Microwave-Assisted Transesterification of Macroalgae
Abstract
:1. Introduction
2. Theory of Transesterification
2.1. Basic Transesterification
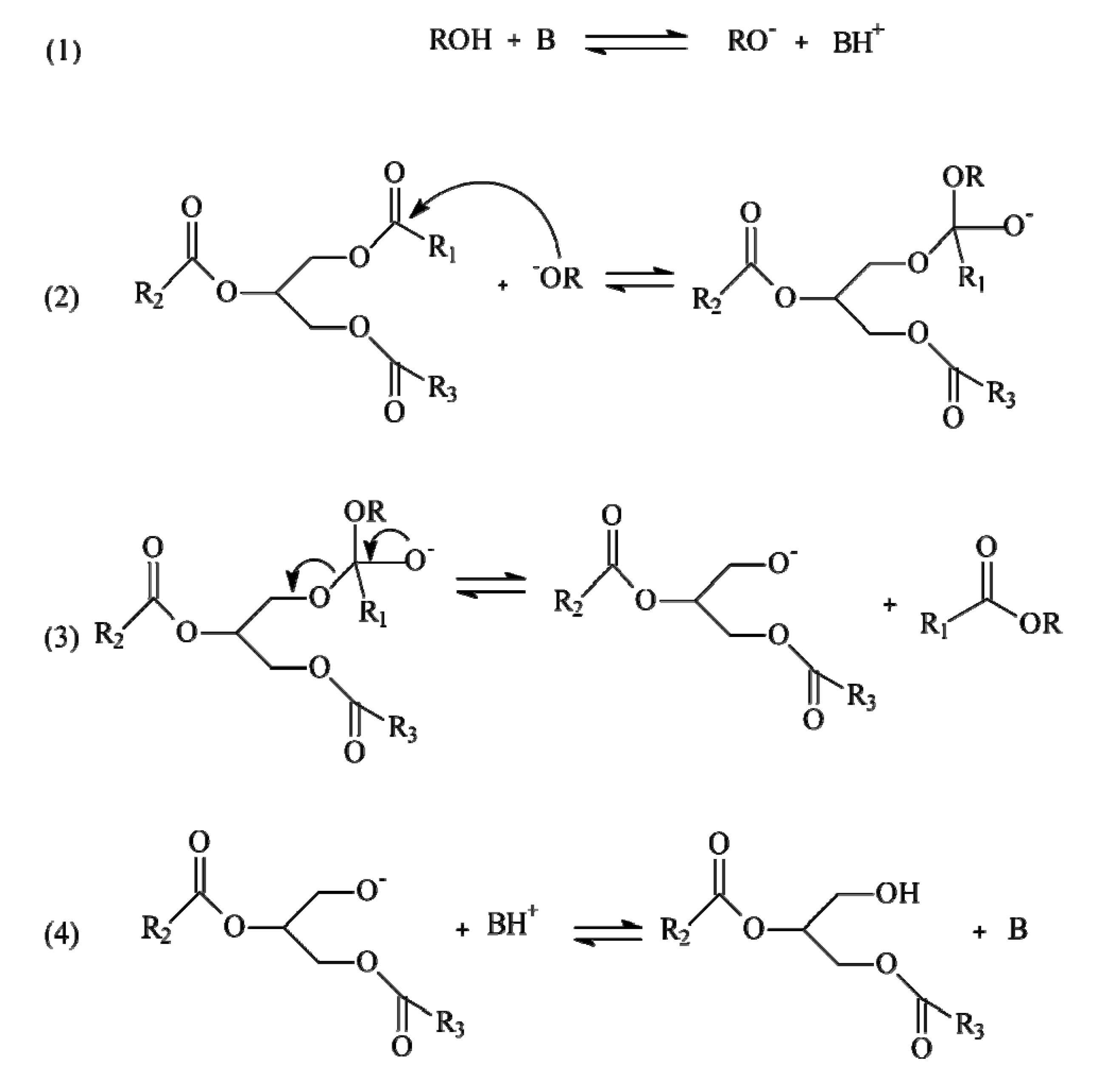
2.2. Microwave Transesterification
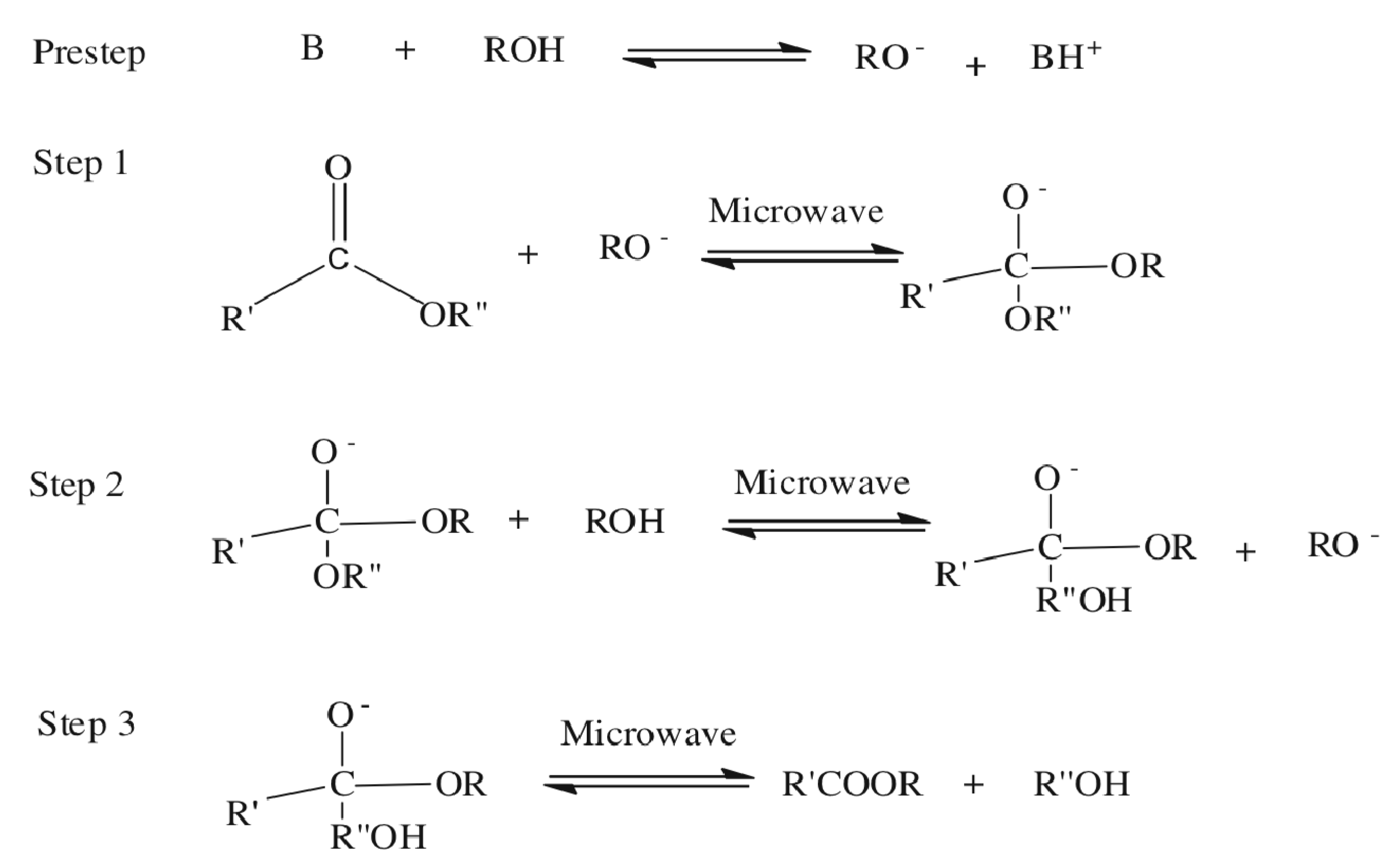
3. Experimental Section
3.1. Characteristics of Algal Species
| Undaria pinnatifida | Himanthalia elongata | Chondrus crispus | |
|---|---|---|---|
| Fatty acids | 2.7 | 2.3 | 1.9 |
| Saturated fatty acids | 1.2 | 0.6 | 1.07 |
| Fiber | 31 | 36 | 43 |
| Na | 3.5 | 3.37 | 2.35 |
| Ca | 0.6932 | 0.6558 | 1.484 |
| Mg | 0.6302 | 0.7323 | 0.5722 |
| P | 0.470 | 0.230 | 0.200 |
| Fe | 0.00785 | 0.00407 | 0.0107 |
| Zn | 0.00386 | - | 0.004 |
| I | 0.009610 | 0.009240 | 0.000650 |
3.2. Experimental Procedure
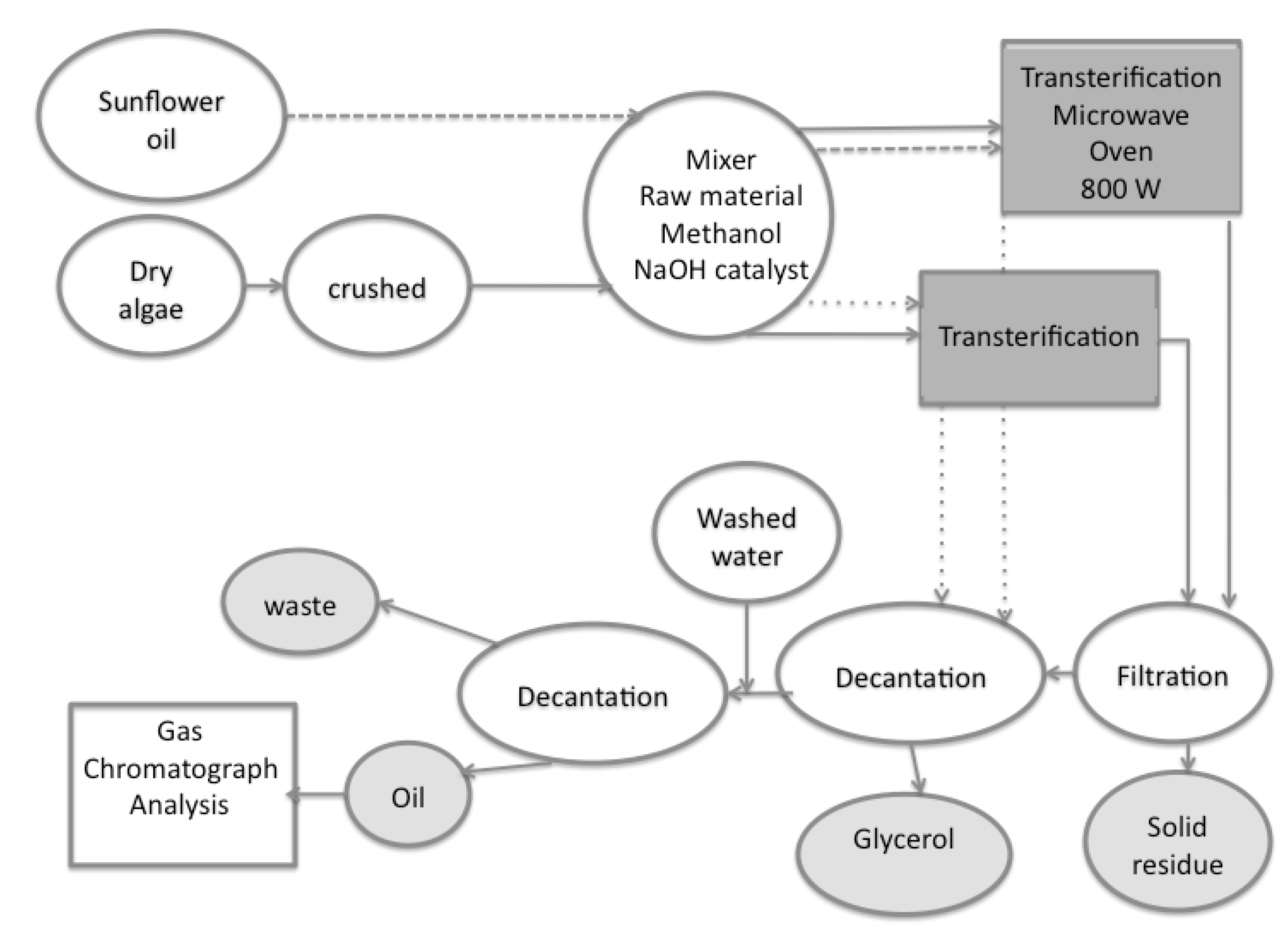
4. Results and Discussion
4.1. Comparison of Transesterification Methods
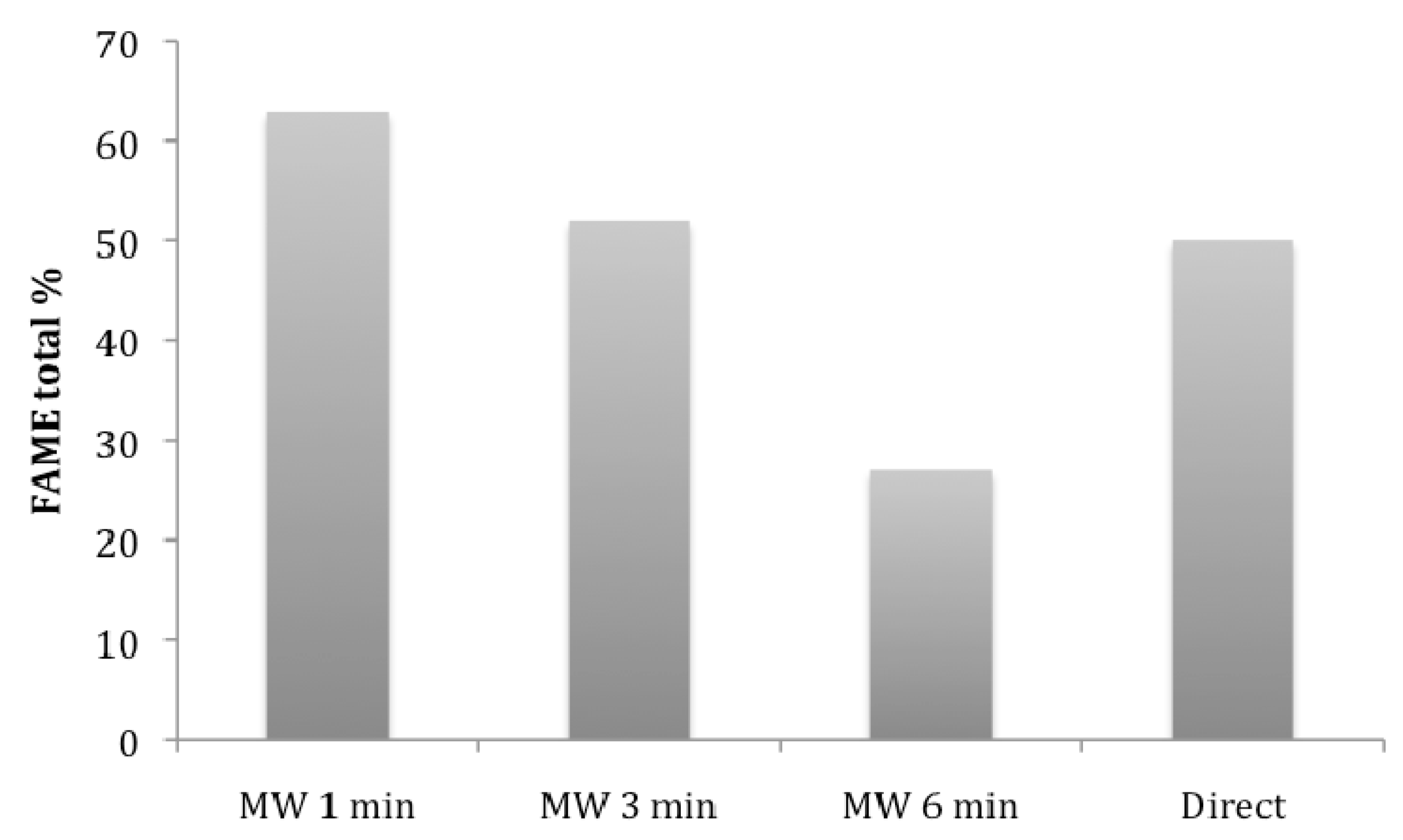
4.2. Transesterification of Macroalgae
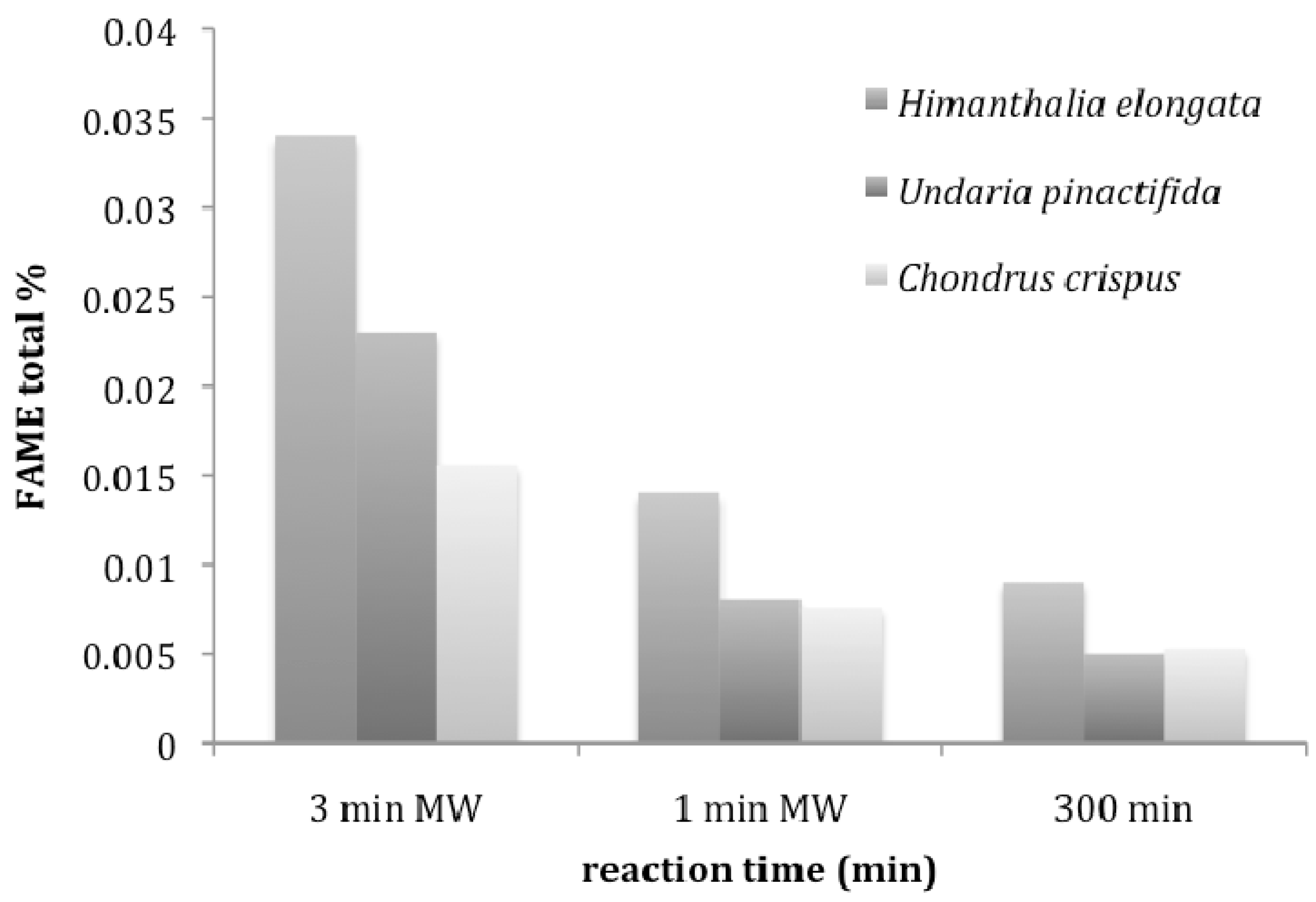
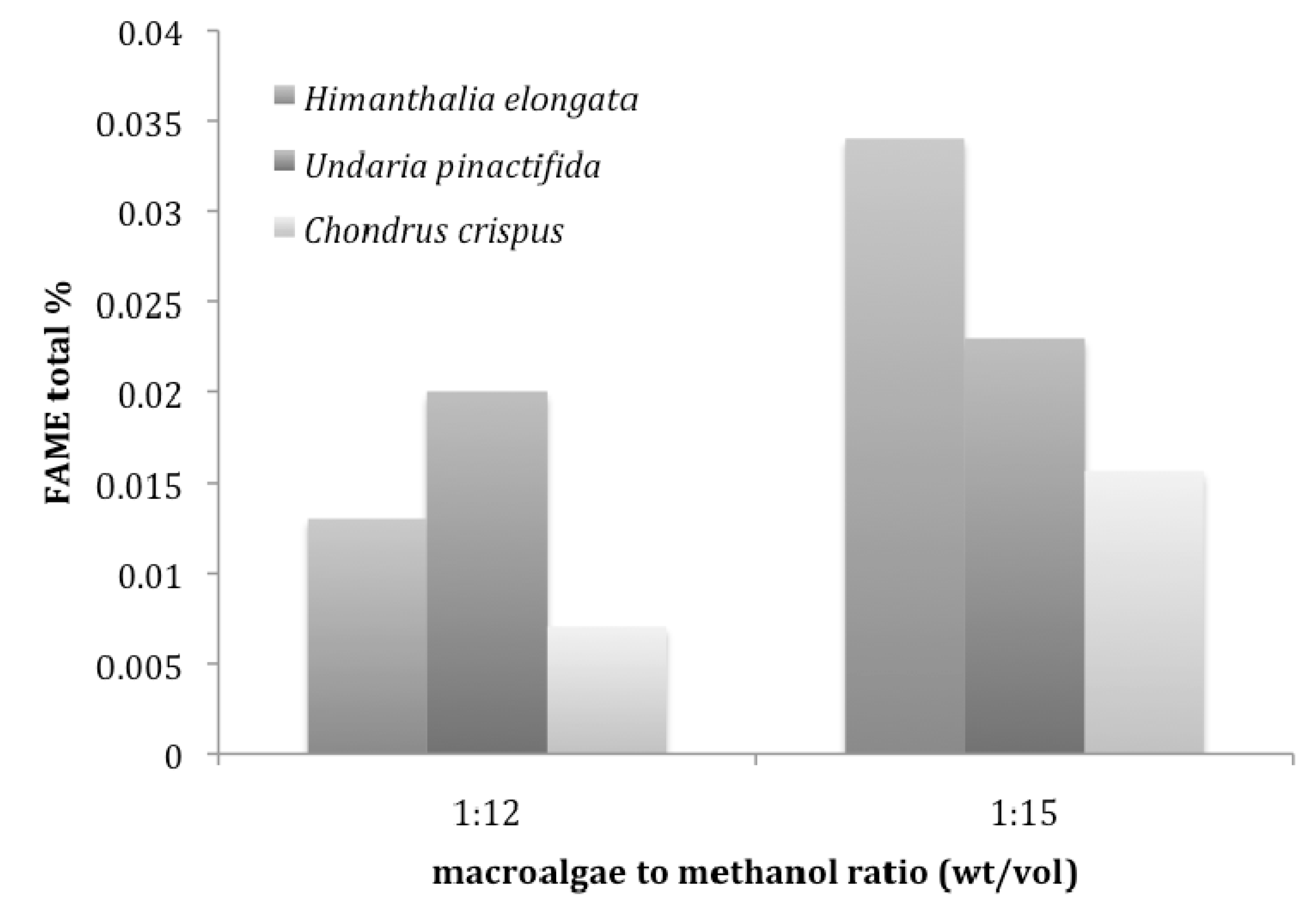
5. Conclusions
References
- Mustafa, B. Potential alternatives to edible oils for biodiesel production—A review of current work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Siddiquee, M.N.; Rohani, S. Lipid extraction and biodiesel production from municipal sewage suldges: A review. Renew. Sustain. Energy Rev. 2011, 5, 1067–1072. [Google Scholar] [CrossRef]
- Balat, M. Production of biodiesel from vegetable oils: A survey. Energy Source Part A 2007, 29, 895–913. [Google Scholar] [CrossRef]
- Giridhar, M.; Chandana, K.; Rajnish, K. Synthesis of biodiesel in supercritical fluids. Fuel 2004, 83, 2029–2033. [Google Scholar] [CrossRef]
- Maceiras, R.; Rodríguez, M.; Cancela, M.A.; Urréjola, S.; Sánchez, A. Macroalgae: Raw material for biodiesel production. Appl. Energy 2011, 88, 3318–3323. [Google Scholar] [CrossRef]
- Johnson, M.B.; Wen, Z. Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuels 2009, 23, 5179–5183. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Z. Biodiesel production by direct methanolysis of oleaginous microbial biomass. J. Chem. Technol. Biotechnol. 2007, 82, 775–780. [Google Scholar] [CrossRef]
- Maceiras, R.; Cancela, A.; Urréjola, S.; Sánchez, A.; Pérez, L. Development of renewable fuels from algae. In Proceedings of European Meeting on Chemical Industry and Environment, Mechelen, Belgium, 17–19 May 2010; pp. 501–508.
- Ross, A.B.; Biler, P.; Kubacki, M.L.; Li, H.; Lea-Langton, A.; Jones, J.M. Hydrothermal processing of microalgae using alkali and organic acids. Fuel 2010, 89, 2234–2243. [Google Scholar] [CrossRef]
- Nezihe, A.; Aysegul, D. Alkali catalyzed transesterification of cottonseed oil by microwave irradiation. Fuel 2007, 86, 2639–2644. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ong, B.H.; Kumar, M.N.S.; Kamarudin, S.K. Microwave-assisted transesterification of jatropha and waste frying palm oil. Int. J. Sustain. Energy 2009, 28, 195–201. [Google Scholar] [CrossRef]
- Yu, D.; Tian, L.; Ma, D.; Wu, H.; Wang, Z.; Wang, L.; Fang, X. Microwave-assisted fatty acid methyl ester production from soybean oil by Novozym 435. Green Chem. 2010, 12, 844–850. [Google Scholar] [CrossRef]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on FAME production processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; López, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Patil, P.D.; Gnaneswar, V.; Mannarswamy, A.; Cooke, P.; Munson-McGee, S.; Nirmalakhandan, N.; Lammers, P.; Deng, S. Optimization of microwave-assisted transesterification of dry algal biomass using response surface methodology. Bioresource Technol. 2011, 102, 1399–1405. [Google Scholar] [CrossRef]
- Refaat, A.A.; El Sheltawy, S.T.; Sadek, K.U. Optimum reaction time, performance and exhaust emissions of biodiesel produced by microwave irradiation. Int. J. Environ. Sci. Technol. 2008, 5, 315–322. [Google Scholar] [CrossRef]
- Sánchez, A.; Maceiras, R.; Cancela, A.; Rodríguez, M. Influence of n-hexane on in situ transesterification of marine macroalgae. Energies 2012, 5, 243–257. [Google Scholar] [CrossRef]
- Mulbry, W.; Kondrad, S.; Buyer, J.; Luthria, D. Optimization of an oil extraction process for algae from the treatment of manure effluent. J. Am. Oil Chem. Soc. 2009, 86, 909–915. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, B.L.; Zhu, G.L. Synthesis of biodiesel using microwave absorption catalysts. Energy Fuels 2009, 23, 548–552. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cancela, A.; Maceiras, R.; Urrejola, S.; Sanchez, A. Microwave-Assisted Transesterification of Macroalgae. Energies 2012, 5, 862-871. https://doi.org/10.3390/en5040862
Cancela A, Maceiras R, Urrejola S, Sanchez A. Microwave-Assisted Transesterification of Macroalgae. Energies. 2012; 5(4):862-871. https://doi.org/10.3390/en5040862
Chicago/Turabian StyleCancela, Angeles, Rocio Maceiras, Santiago Urrejola, and Angel Sanchez. 2012. "Microwave-Assisted Transesterification of Macroalgae" Energies 5, no. 4: 862-871. https://doi.org/10.3390/en5040862
APA StyleCancela, A., Maceiras, R., Urrejola, S., & Sanchez, A. (2012). Microwave-Assisted Transesterification of Macroalgae. Energies, 5(4), 862-871. https://doi.org/10.3390/en5040862





