Recent Advances in Second Generation Ethanol Production by Thermophilic Bacteria
Abstract
:1. Introduction
2. Ethanol Production from Biomass
2.1. Difference between First and Second Generation Ethanol Production
2.2. Comparison between Mesophilic and Thermophilic Microbes Producing Ethanol
- Minimal by-product formation;
- High productivity (>1 g/L/h);
- GRAS-status;
- Broad tolerance to environmental conditions;
- High ethanol tolerance;
- Broad substrate spectra;
- No “glucose effect”;
- High cellulolytic activity;
- Tolerant to inhibitory compounds;
- Tolerate high solid and substrate loadings;
- Simple nutritional needs;
- Low biomass production;
- Ease of genetic manipulation.
- >90% of theoretical yield;
- High ethanol titers (>5% (v/v));
- Minimum number of process steps;
- Minimal process cooling;
- Recyclable cells;
- Co-fermentation of substrates;
- Limited or no pretreatment;
- Limited or no pretreatment.
3. Thermophilic Ethanol Producers
| Microorganism | Selected substrate spectra | Polymer degrading ability | Ethanol tolerance | Inhibitor tolerance | Fermentation products | Max ethanol yields (mol/mol sugar) | Topt (°C) | References |
|---|---|---|---|---|---|---|---|---|
| Caloramator boliviensis | g, gal, man, x, a, cel, suc | X | ND | ND | E, A, L, P, H | 1.53 (x) | 60 | [28,43] |
| Clostridium thermocellum | g *, cel | C | 4%–5% | ND | E, A, L, F, H | 1.53 (g) | 60 | [44,45,46,47] |
| Clostridium AK1 | g, gal, man, x, suc | S, X, P | ND | ND | E, A, H | 1.50 (g), 0.85 (x) | 50 | [48] |
| Thermoanaerobacterium saccharolyticum | g, gal, man, x, a, cel, suc | S, X | NR | NR | E, A, L, H | 1.18 (x) | 60 | [40,49] |
| Thermoanaerobacterium AK17 | g, gal, man, x, a, suc | P | 3.2% | 4 g/L FF 6 g/L HMF | E, A, H | 1.50 (g), 1.33 (x) | 60 | [42,50] |
| Thermoanaerobacter ethanolicus | g, gal, man, x, cel, suc | S, X | 0.5%–5.0% | ND | E, A, L, H | 1.90 (g), 1,64 (x) | 70 | [16,51,52] |
| Thermoanaerobacter pseudoethanolicus | g, cel, suc | S, X, P | 4% | ND | E, A, L, H | 1.88 (g) | 67–69 | [53,54] |
| Thermoanaerobacter mathranii | g, man, x, a, cel, suc | S, X, P, I | 5% | 2% ArC | E, A, L, H | 1.37 (x) | 70 | [17,55] |
| Thermoanaerobacter pentosaceus | g, gal, man, x, a, cel, suc | S, X, P, I | 0.5% | 3.4 g/L FF 3.4 g/L HMF | E, A, L, H | 1.68 (x) | 70 | [56,57] |
| Thermoanaerobacter AK5 | g, gal, man, x, cel | S | ND | ND | E, A, H | 1.70 (g), 1.35 (x) | 65 | [58] |
| Thermoanaerobacter J1 | g, gal, man, x, a, cel | S | ND | ND | E, A, H | 1.70 (g), 1.25 (x) | 65 | [18] |
4. Physiology of Thermophilic Anaerobic Bacteria
4.1. Central Metabolism of Sugars to Various end Products
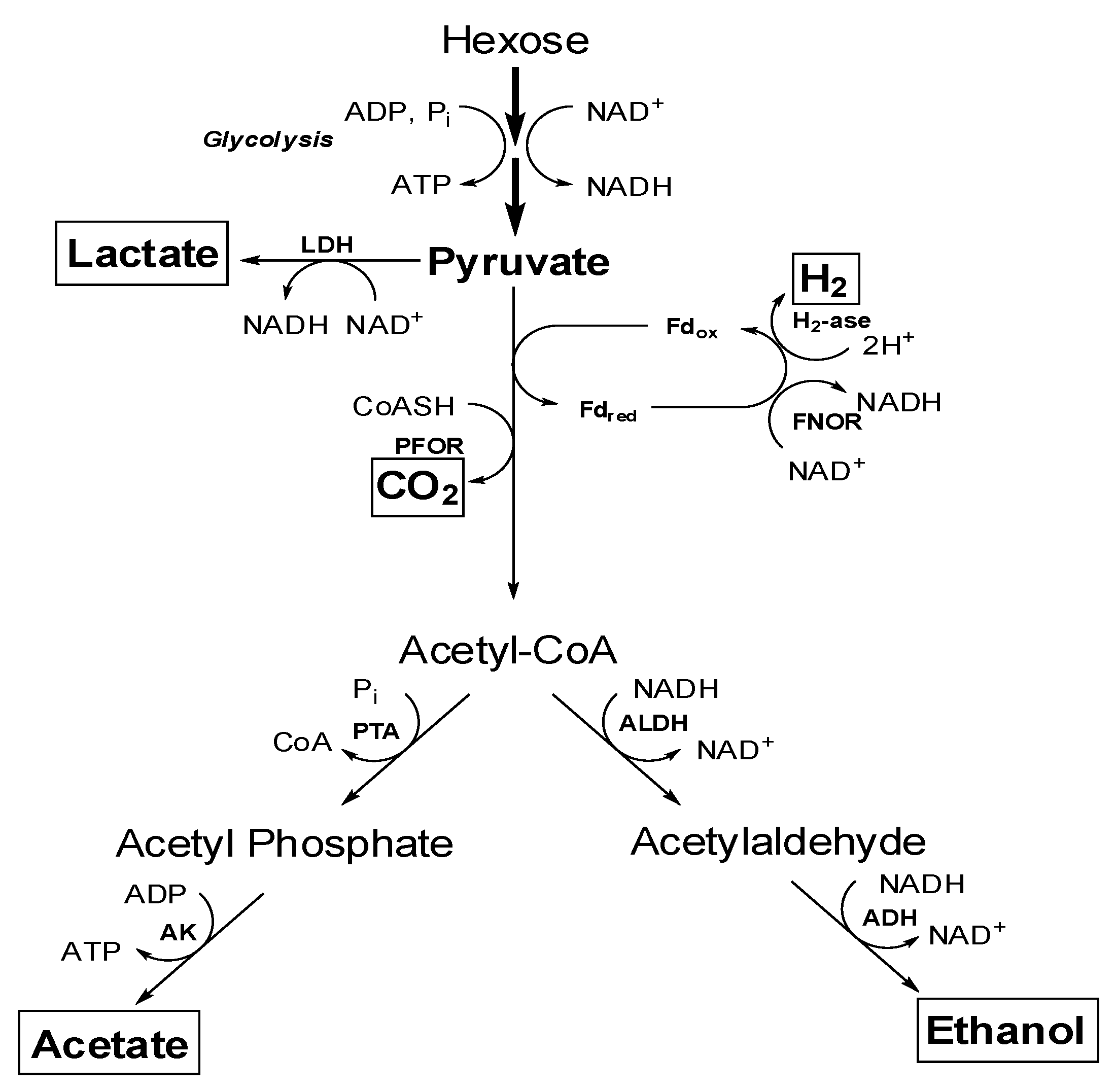
4.2. Effect of Various Factors for Ethanol Production
4.2.1. Partial Pressure of Hydrogen
4.2.2. Substrate Loadings
4.2.3. Ethanol Tolerance
4.2.4. Other Culture Parameters
5. Production of Ethanol from Lignocellulose
| Organisms | Substrate | Fermentation mode | Substrate (g/L) | Pre-treatment | Ethanol yields (mM/g) | T (°C) | References |
|---|---|---|---|---|---|---|---|
| Clostridium thermocellum | Avicel | Batch | 2.5 | A | 5.00 | 60 | [93] |
| Clostridium thermocellum | Avicel | Con | 5.0 | A | 5.48 | 60 | [94] |
| Clostridium thermocellum | Whatman paper | Batch | 8.0 | None | 7.20–8.00 | 60 | [95] |
| Clostridium thermocellum | Paddy straw | Batch | 8.0 | None | 6.10–8.00 | 60 | [95] |
| Clostridium thermocellum | Sorghum stover | Batch | 8.0 | None | 4.80–8.10 | 60 | [95] |
| C. thermocellum and C. thermolacticum | Microcrystal cellulose | Batch | 10.0 | None | 9.1 | 57 | [96] |
| Clostridium AK1 | Hemp | Batch | 5.0 | A/Alk | 3.5 | 50 | [48] |
| Thermoanaerobacter pentosaceus | Rapeseed straw | Con | 50 | Alk | 1.40 | 70 | [57] |
| Thermoanaerobacter mathranii | Wheat straw | Batch | 6.7 | WO/E | 2.61 | 70 | [87] |
| Thermoanaerobacter mathranii | What straw | Batch | 60.0 | WO/E | 5.30 | 70 | [97] |
| Thermoanaerobacter ethanolicus | Beet molasses | Batch | 30.0 | None | 4.81 | 65 | [98] |
| Thermoanaerobacter BG1L1 | Corn stover | Batch | 25.0–150.0 | WO/E | 8.50–9.20 | 70 | [60] |
| Thermoanaerobacter BG1L1 | Wheat straw | Batch | 30.0–120.0 | WO/E | 8.50–9.20 | 70 | [92] |
| Thermoanaerobacter BG1L1 | Corn stover | Con | 25.0–150.0 | WO/E | 8.50–9.20 | 70 | [60] |
| T. ethanolicus | Wood HL | Batch | 8.0 | E | 3.30–4.50 | 70 | [89] |
| Thermoanaerobacter AK5 | Whatman paper | Batch | 2.25 | E | 7.7 | 65 | [58] |
| Thermoanaerobacter AK5 | Grass | Batch | 4.5 | A/E | 4.31 | 65 | [58] |
| Thermoanaerobacter J1 | Whatman paper | Batch | 4.5 | E | 7.5 | 65 | [18] |
| Thermoanaerobacter J1 | Hemp | Batch | 4.5 | A/E | 4.3 | 65 | [18] |
| T. saccharoylticum | Xylan | Batch | 10.0 | WO | 6.30 | 60 | [86] |
| Thermoanaerobacterium AK17 | Cellulose | Batch | 2.5 | E | 8.6 | 60 | [42] |
| Thermoanaerobacterium AK17 | Grass | Batch | 2.5 | A/Alk/E | 5.5 | 60 | [42] |
6. Evolutionary Adaptation and Genetic Engineering of Thermophiles
6.1. Evolutionary Adaptation
6.2. Genetic Engineering
| Strain | Genotype | Substrate | Concentration (g/L) | Mode | Ethanol yields (mol/mol) | References |
|---|---|---|---|---|---|---|
| C. thermocellum | ΔpyrF, Δpta::gapDHp-cat | Cellobiose | 5.0 | Batch | 0.59 | [111] |
| C. thermocellum | ΔpyrF, Δpta::gapDHp-cat | Avicel | 5.0 | Batch | 0.71 | [111] |
| C. thermocellum adhE*(EA) Δldh | Δhpt, Δldh | Cellobiose | 5.0 | Batch | 0.37 | [112] |
| C. thermocellum | Δhpt, Δldh, Δpta (evolved) | Avicel | 19.5 | Batch | 1.08 | [112] |
| C. thermocellum/T. saccharolyticum | Δhpt, Δldh, Δpta (evolved) and Δpta, Δack, Δldh | Avicel | 19.5 | Batch | 1.26 | [112] |
| T. saccharolyticum TD1 | Δldh | Xylolse | 5.0 | Batch | 0.98 | [112] |
| T. saccharolyticum ALK2 | Δpta, Δack, Δldh | Cellobiose | 70.0 | Con | ND | [49] |
| T. saccharolyticium HK07 | Δldh , Δhfs | Cellobiose | 1.8 | Batch | 0.86 | [110] |
| T. saccharolyticium M0355 | Δldh , Δack Δpta | Cellobiose | 50.0 | Batch | 1.73 | [106] |
| T. saccharolyticum M1051 | Δldh , Δack Δpta, ureABCDEFG | Cellobiose | 27.5 | Batch | 1.73 | [110] |
| G. thermoglucosidasius TM242 | Δldh-, pdh up, pflB- | Glucose | 34.0 | Batch | 1.73 | [70] |
| G. thermoglucosidasius TM242 | Δldh-, pdh up, ΔpflB- | Glucose | 34.0 | Batch | 1.84 | [70] |
| G. thermoglucosidasius TM242 | Δldh-, Δpdh up, ΔpflB- | Xylose | 29.0 | Batch | 1.37 | [70] |
| T. mathranii BG1L1 | Δldh | Wheat straw | 30–120 | Con | 1.53–1.67 | [92] |
| T. mathranii BG1G1 | Δldh, GldA | Glucose + glycerol | 5.0 | Batch | 1.68 | [55] |
| T. mathranii BG1G1 | Δldh, GldA | Xylose + glycerol | 5.0 | Batch | 1.57 | [55] |
| T. mathranii BG1G1 | Δldh, GldA | Xylose + glycerol | 12.8 and 7.2 | Con | 1.53 | [55] |
7. Process Technology for Thermophilic Bioethanol Production
- 1)
- Physical and chemical pretreatment of biomass;
- 2A)
- Hydrolases production (cellulases, hemicellulases, etc.);
- 2B)
- accharification (enzymatic hydrolysis of polymers to hexoses and pentoses);
- 3)
- Fermentation (of both pentoses and hexoses);
- 4)
- Product recovery.
7.1. Process Steps for the Conversion of Lignocellulosic Biomass to Ethanol
7.1.1. Pretreatment of Biomass
7.1.2. Enzymatic Hydrolysis and Saccharification
7.1.3. Fermentation
7.1.4. Product Recovery
7.2. Integrated Processes for Ethanol Production from Lignocellulose
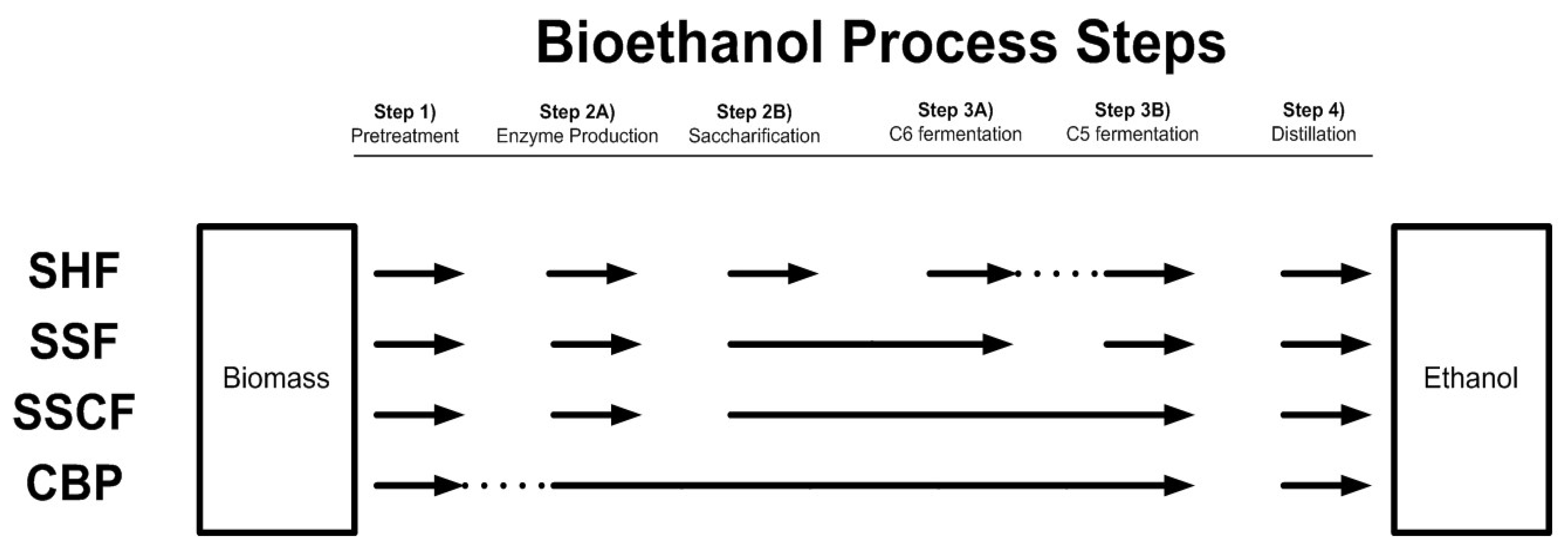
7.2.1. Separate Hydrolysis and Fermentation
7.2.2. Simultaneous Saccharification and Fermentation and Simultaneous Saccharification and Co-Fermentation
7.2.3. Consolidated Bioprocessing
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- European Commission. Directive 2003/30/EC of the European Parliament and of the Council of 8 May 2003 on the promotion of the use of biofuels or other renewable fuels for transport. Off. J. Eur. Union 2003, L123, 42–46. [Google Scholar]
- European Commission. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. Off. J. Eur. Union 2009, L140, 16–47. [Google Scholar]
- RFA: Renewable Fuels Association. World Fuel Ethanol Production. 2013. Available online: http://ethanolrfa.org/pages/World-Fuel-Ethanol-Production (accessed on 30 September 2014).
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Taylor, M.P.; Eley, K.L.; Martin, S.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Thermophilic ethanologenesis: Future prospects for second-generation bioethanol production. Trends Biotechnol. 2009, 27, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Newcomb, M.; Wu, J.H.D. Cellulase, clostridia, and ethanol. Microbiol. Mol. Biol. Rev. 2005, 69, 124–154. [Google Scholar] [CrossRef] [PubMed]
- Hanh-Hagerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Liden, G.; Zacchi, G. Bio-ethanol the fuel of tomorrow from residues today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.F.; Plevin, R.J.; Turner, B.T.; Jones, A.D.; O’Hare, M.; Kammen, D.M. Ethanol can contribute to energy and environmental goals. Science 2006, 311, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Gnansounou, E.; Dauriat, A. Techno-economic analysis of lignocellulosic ethanol: A review. Bioresour. Biotechnol. 2010, 101, 4980–4991. [Google Scholar] [CrossRef]
- Chang, T.; Yao, S. Thermophilic, lignocellulolytic bacteria for ethanol production: Current state and persepectives. Appl. Microbiol. Biotechnol. 2011, 92, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, S.; Olsson, L.; Nielsen, J. Metabolic engineering of Saccharomyces cerevisae. Microbiol. Mol. Biol. Rev. 2000, 64, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, T.W. Engineering yeasts for xylose metabolism. Curr. Opin. Biotechnol. 2006, 17, 320–326. [Google Scholar] [CrossRef] [PubMed]
- He, M.X.; Wu, B.; Qin, H.; Ruan, Z.Y.; Tan, F.R.; Wang, J.L.; Shui, Z.X.; Dai, L.C.; Zhu, Q.L.; Pan, K.; et al. Zymomonas mobilis: A novel platform for future biorefineries. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 2007, 6. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.D.; Wiegel, J. Diversity of thermophilic anaerobes. Ann. N. Y. Acad. Sci. 2008, 1125, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, J.; Ljungdahl, L.G. Thermoanaerobacter ethanolicus gen. nov., spec. nov., a new, extreme thermophilic, anaerobic bacterium. Arch. Microbiol. 1981, 128, 343–348. [Google Scholar] [CrossRef]
- Larsen, L.; Nielsen, P.; Ahring, B.K. Thermoanaerobacter mathranii sp. nov, an ethanol-producing, extremely thermophilic anaerobic bacterium from a hot spring in Iceland. Arch. Microbiol. 1997, 168, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Jessen, J.E.; Orlygsson, J. Production of ethanol from sugars and lignocellulosic biomass by Thermoanaerobacter J1 isolated from a hot spring in Iceland. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Slobodkin, A.I.; Tourova, T.P.; Kuznetsov, B.B.; Kostrikina, N.A.; Chernyh, N.A.; Bonch-Osmolovskaya, E.A. Thermoanaerobacter siderophilus sp. nov., a novel dissimilatory Fe(III)-reducing, anaerobic, thermophilic bacterium. Int. J. Syst. Bacteriol. 1999, 49, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Mendez, B.S.; Pettinari, M.J.; Ivanier, S.E.; Ramos, C.A.; Sineriz, F. Clostridium thermopapyrolyticum sp. nov., a cellulolytic thermophile. Int. J. Syst. Bacteriol. 1991, 41, 281–283. [Google Scholar] [CrossRef]
- Fong, J.C.N.; Svenson, C.J.; Nakasugi, K.; Leong, C.T.C.; Bowman, J.P.; Chen, B.; Glenn, D.R.; Neilan, B.A.; Rogers, P.L. Isolation and characterization of two novel ethanol-tolerant facultative-anaerobic thermophilic bacteria strains from waste compost. Extremophiles 2006, 10, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Imachi, H.; Susilorukmi, A.; Muramatsu, M.; Ohashi, A.; Harada, H.; Hanada, S.; Kamagata, Y. Tepidanaerobacter syntrophicus gen. nov., sp. nov., an anaerobic, moderately thermophilic, syntrophic alcohol- and lactate-degrading bacterium isolated from thermophilic digested sludges. Int. J. Syst. Evol. Microbiol. 2006, 56, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Cayol, J.L.; Ollivier, B.; Patel, B.K.C.; Ravot, G.; Magot, M.; Ageron, E.; Grimont, P.A.D.; Garcia, J.L. Description of Thermoanaerobacter brockii subsp. lactiethylicus subsp. nov., isolated from a deep subsurface French oil well, a proposal to reclassify Thermoanaerobacter finnii as Thermoanaerobacter brockii subsp. finnii comb. nov., and an emended description of Thermoanaerobacter brockii. Int. J. Syst. Bacteriol 1995, 45, 783–789. [Google Scholar]
- Cann, I.K.; Stroot, P.G.; Mackie, K.R.; White, B.A.; Mackie, R.I. Characterization of two novel saccharolytic, anaerobic thermophiles, Thermoanaerobacterium polysaccharolyticum sp. nov. and Thermoanaerobacterium zeae sp. nov., and emendation of the genus Thermoanaerobacterium. Int. J. Syst. Evol. Microbiol. 2001, 51, 293–302. [Google Scholar] [PubMed]
- Amend, J.P.; Shock, E.L. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 2001, 25, 175–243. [Google Scholar] [CrossRef] [PubMed]
- Tomás, A.F. Optimization of Bioethanol Production from Carbohydrate Rich Wastes by Extreme Thermophilic Microorganisms. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, 2013. [Google Scholar]
- Sveinsdottir, M.; Baldursson, S.R.B.; Orlygsson, J. Ethanol production from monosugars and lignocellulosic biomass by thermophilic bacteria isolated from Icelandic hot springs. Icel. Agric. Sci. 2009, 22, 45–58. [Google Scholar]
- Crespo, C.; Pozzo, T.; Karlsson, E.N.; Alvarez, M.P.; Mattiasson, B. Caloramator boliviensis sp. nov., a thermophilic, ethanol-producing bacterium isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2012, 62, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Euzéby, J.P. List of Bacterial Names with Standing in Nomenclature: A folder available on the Internet. Int. J. Syst. Evol. Microbiol. 1997, 47, 590–592. [Google Scholar]
- Parte, A.C. LPSN—List of prokaryotic names with standing in nomenclature. Nucleic Acids Res. 2014, 42, D613–D616. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, J.; Tanner, R.; Rainey, F.A. An introduction to the family clostridiae. In The Prokaryotes, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Part 1; pp. 654–678. [Google Scholar]
- Canganella, F.; Wiegel, J. The potential of thermophilic clostridia in biotechnology. In The Clostridia and Biotechnology; Woods, D.R., Ed.; Butterworth-Heinemann: Freepost, UK, 1993; Volume 23, pp. 394–429. [Google Scholar]
- Carreira, L.H.; Ljungdahl, L.G. Production of ethanol from biomass using anaerobic thermophilic bacteria. In Liquid Fuel Developments; Wise, D.L., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 1–28. [Google Scholar]
- Nölling, J.; Breton, G.; Omelchenko, M.V.; Makarova, K.S.; Zeng, Q.; Gibson, R.; Lee, H.M.; Dubois, J.; Qiu, D.; Hitti, J.; et al. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 2001, 183, 4823–4838. [Google Scholar] [CrossRef] [PubMed]
- Sabathé, F.; Belaich, A.; Soucaille, P. Characterization of the cellulolytic complex (cellulosome) of Clostridium acetobutylicum. FEMS Microbiol. Lett. 2002, 217, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Han, S.O.; Yukawa, H.; Inui, M.; Doi, R.H. Transcription of Clostridium cellulovorans cellulosomal cellulase and hemicellulase genes. J. Bacteriol. 2003, 185, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Rydzak, T.; Grigoryan, M.; Cunningham, Z.J.; Krokhin, O.V.; Ezzati, P.; Cicek, N.; Levin, D.B.; Wilkins, J.A.; Sparling, R. Insights into electron flux manipulations of fermentation conditions and assessment of protein expression profiles in Clostridium thermocellum. Appl. Microbiol. Biotechnol. 2014, 98, 6497–6510. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Lawson, P.A.; Willems, A.; Cordoba, J.J.; Fernandez-Garayzabal, J.; Garcia, P.; Cai, J.; Hippe, H.; Farrow, J.A. The phylogeny of the genus Clostridium: Proposal of five new genera and eleven new species combinations. Int. J. Syst. Evol. Microbiol. 1994, 44, 812–826. [Google Scholar]
- Schink, B.; Zeikus, J.G. Clostridium thermosulfurogenes sp. nov, a new thermophile that produces elementar sulfur from thiosulfate. Microbiology 1983, 129, 1145–1158. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Jain, M.K.; Lee, C.; Lowe, S.E.; Zeikus, J.G. Taxonomic distinction of saccharolytic thermophilic anaerobes: Description of Thermoanaerobacterium xylanolyticum gen. nov., sp. nov., and Thermoanaerobacterium saccharolyticum gen. nov., sp. nov.; reclassification of Thermoanaerobium brockii, Clostridium thermosulfurogenes, and Clostridium thermohydrosulfuricum E100-69 as Thermoanaerobacter brockii comb. nov., Thermoanaerobacterium thermosulfurigenes comb. nov., and Thermoanaerobacter thermohydrosulfuricus comb. nov., respectively; and transfer of Clostridium thermohydrosulfuricum 39E to Thermoanaerobacter ethanolicus. Int. J. Syst. Evol. Microbiol. 1993, 43, 41–51. [Google Scholar]
- Kublanov, I.V.; Prokofeva, M.I.; Kostrikina, N.A.; Kolganova, T.V.; Tourova, T.P.; Wiegel, J.; Bonch-Osmolovskaya, E.A. Thermoanaerobacterium aciditolerans sp. nov., a moderate thremoacidophile from a Kamchatka hot spring. Int. J. Syst. Evol. Microbiol. 2007, 57, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Almarsdottir, A.R.; Sigurbjornsdottir, M.A.; Orlygsson, J. Effects of various factors on ethanol yields from lignocellulosic biomass by Thermoanaerobacterium AK17. Biotechnol. Bioeng. 2012, 109, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Crespo, R.E.; Badshah, M.; Alvarez, M.T.; Mattiasson, B. Ethanol production by continuous fermentation of d-(+)-cellobiose, d-(+)-xylose and sugarcane bagasse hydrolysate using the thermoanaerobe Caloramator boliviensis. Bioresour. Technol. 2012, 103, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Balusu, R.; Paduru, R.M.R.; Seenyya, G.; Reddy, G. Production of ethanol from cellulosic biomass by Clostridium thermocellum SS19 in submerged fermentation: Screening of nutrients using Plackett-Burman desing. Appl. Biochem. Biotechnol. 2004, 117, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.S.; Seenayya, G. High ethanol tolerance of new isolates of Clostridium thermocellum strains SS21 and SS22. World J. Microbiol. Biotechnol. 1999, 15, 173–178. [Google Scholar] [CrossRef]
- Roberts, S.B.; Gowen, C.M.; Brooks, J.P.; Fong, S.S. Genome-scale metabolic analysis of Clostridium thermocellum for bioethanol production. BMC Syst. Biol. 2010, 4. [Google Scholar] [CrossRef]
- Tyurin, M.; Desai, S.; Lynd, L.R. Electrotransformation of Clostridium thermocellum. Appl. Environ. Microbiol. 2012, 70, 883–890. [Google Scholar] [CrossRef]
- Orlygsson, J. Ethanol production from biomass by a moderate thermophile, Clostridium AK1. Icel. Agric. Sci. 2012, 25, 25–35. [Google Scholar]
- Shaw, A.J.; Podkaminer, K.K.; Desai, S.G.; Bardsley, J.S.; Rogers, S.R.; Thorne, P.G.; Hogsett, D.A.; Lynd, L.R. Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield. Proc. Natl. Acad. Sci. USA 2008, 105, 13769–13774. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, P.E.P.; Beck, S.R.; Orlygsson, J.; Puhakka, J.A. Ethanol and hydrogen production by two thermophilic, anaerobic bacteria isolated from Icelandic geothermal areas. Biotechnol. Bioeng. 2008, 101, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Hild, H.M.; Stuckey, D.C.; Leak, D.J. Effect of nutrient limitation on product formation during continuous fermentation of xylose with Thermoanaerobacter ethanolicus JW200 Fe(7). Appl. Microbiol. Biotechnol. 2003, 60, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lacis, L.S.; Lawford, H.G. Ethanol-production from xylose by Thermoanaerobacter ethanolicus in batch and continuous culture. Arch. Microbiol. 1988, 150, 48–55. [Google Scholar] [CrossRef]
- Lovitt, R.W.; Shen, G.J.; Zeikus, J.G. Ethanol production by thermophilic bacteria: Biochemical basis for ethanol and hydrogen tolerance in Clostridium thermohydrosulfuricum. J. Bacteriol. 1988, 170, 2809–2815. [Google Scholar] [PubMed]
- Zeikus, J. Microbiology of methanogenesis in thermal, volcanic environments. J. Bacteriol. 1980, 143, 432–440. [Google Scholar] [PubMed]
- Yao, S.; Mikkelsen, M.J. Metabolic engineering to improve ethanol production in Thermoanaerobacter mathranii. Appl. Microbiol. Biotechnol. 2010, 88, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Tomás, A.F.; Karakashev, D.; Angelidaki, I. Thermoanaerobacter pentosaceus sp. nov., an anaerobic, extreme thermophilic, high ethanol-yielding bacterium isolated from household waste. Int. J. Syst. Evol. Microbiol. 2013, 63, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Tomás, A.F.; Karagöz, P.; Karakashev, D.; Angelidaki, I. Extreme thermophilic ethanol production from rapeseed straw: Using the newly isolated Thermoanaerobacter pentosaceus and combining it with Saccharomyces cerevisiae in a two-step process. Biotechnol. Bioeng. 2013, 110, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Brynjarsdottir, H.; Wawiernia, B.; Orlygsson, J. Ethanol production from sugars and complex biomass by Thermoanaerobacter AK5: The effect of electron-scavenging systems on end-product formation. Energy Fuels 2012, 26, 4568–4574. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Dashti, M.; Prange, A.; Rainey, F.A.; Rohde, M.; Whitman, W.B.; Wiegel, J. Thermoanerobacter sulfurigignens sp. nov., an anaerobic thermophilic bacerium that reduces 1 M thiosulfate to elemental sulfur and tolerates 90 mM sulfite. Int. J. Syst. Evol. Microbiol. 2007, 57, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, T.I.; Ahring, B.K. Evaluation of continuous ethanol fermentation of dilute-acid corn stover hydrolysate using thermophilic anaerobic bacterium Thermoanaerobacter BG1L1. Appl. Microbiol. Biotechnol. 2007, 77, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, T.I.; Mikkelsen, M.J.; Ahring, B.K. High ethanol tolerance of the thermophilic anaerobic ethanol producer Thermoanaerobacter BG1L1. Cent. Eur. J. Biol. 2007, 2, 364–377. [Google Scholar] [CrossRef]
- Georgieva, T.I.; Skiadas, I.V.; Ahring, B.K. Effect of temperature on ethanol tolerance of a thermophilic anaerobic ethanol producer Thermoanaerobacter A10: Modeling and simulation. Biotechnol. Bioeng. 2007, 98, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Lacis, L.S.; Lawford, H.G. Effect of growth rate on ethanol production by T. ethanolicus in glucose or xylose limited continuous culture. Biotechnol. Lett. 1988, 10, 603–608. [Google Scholar] [CrossRef]
- Lacis, L.S.; Lawford, H.G. Analysis of the variation in ethanol yield from glucose or xylose with continuously grown Thermoanaerobacter ethanolicus. Appl. Biochem. Biotechnol. 1989, 20–21, 479–490. [Google Scholar] [CrossRef]
- Lacis, L.S.; Lawford, H.G. Thermoanaerobacter ethanolicus growth and product yield from elevated levels of xylose or glucose in continuous cultures. Appl. Environ. Microbiol. 1991, 57, 579–585. [Google Scholar] [PubMed]
- Lamed, R.; Zeikus, J.G. Ethanol-production by thermophilic bacteria: Relationship between fermentation product yields of and catabolic enzyme-activities in Clostridium thermocellum and Thermoanaerobium brockii. J. Bacteriol. 1980, 144, 569–578. [Google Scholar] [PubMed]
- Lamed, R.; Zeikus, J.G. Glucose fermentation pathway of Thermoanaerobium brockii. J. Bacteriol. 1980, 141, 1251–1257. [Google Scholar] [PubMed]
- Scully, S.M.; Orlygsson, J. Branched-chain alcohol formation from branched-chain amino acids by Thermoanaerobacter brockii and Thermoanaerobacter yonseiensis. Anaerobe 2014, 30, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Svetlitchnyi, V.A.; Kensch, O.; Falkenhan, D.A.; Korseska, S.G.; Lippert, N.; Prinz, M.; Sassi, J.; Schickor, A.; Curvers, S. Single-step ethanol production from lignocellulose using novel extremely thermophilic bacteria. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Cripps, R.E.; Eley, K.; Leak, D.J.; Rudd, B.; Taylor, M.; Todd, M.; Boakes, S.; Martin, S.; Atkinson, T. Metabolic engineering of Geobacillus thermoglucosidasius for high yield ethanol production. Metab. Eng. 2009, 11, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Zambare, V.; Bhalla, A.; Muthukumarappan, K.; Sani, R.K.; Christopher, L. Bioprocessing of agricultural waste to ethanol utilizing a cellulolytic extremophile. Extremophiles 2011, 15, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J. Use of thermophiles for production of fuels and chemicals. In Thermophiles: General, Molecular and Applied Microbiology; Brock, T.D., Ed.; John Wiley & Sons: New York, NY, USA, 1986; pp. 217–255. [Google Scholar]
- Fardeau, M.L.; Faudon, C.; Cayol, J.L.; Magot, M.; Patel, B.K.C.; Ollivier, B. Effect of thiosulphate as electron acceptor on glucose and xylose oxidation by Thermoanaerobacter finnii and a Thermoanaerobacter sp. isolated from oil field water. Res. Microbiol. 1996, 147, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Jones, P. Improving fermentative biomass-derived H2-production by engineered microbial metabolism. Int. J. Hydrog. Energy 2008, 33, 5122–5130. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Fermentative hydrogen production: Principles, progress and prognosis. Int. J. Hydrog. Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R.; Seitz, H.J.; Conrad, R. The capacity of hydrogenotrophic anaerobic bacteria for compete for traces of hydrogen depends on the redox potential of the terminal electron-acceptor. Arch. Microbiol. 1988, 149, 350–357. [Google Scholar] [CrossRef]
- Fardeau, M.L.; Patel, B.K.C.; Magot, M.; Ollivier, B. Utilization of serine, leucine, isoleucine and valine by Thermoanerobacter brockii in the presence of thiosulfate or Methanobacterium sp. as electron acceptors. Anaerobe 1997, 3, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Tomas, A.F.; Karakashev, D.; Angelidaki, I. Effect of xylose and nutrients concentrations on ethanol production by newly isolated extreme thermophilic bacterium. Water Sci. Technol. 2011, 64, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, R.W.; Longin, R.; Zeikus, J.G. Ethanol production by thermophilic bacteria: Physiological comparison of solvent effects on parent and alcohol-tolerant strains of Clostridium thermohydrosulfuricum. Appl. Environ. Microbiol. 1984, 48, 171–177. [Google Scholar] [PubMed]
- Wang, D.I.C.; Avgerinos, G.C.; Biocic, I.; Wang, S.D.; Fang, H.Y. Ethanol from cellulosic biomass [and discussion]. Philos. Trans. R. Soc. Lond. Ser. B 1983, 300, 323–333. [Google Scholar] [CrossRef]
- Klapatch, T.R.; Hogsett, D.A.L.; Baskaran, S.; Pal, S.; Lynd, L.R. Organism development and characterization for ethanol production using thermophilic bacteria. Appl. Biochem. Biotechnol. 1994, 45–46, 209–223. [Google Scholar] [CrossRef]
- Baskaran, S.; Ahn, H.J.; Lynd, L.R. Investigation of the ethanol tolerance of Clostridium thermosaccharolyticum in continuous culture. Biotechnol. Progress. 1995, 11, 276–281. [Google Scholar] [CrossRef]
- Carreira, L.H.; Wiegel, J.; Ljungdahl, L.G. Production of ethanol from bio-polymers by anaerobic, thermophilic, and extreme thermophilic bacteria. I. Regulation of carbohydrate utilization in mutants of Thermoanaerobacter ethanolicus. In Proceedings of the 5th Symposium on Biotechnology for Fuels and Chemicals, Gatlinburg, TN, USA, 10–13 May 1983; Volume 13, pp. 183–191.
- Herrero, A.A.; Gomez, R.F. Development of ethanol tolerance in Clostridium thermocellum: Effect of growth temperature. Appl. Environ. Microbiol. 1980, 40, 571–577. [Google Scholar] [PubMed]
- Timmons, M.D.; Knutson, B.L.; Nokes, S.E.; Strobel, H.J.; Lynn, B.C. Analysis of composition and structure of Clostridium thermocellum membranes from wild-type and ethanol-adapted strains. Appl. Microbiol. Biotechnol. 2009, 82, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Jensen, K.; Nielsen, P.; Bjerre, A.B.; Schmidt, A.S. Pretreatment of wheat straw and conversion of xylose and xylan to ethanol by thermophilic anaerobic bacteria. Bioresour. Technol. 1996, 58, 107–113. [Google Scholar] [CrossRef]
- Ahring, B.K.; Licht, D.; Schmidt, A.S.; Sommer, P.; Thomsen, A.B. Production of ethanol from wet oxidised wheat straw by Thermoanaerobacter mathranii. Bioresour. Technol. 1999, 68, 3–9. [Google Scholar] [CrossRef]
- Zhao, C.; O-Thong, S.; Karakashev, D.; Angelidaki, I.; Lu, W.; Wang, H. High yield simultaneus hydrogen and ethanol production under extreme-thermophilic (70 °C) mixed culture environment. Int. J. Hydrog. Energy 2009, 34, 5657–5665. [Google Scholar] [CrossRef]
- Wiegel, J.; Carreira, L.H.; Mothershed, C.P.; Puls, J. Production of ethanol from bio-polymers by anaerobic, thermophilic, and extreme thermophilic bacteria. II. Thermoanaerobacter ethanolicus JW200 and its mutants in batch cultures and resting cell experiments. In Proceedings of the 5th Symposium on Biotechnology for Fuels and Chemicals, Gatlinburg, TN, USA, 10–13 May 1983; Volume 13, pp. 193–205.
- Rani, K.S.; Swamy, M.V.; Seenayya, G. Production of ethanol from various pure and natural cellulosic biomass by Clostridium thermocellum strains SS21 and SS22. Process Biochem. 1988, 33, 435–440. [Google Scholar] [CrossRef]
- Lin, C.W.; Wu, C.H.; Tran, D.T.; Shih, M.C.; Li, W.H.; Wu, C.F. Mixed culture fermentation from lignocellulosic materials using thermophilic lignocellulose-degrading anaerobes. Process Biochem. 2010, 46, 489–493. [Google Scholar] [CrossRef]
- Georgieva, T.I.; Mikkelsen, M.J.; Ahring, B.K. Ethanol production from wet-exploded wheat straw hydrolysate by thermophilic anaerobic bacterium Thermoanaerobacter BG1L1 in a continuous immobilized reactor. Appl. Biochem. Biotechnol. 2008, 145, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Lynd, L.R.; Grethlein, H.E.; Wolkin, R.H. Fermentation of cellulosic substrates in batch and continuous culture by Clostridium thermocellum. Appl. Environ. Microbiol. 1989, 55, 3131–3139. [Google Scholar] [PubMed]
- Ahn, H.J.; Lynd, L.R. Cellulose degradation and ethanol production by thermophilic bacteria using mineral growth medium. Appl. Biochem. Biotechnol. 1996, 57, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.S.; Swamy, M.V.; Seenayya, G. Increased ethanol production by metabolic modulation of cellulose fermentation in Clostridium thermocellum. Biotechnol. Lett. 1997, 19, 819–823. [Google Scholar] [CrossRef]
- Xu, L.; Tschirner, U. Immobilized anaerobic fermentation for bio-fuel production by Clostridium co-culture. Bioprocess Biosys. Eng. 2014, 37, 1551–1559. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Potential inhibitors from wet oxidation of wheat straw and their effect on growth and ethanol production by Thermoanaerobacter mathranii. Appl. Microbiol. Biotechnol. 2001, 57, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Avci, A.; Donmez, S. Effect of zinc on ethanol production by two Thermoanaerobacter strains. Process Biochem. 2006, 41, 984–989. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Lokken, P.M.; Chen, S.; Zhou, J. Characterization of the impact of acetate and lactate on ethanolic fermentation by Thermoanaerobacter ethanolicus. Bioresour. Technol. 2009, 100, 5955–5965. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Raman, B.; Zhu, M.; Mielenz, J.R.; Brown, S.D.; Guss, A.M.; Lynd, L.R. Mutant selection and phenotypic and genetic characterization of ethanol-tolerant strains of Clostridium thermocellum. Appl. Microbiol. Biotechnol. 2011, 92, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Sittijunda, S.; Tomas, A.F.; Reungsang, A.; O-Thong, S.; Angelidaki, I. Ethanol production from glucose and xylose by immobiliezed Thermoanaerobacter pentosaceus at 70 °C in an up-flow anaerobic sludge blanket (UASB) reactor. Bioresour. Technol. 2013, 143, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.J.; Hogsett, D.A.; Lynd, L.R. Natural competence in Thermoanaerobacter and Thermoanaerobacterium species. Appl. Environ. Microbiol. 2010, 76, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.J.; Covalla, S.F.; Miller, B.B.; Firliet, B.T.; Hogsett, D.; Herring, C.D. Urease expression in a Thermoanaerobacterium saccharolyticum ethanologen allows high titer ethanol production. Metab. Eng. 2012, 14, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.G.; Guerinot, M.L.; Lynd, L.R. Cloning of l-lactate dehydrogenase and elimination of lactic acid production via gene knockout in Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl. Microbiol. Biotechnol. 2004, 65, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Argyros, D.A.; Tripathi, S.A.; Barrett, T.F.; Rogers, S.R.; Feinberg, L.F.; Olson, D.G.; Foden, J.M.; Miller, B.B.; Lynd, L.R.; Hogsett, D.A.; et al. High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes. Appl. Environ. Microbiol. 2011, 77, 8288–8294. [Google Scholar] [CrossRef] [PubMed]
- Tyurin, M.V.; Lynd, L.R.; Wiegel, J. 13 Gene transfer systems for obligately anaerobic thermophilic bacteria. Methods Microbiol. 2006, 35, 309–330. [Google Scholar]
- Mai, V.; Lorenz, W.W.; Wiegel, J. Transformation of Thermoanaerobacterium sp. strain JW/SL-YS485 with plasmid pIKM1 conferring kanamycin resistance. FEMS Microbiol. Lett. 1997, 148, 163–167. [Google Scholar] [CrossRef]
- Mai, V.; Wiegel, J. Advances in development of genetic system for Thermoanaerobacterium spp.: Expression of genes encoding hydrolytic enzymes, development of second shuttle vector, and integration of genes into the chromosome. Appl. Environ. Microbiol. 2000, 66, 4817–4821. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.J.; Hogsett, D.A.; Lynd, L.R. Identification of the [FeFe]-hydrogenase responsible for hydrogen generation in Thermoanaerobacterium saccharolyticum and demonstration of increased ethanol yield via hydrogenase knockout. J. Bacteriol. 2009, 191, 6457–6464. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.A.; Olson, D.G.; Argyros, D.A.; Miller, B.B.; Barrett, T.F.; Murphy, D.M.; Mccool, J.D.; Warner, A.K.; Rajgarhia, V.B.; Lynd, L.R.; et al. Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant. Appl. Environ. Microbiol. 2010, 76, 6591–6599. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Prabhu, S.; Lynd, L.R.; Guss, A.M. Increase in ethanol yield via elimination of lactate production in an ethanol-tolerant mutant of Clostridium thermocellum. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Sommer, P.; Georgieva, T.; Ahring, B.K. Potential for using thermophilic anaerobic bacteria for bioethanol production from hemicellulose. Biochem. Soc. Trans. 2004, 32, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Mikkelsen, M.J. Identification and overexpression of a bifunctional aldehyde/alcohol dehydrogenase responsible for ethanol production in Thermoanaerobacter mathranii. J. Mol. Microbiol. Biotechnol. 2010, 19, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, L.J.; Taylor, M.P.; Eley, K.; Tuffin, M.; Cowan, D.A. Engineering pyruvate decarboxylase-mediated ethanol production in the thermophilic host Geobacillus thermoglucosidasius. Appl. Microbiol. Biotechnol. 2014, 98, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Landisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Pan, X.; Zalesny, R.S. Pretreatment of woody biomass for biofuel production: Energy efficiency, technologies and recalcitrance. Appl. Microbiol. Biotechnol. 2010, 87, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonti, D.; Prussi, M.; Ferrero, S.; Oriani, L.; Ottonello, P.; Torre, P.; Cherchi, F. Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 2012, 46, 25–35. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Elleuche, S.; Schröder, C.; Sahm, K.; Antranikian, G. Extremozymes—Biocatalysts with unique properties from extremophilic microorganisms. Curr. Opin. Biotechnol. 2014, 29, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.; Bansal, N.; Kumar, S.; Bischoff, K.M.; Sani, R.K. Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour. Technol. 2013, 128, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Podkaminer, K.K.; Kenealy, W.R.; Herring, C.D.; Hogsett, D.A.; Lynd, L.R. Ethanol and anaerobic conditions reversibly inhibit commercial cellulase activity in thermophilic simultaneous saccharification and fermentation (tSSF). Biotechnol. Biofuels 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Babiker, M.A.A.; Hosida, H.; Ano, A.; Nonklang, S.; Akada, R. High-temperature fermentation: How can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl. Microbiol. Biotechnol. 2010, 85, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol production: Feedstock and current technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Lynd, L.R. Overview and evaluation of fuel ethanol from cellulosic biomass: Technology, economics, the environment, and policy. Annu. Rev. Energy Environ. 1996, 21, 403–465. [Google Scholar] [CrossRef]
- Lynd, L.R.; van Zyl, W.H.; McBride, J.E.; Laser, M. Consolidated bioprocessing of cellulosic biomass: An update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Singh, A.; Himmel, M.E. Perspectives and new directions for the production of bioethanol using consolidated bioprocessing of lignocellulose. Curr. Opin. Biotechnol. 2009, 20, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Amore, A.; Faraco, V. Potential of fungi as category I Consolidated BioProcessing organisms for cellulosic ethanol production. Renew. Sustain. Energy Rev. 2012, 16, 3286–3301. [Google Scholar] [CrossRef]
- Olson, D.G.; McBride, J.E.; Shaw, A.J.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, S.; Singh, O.V. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 2001, 56, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.R.; Fidantsef, A.L. Directed evolution of industrial enzymes: An update. Curr. Opin. Biotechnol. 2003, 14, 438–443. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scully, S.M.; Orlygsson, J. Recent Advances in Second Generation Ethanol Production by Thermophilic Bacteria. Energies 2015, 8, 1-30. https://doi.org/10.3390/en8010001
Scully SM, Orlygsson J. Recent Advances in Second Generation Ethanol Production by Thermophilic Bacteria. Energies. 2015; 8(1):1-30. https://doi.org/10.3390/en8010001
Chicago/Turabian StyleScully, Sean Michael, and Johann Orlygsson. 2015. "Recent Advances in Second Generation Ethanol Production by Thermophilic Bacteria" Energies 8, no. 1: 1-30. https://doi.org/10.3390/en8010001
APA StyleScully, S. M., & Orlygsson, J. (2015). Recent Advances in Second Generation Ethanol Production by Thermophilic Bacteria. Energies, 8(1), 1-30. https://doi.org/10.3390/en8010001






