Increasing Hydrogen Density with the Cation-Anion Pair BH4−-NH4+ in Perovskite-Type NH4Ca(BH4)3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formation of NH4Ca(BH4)3
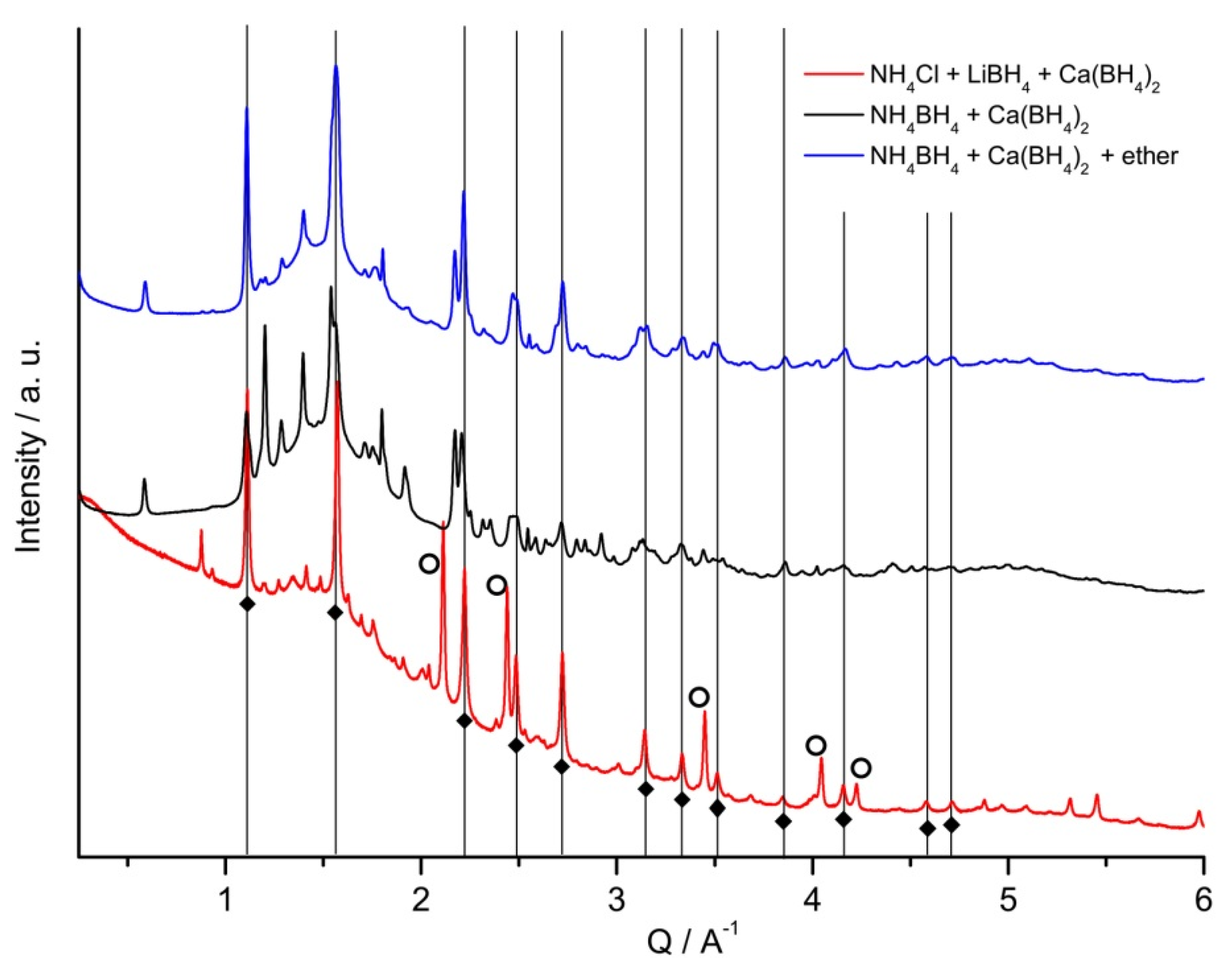
2.2. Thermolysis of NH4Ca(BH4)3
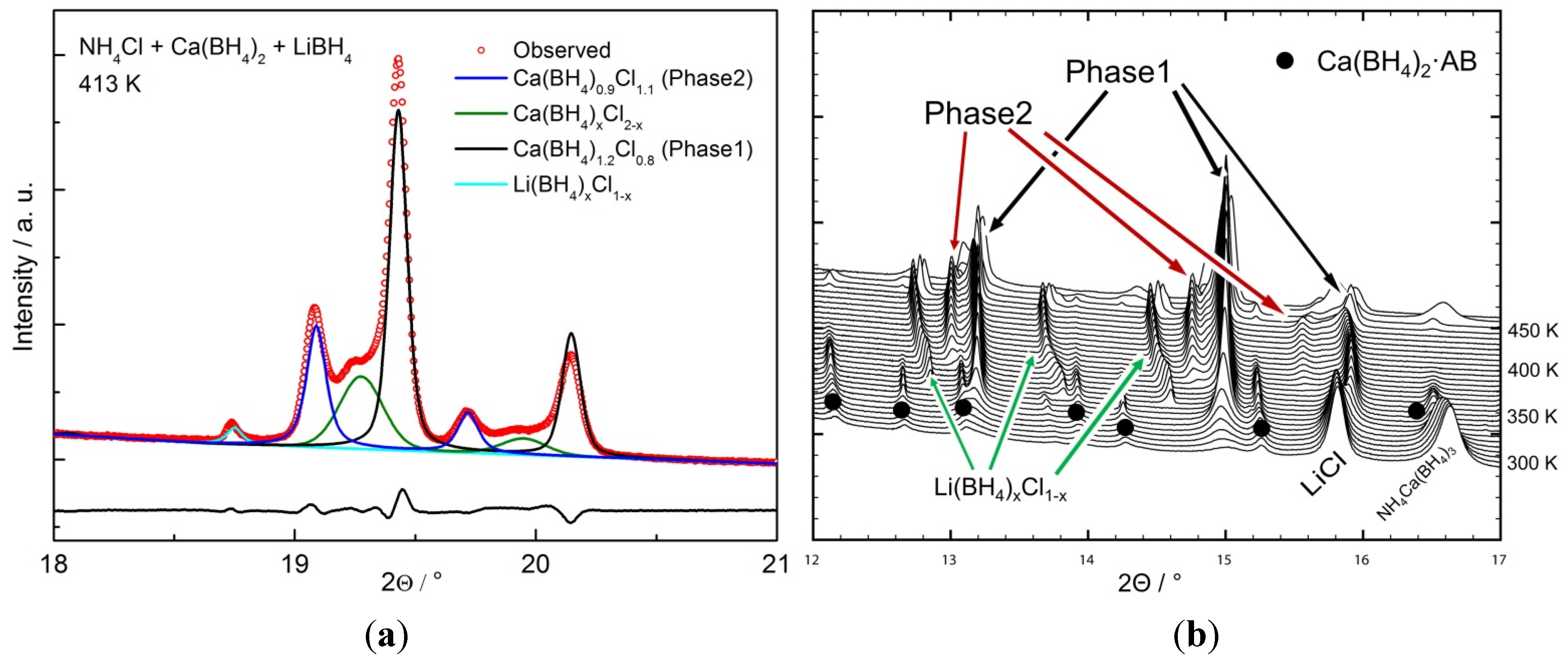
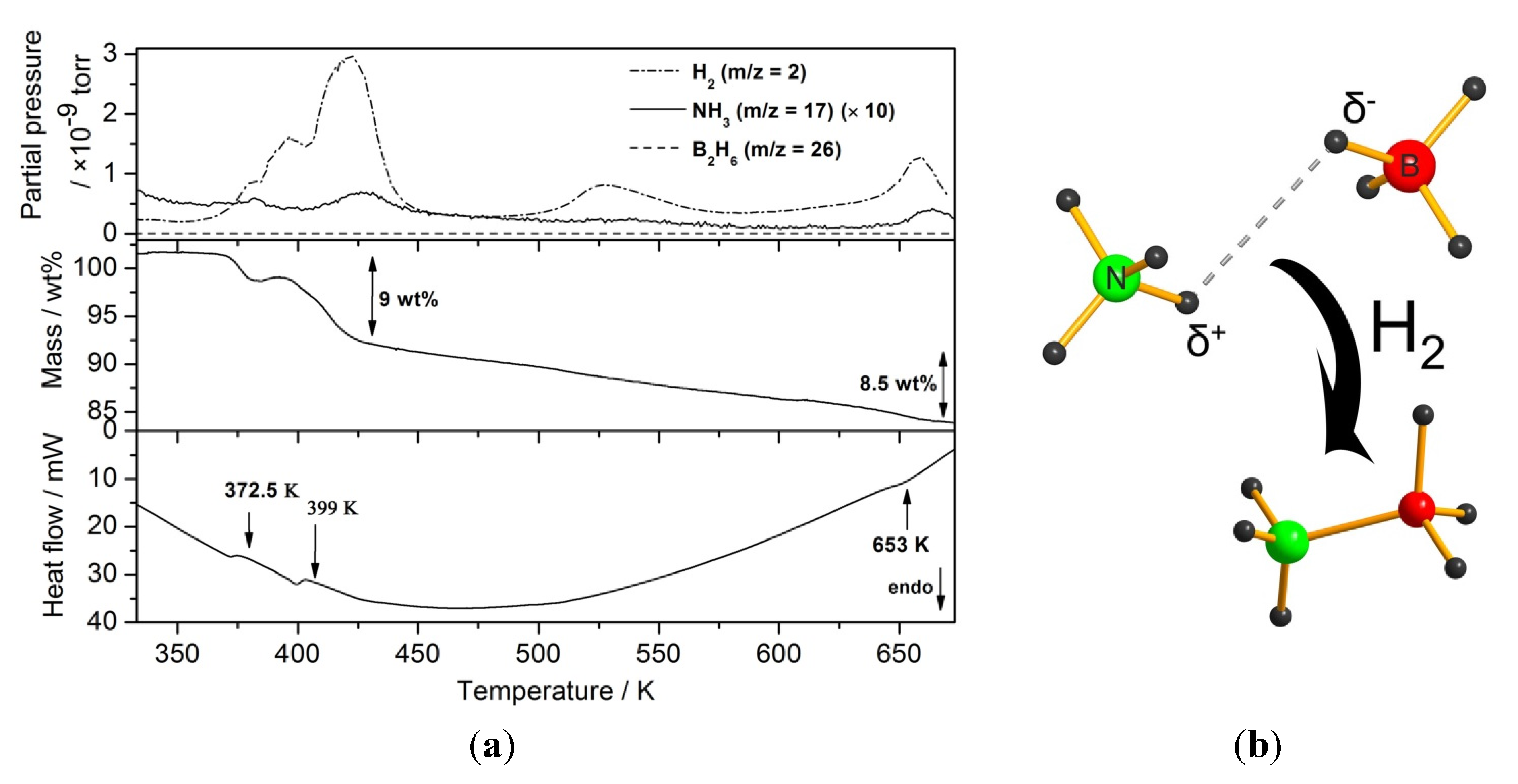
2.2.1. Chloride Substitution in β-Ca(BH4)2
2.2.2. Ether-Samples
2.3. Crystal Structure of NH4Ca(BH4)3
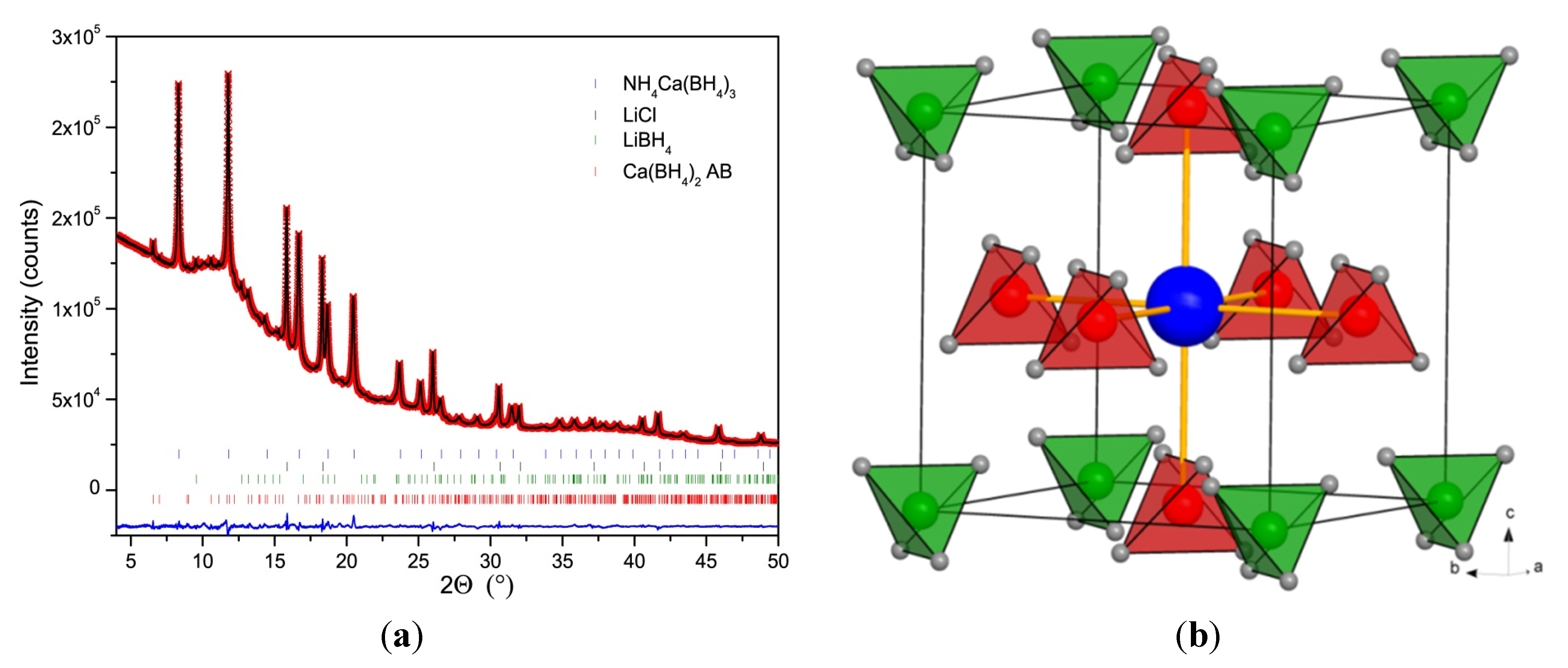
2.4. Crystal Structure of Ca(BH4)2·NH3BH3
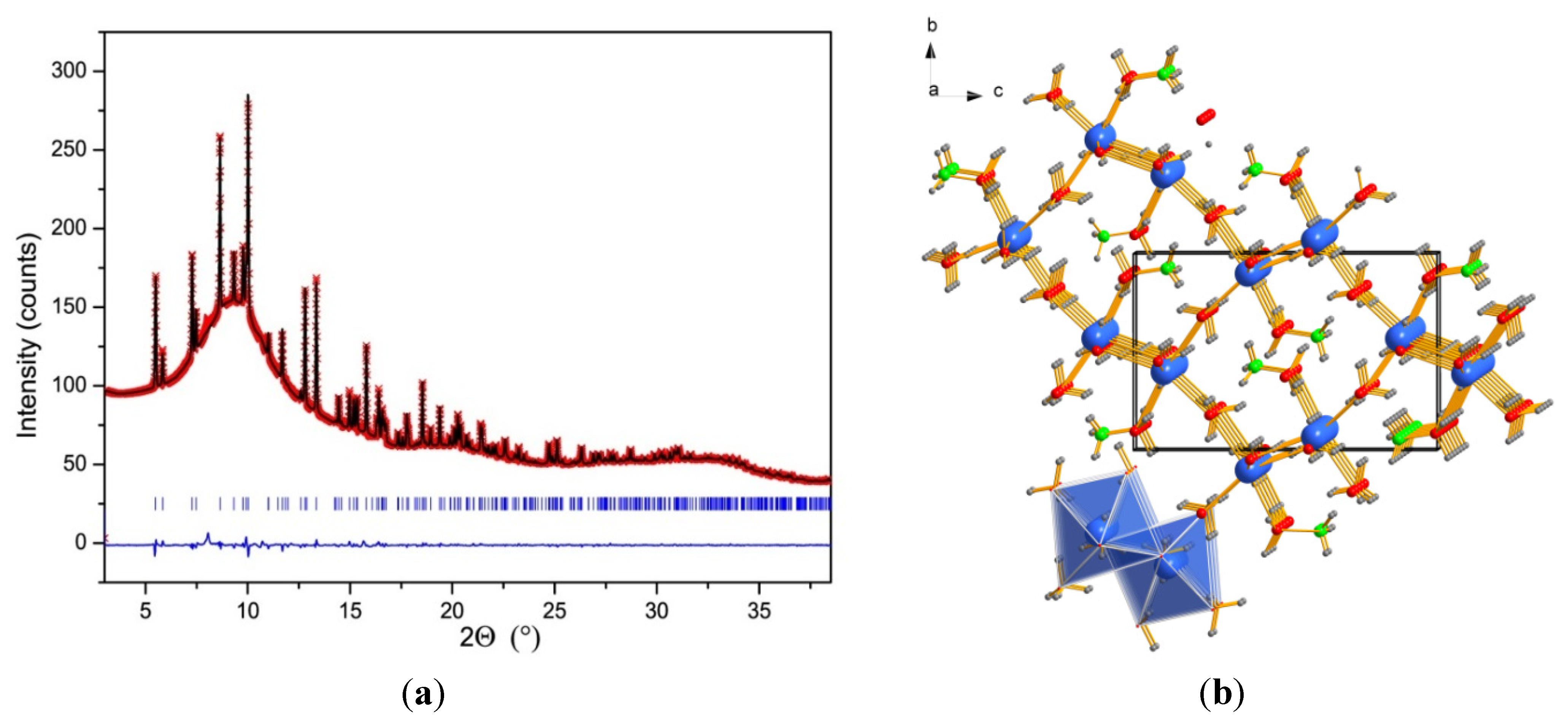
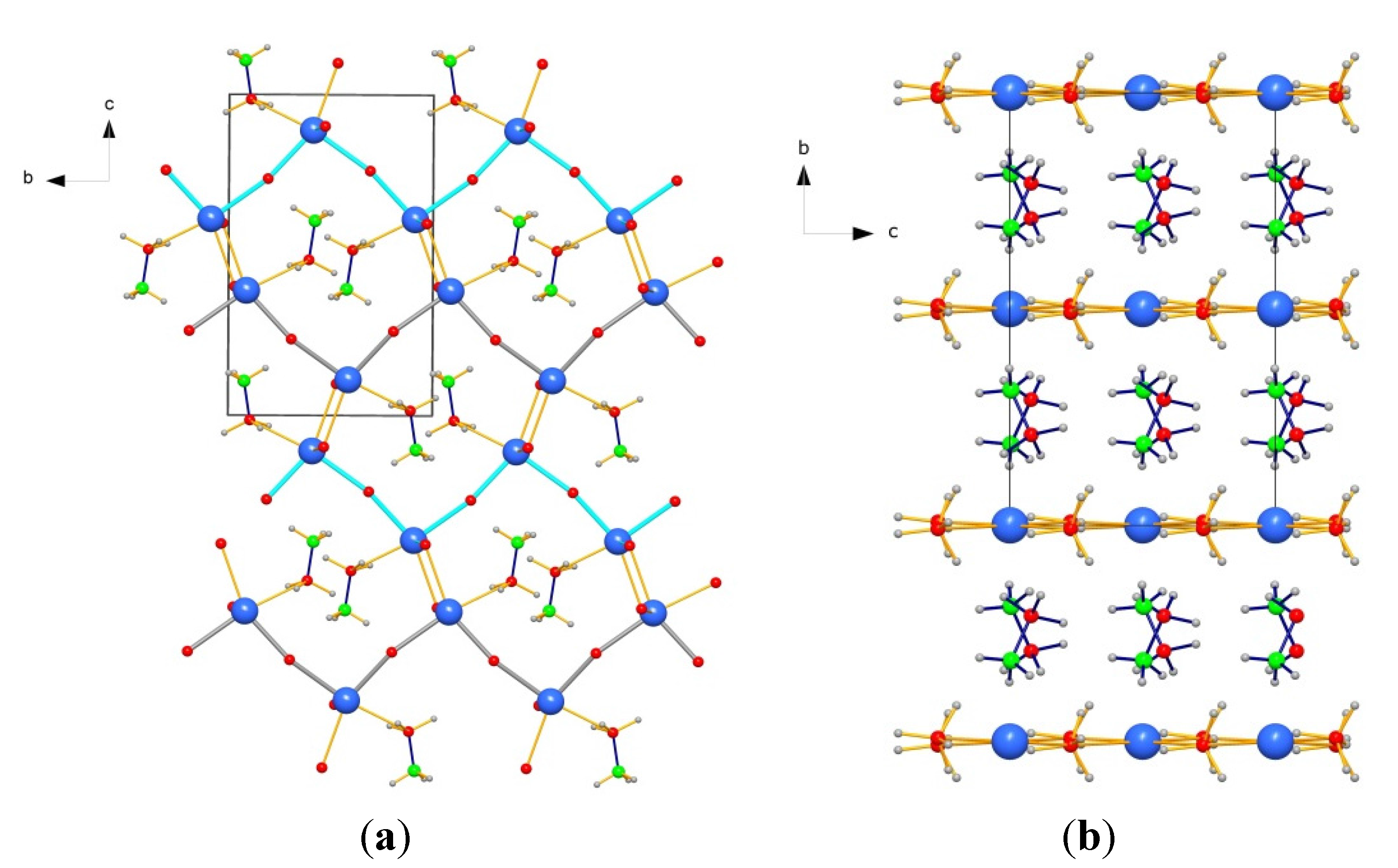
3. Experimental Section
3.1. Synthesis
3.2. Synchrotron Radiation X-ray Powder Diffraction (SR-XPD)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Ueber ein periodensystem der metallboranate. Naturwissenschaften 1955, 42, 438. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Ley, M.B.; Su-Lee, Y.; Cho, Y.W.; Dornheim, M.; Jensen, J.O.; Filinchuk, Y.; Jorgensen, J.E.; Besenbacher, F.; Jensen, T.R. Boron-nitrogen based hydrides and reactive composites for hydrogen storage. Mater. Today 2014, 17, 129–135. [Google Scholar] [CrossRef]
- Schouwink, P.; Ley, M.B.; Tissot, A.; Hagemann, H.; Jensen, T.R.; Smrčok, L.; Černý, R. Structure and properties of a new class of complex hydride perovskite materials. Nat. Commun. 2014, 5, 5706. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.A.; Gutowski, M. Thermodynamic Properties of Molecular Borane Amines and the [BH4−][NH4+] Salt for Chemical Hydrogen Storage Systems from ab Initio Electronic Structure Theory. J. Phys. Chem. A 2005, 109, 5129–5139. [Google Scholar] [CrossRef] [PubMed]
- Karkamkar, A.; Kathmann, S.M.; Schenter, G.K.; Heldebrant, D.J.; Hess, N.; Gutowski, M.; Autrey, T. Thermodynamic and Structural Investigations of Ammonium Borohydride, a Solid with a Highest Content of Thermodynamically and Kinetically Accessible Hydrogen. Chem. Mater. 2009, 21, 4356–4358. [Google Scholar] [CrossRef]
- Flacau, R.; Ratcliffe, C.I.; Desgreniers, S.; Yao, Y.; Klug, D.D.; Pallister, P.; Moudrakowski, I.L.; Ripmeester, J.A. Structure and dynamics of ammonium borohydride. Chem. Commun. 2010, 46, 9164–9166. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, X.; Yisgedu, T.; Meyers, E.A.; Shore, S.G.; Zhao, J.-C. Ammonium Octahydrotriborate (NH4B3H8): New Synthesis, Structure, and Hydrolytic Hydrogen Release. Inorg. Chem. 2011, 50, 3738–3742. [Google Scholar] [CrossRef] [PubMed]
- Yisgedu, T.B.; Huang, Z.; Chen, X.; Lingam, H.K.; King, G.; Highley, A.; Maharrey, S.; Woodward, P.M.; Behrens, R.; Shore, S.G.; et al. The structural characterization of (NH4)2B10H10 and thermal decomposition studies of (NH4)2B10H10 and (NH4)2B12H12. Int. J. Hydrog. Energy 2012, 37, 4267–4273. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Karkamkar, A.; Bowden, M.; Besenbacher, F.; Jensen, T.R.; Autrey, T. Methods to stabilize and destabilize ammonium borohydride. Dalton Trans. 2013, 42, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Rude, L.H.; Nielsen, T.K.; Ravnsbæk, D.B.; Bösenberg, U.; Ley, M.B.; Richter, B.; Arnbjerg, L.M.; Dornheim, M.; Filinchuk, Y.; Besenbacher, F.; et al. Tailoring properties of borohydrides for hydrogen storage: A review. Phys. Status Solidi 2011, 208, 1754–1773. [Google Scholar] [CrossRef]
- Rude, L.H.; Filinchuk, Y.; Sørby, M.H.; Hauback, B.C.; Besenbacher, F.; Jensen, T.R. Anion Substitution in Ca(BH4)2-CaI2: Synthesis, Structure and Stability of Three New Compounds. J. Phys. Chem. C 2011, 115, 7768–7777. [Google Scholar] [CrossRef]
- Xiong, Z.; Keong Yong, C.; Wu, G.; Chen, P.; Shaw, W.; Karkamkar, A.; Autrey, T.; Owen Jones, M.; Johnson, S.R.; Edwards, P.P.; et al. High-capacity hydrogen storage in lithium and sodium amidoboranes. Nat. Mater. 2007, 7, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, W.; Yildirim, H. Alkali and Alkaline-Earth Metal Amidoboranes: Structure, Crystal Chemistry, and Hydrogen Storage Properties. J. Am. Chem. Soc. 2008, 130, 14834–14839. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, W.; Pinkerton, F.E.; Meyer, M.S.; Srinivas, G.; Yildirim, T.; Udovic, T.J.; Rush, J.J. A new family of metal borohydride ammonia borane complexes: Synthesis, structures, and hydrogen storage properties. J. Mater. Chem. 2010, 20, 6550–6556. [Google Scholar] [CrossRef]
- Grove, H.; Rude, L.H.; Jensen, T.R.; Corno, M.; Ugliengo, P.; Baricco, M.; Sørby, M.H.; Hauback, B.C. Halide substitution in Ca(BH4)2. RSC Adv. 2014, 4, 4736–4742. [Google Scholar] [CrossRef]
- Finlinchuk, Y.; Rönnebro, E.; Chandra, D. Crystal structures and phase transformations in Ca(BH4)2. Acta Mater. 2009, 57, 732–738. [Google Scholar] [CrossRef]
- Bowden, M.; Autrey, T. Characterization and mechanistic studies of the dehydrogenation of NHxBHx materials. Curr. Opin. Solid State Mater. Sci. 2011, 15, 73–79. [Google Scholar] [CrossRef]
- Schouwink, P.; Hagemann, H.; Embs, J.P.; D’Anna, V.; Černý, R. Di-hydrogen contact induced lattice instabilities and structural dynamics in complex hydride perovskites. J. Phys. Condens. Matt. 2015, 27, 265403. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.W.; Schultz, D.R.; Girardot, P.R. The Preparation and Properties of Hexamminecobalt (III) Borohydride, Hexamminechromium (III) borohydride and Ammonium Borohydride. J. Am. Chem. Soc. 1958, 1, 1–3. [Google Scholar] [CrossRef]
- Favre-Nicolin, V.; Černý, R. FOX, “free objects for crystallography”: A modular approach to ab initio structure determination from powder diffraction. J. Appl. Cryst. 2002, 35, 734–743. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. FULLPROF SUITE; LLB Sacley & LCSIM: Rennes, France, 2003. [Google Scholar]
- Coelho, A.A. TOPAS-Academic V5. 2012. Available online: http://www.topas-academic.net/ (accessed on 15 February 2013).
- Schouwink, P.; D’Anna, V.; Ley, M.B.; Lawson Daku, L.M.; Richter, B.; Jensen, T.R.; Hagemann, H.; Černý, R. Bimetallic Borohydrides in the System M(BH4)2-KBH4 (M = Mg, Mn): On the Structural Diversity. J. Phys. Chem. C 2012, 116, 10829–10840. [Google Scholar] [CrossRef]
- Dovgaliuk, I.; Ban, V.; Sadikin, Y.; Černý, R.; Aranda, L.; Casati, N.; Devillers, M.; Filinchuk, Y. The first halide-free bimetallic aluminum borohydride: Synthesis, structure, stability, and decomposition pathway. J. Phys. Chem. C 2014, 118, 145–153. [Google Scholar] [CrossRef]
- Knight, D.A.; Zidan, R.; Lascola, R.; Mohtadi, R.; Ling, C.; Sivasubramanian, P.; Kaduk, J.A.; Hwang, S.-J.; Samanta, D.; Jena, P. Synthesis, Characterization, and Atomistic Modeling of Stabilized Highly Pyrophoric Al(BH4)3 via the Formation of the Hypersalt K[Al(BH4)4]. J. Phys. Chem. C 2013, 117, 19905–19915. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schouwink, P.; Morelle, F.; Sadikin, Y.; Filinchuk, Y.; Černý, R. Increasing Hydrogen Density with the Cation-Anion Pair BH4−-NH4+ in Perovskite-Type NH4Ca(BH4)3. Energies 2015, 8, 8286-8299. https://doi.org/10.3390/en8088286
Schouwink P, Morelle F, Sadikin Y, Filinchuk Y, Černý R. Increasing Hydrogen Density with the Cation-Anion Pair BH4−-NH4+ in Perovskite-Type NH4Ca(BH4)3. Energies. 2015; 8(8):8286-8299. https://doi.org/10.3390/en8088286
Chicago/Turabian StyleSchouwink, Pascal, Fabrice Morelle, Yolanda Sadikin, Yaroslav Filinchuk, and Radovan Černý. 2015. "Increasing Hydrogen Density with the Cation-Anion Pair BH4−-NH4+ in Perovskite-Type NH4Ca(BH4)3" Energies 8, no. 8: 8286-8299. https://doi.org/10.3390/en8088286
APA StyleSchouwink, P., Morelle, F., Sadikin, Y., Filinchuk, Y., & Černý, R. (2015). Increasing Hydrogen Density with the Cation-Anion Pair BH4−-NH4+ in Perovskite-Type NH4Ca(BH4)3. Energies, 8(8), 8286-8299. https://doi.org/10.3390/en8088286






