A Novel Grouping Method for Lithium Iron Phosphate Batteries Based on a Fractional Joint Kalman Filter and a New Modified K-Means Clustering Algorithm
Abstract
:1. Introduction
2. Battery Parameter Extraction
2.1. Battery Model Selection
2.1.1. Comparison of Battery Models
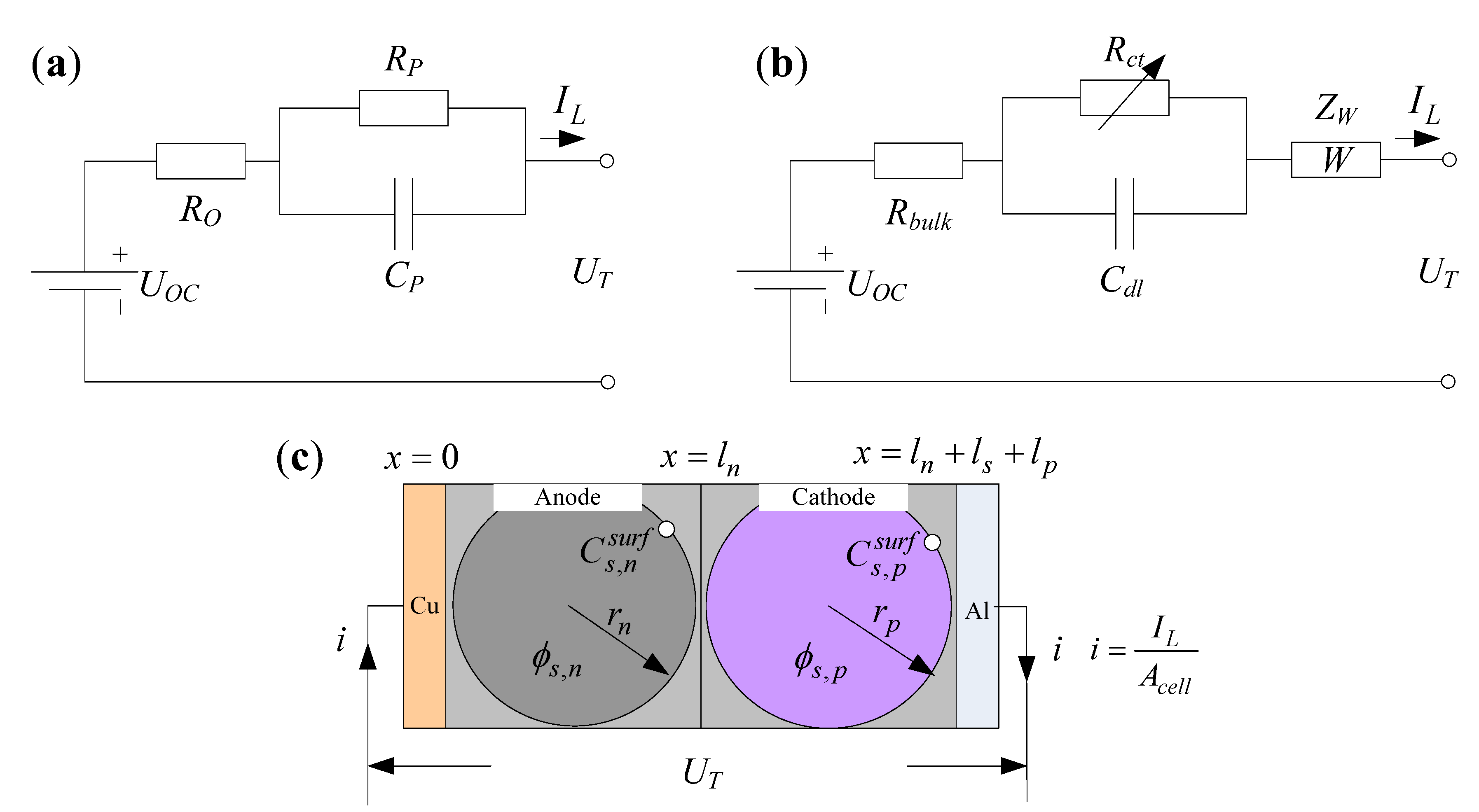
2.1.2. Simplified EIS Model

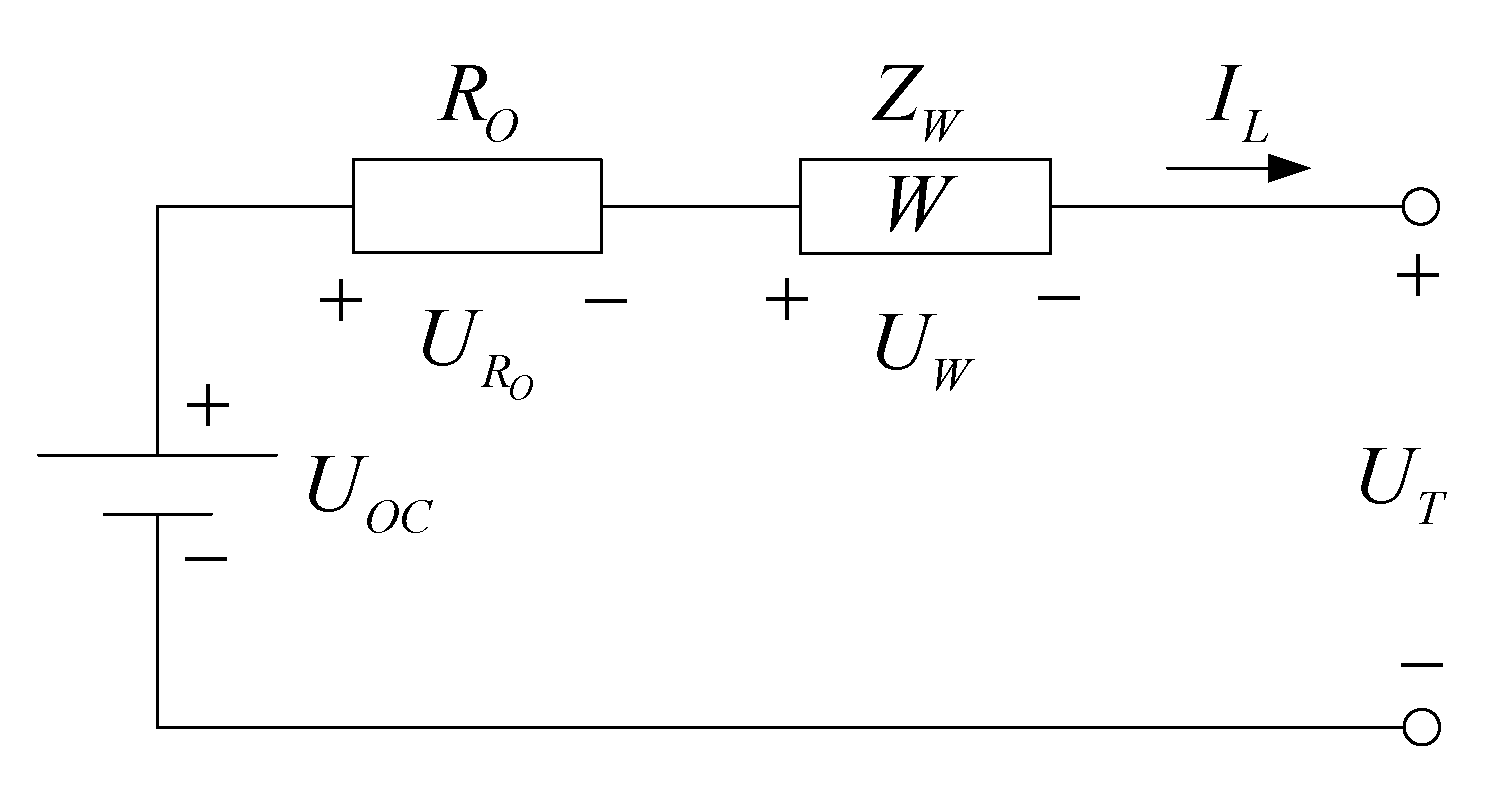
2.2. Model Parameter Identification
2.2.1. Model State Equation Establishment
2.2.2. Model Parameter Identification
| Algorithm 1 Model parameter identification based on FJKF. |
| Definitions: |
| Step 1: Initialization, Qk is the covariance of ωk, Rk is the covariance of noise υk. Pk is the error covariance of the state and parameter estimated values, the initial value for each parameter is give as: |
| , |
| , |
| Step 2: Time update: |
| State and parameter time update: |
| Error covariance time update: |
| Step 3: Measurement update: |
| Kalman gain matrix update: |
| State and parameter measurement update: |
| Error covariance measurement update: |
| Step 4: k = k + 1, repeat Step 2 and Step 3, until all data is processed. |
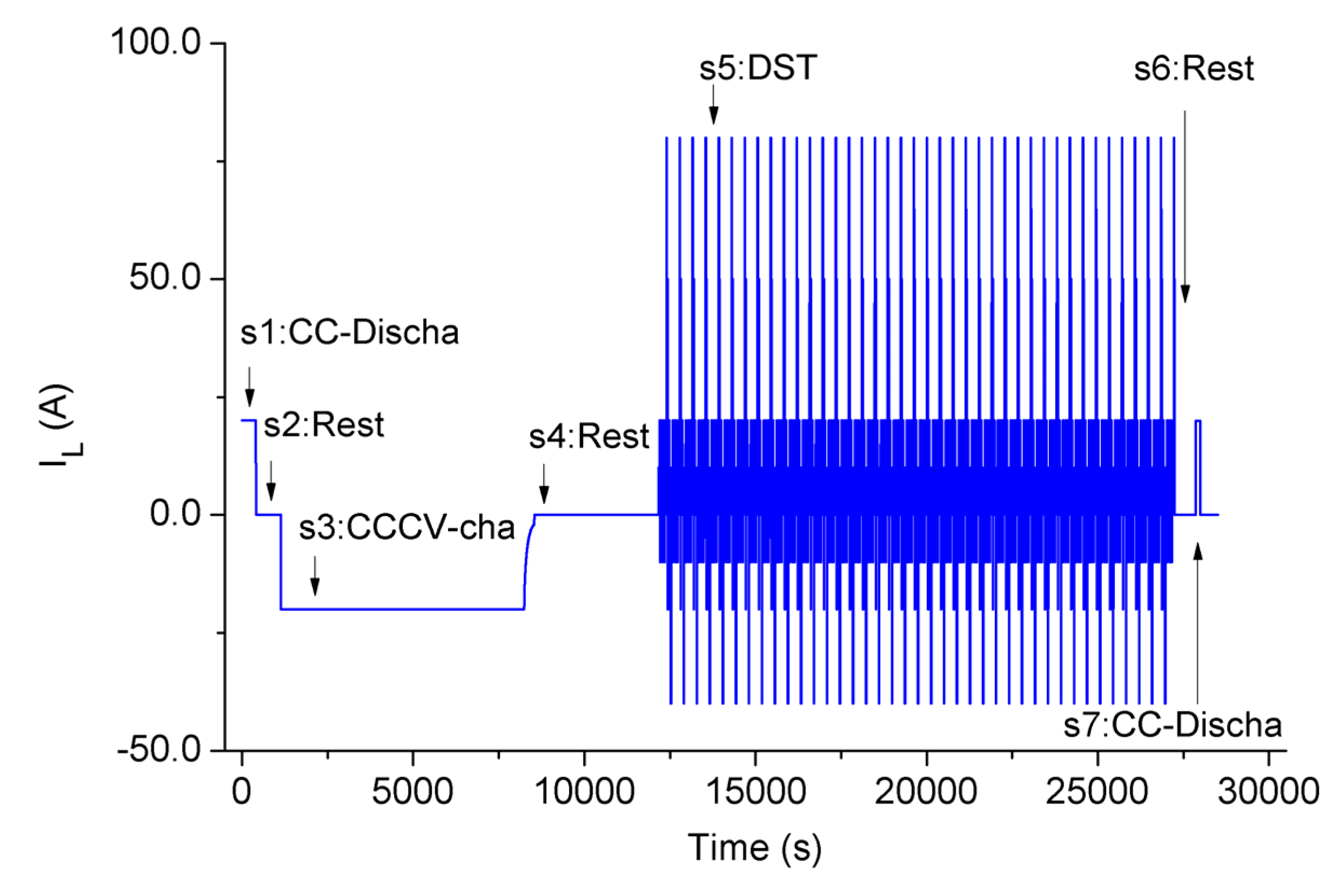
2.3. Parameter Identification Experiment Sequence
| Algorithm 2 Experimental sequence for battery parameter identification. |
| Step 1: Constant current discharge, until battery terminal voltage reaches the lower cut-off voltage. |
| Step 2: Rest for one hour. |
| Step 3: Charge with the battery manufacturer’s recommended procedures. |
| Step 4: Rest for one hour. |
| Step 5: Apply Dynamic Stress Test on the battery. The maximum discharge current is 2C in this paper. Stop when the battery terminal voltage reaches the lower cut-off voltage. The details of DST are described in FreedomCAR battery test manual. |
| Step 6: Rest for several minutes. |
| Step 7: Discharge with a low constant current, until the battery terminal voltage reaches the lower cut-off voltage. This step causes the battery to be fully discharged, this is essential for battery grouping. |
3. Battery Feature Clustering
3.1. Data Preprocess
3.1.1. Data Down-Sampling
3.1.2. Crude Data Exclusion
| Algorithm 3 Crude data excluding process. |
| Step 1: Calculate the average curve of each model parameters of the samples, additionally, calculate the average value of battery capacities. |
| Where, X represents UOC, RO or XW. |
| Step 2: Calculate the average distance between the parameter curve and the average curve, that is the distance between battery capacities and the average capacity. |
| Step 3: Calculate the standard deviation of each distance parameter. |
| Step 4: Discard the samples that do not meet the requirement of Laiyite criterion. The Laiyite criterion is: |
| Step 5: Regard the remaining samples as the evolution objects. Reset the number of evolution objects n, and repeat Steps 1–4, until all the remaining parameters are eligible with the requirement of Laiyite criterion. |
| Step 6: If , randomly discard the samples which have maximum value of until the number of remaining samples is equal to . Ultimately, the samples used for battery grouping are obtained. |
3.2. Consistency Evaluation Parameter Generation
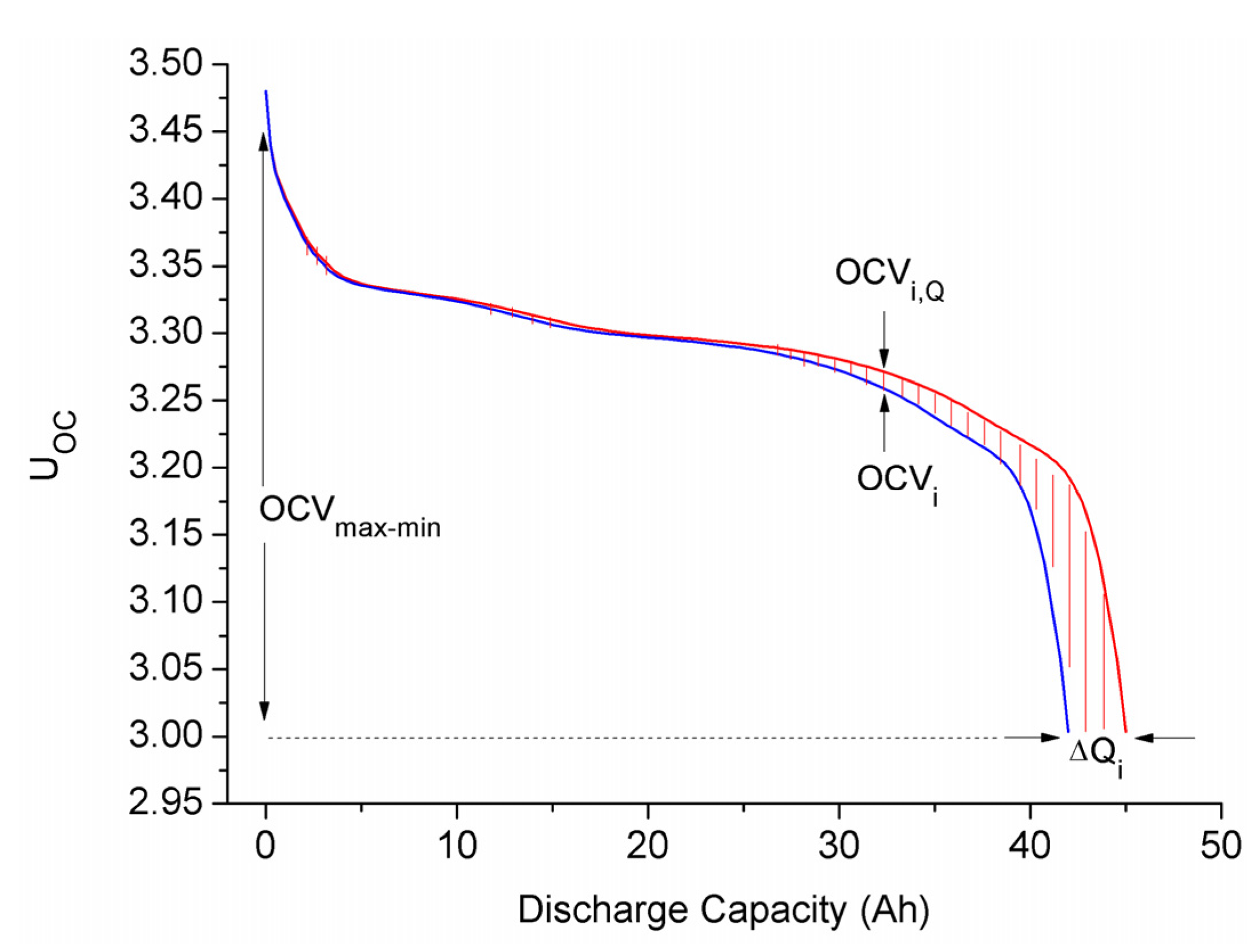
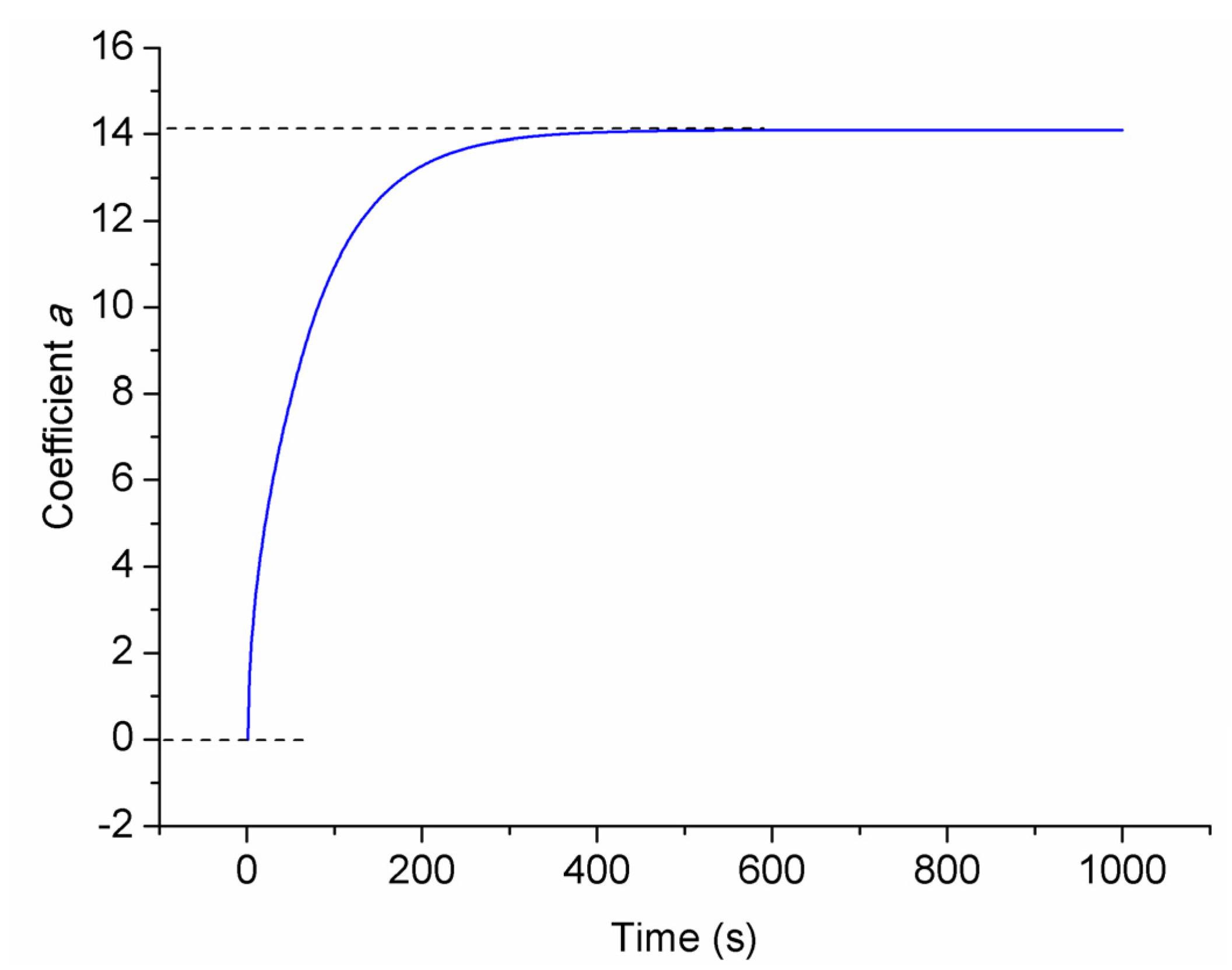
3.3. Battery Equal-Number Clustering
3.3.1. K-Means Clustering Method
| Algorithm 4 Main steps of K-means clustering method. |
| Step 1: Initialize the cluster centers. Select N cluster centers randomly. . |
| Step 2: Calculate the distance between each sample and each cluster center. Clustering the samples to the nearest cluster based on the minimum distance principle. For sample p, it is belong to the cluster which is obtained by: |
| Step 3: Recalculate the cluster centers. The mean value of each cluster is used as the new cluster center: |
| Step 4: Repeat Step 2 and Step 3, until the cluster centers are no longer changing or only changes minimal. |
3.3.2. Kd-Tree Cluster Center Initialization
3.3.3. The New Modified K-Means Clustering Method
| Algorithm 5 Steps of equal-number clustering method. |
| Step 1: Cluster centers initialization. Cluster center is calculated using the kd-tree cluster center initialization method. |
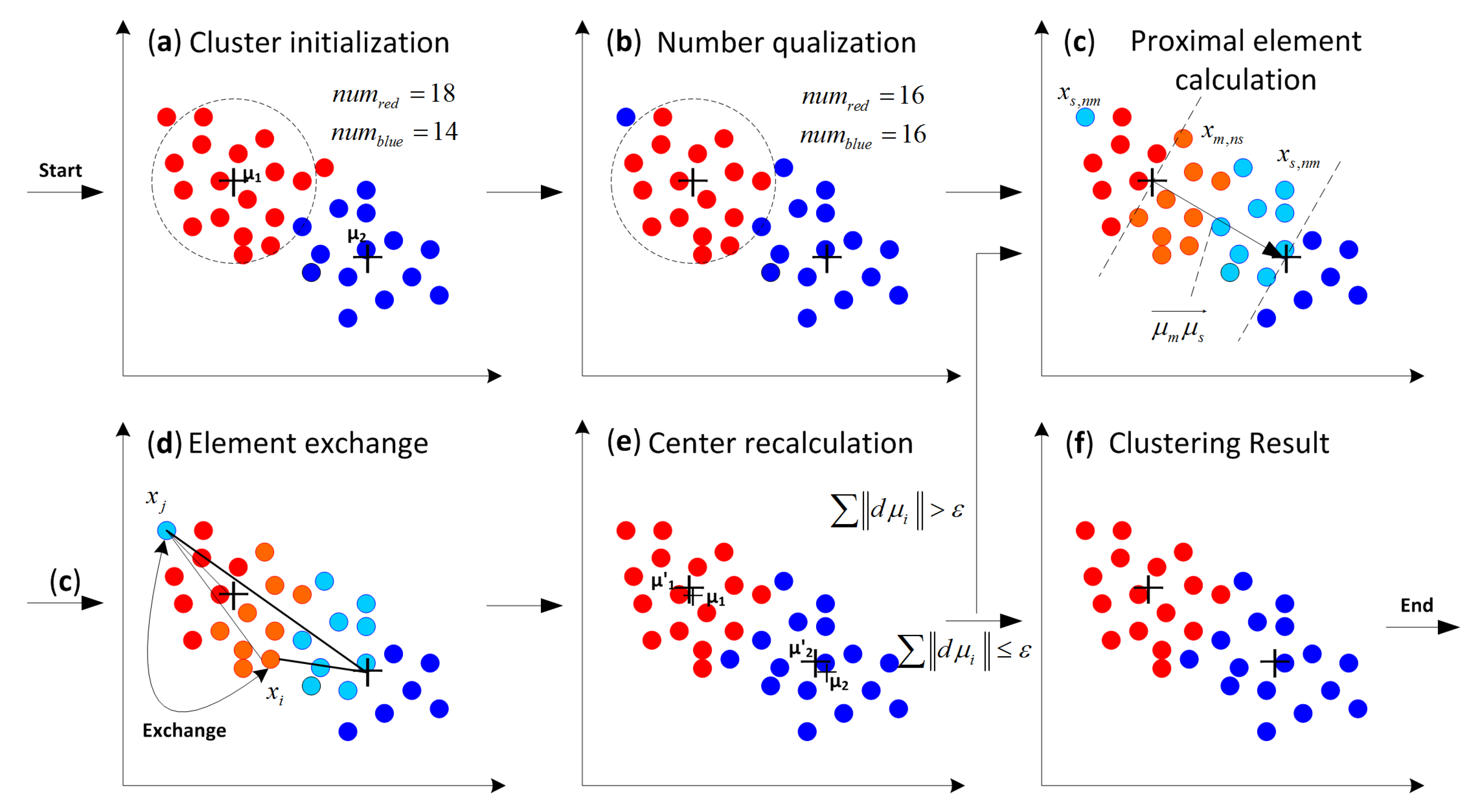
| Step 2: Cluster initialization. The elements are clustered to each cluster according to the minimum distance criterion described in K-means clustering method. Step1 and Step 2 are shown in Figure 7a. |
| Step 3: Element number equalization. Equalize the number of each cluster according to rule 1. This step is shown in Figure 7b. |
| Step 4: Distance data storage. Two arrays are set up for each cluster, named main storage array and auxiliary storage array. Calculate the distances from each element to all centers. The results are stored in the main storage arrays, but auxiliary storage array is not used here. |
| Step 5: Elements exchange among the clusters. |
| Step 5.1: Set one of the clusters as a master cluster Cm, another one is regard as a slave cluster Cs. Find the proximal elements in these two clusters. This step is shown in Figure 7c. |
| Step 5.2: Select a proximal element in Cm and a proximal element in Cs as the quasi-exchange elements, If the two elements can meet the requirements of Equation (28), take out the two elements from their pervious clusters, exchange the belonging of elements and store their feature parameters in the auxiliary storage array. If not, select another proximal element in Cs as the quasi-exchange element and repeat the calculation of Equation (28) and determine whether to exchange elements or not, until all xs,nms are traversed. This step is shown in Figure 7d. |
| Step 5.3: Select another proximal element in Cm as the quasi-exchange element, repeat Step 5.2, until all xm,nss are traversed. |
| Step 5.4: Set another cluster as a new Cs, repeat Step 5.2 and Step 5.3, until all clusters apart from the master cluster are treated as a slave cluster one time for element exchange. |
| Step 5.5: Set another cluster as a new Cm, repeat Step 5.2, Step 5.3 and Step 5.4, until all the clusters are treated as a master cluster one time. |
| Step 6: Take out the data stored in the auxiliary storage array, and push them onto the main storage array of each cluster. Step 6: Recalculate the center of each cluster. This step is shown in Figure 7e. |
| Step 7: Repeat Steps 4–6, until cluster centers do not change or only change a little. |
| Where, ε is the threshold value of process stopping, the equal-number clusters are obtained. |
4. Experimental Details
4.1. Parameter Identification Experimental Details
4.2. Verification Experimental Details
4.2.1. Model and Parameter Identification Verification Experiments
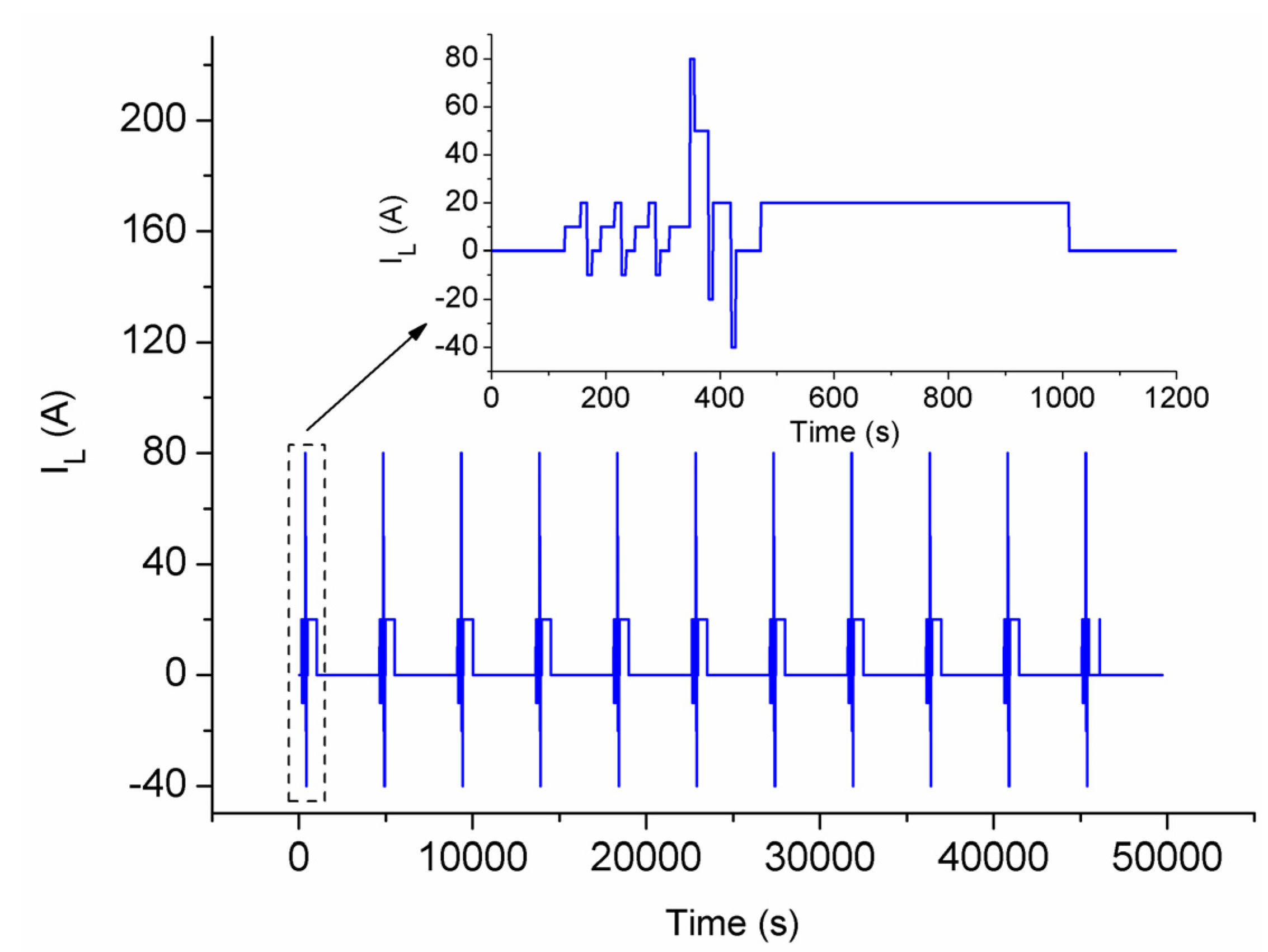
4.2.2. Battery Grouping Verification Experiments
5. Results and Discussion
5.1. Battery Grouping Result Analysis
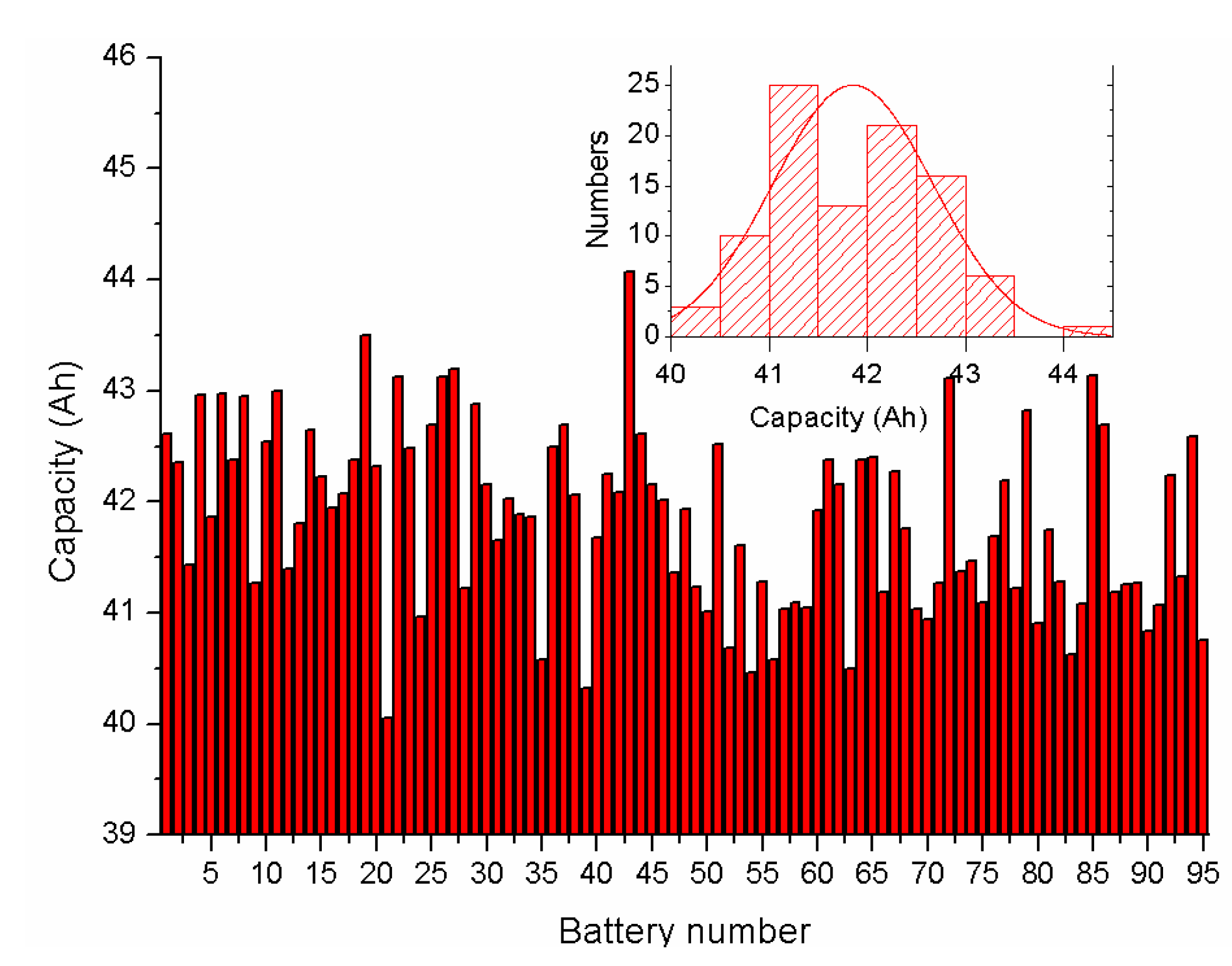
5.1.1. Battery Parameter Identification Result Analysis
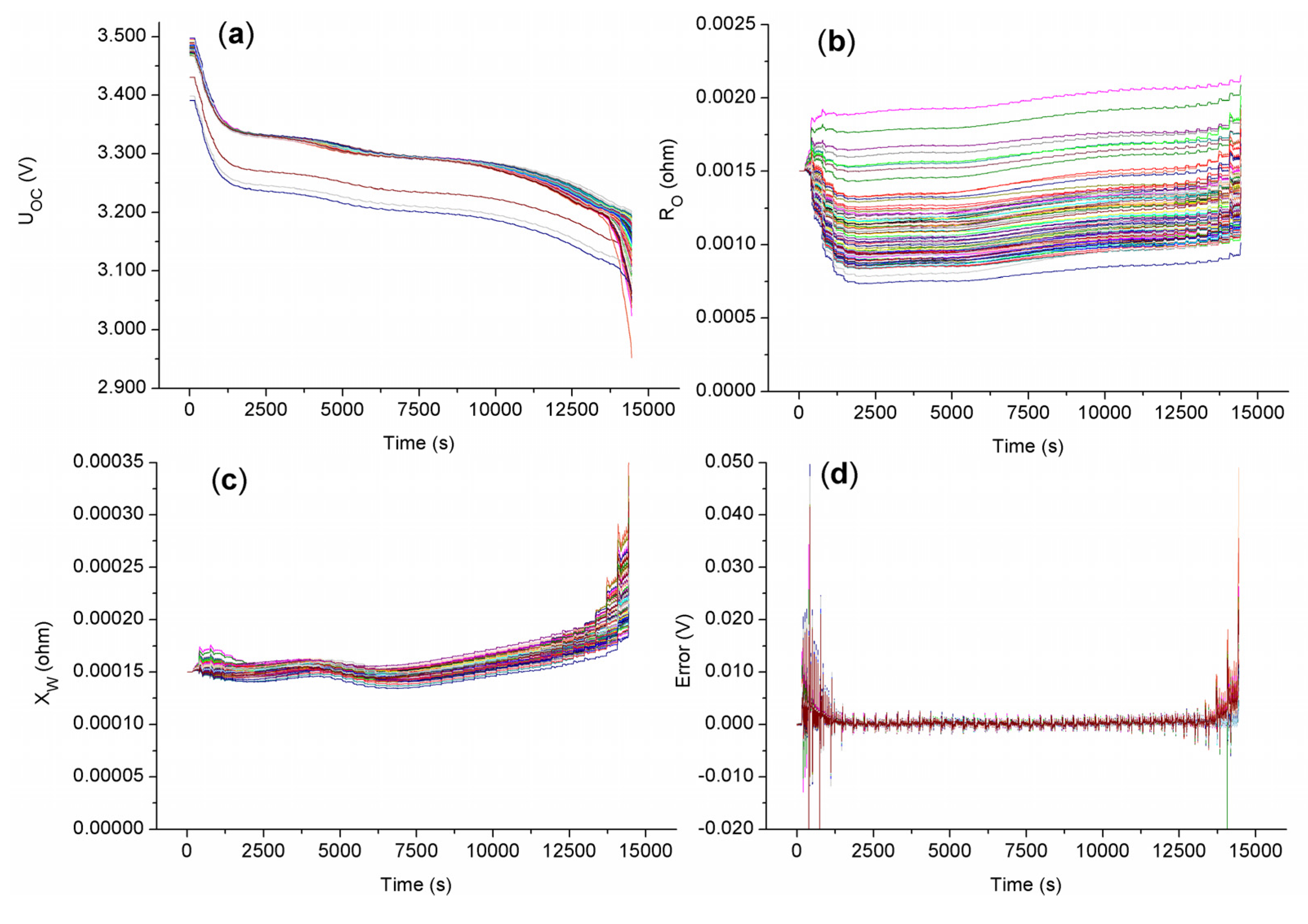
5.1.2. Battery Clustering Result Analysis
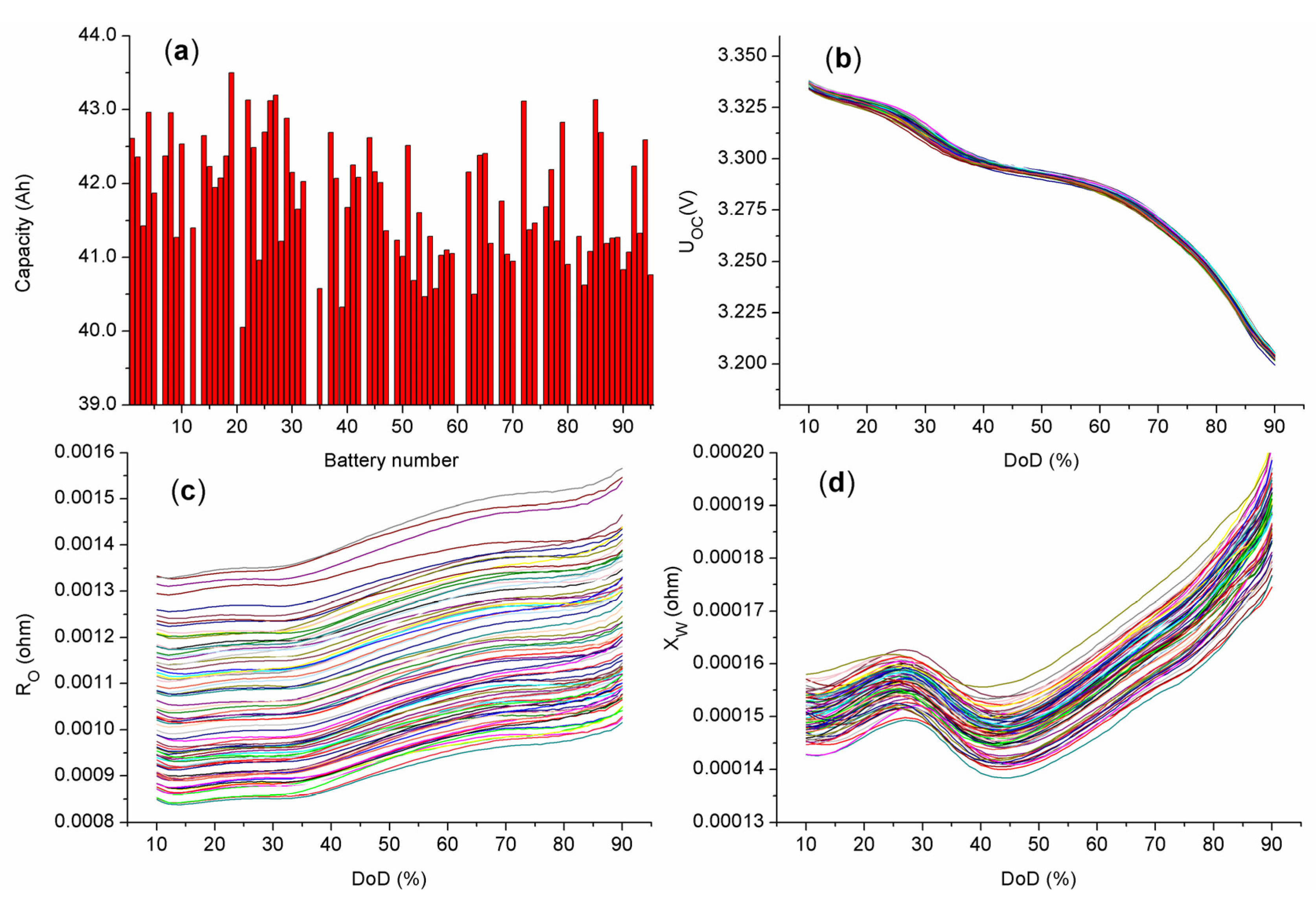
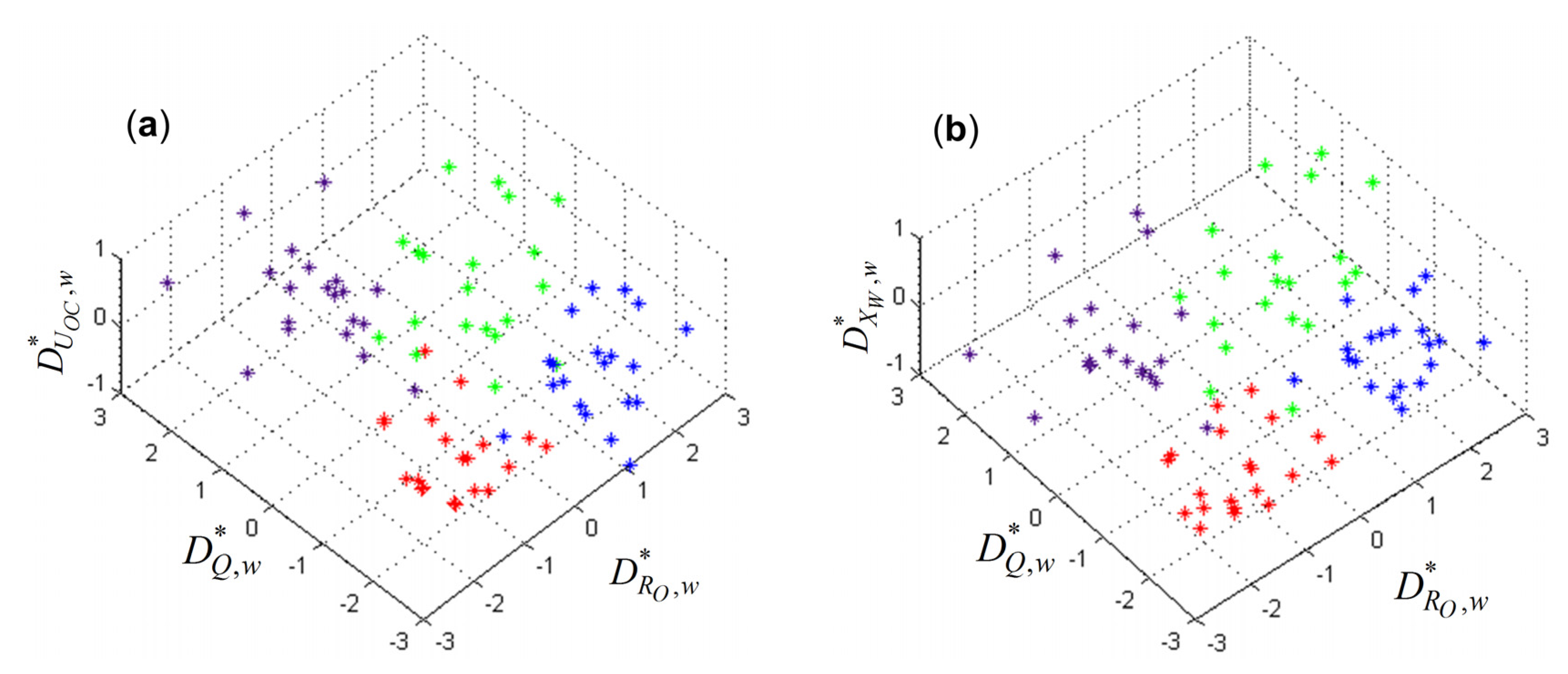
5.2. Verification of the Battery Grouping Method
5.2.1. Model Accuracy Verification

5.2.2. Model Parameter Identification Accuracy Verification

5.2.3. Battery Grouping Accuracy Verification
| Method | Capacity Matching (×10−3 V) | RO Matching (×10−3 V) | Curve Matching (×10−3 V) | Proposed Method (×10−3 V) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dispersion | Max | Min | Avg. | Max | Min | Avg. | Max | Min | Avg. | Max | Min | Avg. |
| DST | 21.56 | 9.483 | 15.40 | 38.43 | 19.18 | 25.94 | 16.50 | 6.910 | 13.41 | 15.80 | 5.682 | 10.78 |
| 0.5 C charge | 26.02 | 9.406 | 17.00 | 43.61 | 27.31 | 32.71 | 22.78 | 7.803 | 18.22 | 19.60 | 6.425 | 13.98 |
| 0.5 C discharge | 21.53 | 9.391 | 15.31 | 41.90 | 26.13 | 31.23 | 19.40 | 7.776 | 15.82 | 19.17 | 7.548 | 14.21 |
| 1 C charge | 30.35 | 15.88 | 24.40 | 42.65 | 28.98 | 33.67 | 22.74 | 17.89 | 20.32 | 22.93 | 16.85 | 19.64 |
| 1 C discharge | 24.40 | 16.13 | 20.46 | 42.92 | 27.97 | 32.32 | 23.32 | 9.052 | 17.03 | 21.80 | 9.939 | 16.71 |
6. Conclusions
- A simplified EIS battery model is adopted for battery characteristic description. The accuracy of this model is relatively higher and the number of parameters is less than in the first order RC model under the tested conditions. The model is suitable for battery grouping.
- A fractional joint Kalman filter is used for parameter identification. Fractional parameters XW can be estimated jointly with the state UW of the fractional element ZW. This algorithm is appropriate and effective for fractional model parameter identification.
- A new modified K-means clustering method is proposed for battery equal-number grouping. In this method, a feature vector with four parameters is used to describe the performance of the battery from different angles. Two rules are designed to equalize the numbers of elements in each group and exchange samples among groups. The rules are added to the K-means clustering method and replace the criterion of element attribution calculation.
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
Symbols
| UT | terminal voltage of a battery, V |
| IL | load current, A |
| OCV | open circuit voltage, V |
| UOC | open circuit voltage, V |
| EOC | open circuit potential of electrode, V |
| RO | ohmic resistance, Ω |
| Rbulk | bulk resistance, Ω |
| Rext | connect resistance, Ω |
| Rsei | SEI resistance, Ω |
| Rct | charge transfer resistance, Ω |
| Cdl | double layer capacitance, F |
| ZW | Warburg impedance, Ωs−0.5 |
| XW | Warburg resistance, Ω |
| UW | terminal voltage of ZW, V |
| Q | nominal capacity, Ah |
| Q0 | typical capacity of the battery, Ah |
| w | weight of a parameter, 1 |
| σ | standard deviation, V or Ω |
| D | distance, V or Ω |
| E | evaluation parameter, V or Ω |
| C | cluster |
| N | total number of clusters |
| n | cluster n |
| M | total number of samples |
| p | sample p |
| μ | cluster center |
Subscripts, superscript
| eff | effective value |
| avg | average value |
| k | time step index |
| m | master |
| s | slave |
| (p) | sample p |
estimation value | |
prior estimation value | |
posteriori estimation value | |
vector | |
normalized value |
Abbreviations
| EV | electric vehicle |
| EIS | electrochemical impedance spectroscopy |
| FJKF | fractional joint Kalman filter |
| DST | dynamic stress test |
| SoC | state of charge |
| DoD | depth of discharge |
| SEI | solid electrolyte interface |
| RMS | root mean square |
References
- Wang, T.; Tseng, K.J.; Zhao, J.; Wei, Z. Thermal investigation of lithium-ion battery module with different cell arrangement structures and forced air-cooling strategies. Appl. Energy 2014, 134, 229–238. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, J.; Wei, Z.; Skyllas-Kazacos, M. Extended Kalman filter method for state of charge estimation of vanadium redox flow battery using thermal-dependent electrical model. J. Power Sources 2014, 262, 50–61. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, C.; Pei, L.; Lu, R.; Xu, B. The State of Arts and Development Trend of SOH Estimation for Lithium-Ion Batteries. In Proceedings of the 2013 IEEE Vehicle Power and Propulsion Conference (VPPC), Beijing, China, 15–18 October 2013; pp. 1–6.
- Andre, D.; Appel, C.; Soczka-Guth, T.; Sauer, D.U. Advanced mathematical methods of SOC and SOH estimation for lithium-ion batteries. J. Power Sources 2013, 224, 20–27. [Google Scholar] [CrossRef]
- Pei, L.; Zhu, C.; Wang, T.; Lu, R.; Chan, C.C. Online peak power prediction based on a parameter and state estimator for lithium-ion batteries in electric vehicles. Energy 2014, 66, 766–778. [Google Scholar] [CrossRef]
- Xiong, R.; He, H.; Sun, F.; Liu, X.; Liu, Z. Model-based state of charge and peak power capability joint estimation of lithium-ion battery in plug-in hybrid electric vehicles. J. Power Sources 2013, 229, 159–169. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.; Chun, C.; Cho, B.H. Stable Configuration of a Li-Ion Series Battery Pack Based on a Screening Process for Improved Voltage/SOC Balancing. IEEE Trans. Power Electron. 2012, 27, 411–424. [Google Scholar] [CrossRef]
- Kim, J.; Cho, B.H. Screening process-based modeling of the multi-cell battery string in series and parallel connections for high accuracy state-of-charge estimation. Energy 2013, 57, 581–599. [Google Scholar] [CrossRef]
- Schneider, E.L.; Oliveira, C.T.; Brito, R.M.; Malfatti, C.F. Classification of discarded NiMH and Li-Ion batteries and reuse of the cells still in operational conditions in prototypes. J. Power Sources 2014, 262, 1–9. [Google Scholar] [CrossRef]
- Fang, K.; Chen, S.; Mu, D.; Wu, B.; Wu, F. Investigation of nickel–metal hydride battery sorting based on charging thermal behavior. J. Power Sources 2013, 224, 120–124. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Pei, L.; Zhu, C.; Xu, B. A comparative study of sorting methods for lithium-ion batteries. In Proceedings of the 2014 IEEE Conference and Expo Transportation Electrification Asia-Pacific (ITEC Asia-Pacific), Beijing, China, 31 August–3 September 2014; pp. 1–6.
- Diamond, R.A.; Wang, H.; Chen, F.; Wilke-Douglas, M. Cell Preparation and Enrichment for FCM Analysis and Cell Sorting. In In Living Color; Springer Lab Manuals; Springer: Berlin/Heidelberg, Germany, 2000; pp. 111–141. [Google Scholar]
- Guo, L.; Liu, G.W. Research of Lithium-Ion Battery Sorting Method Based on Fuzzy C-Means Algorithm. Adv. Mater. Res. 2012, 354–355, 983–988. [Google Scholar] [CrossRef]
- Yun, W.U.; Ge-Chen, L.I.; Chen, S.L. The Application of BP Nerual Network in Battery-sorting. J. Harbin Univ. Sci. Technol. 2001, 5, 54–57. [Google Scholar]
- Feng, F.; Lu, R.; Zhu, C. A Combined State of Charge Estimation Method for Lithium-Ion Batteries Used in a Wide Ambient Temperature Range. Energies 2014, 7, 3004–3032. [Google Scholar] [CrossRef]
- Plett, G.L. Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs Part 1. Background. J. Power Sources 2004, 134, 262–276. [Google Scholar] [CrossRef]
- Plett, G.L. Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs Part 2. Modeling and identification. J. Power Sources 2004, 134, 252–261. [Google Scholar] [CrossRef]
- Plett, G.L. Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs Part 3. State and parameter estimation. J. Power Sources 2004, 134, 277–292. [Google Scholar] [CrossRef]
- Xiong, R.; Sun, F.; He, H.; Nguyen, T.D. A data-driven adaptive state of charge and power capability joint estimator of lithium-ion polymer battery used in electric vehicles. Energy 2013, 63, 295–308. [Google Scholar] [CrossRef]
- Yoon, S.; Hwang, I.; Lee, C.W.; Ko, H.S.; Han, K.H. Power capability analysis in lithium ion batteries using electrochemical impedance spectroscopy. J. Electroanal. Chem. 2011, 655, 32–38. [Google Scholar] [CrossRef]
- Xu, J.; Mi, C.C.; Cao, B.; Cao, J. A new method to estimate the state of charge of lithium-ion batteries based on the battery impedance model. J. Power Sources 2013, 233, 277–284. [Google Scholar] [CrossRef]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental investigation of the lithium-ion battery impedance characteristic at various conditions and aging states and its influence on the application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
- Luo, W.; Lyu, C.; Wang, L.; Zhang, L. A new extension of physics-based single particle model for higher charge–discharge rates. J. Power Sources 2013, 241, 295–310. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Relaxation phenomena in lithium-ion-insertion cells. J. Electrochem. Soc. 1994, 141, 982–990. [Google Scholar] [CrossRef]
- Zhang, D. Modeling Lithium Intercalation of a Single Spinel Particle under Potentiodynamic Control. J. Electrochem. Soc. 1999, 147, 831–838. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, C.; Hinds, G.; Wang, L.; Luo, W.; Zheng, J.; Ma, K. Parameter Sensitivity Analysis of Cylindrical LiFePO4 Battery Performance Using Multi-Physics Modeling. J. Electrochem. Soc. 2014, 161, A762–A776. [Google Scholar] [CrossRef]
- Fleischer, C.; Waag, W.; Heyn, H.; Sauer, D.U. On-line adaptive battery impedance parameter and state estimation considering physical principles in reduced order equivalent circuit battery models part 2. Parameter and state estimation. J. Power Sources 2014, 262, 457–482. [Google Scholar] [CrossRef]
- Fleischer, C.; Waag, W.; Heyn, H.; Sauer, D.U. On-line adaptive battery impedance parameter and state estimation considering physical principles in reduced order equivalent circuit battery models : Part 1. Requirements, critical review of methods and modeling. J. Power Sources 2014, 260, 276–291. [Google Scholar] [CrossRef]
- Li, X.; Zhu, C.; Wei, G.; Lu, R. Online Parameter Estimation of LiFePO4 Battery Simplified Impedance Spectroscopy Model Based on Fractional Joint Kalman Filter. Trans. Chin. Electrotechnical Soc. 2015, in press. [Google Scholar]
- Sierociuk, D.; DzielińSki, A. Fractional Kalman filter algorithm for the states, parameters and order of fractional system estimation. Int. J. Appl. Math. Comput. Sci. 2006, 16, 129–140. [Google Scholar]
- FreedomCAR Battery Test Manual for Power-Assist Hybrid Electric Vehicles; U.S. Department of Energy: Washington, DC, USA, 2003.
- Mcqueen, J. Some methods for classification and analysis of ultivariate observations. In Proceedings of the Berkeley Symposium Mathematical Statistics Probability; University of California Press: Berkeley, CA, USA, 1967; pp. 281–297. [Google Scholar]
- Redmond, S.J.; Heneghan, C. A Method for Initialising the K-Means Clustering Algorithm Using Kd-Trees. Pattern Recognit. Lett. 2007, 28, 965–973. [Google Scholar] [CrossRef]
- Hanze, D.Z.L.G. Cell classification system based on automatic curve-recognition. Chin. J. Power Sources 2000, 2, 65–69. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Song, K.; Wei, G.; Lu, R.; Zhu, C. A Novel Grouping Method for Lithium Iron Phosphate Batteries Based on a Fractional Joint Kalman Filter and a New Modified K-Means Clustering Algorithm. Energies 2015, 8, 7703-7728. https://doi.org/10.3390/en8087703
Li X, Song K, Wei G, Lu R, Zhu C. A Novel Grouping Method for Lithium Iron Phosphate Batteries Based on a Fractional Joint Kalman Filter and a New Modified K-Means Clustering Algorithm. Energies. 2015; 8(8):7703-7728. https://doi.org/10.3390/en8087703
Chicago/Turabian StyleLi, Xiaoyu, Kai Song, Guo Wei, Rengui Lu, and Chunbo Zhu. 2015. "A Novel Grouping Method for Lithium Iron Phosphate Batteries Based on a Fractional Joint Kalman Filter and a New Modified K-Means Clustering Algorithm" Energies 8, no. 8: 7703-7728. https://doi.org/10.3390/en8087703
APA StyleLi, X., Song, K., Wei, G., Lu, R., & Zhu, C. (2015). A Novel Grouping Method for Lithium Iron Phosphate Batteries Based on a Fractional Joint Kalman Filter and a New Modified K-Means Clustering Algorithm. Energies, 8(8), 7703-7728. https://doi.org/10.3390/en8087703








