Design, Optimization and Energetic Efficiency of Producing Hydrogen-Rich Gas from Biomass Steam Gasification
Abstract
:1. Introduction
2. BSG Process Description
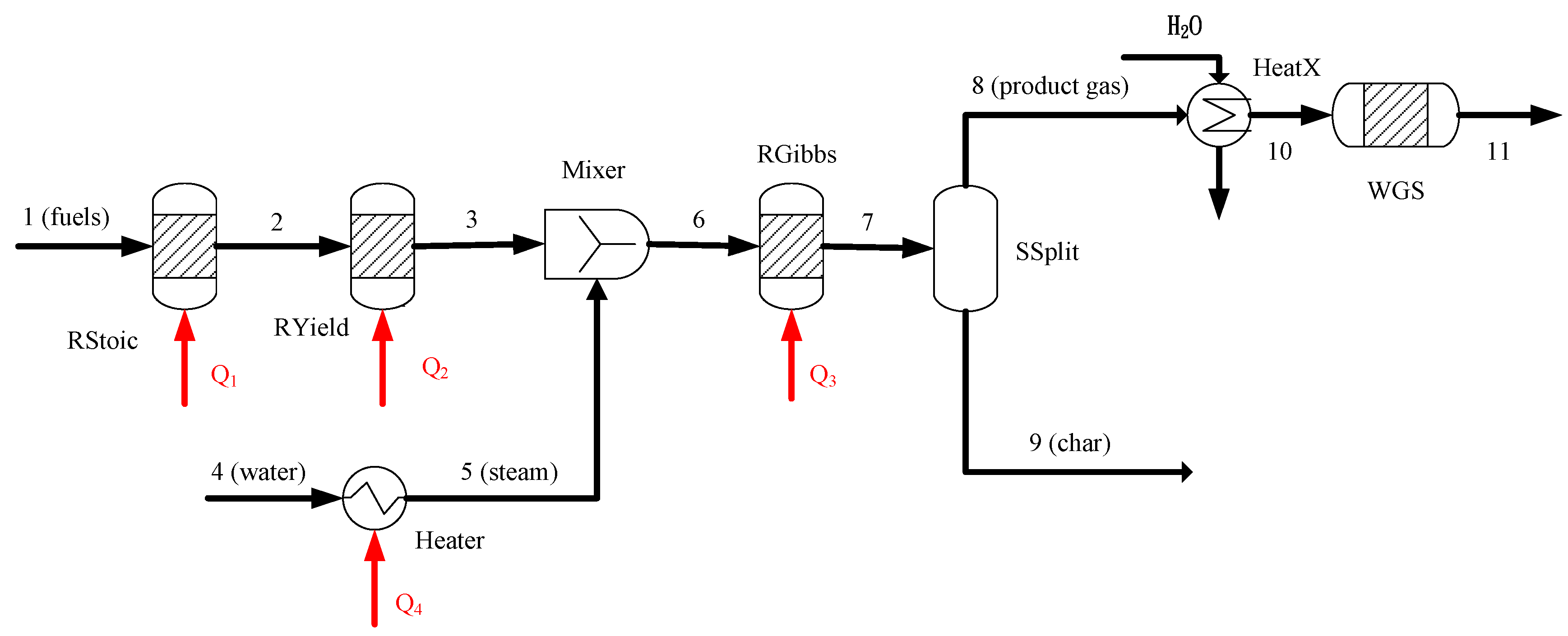
2.1. Model Validation
| Air/fuel ratio | Measurement | Gas composition (vol %) | ||
|---|---|---|---|---|
| H2 | CO | CO2 | ||
| 2.03 | Experimental | 17.20 | 19.60 | 9.90 |
| Model | 18.64 | 21.14 | 9.90 | |
| Relative error (%) | 8.37 | 7.86 | 0 | |
| 2.20 | Experimental | 18.30 | 20.20 | 9.70 |
| Model | 17.26 | 20.59 | 10.00 | |
| Relative error (%) | 5.68 | 1.93 | 3.09 | |
| 2.37 | Experimental | 17.20 | 19.40 | 9.70 |
| Model | 15.85 | 19.57 | 10.39 | |
| Relative error (%) | 7.85 | 0.88 | 7.11 | |
2.2. Mass Balance
| Reaction | Process | Reaction number |
|---|---|---|
| Drying | R1 | |
| Devolatilization | R2 | |
| Oxidation | R3 R4 | |
| Water gas reaction | R5 | |
| Boudouard reaction | R6 | |
| Shift reaction | R7 | |
| Methanation reaction | R8 R9 |
2.3. Energy Balance
3. Results and Discussion
| Feedstocks | Raw oil palm | Torrefied oil palm at 250 °C |
|---|---|---|
| Proximate analysis (wt%) | ||
| Moisture | 7.20 | 3.69 |
| Volatile matter (VM) | 67.25 | 53.76 |
| Fixed carbon (FC) | 19.03 | 32.58 |
| Ash | 6.52 | 9.97 |
| Elemental analysis (wt%) | ||
| C | 44.81 | 54.41 |
| H | 4.10 | 4.54 |
| N | 2.10 | 1.54 |
| O * | 42.47 | 29.62 |
| Higher heating value (MJ kg−1) | 17.10 | 20.59 |
| Items | Parameters | ||
|---|---|---|---|
| Stream class | MCINCPSD | ||
| Thermodynamic property | Peng-Robinson | ||
| Nonconventional properties | Enthalpy | HCOALGEN | |
| Feedstocks | Raw oil palm | CH1.10O0.71N0.040 | |
| Ambient conditions | 25 °C and 1 atm | ||
| Input conditions | Fuel: | 25 °C and 1 atm | |
| Gasifier | 700 °C and 1 atm | ||
| Sensitivity analysis | S/C ratio (mass flow rate) | 0.4–2 | |
3.1. Effect of S/C Ratio
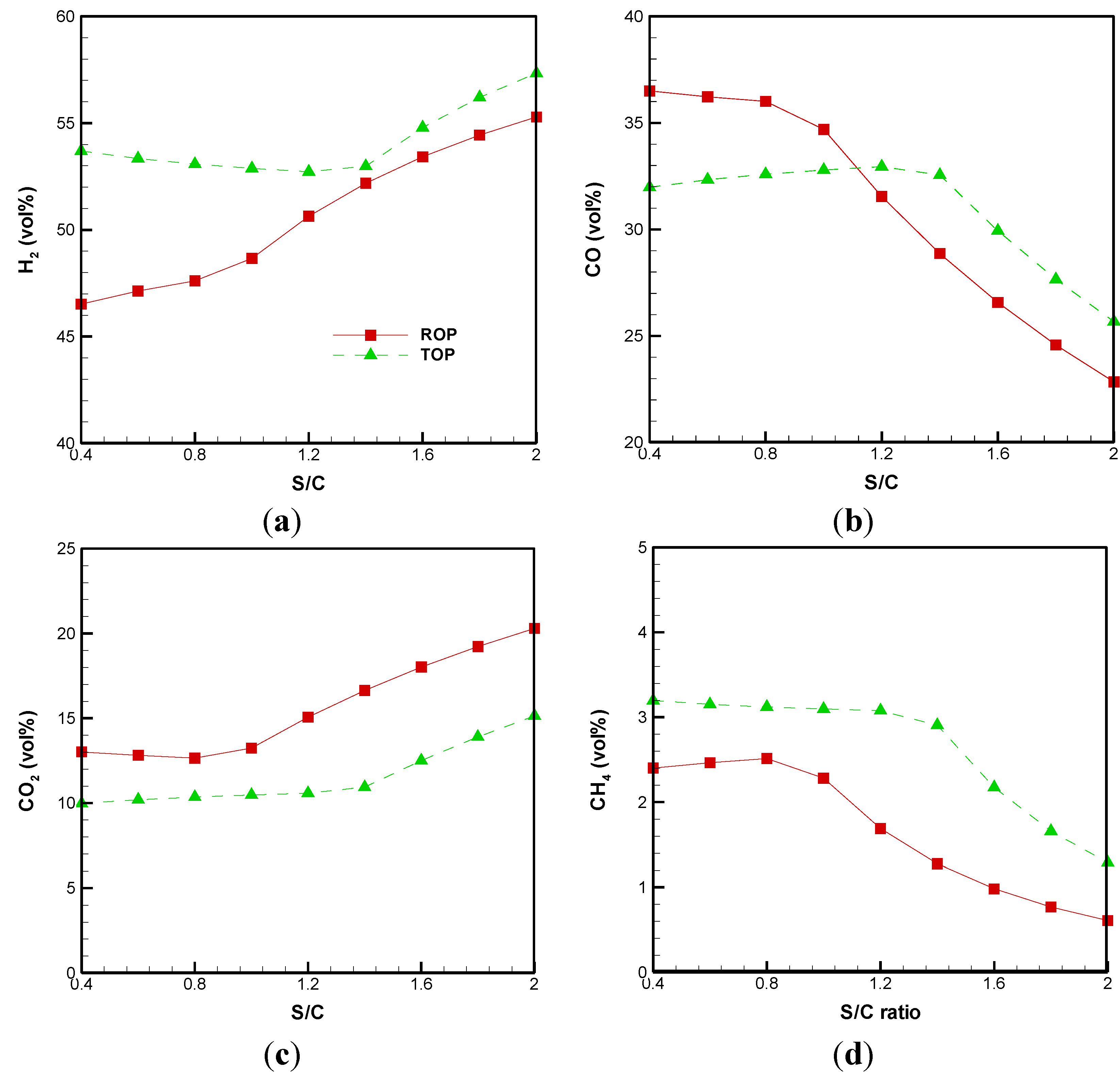
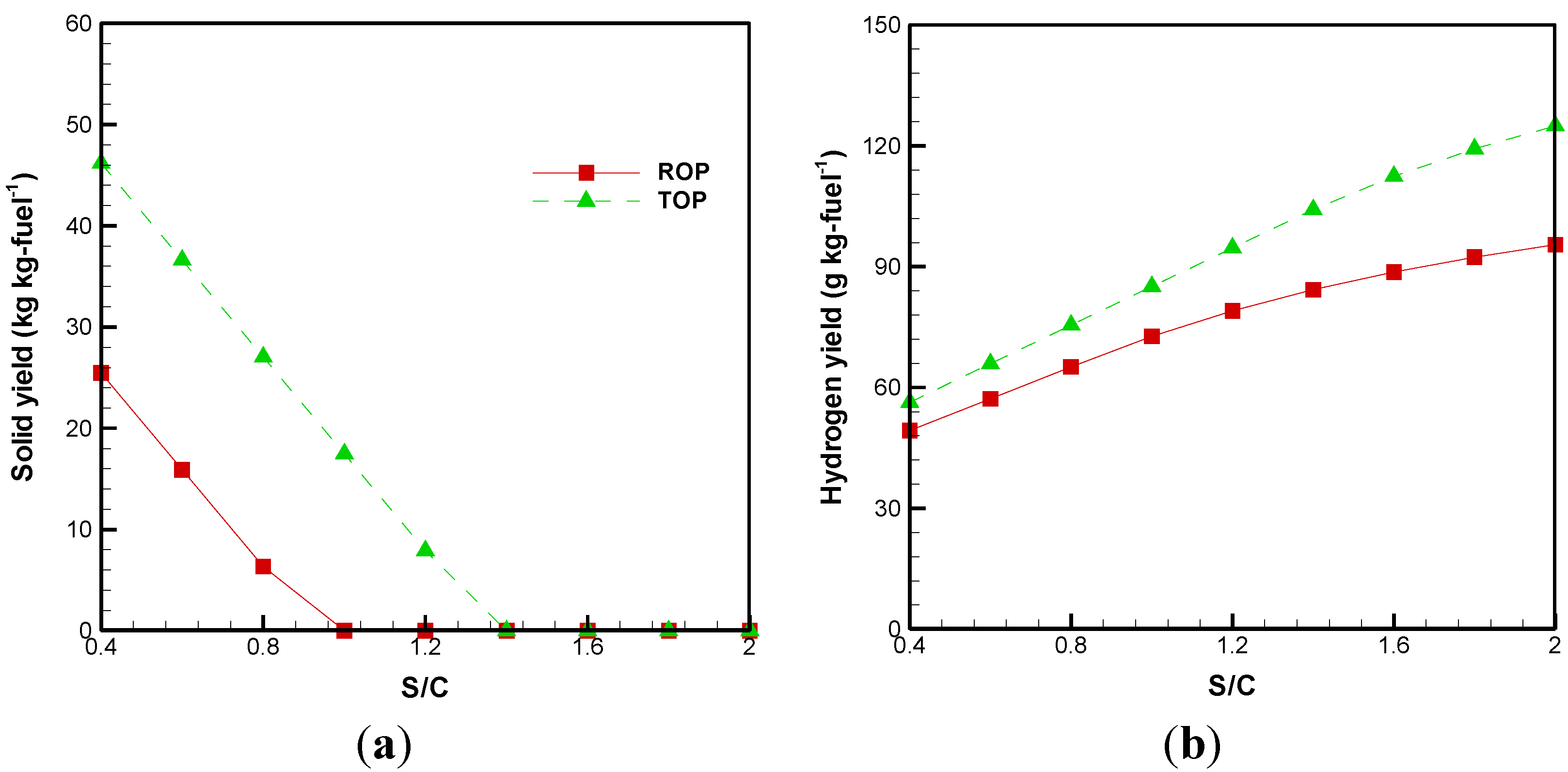
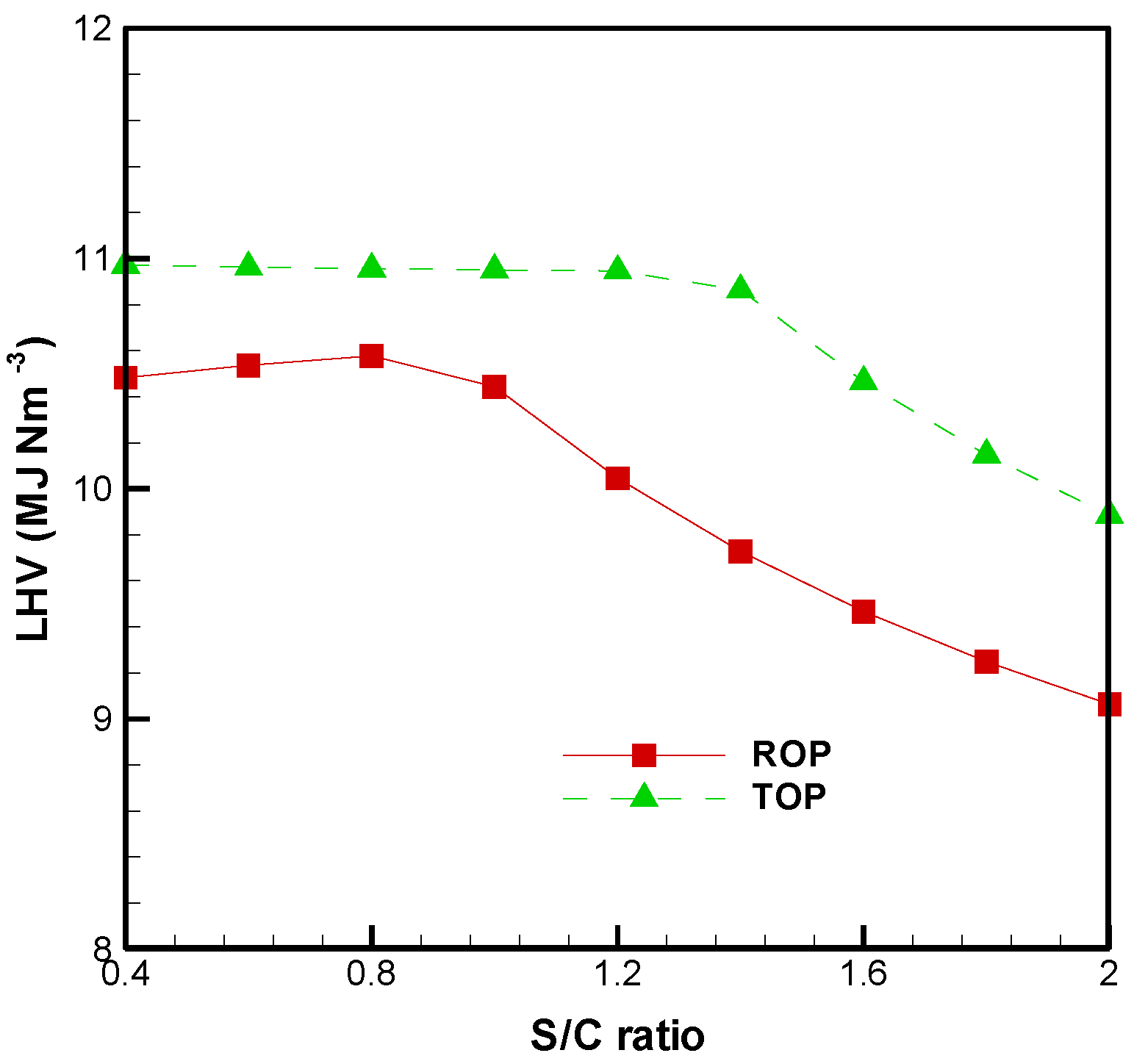
3.2. Cold Gas Efficiency and System Energetic Efficiency

3.3. BSG Process with Heat Recovery
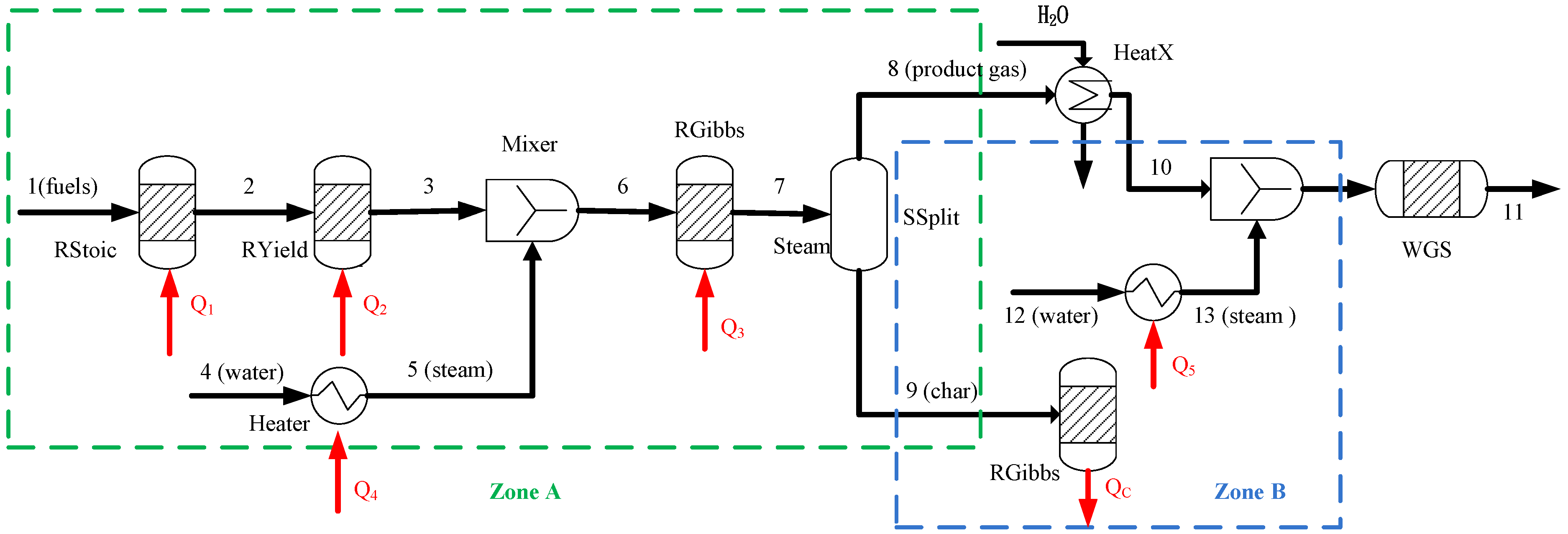
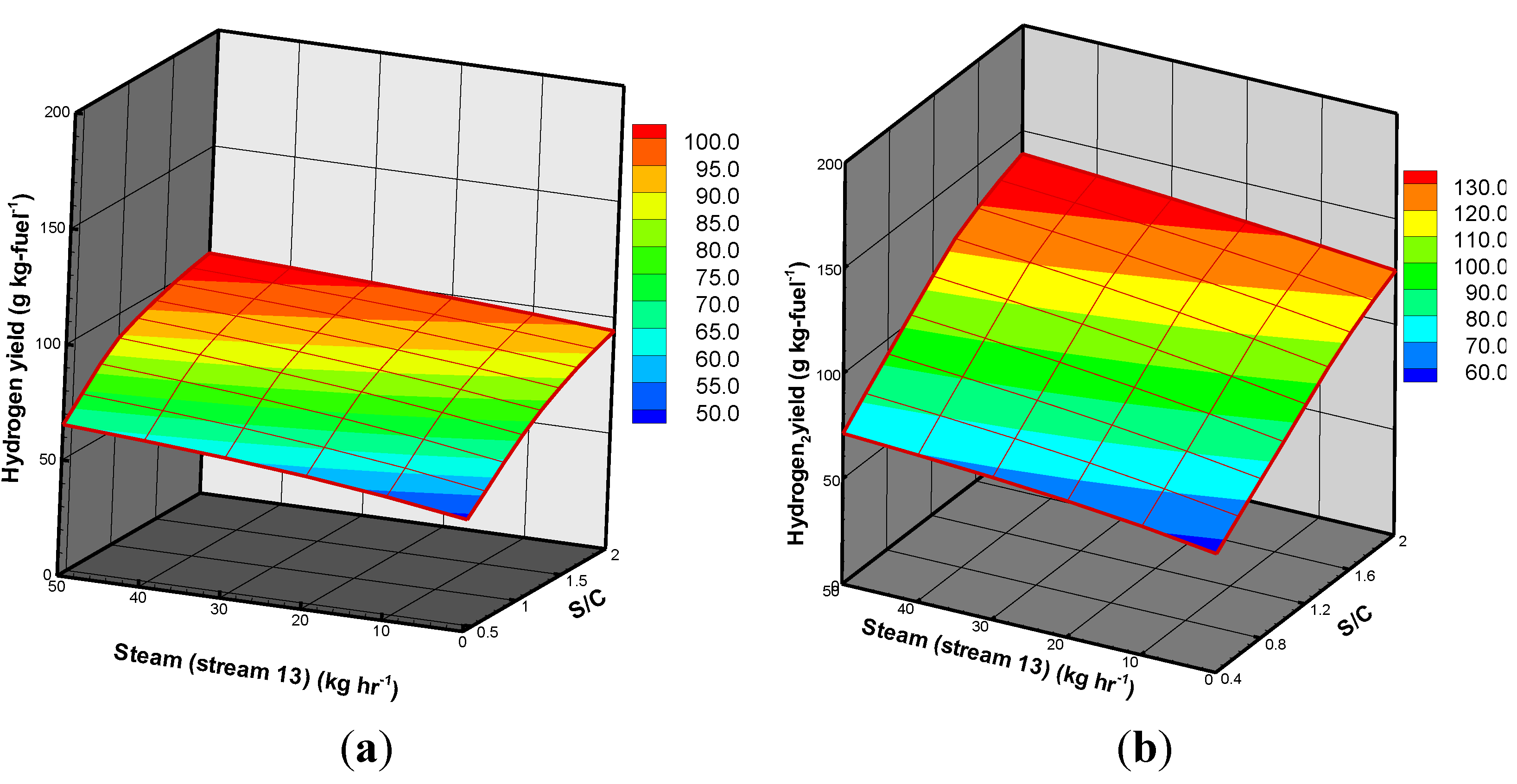
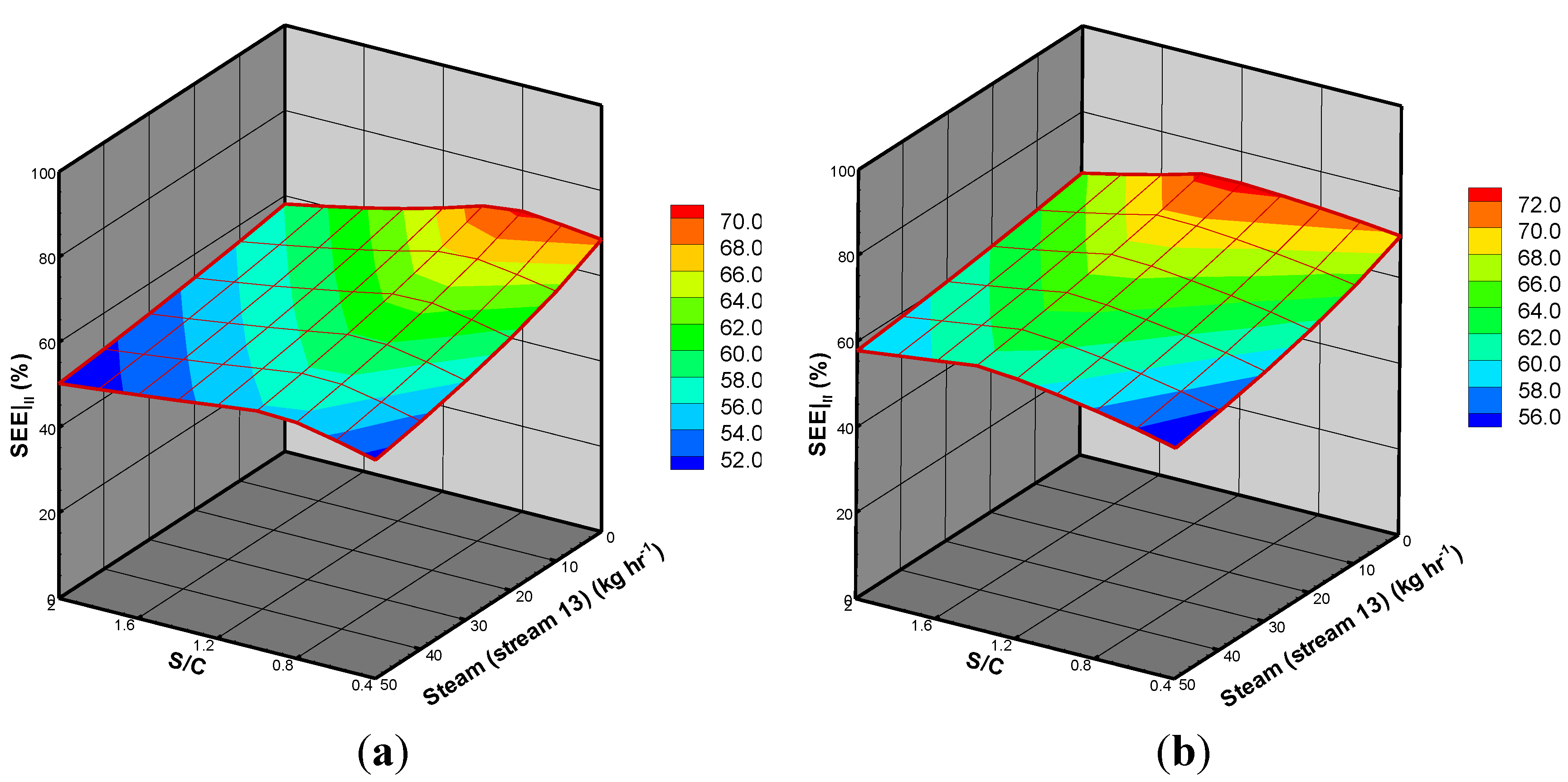

| Process | Design I | Design II (stream 13 = 20 kg/hr) | ||
|---|---|---|---|---|
| Feedstocks | ROP | TOP | ROP | TOP |
| Hydrogen yield (g/kg feedstock) | 79.04 | 94.67 | 85.52 | 103.84 |
| Increasing factor * (%) | - | 19.77 | - | 21.42 |
| SEE (%) | 70.68 | 70.37 | 60.62 | 66.74 |
| Increasing factor * (%) | - | −0.44 | - | 10.10 |
3.4. Process Optimization
| 0 | 120 | |
| 44.81 | 54.41 | |
| 0 | 120 | |
| 44.81 | 54.41 | |
| 0 | 120 | |
| 44.81 | 54.41 | |
| 0 | 50 |
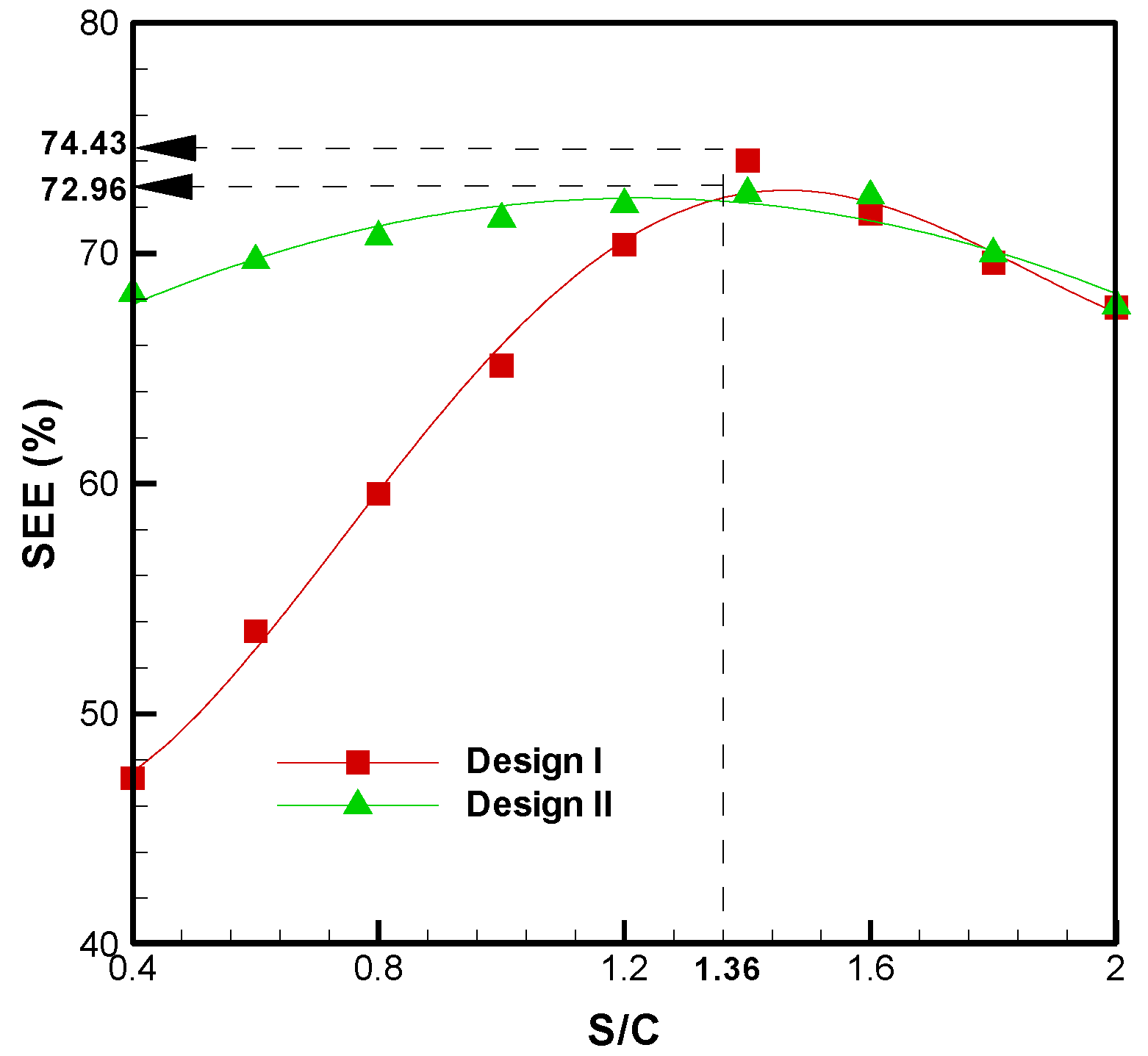
| Process | Design I | Design II | ||
|---|---|---|---|---|
| Before optimization | After optimization | Before optimization | After optimization | |
| S/C ratio | 1.4 | 1.36 | 1.4 | 1.36 |
| SEE (%) | 74.03 | 74.43 | 72.49 | 72.96 |
| Increasing factor * (%) | - | 0.54 | - | 0.65 |
| Hydrogen yield (g/kg feedstock) | 104.18 | 102.53 | 104.20 | 102.59 |
| Increasing factor * (%) | - | −1.58 | - | −1.54 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Howaniec, N.; Smolinski, A. Steam co-gasification of coal and biomass-Synergy in reactivity of fuel blends chars. Int. J. Hydrog. Energy 2013, 38, 16152–16160. [Google Scholar] [CrossRef]
- Taba, L.E.; Irfan, M.F.; Daud, W.A.M.W.; Chakrabarti, M.H. The effect of temperature on various parameters in coal, biomass and CO-gasification: A review. Renew. Sustain. Energy Rev. 2012, 16, 5584–5596. [Google Scholar] [CrossRef]
- Trippe, F.; Fröhling, M.; Schultmann, F.; Stahl, R.; Henrich, E. Techno-economic assessment of gasification as a process step within biomass-to-liquid (BtL) fuel and chemicals production. Fuel Process. Technol. 2011, 92, 2169–2184. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 2: Gasification systems. Energy Convers. Manag. 2009, 50, 3158–3168. [Google Scholar] [CrossRef]
- Normann, F.; Thunman, H.; Johnsson, F. Process analysis of an oxygen lean oxy-fuel power plant with co-production of synthesis gas. Energy Convers. Manag. 2009, 50, 279–286. [Google Scholar] [CrossRef]
- Pentananunt, R.; Rahman, A.N.M.M.; Bhattacharya, S.C. Upgrading of biomass by means of torrfaction. Energy 1990, 15, 1175–1179. [Google Scholar] [CrossRef]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Kuo, P.C. Torrefaction and co-torrefaction characterization of hemicellulose, cellulose and lignin as well as torrefaction of some basic constituents in biomass. Energy 2011, 36, 803–811. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass Bioenergy 2004, 27, 339–352. [Google Scholar] [CrossRef]
- Chew, J.J.; Doshi, V. Recent advances in biomass pretreatment-Torrefaction fundamentals and Technology. Renew. Sustain. Energy Rev. 2011, 15, 4212–4222. [Google Scholar] [CrossRef]
- van der Stelet, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar]
- Chen, Q.; Zhou, J.S.; Liu, B.J.; Mei, Q.F.; Luo, Z.Y. Influence of torrefaction pretreatment on biomass gasification technology. Chinese Sci. Bull. 2011, 56, 1449–1456. [Google Scholar] [CrossRef]
- Kuo, P.C.; Wu, W.; Chen, W.H. Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis. Fuel 2013, 117, 1231–1241. [Google Scholar] [CrossRef]
- Sarkar, M.; Kumar, A.; Tumuluru, J.S.; Patil, K.N.; Bellmer, D.D. Gasification performance of switchgrass pretreated with torrefaction and densification. Appl. Energy 2014, 127, 194–201. [Google Scholar] [CrossRef]
- Berrueco, C.; Recari, J.; Matas Güell, B.; del Alamo, C. Pressurized gasification of torrefied woody biomass in a lab scale fluidized bed. Energy 2014, 70, 68–78. [Google Scholar] [CrossRef]
- Weiland, F.; Nordwaeger, M.; Olofsson, I.; Wiinikka, H.; Nordin, A. Entrained flow gasification of torrefied wood residues. Fuel Process. Technol. 2014, 125, 51–58. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.I.; Kerdsuwan, S.; Gupta, A.K. Hydrogen and syngas production from sewage sludge via steam gasification. Int. J. Hydrog. Energy 2010, 35, 11738–11745. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda, G.; Barba, J.; Mendoza, J.M. Effect of steam content in the air-steam flow on biomass entrained flow gasification. Fuel Process. Technol. 2012, 99, 43–55. [Google Scholar] [CrossRef]
- Franco, C.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. The study of reactions influencing the biomass steam gasification process. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air-steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Schuster, G.; Loffler, G.; Weigl, K.; Hofbauer, H. Biomass steam gasificationan-extensive parametric modeling study. Bioresour. Technol. 2001, 77, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Doherty, W.; Reynolds, A.; Kennedy, D. Aspen plus simulation of biomass gasification in a steam blown dual fluidised bed. In Materials and Processes for Energy: Communicating Current Research and Technological Developments; Méndez-Vilas, A., Ed.; Formatex Research Centre: Badajoz, Spain, 2013. [Google Scholar]
- Ramzan, N.; Ashraf, A.; Naveed, S.; Malik, A. Simulation of hybrid biomass gasification using Aspen plus: A comparative performance analysis for food, municipal solid and poultry waste. Biomass Bioenergy 2011, 35, 3962–3969. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, D.; Guo, J.; Liu, J.; Ji, P. Energy analysis for low-rank coal based process system to co-produce semicoke, syngas and light oil. Energy 2013, 52, 279–288. [Google Scholar] [CrossRef]
- Hla, S.S.; Morpeth, L.D.; Sun, Y.; Duffy, G.J.; Ilyushechkin, A.Y.; Roberts, D.G.; Edwards, J.H. A CeO2–La2O3-based Cu catalyst for the processing of coal-derived syngases via high-temperature water–gas shift reaction. Fuel 2013, 114, 178–186. [Google Scholar] [CrossRef]
- Jayah, T.H.; Aye, L.; Fuller, R.J.; Stewart, D.F. Computer simulation of a downdraft wood gasier for tea drying. Biomass Bioenergy 2003, 25, 459–469. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Gao, F. Hydrogen-rich gas production from biomass steam gasification in an updraft fixed-bed gasifier combined with a porous ceramic reformer. Int. J. Hydrog. Energy 2008, 33, 5430–5438. [Google Scholar] [CrossRef]
- Chen, W.H.; Du, S.W.; Tsai, C.H.; Wang, Z.Y. Torrefied biomasses in a drop tube furnace to evaluate their utility in blast furnaces. Bioresour. Technol. 2012, 111, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.T.; Bej, S.K.; Bakhshi, N.N.; Dalai, A.K. Steam gasification of biomass-derived char for the production of carbon monoxide-rich synthesis gas. Energy Fuels 2001, 15, 736–742. [Google Scholar] [CrossRef]
- Yan, F.; Luo, S.Y.; Hu, Z.Q.; Xiao, B.; Cheng, G. Hydrogen-rich gas production by steam gasification of char from biomass fast pyrolysis in a fixed-bed reactor: Influence of temperature and steam on hydrogen yield and syngas composition. Bioresour. Technol. 2010, 101, 5633–5637. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Leeke, G.A.; Hornung, A.; Wood, J. Steam gasification of rapeseed, wood, sewage sludge and miscanthus biochars for the production of a hydrogen-rich syngas. Biomass Bioenergy 2014, 69, 276–286. [Google Scholar] [CrossRef]
- Kaewpanha, M.; Guan, G.; Hao, X.; Wang, Z.; Kasai, Y.; Kusakabe, K.; Abudula, A. Steam co-gasification of brown seaweed and land-based biomass. Fuel Process. Technol. 2014, 120, 106–112. [Google Scholar] [CrossRef]
- Vivanpatarakij, S.; Assabumrungrat, S. Thermodynamic analysis of combined unit of biomass gasifier and tar steam reformer for hydrogen production and tar removal. Int. J. Hydrog. Energy 2013, 38, 3930–3936. [Google Scholar] [CrossRef]
- Granados, D.A.; Vel asquez, H.I.; Chejne, F. Energetic and exergetic evaluation of residual biomass in a torrefaction process. Energy 2014, 74, 181–189. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, P.-C.; Wu, W. Design, Optimization and Energetic Efficiency of Producing Hydrogen-Rich Gas from Biomass Steam Gasification. Energies 2015, 8, 94-110. https://doi.org/10.3390/en8010094
Kuo P-C, Wu W. Design, Optimization and Energetic Efficiency of Producing Hydrogen-Rich Gas from Biomass Steam Gasification. Energies. 2015; 8(1):94-110. https://doi.org/10.3390/en8010094
Chicago/Turabian StyleKuo, Po-Chih, and Wei Wu. 2015. "Design, Optimization and Energetic Efficiency of Producing Hydrogen-Rich Gas from Biomass Steam Gasification" Energies 8, no. 1: 94-110. https://doi.org/10.3390/en8010094
APA StyleKuo, P.-C., & Wu, W. (2015). Design, Optimization and Energetic Efficiency of Producing Hydrogen-Rich Gas from Biomass Steam Gasification. Energies, 8(1), 94-110. https://doi.org/10.3390/en8010094






