomass Case Study
3. Developed Conceptual Plant Designs
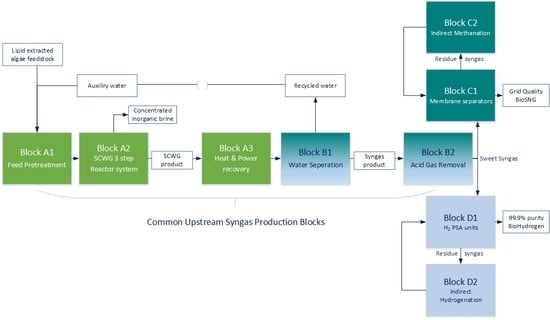
3.1. Common Upstream Syngas Production Blocks
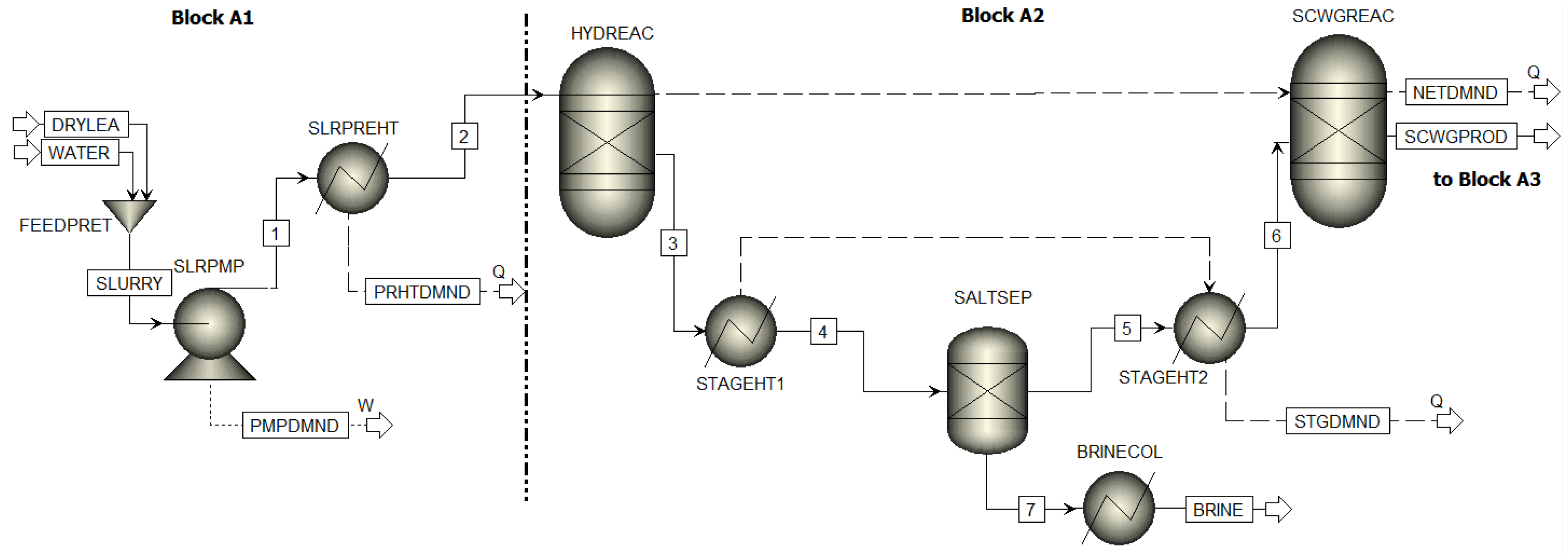
3.1.1. Three Step Supercritical Water Refining Reactor System
3.1.2. Product Recovery Blocks
3.2. Synthetic Natural Gas Production Pathway
3.3. Hydrogen Production Pathway
3.4. Case Studies
3.5. Conceptual Plant Evaluation
4. Results and Discussion
4.1. Three Step Reactor Model Validation
4.2. Conceptual Plant Simulation—Mass Balance
4.2.1. Upstream Processing
4.2.1.1. Influence of Solid Throughput
4.2.1.2. Influence of SCWG Temperature
4.2.2. Synthetic Natural Gas Cases
4.2.3. Hydrogen Cases
4.3. Conceptual Plant Simulation–Energy Balance
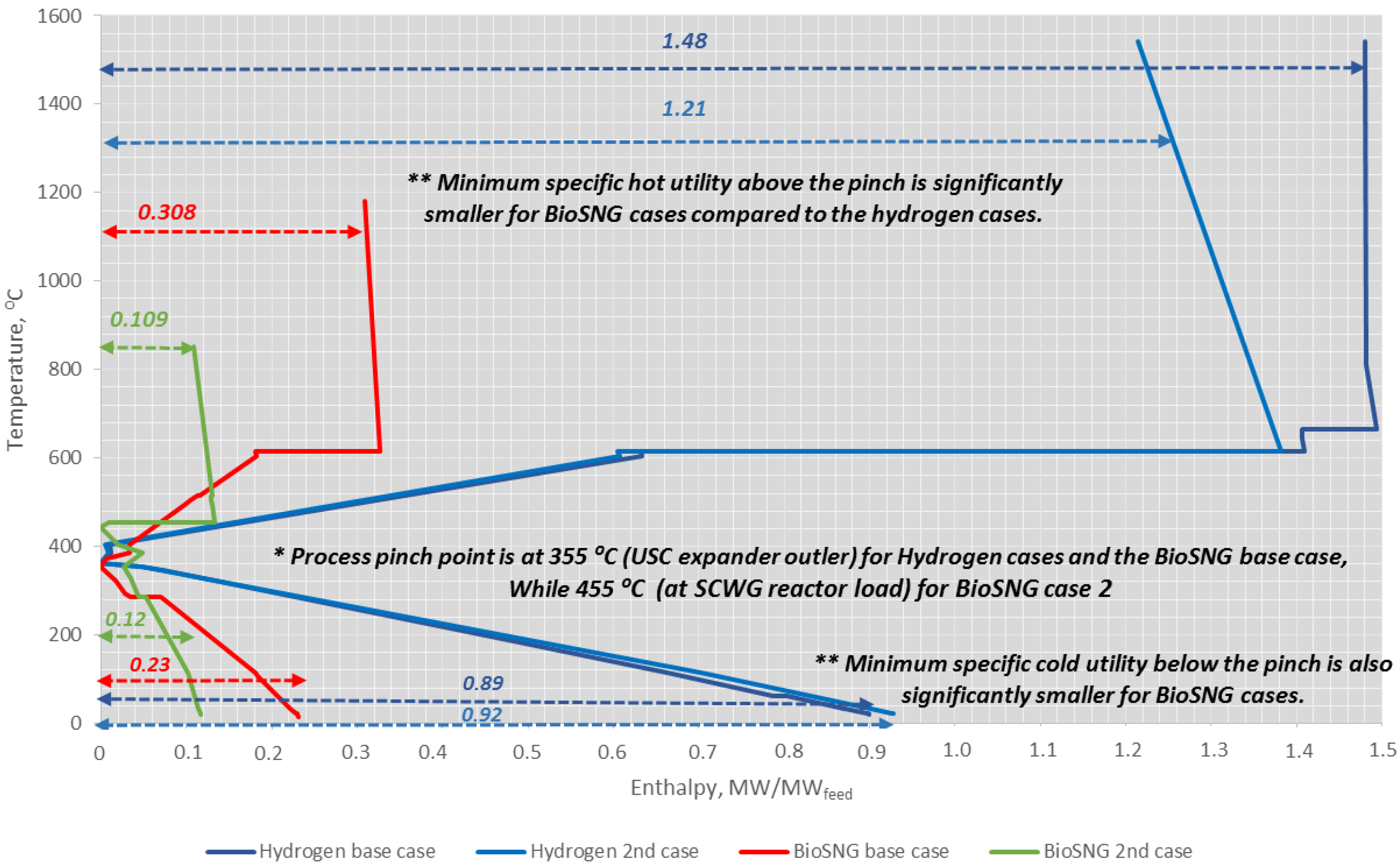
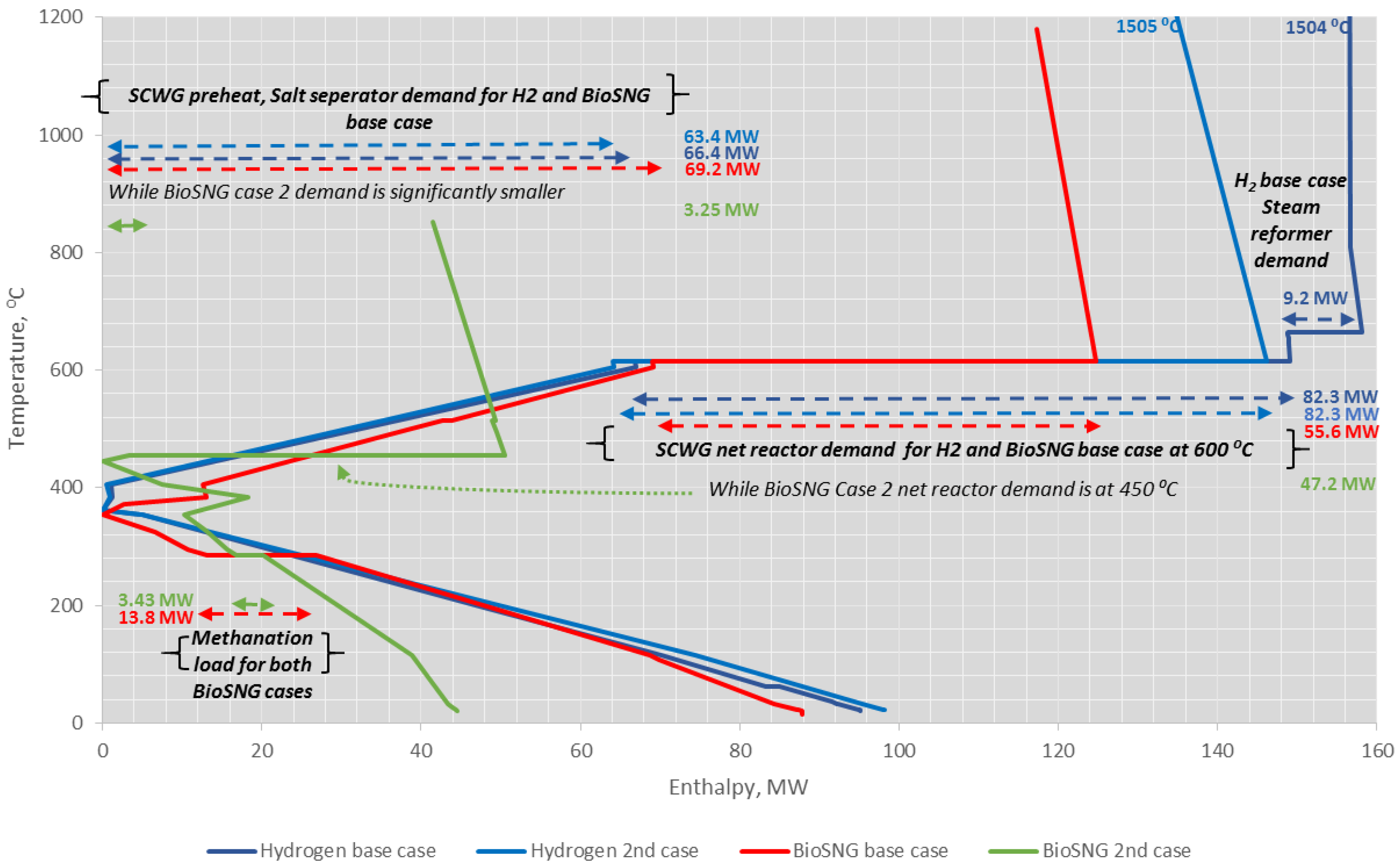
4.4. Heat Integration Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations Framework Convention on Climate Change—Paris Climate Change Conference—November 2015. Available online: http://unfccc.int/meetings/paris_nov_2015/meeting/8926.php (accessed on 6 January 2016).
- Chum, H.; Faaij, A.; Moreira, J.; Berndes, G.; Dhamija, P.; Dong, H.; Gabrielle, B.; Eng, A.G.; Lucht, W.; Mapako, M.; et al. IPCC Special Report on Renewable Energy Sources and Climate Change Mitigation; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Seyboth, K., Matschoss, P., Kadner, S., Zwickel, T., Eickemeier, P., Hansen, G., Schlömer, S., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2011; pp. 46–59. [Google Scholar]
- Kainiemi, L.; Eloneva, S.; Järvinen, M. An assessment of the uncertainties related to bioenergy applications. Manag. Environ. Qual. 2014, 25, 301–312. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Harinck, J.; Smit, K.G.; de Jong, W. Supercritical water gasification of biomass: A literature and technology overview. Energies 2015, 8, 859–894. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of water for chemical reactions in high-temperature water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Biorefin. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Brunner, G. Applications of supercritical fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Loppinet-Serani, A.; Aymonier, C.; Cansell, F. Current and foreseeable applications of supercritical water for energy and the environment. ChemSusChem 2008, 1, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Knez, Ž.; Markočič, E.; Hrnčič, M.K.; Ravber, M.; Škerget, M. High pressure water reforming of biomass for energy and chemicals: A short review. J. Supercrit. Fluids 2015, 96, 46–52. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Michael, J.; Antal, J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Patel, B.; Guo, M.; Izadpanah, A.; Shah, N.; Hellgardt, K. A review on hydrothermal pre-treatment technologies and environmental profiles of algal biomass processing. Bioresour. Technol. 2016, 199, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Williams, P.T. A comparison of product yields and inorganic content in process streams following thermal hydrolysis and hydrothermal processing of microalgae, manure and digestate. Bioresour. Technol. 2016, 200, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Modell, M. Gasification and Liquefaction of Forest Products in Supercritical Water. In Fundamentals of Thermochemical Biomass Conversion; Overend, R.P., Milne, T.A., Mudge, L.K., Eds.; Springer: Amsterdam, The Netherlands, 1985; pp. 95–119. [Google Scholar]
- Antal, M.J., Jr.; Allen, S.; Lichwa, J.; Schulman, D.; Xu, X. Hydrogen production from high moisture content biomass in supercritical water. In Proceedings of the 1999 U.S. DOE Hydrogen Program Review, NREL/CP-570-26938, Golden, CO, USA, 4–6 May 1999; pp. 1–24.
- De Blasio, C.; Lucca, G.; Özdenkci, K.; Mulas, M.; Lundqvist, K.; Koskinen, J.; Santarelli, M.; Westerlund, T.; Järvinen, M. A study on supercritical water gasification of black liquor conducted in stainless steel and nickel-chromium-molybdenum reactors. J. Chem. Technol. Biotechnol. 2016, 91, 2664–2678. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Dowaki, K.; Matsumura, Y.; Matsuhashi, R.; Li, D.; Ishitani, H.; Komiyama, H. Comprehensive comparison of efficiency and CO2 emissions between biomass energy conversion technologies—Position of supercritical water gasification in biomass technologies. Biomass Bioenergy 2003, 25, 257–272. [Google Scholar] [CrossRef]
- Cantero, D.A.; Bermejo, M.D.; Cocero, M.J. Reaction engineering for process intensification of supercritical water biomass refining. J. Supercrit. Fluids 2015, 96, 21–35. [Google Scholar] [CrossRef]
- Magdeldin, M.; Kohl, T.; Jarvinen, M. Process modeling, synthesis and thermodynamic evaluation of hydrogen production from hydrothermal processing of lipid extracted algae integrated with a downstream reformer conceptual plant. Biofuels 2016, 7, 97–116. [Google Scholar] [CrossRef]
- Magdeldin, M.; Kohl, T.; De Blasio, C.; Järvinen, M. Heat integration assessment for the conceptual plant design of synthetic natural gas production from supercritical water gasification of spirulina algae. In Proceedings of ECOS 2015—The 28th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Pau, France, 29 June–3 July 2015.
- Boukis, N.; Galla, U.; Müller, H.; Dinjus, E. Biomass gasification in supercritical water. Experimental progress achieved with the VERENA pilot plant. In Proceedings of the 15th European Conference & Exhibition, Berlin, Germany, 7–11 May 2007; pp. 1013–1016.
- Xiao, P.; Guo, L.; Zhang, X.; Zhu, C.; Ma, S. Continuous hydrogen production by biomass gasification in supercritical water heated by molten salt flow: System development and reactor assessment. Int. J. Hydrog. Energy 2013, 38, 12927–12937. [Google Scholar] [CrossRef]
- Marias, F.; Letellier, S.; Cezac, P.; Serin, J.P. Energetic analysis of gasification of aqueous biomass in supercritical water. Biomass Bioenergy 2011, 35, 59–73. [Google Scholar] [CrossRef]
- Feng, W.; van der Kooi, H.J.; de Swaan Arons, J. Biomass conversions in subcritical and supercritical water: Driving force, phase equilibria, and thermodynamic analysis. Chem. Eng. Process. Process Intensif. 2004, 43, 1459–1467. [Google Scholar] [CrossRef]
- Tang, H.; Kitagawa, K. Supercritical water gasification of biomass: Thermodynamic analysis with direct Gibbs free energy minimization. Chem. Eng. J. 2005, 106, 261–267. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, L.; Zhang, X.; Yan, Q. Thermodynamic modeling and analysis of biomass gasification for hydrogen production in supercritical water. Chem. Eng. J. 2007, 131, 233–244. [Google Scholar] [CrossRef]
- Voll, F.A.P.; Rossi, C.C.R.S.; Silva, C.; Guirardello, R.; Souza, R.O.M.A.; Cabral, V.F.; Cardozo-Filho, L. Thermodynamic analysis of supercritical water gasification of methanol, ethanol, glycerol, glucose and cellulose. Int. J. Hydrog. Energy 2009, 34, 9737–9744. [Google Scholar] [CrossRef]
- Castello, D.; Fiori, L. Supercritical water gasification of biomass: Thermodynamic constraints. Bioresour. Technol. 2011, 102, 7574–7582. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.D.; Guirardello, R. Thermodynamic analysis of supercritical water gasification of microalgae biomass for hydrogen and syngas production. Chem. Eng. Trans. 2013, 32, 553–558. [Google Scholar]
- Louw, J.; Schwarz, C.E.; Knoetze, J.H.; Burger, A.J. Thermodynamic modelling of supercritical water gasification: Investigating the effect of biomass composition to aid in the selection of appropriate feedstock material. Bioresour. Technol. 2014, 174, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Susanti, R.F.; Dianningrum, L.W.; Yum, T.; Kim, Y.; Lee, Y.-W.; Kim, J. High-yield hydrogen production by supercritical water gasification of various feedstocks: Alcohols, glucose, glycerol and long-chain alkanes. Chem. Eng. Res. Des. 2014, 92, 1834–1844. [Google Scholar] [CrossRef]
- Tushar, M.S.H.K.; Dutta, A.; Xu, C. Simulation and kinetic modeling of supercritical water gasification of biomass. Int. J. Hydrog. Energy 2015, 40, 4481–4493. [Google Scholar] [CrossRef]
- Gasafi, E.; Reinecke, M.-Y.; Kruse, A.; Schebek, L. Economic analysis of sewage sludge gasification in supercritical water for hydrogen production. Biomass Bioenergy 2008, 32, 1085–1096. [Google Scholar] [CrossRef]
- Fiori, L.; Valbusa, M.; Castello, D. Supercritical water gasification of biomass for H2 production: Process design. Bioresour. Technol. 2012, 121, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Withag, J.A.M.; Smeets, J.R.; Bramer, E.A.; Brem, G. System model for gasification of biomass model compounds in supercritical water—A thermodynamic analysis. J. Supercrit. Fluids 2012, 61, 157–166. [Google Scholar] [CrossRef]
- Galera, S.; Gutiérrez Ortiz, F.J. Techno-economic assessment of hydrogen and power production from supercritical water reforming of glycerol. Fuel 2015, 144, 307–316. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Ollero, P.; Serrera, A.; Sanz, A. Thermodynamic study of the supercritical water reforming of glycerol. Int. J. Hydrog. Energy 2011, 36, 8994–9013. [Google Scholar] [CrossRef]
- Aziz, M. Integrated supercritical water gasification and a combined cycle for microalgal utilization. Energy Convers. Manag. 2015, 91, 140–148. [Google Scholar] [CrossRef]
- Gassner, M.; Vogel, F.; Heyen, G.; Maréchal, F. Optimal process design for the polygeneration of SNG, power and heat by hydrothermal gasification of waste biomass: Thermo-economic process modelling and integration. Energy Environ. Sci. 2011, 4, 1726–1741. [Google Scholar] [CrossRef]
- Valderrama, J.O. The State of the Cubic Equations of State. Ind. Eng. Chem. Res. 2003, 42, 1603–1618. [Google Scholar] [CrossRef]
- Brandenberger, M.; Matzenberger, J.; Vogel, F.; Ludwig, C. Producing synthetic natural gas from microalgae via supercritical water gasification: A techno-economic sensitivity analysis. Biomass Bioenergy 2013, 51, 26–34. [Google Scholar] [CrossRef]
- Haiduc, A.G.; Brandenberger, M.; Suquet, S.; Vogel, F.; Bernier-Latmani, R.; Ludwig, C. SunCHem: An integrated process for the hydrothermal production of methane from microalgae and CO2 mitigation. J. Appl. Phycol. 2009, 21, 529–541. [Google Scholar] [CrossRef]
- Stucki, S.; Vogel, F.; Ludwig, C.; Haiduc, A.G.; Brandenberger, M. Catalytic gasification of algae in supercritical water for biofuel production and carbon capture. Energy Environ. Sci. 2009, 2, 535–541. [Google Scholar] [CrossRef]
- Ji, P.; Feng, W.; Chen, B.; Yuan, Q. Finding appropriate operating conditions for hydrogen purification and recovery in supercritical water gasification of biomass. Chem. Eng. J. 2006, 124, 7–13. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Harinck, J.; Smit, K.G.; de Jong, W. Supercritical water gasification of biomass: A detailed process modeling analysis for a microalgae gasification process. Ind. Eng. Chem. Res. 2015, 54, 5550–5562. [Google Scholar] [CrossRef]
- Mian, A.; Ensinas, A.V.; Marechal, F. Multi-objective optimization of SNG production from microalgae through hydrothermal gasification. Comput. Chem. Eng. 2015, 76, 170–183. [Google Scholar] [CrossRef]
- Elliott, D.C. Review of recent reports on process technology for thermochemical conversion of whole algae to liquid fuels. Algal Res. 2016, 13, 255–263. [Google Scholar] [CrossRef]
- Kouhia, M.; Holmberg, H.; Sonck, M.; Ahtila, P. Energy analysis of algae-to-biofuel production chains integrated with a combined heat and power plant. In Proceedings of the 23rd European Biomass Conference & Exhibition-EUBCE, Vienna, Austria, 1–4 June 2015.
- Berglin, E.J.; Enderlin, C.W.; Schmidt, A.J. Review and Assessment of Commercial Vendors/Options for Feeding and Pumping Biomass Slurries for Hydrothermal Liquefaction; Report Number: PNNL-21981; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2012. [Google Scholar]
- Minowa, T.; Sawayama, S. A novel microalgal system for energy production with nitrogen cycling. Fuel 1999, 78, 1213–1215. [Google Scholar] [CrossRef]
- Chakinala, A.G.; Brilman, D.W.F.; van Swaaij, W.P.M.; Kersten, S.R.A. Catalytic and non-catalytic supercritical water gasification of microalgae and glycerol. Ind. Eng. Chem. Res. 2010, 49, 1113–1122. [Google Scholar] [CrossRef]
- Patzelt, D.J.; Hindersin, S.; Elsayed, S.; Boukis, N.; Kerner, M.; Hanelt, D. Hydrothermal gasification of Acutodesmus obliquus for renewable energy production and nutrient recycling of microalgal mass cultures. J. Appl. Phycol. 2015, 27, 2239–2250. [Google Scholar] [CrossRef]
- Frank, E.D.; Elgowainy, A.; Han, J.; Wang, Z. Life cycle comparison of hydrothermal liquefaction and lipid extraction pathways to renewable diesel from algae. Mitig. Adapt. Strateg. Glob. Chang. 2012, 18, 137–158. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Lea-Langton, A.R.; Ross, A.B.; Williams, P.T. Catalytic hydrothermal gasification of algae for hydrogen production: Composition of reaction products and potential for nutrient recycling. Bioresour. Technol. 2013, 127, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Phyllis2—Database for Biomass and Waste. Available online: https://www.ecn.nl/phyllis2/ (accessed on 14 July 2015).
- Bejan, A.; Tsatsaronis, G.; Moran, M. Thermal Design and Optimization, 1st ed.; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Yakaboylu, O.; Harinck, J.; Smit, K.G.; de Jong, W. Testing the constrained equilibrium method for the modeling of supercritical water gasification of biomass. Fuel Process. Technol. 2015, 138, 74–85. [Google Scholar] [CrossRef]

| Reference | Feedstock | SCWG Conditions | End of Pipe Product | Modelling Tool & Approach | EOS for Supercritical Properties | Inorganic Modelling | Energy Recovery Assessment |
|---|---|---|---|---|---|---|---|
| Louw et al. [31] | - 5 model compounds - 49 real biomass | 600, 700 & 800 °C; 221 bar; 5, 15 & 25 wt. % solids | Reactor model only | Aspen Plus™ NS-GM | PR-BM 1 | No | Isothermal reactor demand was assessed. |
| Susanti et al. [32] | a list of C1 to C16 model compounds | 650 & 740 °C; 250 bar; 10 & 20 wt. % solids | Reactor model only | Aspen Plus™ NS-GM | PR | No | Not considered. |
| Tushar et al. [33] | - glucose - furfural - phenol | 700 °C; 280 bar; 5 wt. % solids | Reactor model only | Aspen Plus™ NS-GM | Ideal package | No | Not considered. |
| Feng et al. [25] | - cellulose | 600 °C; 350 bar; 20 wt. % solids | High purity H2 | Not reported | SAFT | No | Recuperated heat and combustion of non-H2 syngas. |
| Lu et al. [27] | - wood sawdust | 600 to 1100 °C; 250 bar; 5 to 30 wt. % solids | High purity H2 | Not reported | Duan | No | Recuperated heat. |
| Gasafi et al. [34] | - sewage sludge | 600 °C; 280 bar; 20 wt. % solids | High purity H2 | Not reported | N/A 2 | No | Recuperated heat, combustion of non-H2 and auxiliary gas. |
| Fiori et al. [35] | - glycerol - microalgae - sewage sludge - grape marc - phenol | 500, 700 & 900 °C; 300 bar; 5 to 25 wt. % solids | Power | Aspen Plus™ NS-GM | PR-VdW 3 | No | Combustion of non-H2 syngas. |
| Withag et al. [36] | - methanol - cellulose | 400 to 800 °C; 100 to 400 bar; 5 to 35 wt. % solids | Dry gas Captured CO2 | Aspen Plus™ NS-GM | SRK-MHV2 4 | No | Recuperated heat. |
| Galera et al. [37] | - glycerol | 800 °C; 240 bar; 26 wt. % solids | Power | Aspen Plus™ NS-GM | PSRK 5 | No | Heat exchanger network-HEN. |
| Aziz et al. [39] | - Spirulina algae | 700 °C; 220 to 300 bar; 10 wt. % solids | Power | SimSci PRO/II NS-GM | Not reported | No | Exhaust heat from CC turbines. |
| Gassner et al. [40] | - generalized waste biomass | 350 to 450 °C; 300 bar; 10 to 20 wt. % solids | Grid quality CH4 | Belsim SA Not reported 6 | Duan—Lee Kesler | Yes | Different Scenarios evaluated. |
| SunCHem concept [42,43,44] | - microalgae | 400 °C; 250 bar; 15 wt. % solids | Grid quality CH4 | Not reported 7 | N/A | Yes | Recuperated heat and split combustion of CH4 product. |
| Proximate Analysis, wt. % (as Received Basis) | Ultimate Analysis, wt. % (Dry Ash Free Basis) | ||
|---|---|---|---|
| Ash content | 4.59 | Carbon | 50.99 |
| Moisture | 5.62 | Hydrogen | 7.44 |
| Volatiles | 75.34 | Oxygen | 33.61 |
| Fixed carbon | 14.45 | Sulfur | 0.48 |
| Nitrogen | 7.48 | ||
| Specification | Target |
|---|---|
| Methane | >96 mol% |
| Carbon dioxide and nitrogen | <2.5 mol% |
| Hydrogen and carbonyl sulphide | <5 mg/Nm3 |
| Carbon monoxide | <0.05 mol% |
| Hydrogen | <2 mol% |
| Oxygen | <1 mol% |
| Water content | <3 mg/Nm3 |
| Wobbe Index | 13.76 < x < 15.81 kWh/Nm3 |
| Relative density | 0.555 < x < 0.700 |
| Design Condition | Hydrogen Base Case | Hydrogen 2nd Case | BioSNG Base Case | BioSNG 2nd Case |
|---|---|---|---|---|
| Slurry solid conc., wt. % | 5 | 5 | 18 | 18 |
| SCWG reformer T, °C | 600 | 600 | 600 | 450 |
| In-situ power production | Yes | Yes | Yes | No |
| In-situ indirect production | Yes | No | Yes | Yes |
| Parameter | Lower Temperature CH4 Production, Reference [44] | Higher Temperature H2 production, Reference [52] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data label | D-1 | D-2 | D-3 | D-4 | D-5 | D-6 | D-7 | D-8 | M | |||||
| P, bar | 324 | 336 | 308 | 311 | 240 | |||||||||
| T, °C | 399 | 403 | 400 | 403 | 600 | |||||||||
| conc., wt. % | 2.5 | 2.5 | 5 | 10 | 7.3 | |||||||||
| Residence time, min | 63 | 360 | 63 | 360 | 2 | |||||||||
| Catalyst | Ru/C | Ru/ZrO2 | Ru/C | Ru/ZrO2 | Ni wire | Ru/TiO2 | Ru/TiO2 | PtPd | ||||||
| 0.7 g/gfeed | 2 g/gfeed | |||||||||||||
| Feedstock | Spirulina Platensis | Chlorella Vulgaris | ||||||||||||
| CGE, % | 93 | 90 | 93 | 89 | 45 | 89 | 66 | 88 | 82 | 69 | 100 | 70 | 88 | |
| Gas, mol% | CH4 | 43 | 48 | 52 | 47 | 21 | 52 | 43 | 54 | 25 | 18 | 18 | 20 | 16 |
| H2 | 5.8 | 19 | 8 | 21 | 14 | 12 | 8.8 | 7.2 | 18 | 34 | 46 | 17 | 51 | |
| CO2 | 50 | 32 | 38 | 33 | 58 | 36 | 44 | 38 | 29 | 33 | 28 | 30 | 29 | |
| CO | 0.1 | ND | ND | ND | 0.1 | ND | 0.1 | ND | 13 | 6 | 2 | 16 | 0.1 | |
| C+ | 2.3 | ND | ND | ND | 7.4 | ND | 3.4 | ND | 14 | 10 | 5 | 17 | ND | |
| Case Study | Hydrogen Base Case | Hydrogen 2nd Case | BioSNG Base Case | BioSNG 2nd Case | |
|---|---|---|---|---|---|
| Slurry solid conc., wt. % | 5% | 18% | |||
| Feed Pretreatment—block A1 | |||||
| Slurry outlet P, bar | 250 | ||||
| Slurry outlet T, °C | 350 | ||||
| SCWG Three step Reactor System—block A2 | |||||
| Hydrolysis reactor T, °C | 350 | ||||
| Salt separator inlet T, °C | 380 | ||||
| SCWG reactor inlet T, °C | 600 | 450 | |||
| SCWG product (water content considered), mol% | CH4 | 0.89 | 6.31 | 7.54 | |
| H2 | 4.54 | 5.49 | 1.42 | ||
| CO2 | 2.49 | 6.53 | 5.77 | ||
| CO | 0.06 | 0.21 | 0.02 | ||
| H2S | 0.01 | 0.05 | 0.05 | ||
| Reactive H2O, kg/kgorganicfeed | 0.64 | 0.34 | −0.24 | ||
| CH4 gas yield, kg/kgorganicfeed | 0.14 | 0.27 | 0.31 | ||
| H2 gas yield, kg/kgorganicfeed | 0.09 | 0.03 | 0.01 | ||
| Heat and Power Recovery—block A3 | |||||
| Expander isentropic efficiency-, % | 92 | N/A | |||
| Expander discharge P, bar | 65 | N/A | |||
| Expander discharge T, °C | 367 | 377 | N/A | ||
| Product cooler outlet T, °C | 25 | ||||
| Water Separation—block B1 | |||||
| 1st stage pressurized KO drum, bar | 60 | ||||
| Dry gas composition, mol% | CH4 | 11.5 | 33.5 | 50.2 | |
| H2 | 59.3 | 29.2 | 9.42 | ||
| CO2 | 25.4 | 31.6 | 34.2 | ||
| CO | 0.72 | 1.06 | 0.12 | ||
| H2S | 0.11 | 0.21 | 0.25 | ||
| H2O | 0.12 | 0.14 | 0.15 | ||
| CH4 recovery in dry gas, % | 98.4 | 99.4 | 99.2 | ||
| H2 recovery in dry gas, % | 98.9 | 99.6 | 99.5 | ||
| CO2 recovery in dry gas, % | 77.1 | 90.7 | 87.8 | ||
| H2S recovery in dry gas, % | 63.4 | 83.5 | 79.4 | ||
| Acid Gas Removal—block B2 | |||||
| Sweet gas composition, mol% | CH4 | 15.1 | 51.9 | 75.2 | |
| H2 | 79.1 | 39.8 | 14.7 | ||
| CH4 recovery in sweet gas, % | 97.6 | ||||
| CO2 extracted, % | 96.6 | ||||
| H2S extracted, % | 95.0 | ||||
| Case Study | Base Case | 2nd Case | |
|---|---|---|---|
| Membrane separator system—block C1 | |||
| CH4 recovery rate in product gas, % | 95.1 | ||
| H2 extracted, % | 99.0 | ||
| N-compounds extracted, % | 96.0 | ||
| H2S extracted, % | 99.9 | ||
| Indirect Methanation—block C2 | |||
| Off-gas feed, kg/kgorganicfeed | 0.07 | 0.03 | |
| Off-gas composition to methanation, mol% | CH4 | 1.23 | 4.53 |
| H2 | 93.5 | 87.9 | |
| CO2 | 1.96 | 6.13 | |
| CO | 3.21 | 1.06 | |
| H2S, ppmv | 323 | 219 | |
| COx conversion rate in methanation, % | 97.4 | 95.7 | |
| methanation dry outlet composition, mol% | CH4 | 76.8 | 76.7 |
| H2 | 19.9 | 17.2 | |
| Grid BioSNG product | |||
| Gross production rate, kg/kgorganicfeed | 0.353 | 0.348 | |
| Grid BioSNG composition, mol% | |||
| CH4 | 97.7 | 98.4 | |
| H2 | 0.79 | 0.22 | |
| CO2 | 1.09 | 0.93 | |
| Case Study | Base Case | 2nd Case |
|---|---|---|
| H2 PSA units—block D1 | ||
| H2 recovery in product gas, % | 90.00 | |
| H2 purity in product gas, % | 99.99 | |
| Direct H2 production, kg/kgorganicfeed | 0.082 | |
| Residue gas composition, mol% | ||
| CH4 | 52.8 | |
| H2 | 27.5 | |
| Indirect hydrogenation—block D2 | ||
| Steam reformer | ||
| CH4 conversion rate, % | 98.7 | N/A |
| H2 outlet, mol% | 71.1 | N/A |
| CO outlet, mol% | 21.4 | N/A |
| Water gas shift reactors | ||
| Combined CO conversion rate, % | 97.2 | N/A |
| H2 outlet, mol% | 76.2 | |
| CO outlet, ppmv | 10 | N/A |
| Indirect H2 production, kg/kgorganicfeed | 0.069 | N/A |
| Case Study | Hydrogen Base Case | Hydrogen 2nd Case | BioSNG Base Case | BioSNG 2nd Case |
|---|---|---|---|---|
| Plant feedstock input, MWLHV | 105.8 | 308.9 | ||
| Feed pretreatment block—A1 | ||||
| Slurry pump demand | 0.037 | 0.009 | ||
| Slurry preheater demand | 1.478 | 0.381 | ||
| SCWG Three step reactor block—A2 | ||||
| Net reactor demand | 0.777 | 0.146 | 0.124 | |
| Interstage heating demand | 0.652 | 0.242 | 0.111 | |
| Heat and power recovery block—A3 | ||||
| Expander load | 0.321 | 0.088 | N/A | |
| Product cooler load | 2.382 | 0.578 | 0.569 | |
| Downstream hydrogen pathway blocks—D1 and D2 | ||||
| Steam reformer demand | 0.088 | N/A | ||
| Water gas shift load | 0.016 | N/A | ||
| Steam generation demand | 0.049 | N/A | ||
| Net downstream power load | 0.007 | 0.007 | ||
| Residue gas heat recovery | 0.064 | 0.305 | ||
| Gross H2 production | 0.855 | 0.463 | ||
| Split H2 indirect product, % | 46 | 0 | ||
| Downstream BioSNG pathway blocks—C1 and C2 | ||||
| Methantion reactor load | 0.036 | 0.009 | ||
| Steam generation demand | 0.058 | 0.015 | ||
| Net downstream power demand | 0.016 | 0.004 | ||
| Residue gas heat recovery load | 0.051 | 0.048 | ||
| Gross BioSNG production | 0.738 | 0.728 | ||
| Split BioSNG indirect product, % | 21 | 6 | ||
| Performance Parameter | Hydrogen Base Case | Hydrogen 2nd Case | BioSNG Base Case | BioSNG 2nd Case |
|---|---|---|---|---|
| Gross syngas product, MWLHV/MWfeedLHV | 0.855 | 0.463 | 0.738 | 0.729 |
| Net syngas product, MWLHV/MWfeedLHV | 0.345 | 0.199 | 0.458 | 0.622 |
| Energetic efficiency, % | 55.29 | 42.27 | 59.04 | 66.46 |
| Fuel-equivalent efficiency, % | 65.14 | 52.74 | 63.01 | 66.21 |
| Exergetic efficiency, % | 48.45 | 36.62 | 46.91 | 57.39 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdeldin, M.; Kohl, T.; De Blasio, C.; Järvinen, M.; Won Park, S.; Giudici, R. The BioSCWG Project: Understanding the Trade-Offs in the Process and Thermal Design of Hydrogen and Synthetic Natural Gas Production. Energies 2016, 9, 838. https://doi.org/10.3390/en9100838
Magdeldin M, Kohl T, De Blasio C, Järvinen M, Won Park S, Giudici R. The BioSCWG Project: Understanding the Trade-Offs in the Process and Thermal Design of Hydrogen and Synthetic Natural Gas Production. Energies. 2016; 9(10):838. https://doi.org/10.3390/en9100838
Chicago/Turabian StyleMagdeldin, Mohamed, Thomas Kohl, Cataldo De Blasio, Mika Järvinen, Song Won Park, and Reinaldo Giudici. 2016. "The BioSCWG Project: Understanding the Trade-Offs in the Process and Thermal Design of Hydrogen and Synthetic Natural Gas Production" Energies 9, no. 10: 838. https://doi.org/10.3390/en9100838
APA StyleMagdeldin, M., Kohl, T., De Blasio, C., Järvinen, M., Won Park, S., & Giudici, R. (2016). The BioSCWG Project: Understanding the Trade-Offs in the Process and Thermal Design of Hydrogen and Synthetic Natural Gas Production. Energies, 9(10), 838. https://doi.org/10.3390/en9100838