Abstract
Coastal methane hydrate deposits are globally abundant. There is a need to understand the deep sediment sourced methane energy contribution to shallow sediment carbon relative to terrestrial sources and phytoplankton. Shallow sediment and porewater samples were collected from Atwater Valley, Texas-Louisiana Shelf, Gulf of Mexico near a seafloor mound feature identified in geophysical surveys as an elevated bottom seismic reflection. Geochemical data revealed off-mound methane diffusion and active fluid advection on-mound. Gas composition (average methane/ethane ratio ~11,000) and isotope ratios of methane on the mound (average δ13CCH4(g) = −71.2‰; Δ14CCH4(g) = −961‰) indicate a deep sediment, microbial source. Depleted sediment organic carbon values on mound (δ13CSOC = −25.8‰; Δ14CSOC = −930‰) relative to off-mound (δ13CSOC = −22.5‰; Δ14CSOC = −629‰) suggest deep sourced ancient carbon is incorporated into shallow sediment organic matter. Porewater and sediment data indicate inorganic carbon fixed during anaerobic oxidation of methane is a dominant contributor to on-mound shallow sediment organic carbon cycling. A simple stable carbon isotope mass balance suggests carbon fixation of dissolved inorganic carbon (DIC) associated with anaerobic oxidation of hydrate-sourced CH4 contributes up to 85% of shallow sediment organic carbon.
1. Introduction
Sediment organic carbon (SOC) composition and provenance have been extensively studied in the Gulf of Mexico (GoM). Generally, inputs of terrestrially-derived organic carbon (OC) dominate near shore sediments and decrease with distance offshore [1,2,3,4,5]. Terrestrial-derived OC in surficial sediments of the GoM shifts from C3 plant material near shore to highly degraded, soil-derived material offshore [1,2,3]. Surface sediments on the slope (365–2270 m water depth) have Δ14C values between −309.1‰ and −228.6‰ corresponding to 64% to 78% modern carbon [3]. Mayer et al. [4] calculated that surficial SOC on the slope is 41% to 46% of marine origin. Carbon isotope signatures typical for marine phytoplankton have been measured in surface sediments at water column depths from 74 to 2250 m (mean δ13C value = −20.8‰; [6]). In the same study, depleted (δ13C = −24.1‰) sedimentary organic matter was observed at a cold seep site (688 m water depth) that suggested a contribution of seep hydrocarbons to sediment organic matter.
Methane hydrate deposits are abundant in deep sediments along the Texas-Louisiana Shelf [7,8]. Hydrate-sourced CH4 may contribute to the sediment OC pool and should be considered in investigations of carbon cycling and regional carbon mass balance calculations. During anaerobic oxidation of methane (AOM), bacteria utilize seawater-sourced sulfate (SO42−) as a terminal electron acceptor to oxidize methane as an energy source, and produce sulfide (H2S) and dissolved inorganic carbon (DIC) in the process [9,10]. During this oxidation porewater DIC sourced from downward seawater diffusion, upward deep system advection or diffusion, and AOM is incorporated into microbial biomass [11,12,13,14]. This is a contrast to aerobic CH4 assimilation, where methane-derived carbon is directly incorporated into microbial biomass [15]. Deep sediment hydrocarbons, including CH4, have been shown to contribute to carbon cycling in shallow sediments and the water column [16,17,18,19,20,21]. However, the indirect contribution of hydrate-sourced CH4 to shallow sediment carbon pools through AOM and subsequent carbon dioxide (CO2) fixation has not been thoroughly evaluated.
Shallow sediment CH4 in Atwater Valley, Gulf of Mexico has been shown to be dominated by biogenic gas from deep sediments [17]. In this study, two sites in Atwater Valley were contrasted: A sediment mound with active fluid advection and high vertical CH4 flux and an off-mound site exhibiting steady-state CH4 diffusion [22,23]. Gas speciation and δ13C values at both sites indicate that the shallow sediment gas is primarily biogenic-sourced CH4, absent of higher molecular weight gases [22]. In this study a carbon budget is constructed for each site using hydrocarbon gas concentrations and stable carbon isotope (δ13C) and radiocarbon isotope (Δ14C) signatures of the organic and inorganic carbon pools in solid phase sediment and pore water. The hypothesis is that dissolved inorganic carbon (DIC) assimilated during AOM is a dominant contributor to on-mound shallow sediment organic carbon cycling.
2. Materials and Methods
2.1. Study Location
Atwater Valley is a shallow trough in the Mississippi Canyon, Gulf of Mexico (Figure 1a). This underwater trough is part of the Mississippi Fan Fold-belt with sediment features including basinward-verging anticlines and underlain southern verging thrust faults across a 300 km long and 50 km wide region. Fold strata were formed during Late Jurassic to Miocene geologic periods resulting in formation of substantial salt tongues and sheets [24]. Canyon fill through the gas hydrate stability zone (HSZ) is comprised of fine-grained sediments, mostly interbedded debris flows and hemipelagic sediments overlain by a fine Holocene pelagic drape [25]. The area is characterized by seafloor mounds and basins formed through vertical advection of hydrocarbons, minerals, and CH4-rich fluids [26].
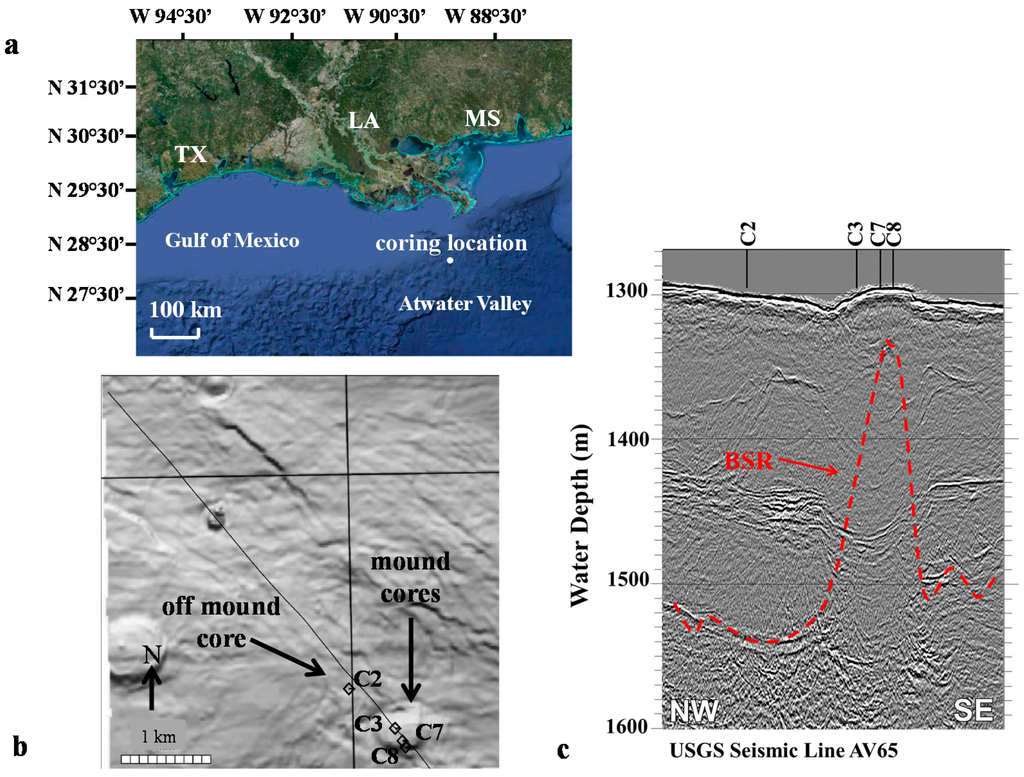
Figure 1.
(a) Atwater Valley, Gulf of Mexico, Texas-Louisiana Shelf coring location (provided by Google Earth); (b) Sediment floor contours through the sample region, with sites on- (cores 3,7,8) and off-mound (core 2), the solid line represents the seismic data transect through the coring locations; (c) Core sites selected for this study were over a strong sediment bottom reflection (BSR) off-mound and on the mound observed with an elevated BSR and vertical seismic blanking above the BSR mound.
A 4 m high seafloor mound, at a water column depth between 1296 and 1300 m, was chosen as a study site based on previous seismic and geochemical studies conducted in May 2004 [22,23,27] (Table 1). A key mound feature (Figure 1b) is a shallowing bell-shaped bottom simulating reflection (BSR) that raises approximately 200 m relative to off-mound BSR at 240 mbsf (Figure 1c, Table 1). At this location, heat flow elevates from a background signature off-mound of 40 to 160 mW·m−2 on-mound (Table 1). These geophysical conditions on mound were interpreted to be a thermal perturbation to the HSZ base, creating vertical fluid fluxes [23].
Porewater chloride (Cl−) concentrations on-mound averaged 934 ± 74 mM, well above the seawater background, indicating active vertical advection of higher salinity porewaters (Table 1) and a rise in the BSR (Figure 1c). This interpretation is supported with the observation of Late Jurassic to Miocene salt tongues and salt diapirism in this region that would reduce deep sediment hydrate stability [24]. An advective CH4 flux of 3250 mM·m−2·year−1 was estimated on the mound (Table 1). In contrast, 750 m off-mound (Figure 1b) a moderate diffusive CH4 flux of 20.6 mM·m−2·year−1 was observed (Table 1). Off-mound, sediment porewater SO42− concentrations were observed to decrease linearly from near seawater values at the sediment-water interface to below detection limits at 410 centimeters below the sea floor (cmbsf). Below 410 cmbsf, sediment headspace methane concentrations increased linearly, indicating a sulfate methane-transition (SMT) supported by the anaerobic oxidation of methane (AOM). Vertical fluid advection limited downward SO42− diffusion on-mound (Table 1).

Table 1.
Review of previously published data for core sites on- and off-mound. Where data is general for the mound and not located at specific stations it is listed under C7.
| Parameter | Cores | ||||
|---|---|---|---|---|---|
| Off Mound | Mound | ||||
| C2 | C7 | C3 | C8 | Ref. | |
| Latitude | 27°56′26.644 | 27°56′26.644 | 27°56′26.644 | 27°56′26.644 | [27] |
| Longitude | 89°16′49.696 | 89°16′49.696 | 89°16′49.696 | 89°16′49.696 | [27] |
| Water Column Depth (m) | 1300 | 1296 | 1301 | 1296 | [27] |
| Core Pentetration (cmbsf) | 470 | 867 | 515 | 370 | [27] |
| SMT (cmbsf) | 410 | no SMT | no SMT | 59 | [22] |
| Methane Flux (mM CH4 m−2·year−1) | 20 | 3250 | ND | 167 | [22] |
| Average Cl− (mM) | 551 ± 6 | 934 ± 44 | 911 ± 79 | 770 ± 102 | [22] |
| Heat Flow (mW·m−2) | 40 | 160 | - | - | [22] |
| BSR depth (mbsf) | 240 | 40 | - | - | [23] |
Close proximity in spatial variation of downward SO42− diffusion and inferred vertical CH4 flux observed in previous studies on this mound makes it a unique study site to investigate CH4 contribution to shallow sediment carbon cycling [22]. Porewater and sediment inorganic and organic carbon concentration and carbon isotope ratio data from an off-mound core (C2) are compared to an on-mound core (C7). Results from two additional sediment mound cores (C3 and C8) located near C7 are used to provide additional data for interpretation of CH4 contribution to sediment carbon pools (Table 1).
2.2. Sediment Core Collection and Processing
Piston cores C7, C3, and C8 were collected on-mound (water depth = 1296 m) and C2 was collected off-mound (water depth = 1300 m, Figure 1b,c). For on-mound cores C7 was located near the center, while C3 and C8 were near the mound edge, 150 and 70 m, respectively, from C7. Sediment cores were collected and processed shipboard as described in Coffin et al. [22]. Briefly, sediment cores were obtained using a 10 m piston coring system with 2.75 polycarbonate core liners. Cores ranged in length from ~300 to 800 cm and were processed immediately onboard the ship.
Core liners were inspected for gas pockets and gas expansion voids. At void spaces, the liner was drilled and gas sampled with a 60 mL polypropylene syringe fitted with a modified 3-way stopcock. Gas samples were then transferred to 30 mL pre-evacuated, glass serum vials fitted with a gastight stopper and aluminum seal. Subsequently, sediment plugs were collected from regular intervals along the core using a 3 mL polypropylene syringe with the tip cut off, transferred to pre-weighed 20 mL serum vials, and capped with gastight stoppers and aluminum seals to determine sediment headspace light hydrocarbon concentrations; (CH4 through C3H8) as well as δ13CCH4(g) ratios.
For additional sampling, core liners were removed and cut in 10 cm sections within an interval of 25–45 cm. Wet sediment from each section was frozen in snap-tight Petri-dishes for laboratory measurements of sediment porosity and percent organic carbon. Porewater pressed from sediment using 70 mL Reeburgh-style PVC press containers pressurized to 400 KPa (60 psi) by a low-pressure air on a latex sheet between core sections and press gas inflow was collected into 60-mL polypropylene syringes [28]. Porewater was filtered from syringes through ashed Whatman GF-F filters into ashed 20 mL vials and subsequently distributed into appropriate vials for each analysis; 2 mL in a 5 mL glass serum vial for [DIC]; 1 mL in a 2 mL glass serum vial for δ13CDIC, and; 2 mL in a 5 mL glass screw-top vial for dissolved organic carbon concentration [DOC] and δ13CDOC. Pressed sediment for inorganic and organic carbon concentration and isotope analyses was wrapped in ashed aluminum foil, sealed in Whirlpack bags, and stored frozen at −20 °C for analyses at the land-based laboratory.
2.3. Shipboard Analyses
To extract volatile hydrocarbons from sediment into vial headspace for gas analysis, 3 mL of nitrogen sparged, deionized water was injected through the septum of the serum vial and vial was shaken for 3 min. After this extraction, the headspace sample was removed from the vial and injected into a sampling loop on a Shimadzu GC-14A gas chromatograph-flame ionization detector (GC-FID) with a Hayesep-Q packed column (Alltech, Deerfield, IL, USA) to measure CH4, ethane (C2H6), and propane (C3H8) concentrations. Sediment CH4 concentrations were corrected for atmospheric background in the vials (95% extraction efficiency was assumed). Core gas pocket C1-C3 alkane concentrations were also measured using the GC-FID. Analytical precision was within 0.1 mM, based on replicate analyses. The limit of detection for methane was 0.009 mM and where concentrations were lower data are presented as 0.0 mM.
Porewater DIC concentrations were determined using a UIC CO2 coulometer (UIC, Inc., Joliet, IL, USA) standardized to a certified seawater reference material (University of California, San Diego, CA, USA). Replicate variability was less than 0.15 mM.
2.4. Post-Cruise Laboratory Analyses
Sediment total carbon and OC (%TC, %SOC) concentrations and δ13C values were determined on a Fisons EA 1108 C/H/N analyzer in line with a Thermo Electron Delta Plus XP Isotope Ratio Mass Spectrometer (IRMS) interface via a Conflo II (Thermo Scientific, Waltham, MA, USA). Pressed sediment was dried at 80 °C, ground with a mortar and pestle, then 15 to 20 mg of sediment was weighed in tin capsules for TC analysis. For SOC analysis, sub-samples were weighed in silver capsules, treated with an excess of 10% HCl and dried in an oven at 70 °C overnight to remove inorganic carbon. Sediment inorganic carbon (%CaCO3) concentrations were determined from the difference between TC and SOC. A concentration calibration curve for carbon concentration analysis was generated daily by analyzing an acetanilide standard. For sediment δ13CSOC values, IAEA-C8 (oxalic acid), IAEA-CH-6 (sucrose) and USGS 40 (l-glutamic acid) were used as calibration standards. Acetanilide standards (USGS-40 and IAEA-C8) were also used as check standards during analysis. All δ13C data presented in this work are in per mil units (‰) and referenced to the Vienna Pee Dee Belemnite (VPDB) scale. Errors were based on triplicate runs. Error for % SOC was within ±0.03%C, %CaCO3 varied by less 0.2%, and δ13CSOC varied by less than 0.2‰.
Sediment δ13CCaCO3, pore water δ13CDIC, and gas pocket and sediment δ13CCH4 ratios were determined using a Thermo Electron Trace GC equipped with a Varian Porapak-Q column and GC-CIII combustion interface in-line with the Delta Plus XP IRMS (Thermo Scientific, Waltham, MA, USA) [22]. For δ13CCaCO3 analysis, 250 mg of sediment in a serum vial was treated with 2 mL of 10% HCl. For δ13CDIC analysis, 2 mL porewater samples were treated with 200 μL of 85% H3PO4. In both cases, CO2 was extracted from the vial headspace and injected into the GC via a split/splitless inlet in split mode. All δ13CCaCO3 and δ13CDIC values were normalized through analysis of CO2 and C1-C5 alkanes in NIST RM 8560 (natural gas, petroleum origin). Samples for δ13CCH4 analysis were introduced via an in-line cryogenic focusing system according to the method of Plummer et al. [29]. A separate δ13C normalization curve was generated for C1-C4 alkanes and used to normalize δ13CCH4 data. Replicate δ13CCaCO3 values varied by less than 0.2‰, δ13CDIC by less than 0.5‰, and δ13CCH4 by less than 1.0‰.
Porewater DOC was measured on an OI Analytical 1010 total organic carbon analyzer (OI Analytical, College Station, TX, USA) using a heated persulfate oxidation method modified for seawater analyses [30]. The samples were kept frozen until ready for analysis, then acidified and nitrogen sparged in the lab prior to analysis to remove DIC. The total organic carbon analyzer was interfaced with the Delta Plus XP IRMS. A DOC calibration curve was generated using standards of potassium hydrogen phthalate. Measured δ13CDOC values were normalized to the VPDB scale by analyzing solutions of IAEA-CH-6 (= −10.449 ± 0.033‰) and USGS 40 (−26.389 ± 0.042) standards. Using triplicate analyses, DOC concentrations varied by less than 0.02 mM, and δ13CDOC varied by less than 0.2‰.
2.5. Radiocarbon Isotope Analysis
Graphite sample preparation for Δ14C analysis of CH4 and SOC are described in detail by Pohlman et al. [31]. Targets were prepared in the U.S. Naval Research Laboratory (NRL) Graphite Lab. Targets were then analyzed at the NRL accelerator mass spectrometer (AMS) facility using an AMS equipped with a high intensity cesium sputter source for 14C analysis [32]. Data analysis was according to standard procedures described in Tumey et al. [33]. Samples were measured against OX II standards and blanks distributed throughout loadings of the sample wheel. On average, each wheel contained 2 AMS blanks for tuning the accelerator, three processing blanks appropriate for the samples on the wheel, and 7 OX II standards. δ13C results were obtained from GC-IRMS analysis of the samples. These values were used to calculate the δC fractionation correction for each sample. The Δ14C data were calculated as:
where R is 14C/13C or 14C/12C, Rsn represents the sample with normalization to a standardized δ13C and Rabs represents oxalic acid standards normalized to a δ13C standard and the standardized atmospheric 14C level in 1950 [34,35,36]. To compare the influence of C cycling with aging of deposited sediment, Δ14C was converted to conventional radiocarbon age (CRA) where:
In this equation, t is the CRA in years before present (1950), using the Libby half-life for 14C (t1/2 = 5568 years). To obtain actual calendar years, a standardized conversion was applied. The Δ14C term is the value obtained after the 13C correction is applied, λ is the 14C constant (1/8267 years), and y is the year of measurement. Thus, for Δ14C of 0, t would be 0 for a measurement performed in 1950 and 1955 for 2007, when the measurement was performed [33].
2.6. δ13C Data Interpretation
The relative contribution of CH4 to the shallow sediment carbon pools on-mound with a high advection and off-mound with a moderate diffusion assumes microbial assimilation of DIC during AOM [37]. Methane contribution at these different flux rates is summarized at specific core depths or averages through the sulfate methane transition zone. Accounting for isotope fractionation during assimilation, DIC contribution to organic carbon pools is estimated using a simple, two end-member isotope mass balance [38]:
where Rx represents δ13C of SOC or DOC and the isotopic composition of marine phytodetritus (PD) and DIC are represented by RPD and RDIC, respectively. The variables CPD and CDIC represent the corresponding fractional contributions of marine PD and DIC such that:
The percent contribution of DIC to each carbon pool (%X) can then be derived from Equations (3) and (4):
where Rs represents the isotopic composition of DIC. Consideration of isotope fractionation during DIC assimilation is presented in the Discussion.
3. Results
Results focus on a comparison of off-mound (C2) and on-mound (C7) CH4 contribution to organic carbon in gas, porewater and sediment samples. Core C3 provides supplementary data for assessing the on-mound CH4 flux and cycling. Core C8, located near the edge of the mound, shown to have active vertical CH4 diffusion [22], is also used in data interpretation.
3.1. Radiocarbon
The Δ14CCH4(g) values measured in gas pockets of near-mound cores C3 and C8 taken close to the primary mound core are presented in Figure 2 and Table 2. The gas pockets in core C8 were slightly enriched in 14C relative to gas pockets in core C3. On-mound SOC in core C7 was substantially more 14C-depleted than SOC in off-mound core C2 (Figure 2). In each core, the shallowest sediments had a more modern radiocarbon age, however, there was a large difference in the Δ14CSOC data between the cores. To estimate a change in apparent sediment age caused by the presence of CH4, a sedimentation rate of 0.037 ± 0.022 cm·year−1 [3] and natural radiocarbon decay starting at shallow sediment off-mound Δ14C value (−283‰) was applied to data to construct a conservative age line (Figure 3). CRA in off-mound and on-mound SOC did not conform to the predicted conservative age line. In the shallow section of the off-mound core, carbon age was older than predicted aging line down to approximately 300 cm and was younger below 300 cm. On-mound sediment CRA was older than the age line through the core.
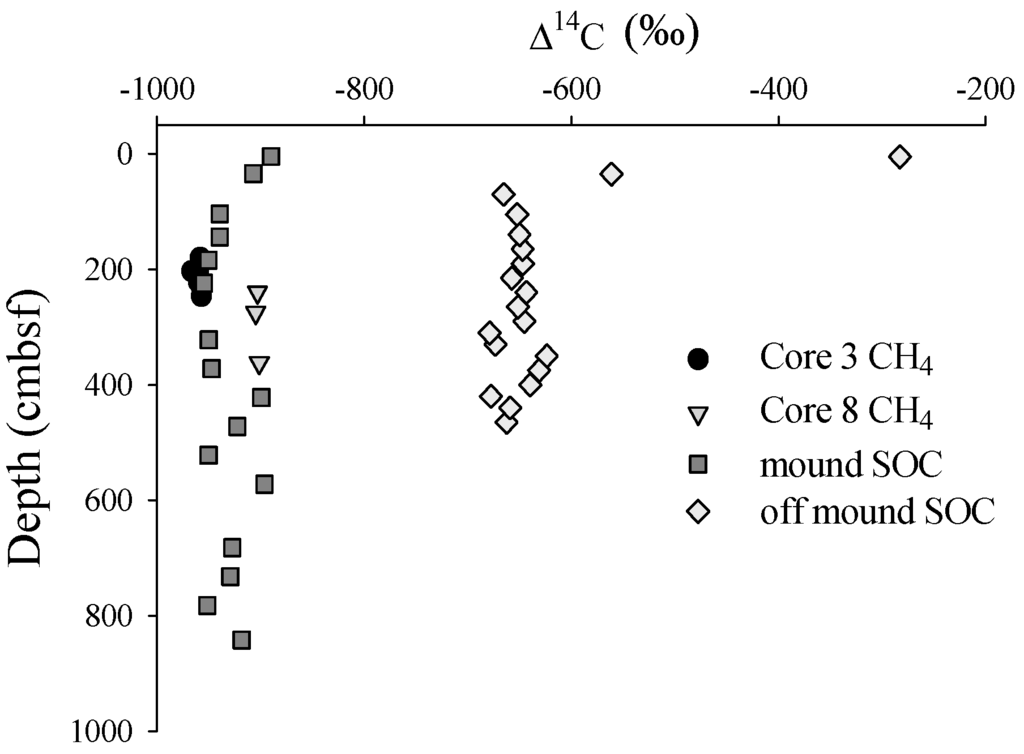
Figure 2.
Δ14CCH4 of free gas and SOC at on-mound and off-mound sites.
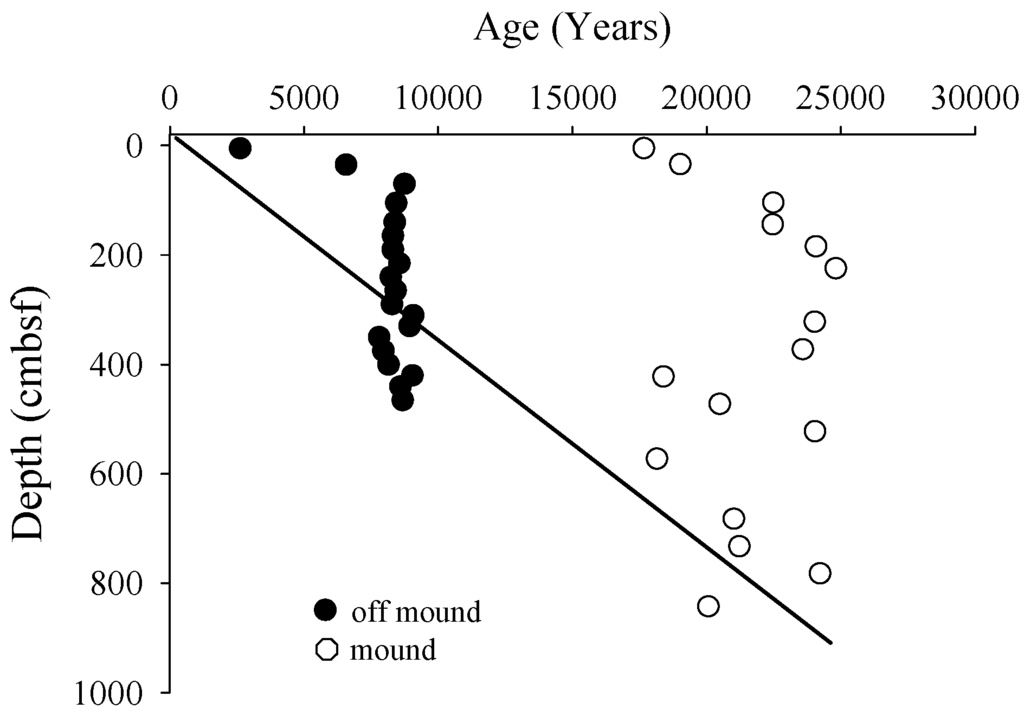
Figure 3.
Conventional radiocarbon age of SOC for cores taken off- and on-mound. The conservative aging line shown was calculated using estimates of annual sedimentation rate for this region.
3.2. Solid Phase Sediment Profiles
An overview of all carbon pool concentration and δ13C data is presented in Table 2. A complete data set is available in Supplementary. Sediment OC concentrations were higher in off-mound C2 core than on-mound C7 core (Figure 4a, Table 2). On-mound δ13CSOC values showed strong 13C-depletion near the seawater interface (SWI, Figure 4b). Off-mound SOC was more 13C-enriched with higher values near surface above the SMT of 410 cmbsf (Figure 4b, Table 1). Profiles of CaCO3 (Figure 4c) and δ13CCaCO3 (Figure 4d) also were substantially different when compared on- and off-mound. CaCO3 as a percentage of total sediment mass was substantially higher on-mound (average = 11.4 ± 1.1%, n = 16) than off-mound (average = 4.1 ± 3.0%, n = 19). Sediment δ13CCaCO3 values were generally 13C-enriched on-mound (average = −2.7 ± 2.8‰, n = 16) relative to off-mound (average = −4.5 ± 2.4‰, n = 19). However, at the SWI, on-mound δ13CCaCO3 value was 13C-depleted while off-mound δ13CCaCO3 was 13C-enriched.
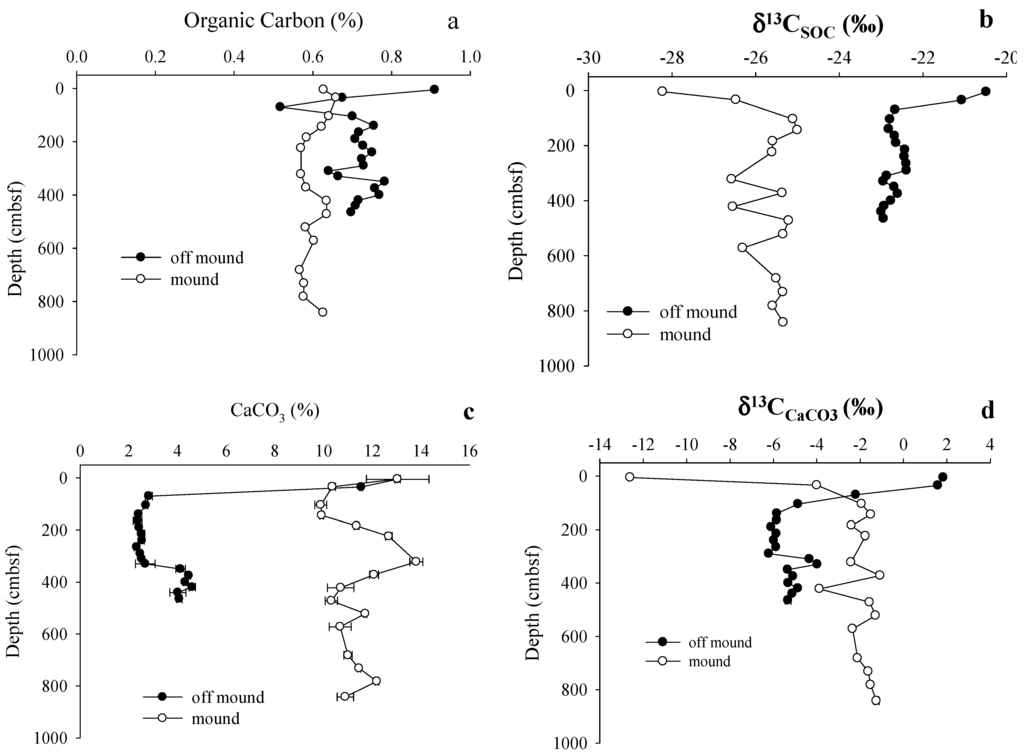
Figure 4.
Sediment profiles at on-mound and off-mound sites: (a) Percent sediment organic carbon (SOC); (b) δ13CSOC; (c) percent CaCO3; and (d) δ13CCaCO3.

Table 2.
Summary of C pool concentrations and δ13C, with minimum, maximum and average values for each core. The full data set for each core is available in Supplementary.
| Core ID | Gas | Porewater | Sediment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ13CCH4 | Δ14CCH4 | CH4 (mM) | δ13CCH4 | DIC (mM) | δ13CDIC | DOC (mM) | δ13CDOC | % SOC | δ13CSOC | Δ14CSOC | % CaCO3 | δ13CTIC | |
| C2 | |||||||||||||
| Min | ND | ND | LOD | −86.9 | 2.8 | −47.1 | 0.82 | −26.2 | 0.91 | −23 | −679 | 2.4 | −6.1 |
| Max | ND | ND | 12.5 | −48.8 | 20 | −9.4 | 3.64 | −20.1 | 0.64 | −20.5 | −283 | 13.04 | 1.8 |
| AVG ± SD | ND | ND | 5.3 ± 5.5 | −74.1 ±11.5 | 10.2 ± 5.3 | −27.6 ± 11.5 | 2.06 ± 1.18 | −23.9 ± 1.5 | 0.72 ± 0.07 | −22.5 ± 0.6 | −629 ± 88 | 4.4 ± 3.2 | −4.6 ± 2.4 |
| C3 | |||||||||||||
| Min | −72.5 | −963 | 0.1 | −75.4 | 6.7 | −42.9 | ND | ND | ND | ND | ND | ND | ND |
| Max | −72 | −957 | 7.9 | −72.8 | 13.6 | 3.7 | ND | ND | ND | ND | ND | ND | ND |
| AVG ± SD | −72.3 ± 0.2 | −960 ± 3 | 4.2 ± 1.8 | −73.7 ± 0.9 | 9.7 ± 2.3 | −3.0 ± 12.6 | ND | ND | ND | ND | ND | ND | ND |
| C7 | |||||||||||||
| Min | −71.4 | ND | 3.3 | −82 | 5.6 | −48.1 | 2.05 | −27.6 | 0.57 | −28.2 | −951 | 9.89 | −12.6 |
| Max | −71.2 | ND | 10.6 | −69 | 12.1 | 4.6 | 3.92 | −24.8 | 0.66 | −25 | −890 | 13.04 | −1.1 |
| AVG ± SD | −71.3 ± 0.3 | ND | 6.2 ± 1.8 | −70.4 ± 3.3 | 7.3 ± 1.6 | −3.4 ± 14.4 | 3.55 ± 0.42 | −25.6 ± 0.8 | 0.60 ± 0.03 | −25.8 ± 0.8 | −929 ± 22 | 11.4 ± 1.1 | −2.7 ± 2.8 |
| C8 | |||||||||||||
| Min | −72.3 | −905 | 0.0 | −85.2 | 5.5 | −44.3 | ND | ND | ND | ND | ND | ND | ND |
| Max | −65.8 | −901 | 13.0 | −71.5 | 13.8 | 6.7 | ND | ND | ND | ND | ND | ND | ND |
| AVG ± SD | −69.7 ± 3.4 | −903 ± 2 | 5.64 ± 3.62 | −76.6 ± 3.9 | 10.6 ± 2.0 | −15.0 ± 21.7 | ND | ND | ND | ND | ND | ND | ND |
3.3. Gas Sources and Sediment and Porewater Carbon Profiles
Through the discussion we assume that shallow CH4 originates from deep sediment CH4 fluxes; deep CH4 is based on on-mound core liner gas pocket data. Assuming void gas from core liner pockets is a deeper source is supported with depleted Δ14CCH4 data (see Section 3.1. Radiocarbon), coupled with observation of elevated Cl− profiles (Table 1) indicating deep vertical advection to the shallow system. Further support for this interpretation is presented below with on-mound porewater DIC data. This δ13CCH4 value is compared to sediment headspace methane (CH4(g)) to assess shallow sediment cycling (Table 2). Measured δ13CCH4(g) values from on-mound core C7 and near-mound core C3 showed little variation within and between cores. Variation of δ13CCH4(g) was greater and moderately depleted in 13C in near-mound core C8. For all cores with gas pockets in which ethane was detected, the C1/C2 ratios were high ([27]; average 11,000, n = 13). As a note, all CH4 concentrations shown in Table 2 are relative (headspace) measurements and underestimate actual sediment methane concentrations as a result of pressure changes during core retrieval from the ocean floor resulting in degassing [21].
Off-mound (C2) CH4 concentrations were highest below the apparent SMT at 410 cmbsf (Table 2) and near the limit of detection above the SMT (Figure 5a). On-mound CH4 concentrations were higher in shallow sediments (C3, C7, and C8), relative to off-mound, and showed a general decrease toward the sediment-water interface (SWI). On-mound sediment δ13CCH4 was relatively uniform through the profile except for 13C depletion at the SWI (Figure 5b). Mound core C3 had a similar profile with moderate 13C depletion through the profile. More variation was observed in on-mound core C8, with 13C-depletion observed at 50 cmbsf (Figure 5b). Off-mound sediment CH4 was 13C-enriched in shallow sediments up to 260 cmbsf and was depleted deeper than 300 cmbsf (Figure 5b). Off-mound δ13CCH4 data are not presented above 260 cmbsf because CH4 concentrations were below the limits of detection for carbon isotope analyses.
On-mound (C7) porewater DIC concentrations increased from the SWI to 34 cmbsf, and subsequently decreased towards the core base (Figure 6a). Off-mound (C2) porewater DIC concentrations gradually increased from a SWI minimum to a maximum at 350 cmbsf and then declined rapidly toward the core base. On-mound, δ13CDIC values decreased to most depleted 13C value at 34 cmbsf where concentration increased; below this point δ13CDIC values increased and remained uniform (Figure 6b). Off-mound δ13CDIC values showed a similar patter with the minimum value at 330 cmbsf.
While on-mound and off-mound porewater DOC concentration ranges were similar, notable differences in vertical profiles were observed (Figure 7a). Off-mound DOC concentrations in porewaters were lowest in near surface sediments and generally increased with depth. On-mound porewater DOC concentrations were relatively consistent throughout the profile, except for a low value near surface. On-mound porewater δ13CDOC values showed little variation, however, porewater DOC was substantially 13C-depleted throughout the core with a minimum value at 34 cmbsf (Figure 7b). In contrast, off-mound porewater δ13CDOC values varied substantially and were generally 13C-enriched; δ13CDOC values were elevated in samples taken less than 100 cmbsf and depleted below 100 cmbsf (Figure 7b).
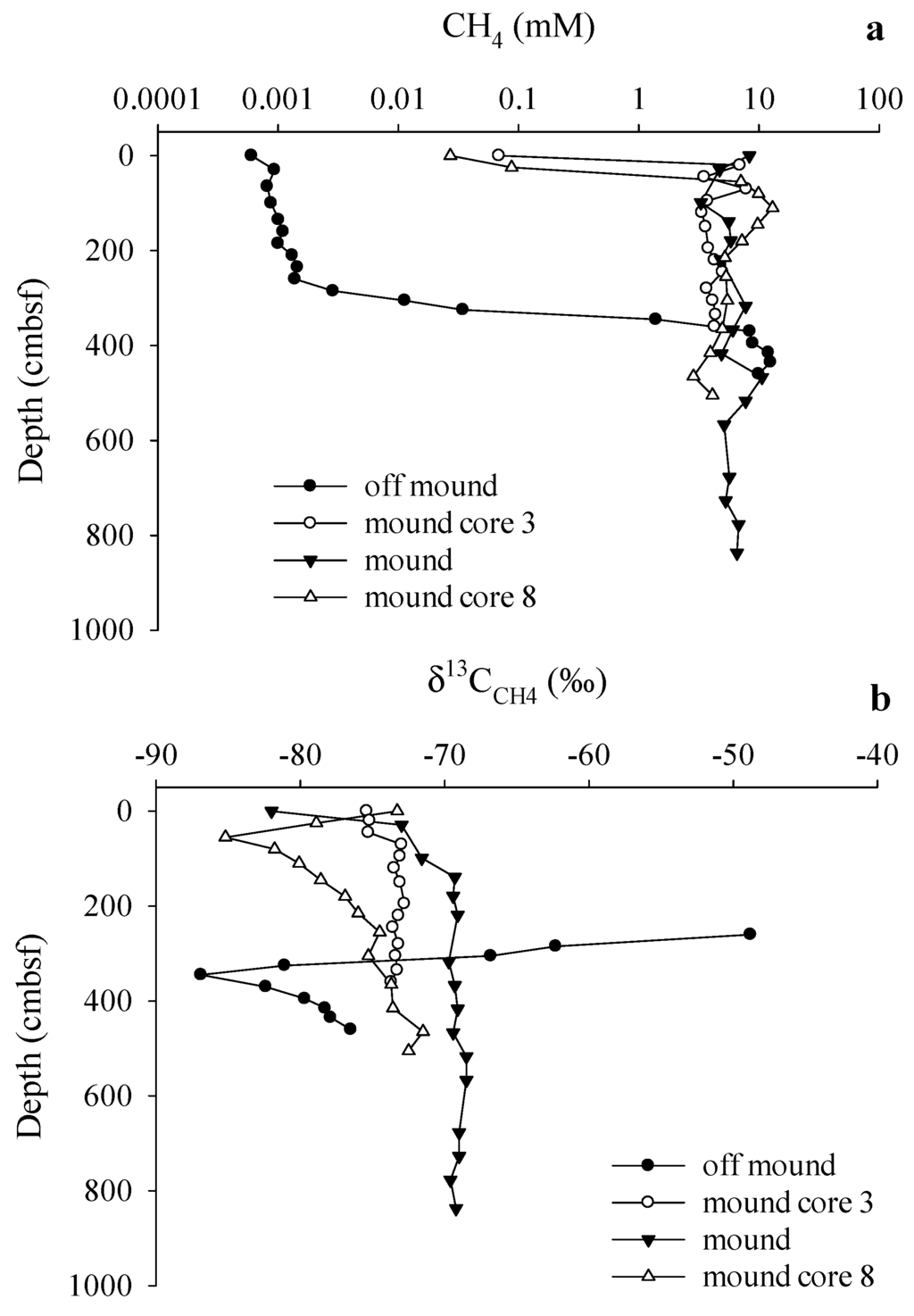
Figure 5.
(a) Sediment CH4 concentrations; and (b) δ13CCH4 values measured for on-mound (C7) and off-mound (C2) core locations. On-mound cores include cores 3 and 8 that are used for estimating the C isotope values for source CH4 and interpretation of mound C cycling.
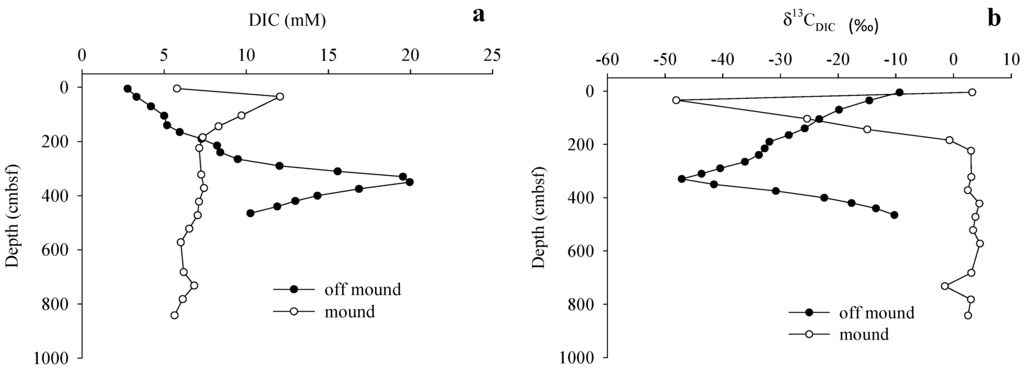
Figure 6.
(a) Sediment pore water dissolved inorganic carbon (DIC) concentrations; and (b) δ13CDIC values measured on-mound and off-mound.
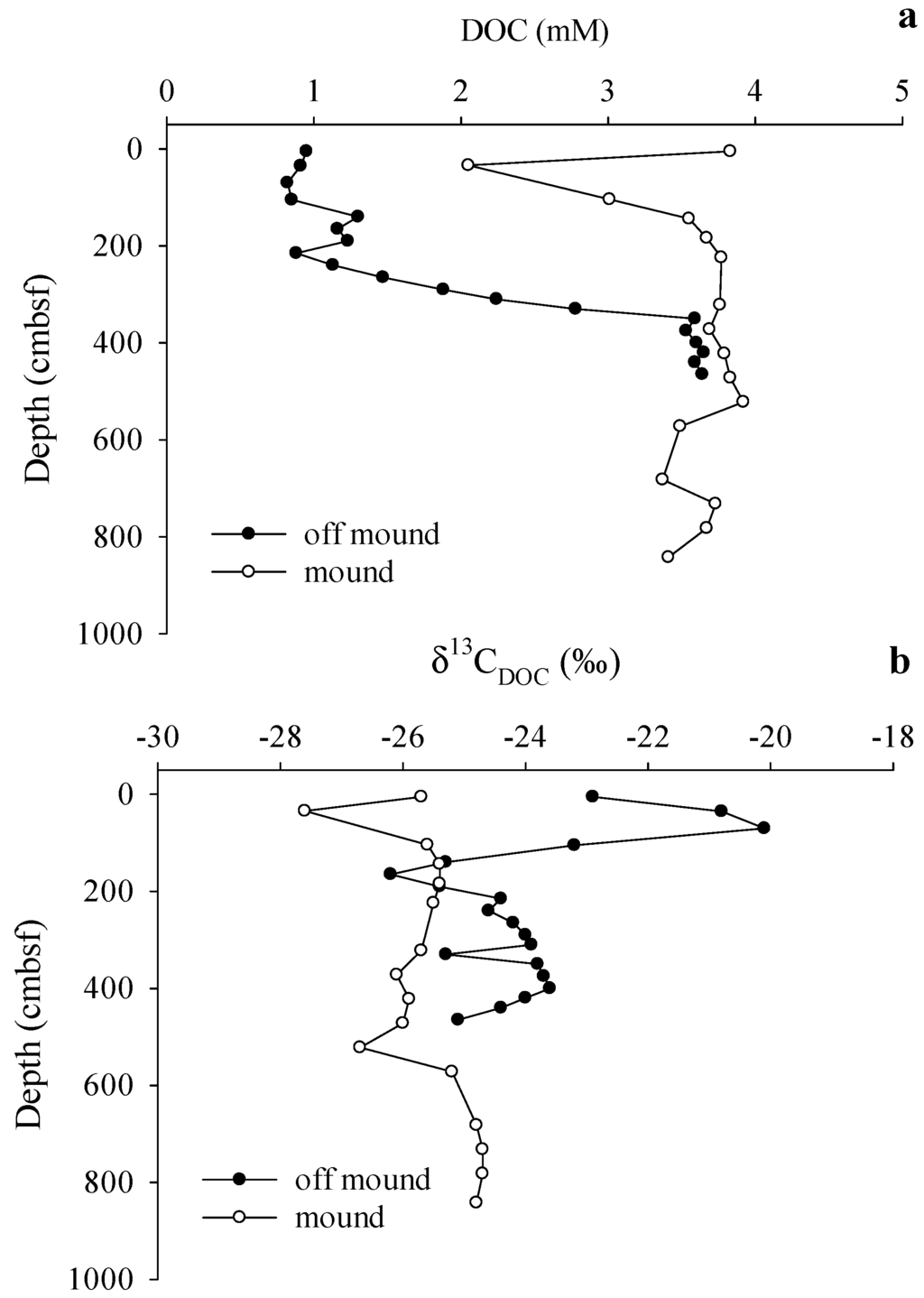
Figure 7.
(a) Vertical profiles of sediment pore water dissolved organic carbon (DOC) concentrations compared on-mound and off-mound; and (b) Vertical profiles of δ13CDOC values measured on-mound and off-mound.
4. Discussion
4.1. Shallow Sediment Carbon
Shallow sediment carbon cycling in Atwater Valley was investigated assuming marine phytodetritus and AOM carbon fixation are the primary sources to SOC and DOC. The difference in Δ14CSOC values between cores on- and off-mound is likely a result of AOM incorporation of isotopically-depleted DIC into OC [11,12,37], with a substantially greater CH4 influence on-mound (Figure 2 and Figure 3). There is a pronounced 14C-depletion in SOC on-mound (−955‰ to −890‰) with a slightly more modern signature in surface sediments. Elevation in Δ14C observed in on-mound (C8) surface sediment results from more modern seawater DIC fixation during AOM, further discussed below. Note that on mound δ13CDIC was relatively uniform near 0‰ (Figure 6b) and represents a deep sourced signature associated with the fluid advection observed in the mound δ13CCH4 (Figure 5b). An alternate interpretation of on-mound aged carbon data could be sediment erosion resulting in uncovering relic SOC. While erosion could contribute to the aged carbon pattern on-mound, elevated CaCO3 concentrations relative to off-mound values indicate active AOM, resulting in over saturated DIC and subsequent precipitation (Figure 4). In contrast to on-mound data, off-mound SOC was substantially more modern (−678‰ to −283‰, Figure 3) implying a lower contribution of AOM DIC fixation to carbon cycling. Gordon and Goñi [3] reported surface sediment Δ14CSOC ranging from −309.1‰ to −228.6‰ in the same general sampling area, with water column depths of 365 to 2270 m. In this study, similar Δ14CSOC on- and off-mound profiles with more 14C-depletion observed on-mound does indicate depleted radiocarbon DIC contribution to carbon cycling (Figure 2 and Figure 3). The near-constant vertical CRA of the sediments sampled in this study, especially off-mound, suggests sediment mixing or rapid deposition, perhaps created by a shelf slump (Figure 3). Subsequent diagenesis of organic matter with different contributions of 14C-depleted CH4 between core locations is evident with differences in CRA between on-mound and off-mound cores. δ13C data in this study provides an estimate of the DIC fixation driven by the AOM of deep sourced CH4 in shallow sediment carbon cycling. Other investigators have shown that Mississippi River particulate organic carbon (POC) changes from a terrigenous (δ13C = −28‰ to −26‰) to phytoplankton source (δ13C = −19‰) near the river mouth [1,3,39,40]. In a previous study, POC-δ13C reported well offshore near Atwater Valley ranged from −22.5‰ to −18.7‰, [41], in the range of phytodetritus [42,43]. For our study off-mound δ13CSOC values (−20.5‰ at the surface to −23‰ near the core bottom, Figure 4) were characteristic of SOC dominated by phytodetritus. In contrast, on-mound δ13CSOC ranged from −25.0‰ down core to −28.2‰ near surface, indicating an alternate carbon source.
A common interpretation in 13C-depleted isotope signatures in coastal waters is carbon sourced from terrestrial plants [39]. However, more recent studies show 13C-depleted δ13CSOC values observed in these sediments likely results from carbon assimilated into bacterial biomass during CH4 cycling [22,37]. In anoxic sediments bacterial biomass is incorporated and preserved in solid phase sediments [44,45] and through time can constitute a significant portion of the SOC pool [46,47]. While our data indicate SOC is initially derived from marine phytoplankton, once deposited it is subject to diagenesis which includes incorporation of deep sediment CH4 into solid phase sediment and pore water carbon pools.
Several additional observations pertaining to sediment carbon pools support interpretation of CH4 contribution to carbon cycling. In the presence of sediment anaerobic CH4 oxidation DIC oversaturation results in formation of CaCO3 [48,49]. On-mound where 13C depleted SOC was observed, CaCO3 concentrations were 3 to 4 times higher than off-mound (Figure 4c). On-mound and off-mound data also suggest CH4 contribution to pore water DOC. On-mound core porewater δ13CDOC values ranged from −27.6‰ to −24.8‰ with 13C-depletion in shallower sediments, though porewater DOC concentrations showed little variation (Figure 7). The shift to lower δ13CDOC values in shallow on-mound sediments coincided with a decrease in pore water SO42− concentrations and apparent AOM [22]. Off-mound porewater DOC concentrations below the SMT were similar to those on-mound and had depleted δ13CDOC (−26.2‰; Figure 7) indicating a CH4 contribution to porewater DOC. In off-mound surface sediments above the SMT, porewater DOC concentrations were lower with an increase in δ13CDOC (−20.1‰) more typical of a marine phytoplankton source. Here it is assumed that DO13C depletion is not a result of diagenesis or selective metabolism during heterotrophic microbial carbon cycling that would result in isotopic fractionation of the total DOC pool [50,51].
4.2. Methane Source and Cycling
Composition and isotopic signatures of gas pocket samples on-mound indicate a microbial CH4 source with average C1/C2 = 11,000 (methane/ethane) and δ13CCH4(g) = −71.2‰ (Table 2) [8,52,53,54]. While data interpretation is contingent on piston core depths, there are indications that on-mound CH4 originates from deep sediment; (1) Δ14CCH4(g) depletion measured in on-mound (C3) (−961‰) is a value characteristic of CH4 that originates in from a deep system [55]; and (2) SO42− not observed below the SWI in cores C3 and C7 suggests active advection of deep sediment CH4 to the surface [22]. In contrast, 14C-enrichment indicated by Δ14CCH4(g) values in on-mound (C8, −903‰, Figure 2) and presence of a shallow SMT (59 cmbsf) suggests modern seawater DIC is being reduced to CH4 during methanogenesis. A biogenic CH4 source, absent of higher molecular weight gases and oil products that are present in a thermogenic source [53], presents an opportunity to study deep sediment CH4 contribution to shallow sediment carbon cycling. On mound core C3 gas pocket carbon isotope ratios is used as a CH4(g) end-member to estimate CH4 contribution to organic carbon pools, presented below.
Strong difference in shallow sediment CH4 cycling was observed within and between cores. Measured on-mound sediment headspace δ13CCH4 values (−70.4‰) deeper than 100 cmbsf were similar to the δ13CCH4(g) value indicating little microbial CH4 consumption or production (Figure 5b). In contrast, in the top 100 cm on-mound, sediment headspace δ13CCH4 values were depleted 11‰ relative to δ13CCH4(g) values (Figure 5, Table 1). The 13C-enrichment indicated by δ13CCH4(g) values in C8 (−65.8‰) at 240 cmbsf likely results from isotopic fractionation during AOM [22,56]. Where the upward CH4 flux due to active vertical fluid advection (such as C7 and C3) impedes downward SO42− diffusion into sediment, AOM or methanogenesis will not occur and there is not a change in the δ13CCH4(g) ratio. Depleted δ13CCH4 values on-mound near surface and corresponding enriched δ13CDIC values do indicate active methanogenesis [57,58]. Methanogenesis typically occurs in marine sediments below AOM [59,60]. However, with advection on-mound, δ13CDIC data indicate shallow methanogenesis near the SWI (Figure 5b). While enriched δ13CCH4 and an associated SMT was not observed there is potential for seawater SO42− to support AOM at the SWI (Figure 5b).
Active CH4 cycling was also observed in the off-mound core. Above the BSR (Figure 1) there was greater variation in the δ13CCH4 profile with lowest value (−86.9‰), potentially resulting from methanogenesis at a mid-core depth (Figure 5b), just below the SMT (Table 1). Note in a recent study carbon isotope equilibration at the point of sulfate-limited anaerobic oxidation was observed and may account for this depleted δ13CCH4 [61]. Above the SMT, decreased CH4 concentrations and enriched sediment headspace δ13CCH4 values up to −48.8‰ result from AOM through the SO42− gradient (Figure 5). A corresponding depletion in δ13CDIC values to −47.1‰ (330 cmbsf) and a subsequent increase to −10.5‰ towards the core base (Figure 6) is characteristic of AOM through the SMT located above the zone of methanogenesis [57,62].
4.3. Estimation of CH4 Contribution to the Shallow Organic Carbon
The relative contributions of phytodetritus and AOM to shallow sediment carbon cycling on-mound and off-mound can be estimate using a simple carbon budget calculation (Equation (5)). We assume sediment CH4 is primarily oxidized during AOM for energy and DIC is assimilated into cellular biomass [11,12,13] with an isotopic fraction of 3.75‰ , a value recently applied in a study on the Hikurangi Margin [37] and an intermediate (2.0‰ to 5.5‰) for isotopic fractionation during DIC assimilation in the reversed tricarboxylic acid cycle [63,64,65]. For estimating the DIC contribution to SOC during AOM it is assumed δ13CPD end-member (Equation (5)) was −20.5‰, the off-mound signature in surface sediments with the most modern Δ14CSOC value (Figure 2). Use of this δ13CPD end-member is supported by other studies on the TX-LA Shelf where offshore to nearshore sediment δ13C values ranged from −21.7‰ to −19.7‰ [1,3,40]. On the Gulf of Mexico abyssal plain, with no substantial terrestrial inputs, δ13CSOC measured values were −20.6‰ [66]. The average porewater δ13CDIC value measured on-mound, through the SMT (between 34 and 144 cm) was −29.5‰. Adjusting this value for fractionation during DIC fixation (3.75‰, [37]) provides a δ13CDIC end member of −25.8‰. On-mound, with a δ13CSOC average of −25.0‰, results in an AOM-DIC contribution to SOC of 85% (Equation (5), Figure 8). We assume, with on-mound advection, AOM occurs at the sediment water column interface where SO42− is available and this signature is buried through time. This observation of high CH4 contribution to shallow sediment carbon cycling is supported with a comparison of regional sedimentation rates and on-mound vertical CH4 flux. Phytodetritus contribution to SOC in this region is estimated on the basis of a sedimentation rate near this location of 0.005 cm·year−1 [1], sediment density of 2.6 gm·cm−3 [67], and SOC mineralization of shallow off-mound sediment relative to deep on-mound sediment of 37% [22,68] to be 40.8 mM·C·m−2·year−1. This suggests a small contribution of carbon relative to CH4 at 3250 mM·C·m−2·year−1 (Table 1). Applying this approach to off-mound sediment δ13CSOC values below 35 cmbsf averaged −22.7‰ and results in a 42% contribution of AOM-derived DIC incorporation to SOC (Figure 8).
Porewater DOC concentrations and δ13CDOC also indicate different carbon sources between on- and off-mound cores (Figure 7). In this analysis we do assume that porewater bacterial production, cell DOC excretion, and degradation of SOC contribute to porewater DOC. Assuming DIC is fixed into bacterial carbon and subsequently cycled to DOC (Figure 8), the contribution of AOM to DOC (Equation (5)) can be estimated using the phytodetritus end-member δ13CPD of −20.5‰ and δ13CDIC of −25.8‰. The δ13CDOC average off-mound (−24.3‰), suggests 72% of DOC below the SMT is AOM derived (Figure 8). The on-mound average δ13CDOC value of −25.6‰ through the entire C7 core indicates an indirect contribution of AOM to porewater DOC of 96%. The production of DOC via AOM in sediment may result in significant contribution relative to phytoplankton derived carbon. It is important to note that patterns in δ13CDIC and δ13CDOC depletion were similar in the vertical core profiles and with high porewater DOC concentrations ogranoclastic degradation of DOC and subsequent production from light DIC from further AOM can result in an overestimate of AOM related DOC production. Furthermore acetogenesis can contribute to the observation of depleted 13C in the DOC [69]. Also, it is interesting that this observation is similar to a recent study off the coast of New Zealand where AOM contribution to porewater DOC was estimated to be up to 71% at a location with lower vertical CH4 flux rates [37].
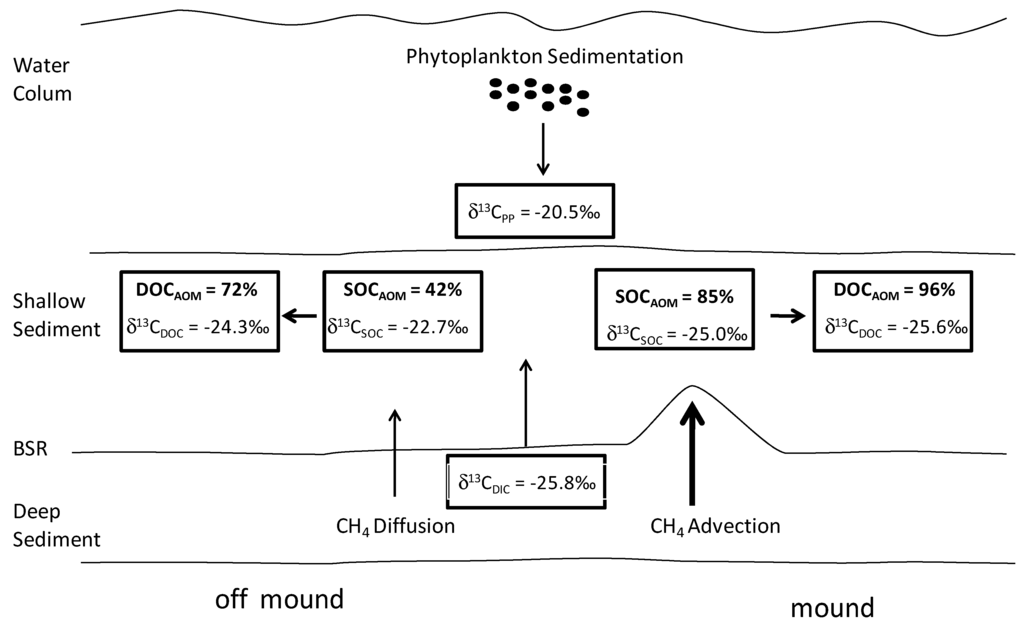
Figure 8.
A summary of dissolved organic carbon (DIC) and phytoplankton biomass contributions to shallow SOC and DOC at the on-mound and off-mound sites using δ13C values.
5. Conclusions
Diffusive and advective fluxes of deep sediment CH4 contribute significantly to shallow sediment carbon cycling in Atwater Valley, Gulf of Mexico. Deep salt diapirs at this study location are predicted to result in destabilization of CH4 hydrates and result in an elevated vertical methane flux [22,23]. This prediction is supported in the observation of a substantial rise in the BSR (Figure 1c).The upward flux of deep sediment CH4 via fluid advection on-mound supports a substantial fraction of sediment carbon production (up to 85%), whereas marine phytoplankton sourced carbon contributes ~50% where there is a low diffusive CH4 flux (Figure 8). Consistent 13C-enriched δ13CDIC values deeper than 100 cmbsf and the 14C-depleted Δ14CSOC and Δ14CCH4 values measured in gas pockets on-mound can be interpreted as long-term mound formation driven by active advection that supported shallow sediment carbon cycling driven by AOM near the SWI where SO42− is abundant. The cycling of CH4 via AOM is reflected in the organic and inorganic carbon pools. The variation in CH4 cycling in an advection dominated on-mound sediment and a diffusion dominated sediment off-mound is evident in the same sediment carbon pools. Observed δ13CDIC profiles followed changes in δ13CCH4 values, as did δ13CCaCO3 values, indicating oxidation of CH4 during AOM was occurring in the sediment pore fluids on the mound. Mass balances using δ13C of CH4 and SOC showed a large difference in the estimated contribution of deep sediment CH4 to the shallow sediment carbon pools off and on-mound relative to marine phytoplankton. The DOC pool in sediment porewaters also reflected a significant contribution (up to 96%) from deep sediment CH4. In another study on the TX-LA Shelf, petroleum seeps were found to contribute 40% to 60% to the total organic C in a shallower slope region [17].
This study suggests a need for global consideration of the distribution of deep sediment CH4, especially hydrate bound methane, and the flux of carbon from this globally-significant carbon pool to shallow sediments. More estimates of deep sediment CH4 and petroleum contributions to shallow sediments and the water column will help refine marine carbon cycling models and budget estimates and improve predictions of the impacts of climate change on these reservoirs. The current estimate for the world coastal CH4 hydrate distribution is 21 × 1015 m3 of CH4 at standard temperature and pressure [8]. A more thorough understanding of the potential for fixation of CH4 into organic carbon and carbonate phases in shallow sediments will assist in evaluating deep sediment sourced CH4 fluxes through the shallow sediments to the water column and the atmosphere.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1996-1073/8/3/1561/s1.
Acknowledgments
Warren Wood, NRL-Stennis Space Center, MS provided seismic profiles and seafloor topography. Ross Downer, Milbar Hydo-Test, Inc. (Shreveport, LA, USA) was lead for all coring operations. We appreciate the technical discussions and reviews of this manuscript from Paula Rose, Jeff Chanton, Thomas Boyd, and Leila Hamdan. David Knies contributed to radiocarbon analyses. We also appreciate the support by the crew of the RV Gyre. Finally, reviewers of this manuscript have provided excellent input to research and the presentation. This research was supported by Department of Energy-National Energy Technology Laboratory, Office of Naval Research and the US Naval Research Laboratory.
Author Contributions
All authors have contributed to writing and revisions of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goñi, M.A.; Ruttenberg, K.C.; Eglington, T.I. A reassesment of the sources and importance of land-derived organic matter in surface sediment from the Gulf of Mexico. Geochem. Cosmochim. Acta 1998, 62, 3055–3075. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Mitra, S.; McKee, B.A. Sources of terrestrially-derived organic carbon in lower Mississippi River and Louisiana shelf sediments: Implications for differential sedimentation and transport at the coastal margin. Mar. Chem. 2002, 77, 211–223. [Google Scholar] [CrossRef]
- Gordon, E.S.; Goñi, M.A. Controls on the distribution and accumulation of terrigenous organic matter in sediments from the Mississippi and Atchafalaya river margin. Mar. Chem. 2004, 92, 331–352. [Google Scholar] [CrossRef]
- Mayer, L.M.; Schick, L.L.; Allison, M.A.; Rutthenberg, K.C.; Bentley, S.J. Marine vs. terrigenous organic matter in Louisiana coastal sediments: The uses of bromine organic carbonratios. Mar. Chem. 2007, 107, 244–254. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Atilla, N.; Normandeau, C.; Turner, R.E. Ecosystem history of Mississippi River influenced continental shelf revealed through preserved phytoplankton pigments. Mar. Pollut. Bull. 2004, 49, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Ruttenberg, K.C.; Goñi, M.A. Phosphourus distribution, C:N:P ratios, and δ13Coc in arctic, temperate, and tropical coastal sediments: Tools for characterizing bulk sedimentary organic matter. Mar. Geol. 1997, 139, 123–145. [Google Scholar] [CrossRef]
- Anderson, R.K.; Scalan, R.S.; Parker, P.L.; Behrens, E.W. Seep oil and gas in Gulf of Mexico slope sediment. Science 1998, 222, 619–621. [Google Scholar] [CrossRef]
- Milkov, A.V. Molecular and stable isotope compositions of natural gas hydraes: A revised global data set and basic interpretations in the context of geological settings. Org. Geochem. 2005, 36, 681–702. [Google Scholar] [CrossRef]
- Borowski, W.S.; Paull, C.K.; Ussler, W., III. Marine porewater sulfate profiles indicate in situ methane flux from underlying gas hydrate. Geology 1996, 24, 655–658. [Google Scholar] [CrossRef]
- Pancost, R.D.; Damste, J.S.S.; de Lint, S.; van der Maarel, M.J.E.C.; Gottschal, J.C.; the Medinaut Shipboard Scientific Party. Biomarker evidence for widespread anaerobic methane oxidation in Mediterranean sediments by a consortium of methanogenic archaea and bacteria. Appl. Environ. Microbiol. 2000, 66, 1126–1132. [Google Scholar] [CrossRef]
- Knittel, K.; Boetius, A. Anaerobic oxidation of methane: Progress with an unknown process. Annu. Rev. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Alperin, M.J.; Hoehler, T.M. Anaerobic methane oxidation by archaea/sulfate-reducing bacteria aggregates: 2. Isotopic constraints. Am. J. Sci. 2009, 309, 958–984. [Google Scholar] [CrossRef]
- Wegener, G.; Niemann, H.; Elvert, M.; Hinrichs, K.-U.; Boetius, A. Assimilation of methane and inorganic carbon by microbial communities mediating the anaerobic oxidation of methane. Environ. Microbiol. 2008, 10, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Kellermann, M.; Wegener, G.; Elvert, M.; Yoshinga, M.Y.; Lin, Y.-S.; Holler, T.; Mollar, X.P.; Knittel, K.; Hinrichs, K.-U. Autotrophy as a predominant mode of carbon fixation in anaerobic methane-oxidizing mincrobial communities. PNAS 2012, 109, 19321–19326. [Google Scholar] [CrossRef] [PubMed]
- Leak, D.J.; Dalton, H. Growth yields of methanotrophs. Appl. Microbiol. Biotechnol. 1986, 23, 470–476. [Google Scholar] [CrossRef]
- Kelley, C.A.; Coffin, R.B.; Cifuentes, L.A. Stable isotope evidence for alternate carbon sources in the Gulf of Mexico. Limnol. Oceanogr. 1998, 43, 1962–1969. [Google Scholar]
- Wang, X.-C.; Chen, R.F.; Whelan, J.; Eglinton, T. Contribution of “old” carbon from natural marine hydrocarbon seeps to sedimentary and dissolved organic carbon pools in the Gulf of Mexico. Geophys. Res. Lett. 2001, 28, 3313–3316. [Google Scholar] [CrossRef]
- Joye, S.B.; Boetius, A.; Orcutt, B.N.; Montoya, R.P.; Schulz, H.N.; Ericson, M.J.; Lugo, S.K. The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chem. Geo. 2004, 205, 219–238. [Google Scholar] [CrossRef]
- Paull, C.K.; Ussler, W., III; Lorenson, T.; Winters, W.; Dougherty, J. Geochemical constraints on the distribution of gas hydrates in the Gulf of Mexico. Geo. Mar. Lett. 2005, 25, 273–280. [Google Scholar] [CrossRef]
- Ruppel, C.; Dickens, G.R.; Castellini, D.G.; Gilhooly, W.; Lisarralde, D. Heat and salt inhibition of gas hydrae formation in the northern Gulf of Mexico. Geophys. Res. Lett. 2005, 32, L04605. [Google Scholar] [CrossRef]
- Lapham, L.L.; Chanton, J.P.; Martens, C.S.; Sleeper, S.; Woolsey, J.R. Microbial activity in surficial sediments overlying acoustic wipeout zones at a Gulf of Mexico cold seep. Geochem. Geophys. Geosyst. 2008, 9. [Google Scholar] [CrossRef]
- Coffin, R.B.; Hamdan, L.; Plummer, R.; Smith, J.; Gardner, J.; Wood, W.T. Analysis of methane and sulfate flux in methane charged sediments from the Mississippi Canyon, Gulf of Mexico. Mar. Pet. Geol. 2008, 25, 977–987. [Google Scholar] [CrossRef]
- Wood, W.T.; Hart, P.E.; Hutchinson, D.R.; Dutta, N.; Snyder, F.; Coffin, R.B.; Gettrust, J.F. Gas and gas hydrate distribution around seafloor seeps in Mississippi Canyon, Northern Gulf of Mexico, using multi-resolution seismic imagery. Mar. Pet. Geol. 2008, 9, 952–959. [Google Scholar] [CrossRef]
- Weimer, P.; Buffler, R.T. Structural geology and evolution of the Mississippi fan fold belt, deep Gulf of Mexico. AAPG Bull. 1992, 76, 225–251. [Google Scholar]
- Goodwin, R.H.; Prior, D.B. Geometry and depositional sequences of the Mississippi Canyon, Gulf of Mexico. J. Sediment. Res. 1989, 59, 318–329. [Google Scholar]
- Ellwood, B.B.; Balsam, W.L.; Roberts, H.H. Gulf of Mexico sediment sources and sediment transport trends from magnetic susceptibility measurements of surface samples. Mar. Geol. 2006, 230, 237–248. [Google Scholar] [CrossRef]
- Coffin, R.B.; Gardner, J.; Pohlman, J.; Downer, R.; Wood, W. Methane Hydrate Exploration, Atwater Valley, Texas-Louisiana Shelf: Geophysical And Geochemical Profiles; Naval Research Laboratory: Washington, DC, USA, 2006. [Google Scholar]
- Reeburgh, W.S. An improved interstitial water sampler. Limnol. Oceanogr. 1967, 12, 163–165. [Google Scholar] [CrossRef]
- Plummer, R.E.; Pohlman, J.; Coffin, R.B. Compound-Specific Stable Carbon Isotope Analysis of Low-Concentration Complex Hydrocarbon Mixtures from Natural Gas Hydrate Systems; American Geophysical Union: Washington, DC, USA, 2005. [Google Scholar]
- Osburn, C.L.; St-Jean, G. The use of wet chemical oxidation with high-amplification isotope ratio mass spectrometry (WCO-IRMS) to measure stable isotope values of dissolved organic carbon in seawater. Limnol. Oceanogr. Methods 2007, 5, 296–308. [Google Scholar] [CrossRef]
- Pohlman, J.W.; Knies, D.L.; Grabowski, K.S.; DeTurck, T.M.; Treacy, D.J.; Coffin, R.B. Sample distillation/graphitization system for carbon pool analysis by accelerator mass spectrometry (AMS). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2000, 172, 428–433. [Google Scholar] [CrossRef]
- Grabowski, K.S.; Knies, D.L.; DeTurck, T.M.; Treacy, D.J.; Pohlman, J.W.; Coffin, R.B.; Hubler, G.K. A report on the Naval Research Laboratory AMS facility. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2000, 172, 34–39. [Google Scholar] [CrossRef]
- Tumey, S.T.; Grabowski, K.S.; Knies, D.L.; Mignerey, A.C. Data collection, filtering and analysis at the Naval Research Laboratory trace element accelerator mass spectrometry facility. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2004, 176, 428–433. [Google Scholar]
- Stuiver, M.; Polach, H.A. Discussion: Reporting of 14C data. Radiocarbon 1977, 19, 355–363. [Google Scholar]
- Stuiver, M. International agreements and the use of the new oxalic acid standard. Radiocarbon 1983, 25, 793–795. [Google Scholar]
- Donahue, D.J.; Linick, T.W.; Jull, A.J.T. Isotope-ratio and background corrections for accelerator mass spectrometry radiocarbon measurements. Radiocarbon 1990, 32, 135–142. [Google Scholar]
- Coffin, R.B.; Hamdan, L.J.; Smith, J.P.; Rose, P.S.; Plummer, R.E.; Yoza, B.; Pecher, I.; Montgomery, M.T. Contribution of vertical methane flux to shallow sediment carbon pools across porangahau ridge, New Zealand. Energies 2014, 7, 5332–5356. [Google Scholar] [CrossRef]
- Macko, S.A.; Ostrom, N.E. Pollution studies using stable isotopes. In Stable Isotopes in Ecology and Environmental Science; Lajtha, K., Michener, R., Eds.; Blackwell Scientific Publications: Hoboken, NJ, USA, 1994; pp. 45–62. [Google Scholar]
- Fry, B. Using stable isotopes to monitor watershed influences on aquatic trophodynamics. Can. J. Fish. Aquat. Sci. 1999, 56, 2167–2171. [Google Scholar] [CrossRef]
- Gordon, E.S.; Goñi, M.A. Source and distribution of terrigenous organic matter delivered by the Atchafalaya River to sediments in the northern Gulf of Mexico. Geochem. Cosmochim. Acta 2003, 67, 2359–2375. [Google Scholar] [CrossRef]
- Wang, X.; Chen, R.F.; Gardner, G.B. Sources and transport of dissolved and particulate organic carbon in the Mississippi River estuary and adjacent coastal waters of the northern Gulf of Mexico. Mar. Chem. 2004, 89, 241–256. [Google Scholar] [CrossRef]
- Peterson, B.J.; Howarth, R.W.; Garritt, R.H. Multiple stable isotopes used to trace the flow of organic matter in estuarine food webs. Science 1985, 277, 1361–1363. [Google Scholar] [CrossRef]
- Chen, X.; Lohrenz, S.E.; Wiesenburg, D.A. Distribution and controlling mechanisms of primary production on the Louisianan-Texas continental shelf. J. Mar. Syst. 2000, 25, 179–207. [Google Scholar] [CrossRef]
- Burdige, D.J.; Martens, C.S. Biogeochemical cycling in an organic-rich coastal marine basin: The sedimentary cycling of dissolved, free amino acids. Geochim. Cosmochim. Acta 1990, 54, 3033–3052. [Google Scholar] [CrossRef]
- Lee, C. Controls on organic carbon preservation: The use of stratified water bodies to compare intrinsic rates of decomposition in oxic and anoxic systems. Geochim. Cosmochim. Acta 1992, 56, 3323–3335. [Google Scholar] [CrossRef]
- Canuel, E.A.; Martens, C.S. Seasonal variations in the sources and alteration of organic matter associated with recently-deposited sediments. Org. Geochem. 1993, 20, 563–577. [Google Scholar] [CrossRef]
- Gong, C.; Hollander, D.J. Differential contribution of bacteria to sedimentary organic matter in oxic and anoxic environments, Santa Monica Basin, California. Org. Geochem. 1997, 26, 545–563. [Google Scholar] [CrossRef]
- Orphan, V.J.; Hinrichs, K.U.; Ussler, W., III; Paull, C.K.; Taylor, L.T.; Sylva, S.P.; Hayes, J.M.; Delong, E.F. Comparative analysis of methane-oxidizing archaea and sulfate-reducing bacteria in anoxic marine sediments. Appl. Environ. Microbiol. 2001, 67, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Reeburgh, W.S. Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef] [PubMed]
- Goevert, D.; Conrad, R. Effect of substrate concentration on carbon isotope fractionation during acetoclastic methanogenesis by Methanosarcina barkeri and M. acetivorans and in rice field soil. Appl. Environ. Microbiol. 2009, 75, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Szynkiewicz, A.M.; Jedrysek, O.; Kurasiewicz, M. Carbon isotope effects during precipitation of barium carbonate: Implications for environmental studies. Environ. Chem. Lett. 2006, 4, 29–35. [Google Scholar] [CrossRef]
- Martens, C.S.; Chanton, J.P.; Paull, C.K. Fossil biogenic methane at the Florida escarpment. Geology 1991, 19, 851–854. [Google Scholar] [CrossRef]
- Sassen, R.; MacDonald, I.R. Hydrocarbons of experimental and natural gas hydrates, Gulf of Mexico continental slope. Org. Geochem. 1997, 26, 289–293. [Google Scholar] [CrossRef]
- Sassen, R.; Sweet, S.T.; DeFreitas, D.A.; Morelos, J.A.; Milkov, A.V. Gas hydrate and crude oil from the Mississippi Fan Foldbelt, downdip Gulf of Mexico Salt Basin: Significance to petroleum system. Org. Geochem. 2001, 32, 999–1008. [Google Scholar] [CrossRef]
- Pohlman, J.W.; Kaneko, M.; Heuer, V.B.; Coffin, R.B.; Whiticar, M. Methane soucrces and production in the north Cascadia Margin gas hydrate system. Earth Planet. Sci. Lett. 2009, 287, 504–512. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 16, 291–314. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gleske, A.; Amann, R.; Jørgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Orphan, V.J.; House, C.H.; Hinrichs, K.-U.; McKeegan, K.D.; DeLong, E.F. Methane-consuming archea revealed by directly coupled isotopic and phylogenetic analysis. Science 2001, 293, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Alperin, M.J.; Blair, N.E.; Albert, D.B.; Hoehler, T.M.; Martens, C.S. Factors that control the stable carbon isotopic composition of methane produced in an anoxic marine sediment. Glob. Biogeochem. Cycles 1992, 6, 271–291. [Google Scholar] [CrossRef]
- Hoehler, T.M.; Alperin, M.J.; Albert, D.B.; Martens, C.S. Field and laboratory studies of methane oxidation in an anoxic marine sediment: Evidence for a methanogen-sulfate reducer consortium. Glob. Biogeochem. Cycles 1994, 8, 451–463. [Google Scholar] [CrossRef]
- Yoshinaga, M.Y.; Holler, T.; Goldhammer, T.; Wegener, G.; Pohlman, J.W.; Brunner, B.; Kuypers, M.M.M.; Hinrichs, K-U.; Elvert, M. Carbon isotope equilibration during sulphate-limited anaerobic oxidation of methane. Nat. Geosci. 2014, 7. [Google Scholar] [CrossRef]
- Borowski, W.S.; Paull, C.K.; Ussler, W., III. Global and local variations of interstitial sulfate gradients in the deep-water, continental margin sediments: Sensitivity to underlying methane and gas hydrates. Mar. Geol. 1999, 159, 131–154. [Google Scholar] [CrossRef]
- House, C.H.; Schopf, J.W.; Stetter, K.O. Carbon isotopic fractionation by Archaens and other thermophilic prokaryotes. Org. Geochem. 2003, 34, 345–356. [Google Scholar] [CrossRef]
- Zhang, C.L.; Fouke, B.W.; Bonheyo, G.T.; Peackock, A.D.; White, D.C.; Huang, Y.; Romanek, C.S. Lipid biomarkers and carbon-isotopes of modern travertine deposits (Yellowstone Natioinal Park, USA): Implications for biogeochemical dynamics in hot-spring systems. Geochem. Cosmochim. Acta 2004, 68, 3157–3169. [Google Scholar] [CrossRef]
- Quandt, L.; Gottschalk, G.; Ziegler, H.; Stichler, W. Isotope discrimination by photosynthetic bacteria. REMS Microbiol. Lett. 1977, 1, 125–128. [Google Scholar] [CrossRef]
- Morse, J.W.; Beazley, M.J. Organic matter in deepwater sediments of the Northern Gulf of Mexico and its relationship to the distribution of benthic organisms. Deep Sea Res. II 2008, 55, 2563–2571. [Google Scholar] [CrossRef]
- Burdige, D.J. Geochemistry of Marine Sediments; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Martens, C.S.; Klump, J.V. Biogeochemical cycling of an organic-rich coastal marine basin 4. An organic carbon budget for sediments dominated by sulfate reduction and methanogenesis. Geochem. Cosmochim. Acta 1984, 48, 1987–2004. [Google Scholar] [CrossRef]
- Hoehler, T.M.; Albert, D.B.; Alperin, M.J.; Martens, C.S. Acetogenesis from CO2 in an anoxic marine sediment. Limnol. Oceanogr. 1999, 44, 662–667. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).