Available Resources for Algal Biofuel Development in China
Abstract
:1. Introduction
2. Algal Biodiversity in China
| Category | In the World | In China | Freshwater (%) | Seawater (%) | ||
|---|---|---|---|---|---|---|
| Freshwater | Seawater | Freshwater | Seawater | |||
| Cyanobateria | 1600 | 2000 | 1000 | 99 | 63 | 5 |
| Euglenophyta | 800 | 0 | 600 | 0 | 75 | 0 |
| Diatomeae | 8000 | 8336 | 4000 | 1485 | 50 | 18 |
| Chrysophyta | 701 | 49 | 100 | 17 | 14 | 35 |
| Xanthophyta | 381 | 20 | 25 | 5 | 7 | 25 |
| Cryptophyta | 60 | 19 | 15 | 6 | 25 | 32 |
| Prymnesiophyta | 0 | 500 | 0 | 34 | 0 | 7 |
| Phaeophyta | 8 | 1500 | 3 | 260 | 38 | 17 |
| Dinozoa | 50 | 4000 | 30 | 302 | 60 | 8 |
| Rhodophyta | 0 | 4100 | 0 | 569 | 0 | 14 |
| Chlorophyta | 8000 | 600 | 3000 | 163 | 38 | 27 |
| Total | 19,600 | 21,124 | 8773 | 2940 | 45 | 14 |
2.1. Algal Biodiversity in China Seas
2.2. Algal Biodiversity in China Freshwater Bodies
2.3. Algal Production in Open Ponds
| Species | Yield g/(L·d) | Yield g/(m2·d) | References |
|---|---|---|---|
| Spirulina maxima | 0.21 | 25 | [10,11] |
| Chlorella vulgaris | 0.18 | [10] | |
| Scenedesmus obliquus | 0.09 | [10] | |
| Dunaliella tertiolecta | 0.12 | [10] | |
| Nannochloropsis sp. | 0.09–0.31 | [10] | |
| Neochloris oleabundans | 0.09 | [10] | |
| Haematococcus pluvialis | 15.1–70.4 | [12] | |
| Spirulina platensis | 0.06–0.18 | 15–51 | [11,13] |
| Scenedesmus sp. | 0.03–0.13 | 2.43–13.5 | [14] |
| Chlorella sp. | 0.02–2.9 | 1.61–25.0 | [14,15] |
| Pleurochrysis carterae | 0.02–0.22 | 3.20–35.2 | [16] |
| Dunaliella salina | 0.22–0.34 | 1.6–37.7 | [17] |
| Spirulina sp. | 0.006–0.07 | 2–17 | [18,19,20] |
| Anabaena sp. | 0.031–0.078 | 4.9–23.5 | [14,21] |
| Phaeodactylum tricornutum | 0.0028–0.16 | 2.4–11.3 | [22,23] |
| Nannochloris sp. | 0.29–0.32 | [23] |
2.4. Algal Culture Collections in China
3. Resource Availability in China
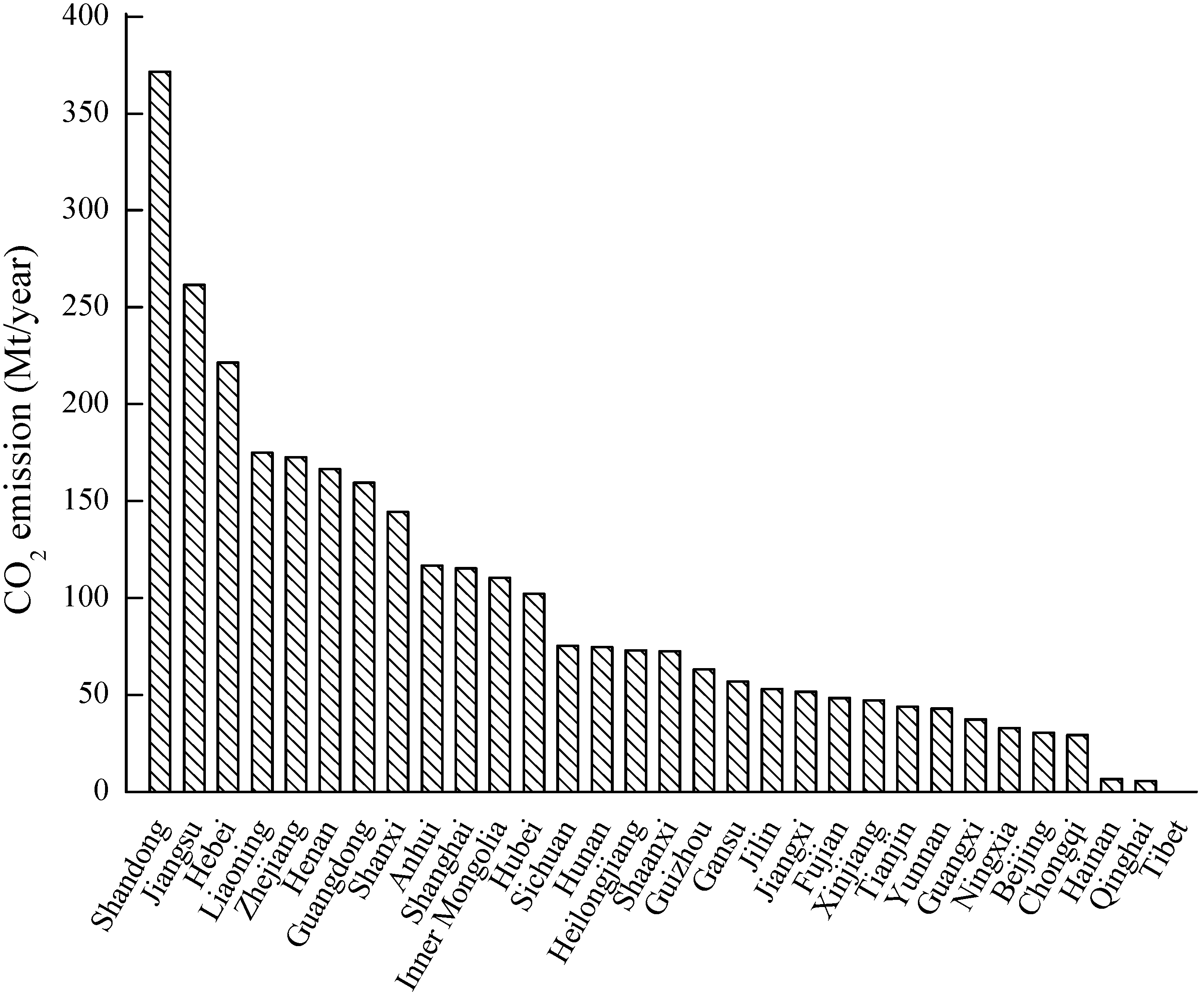
3.1. CO2 Sources
| CO2 Sources | CO2 Relative Emissions (%) | Concentration (%) |
|---|---|---|
| Power plants | 63 | 15 |
| Cement production | 19 | 20 |
| Ethylene | 1 | 12 |
| Steel works | 10 | 15 |
| Refineries | 3 | 8 |
| Ammonia | 4 | 100 |
| Species | CO2 (%) | Biomass Productivity (mg L−1 d−1) | CO2 Fixation Rates (mg L−1 d−1) | μ (d−1) | References |
|---|---|---|---|---|---|
| Botryococcus braunii | 5–10 or flue gas | 26.55 | 496.98 | 0.24 | [26,27,28] |
| Scenedesmus sp. | 10 or flue gas | 217.50 | [26] | ||
| Chlorella vulgaris | 5–10 | 104.76–310 | 251.64 | 0.29 | [26,28] |
| Spirulina platensis | 5 | 730 | 318.61 | 0.22 | [28] |
| Dunaliella tertiolecta | 5 | 420 | 272.4 | 0.21 | [28] |
| Chlorella sp. | 0.03–15 | 191–1484 | 350–13,700 | 0.58–0.66 | [27,29,30] |
| Chlorococcum littorale | 10–20 or flue gas | 400–2500 | 650–17,300 | 1.8–1.9 | [27,31,32,33] |
| Scenedesmus obliquus | 6–12 | 40–140 | 0.19–0.261 | [34,35] | |
| Chlorella kessleri | 6 | 61–90 | [34] | ||
| Spirulina sp. | 6–12 | 40–220 | 0.27–0.44 | [35] | |
| Aphanothece microscopica | 15 | 5612–14,495 | [36] | ||
| Euglena gracilis | 5–10 | 1.44 | [37] |
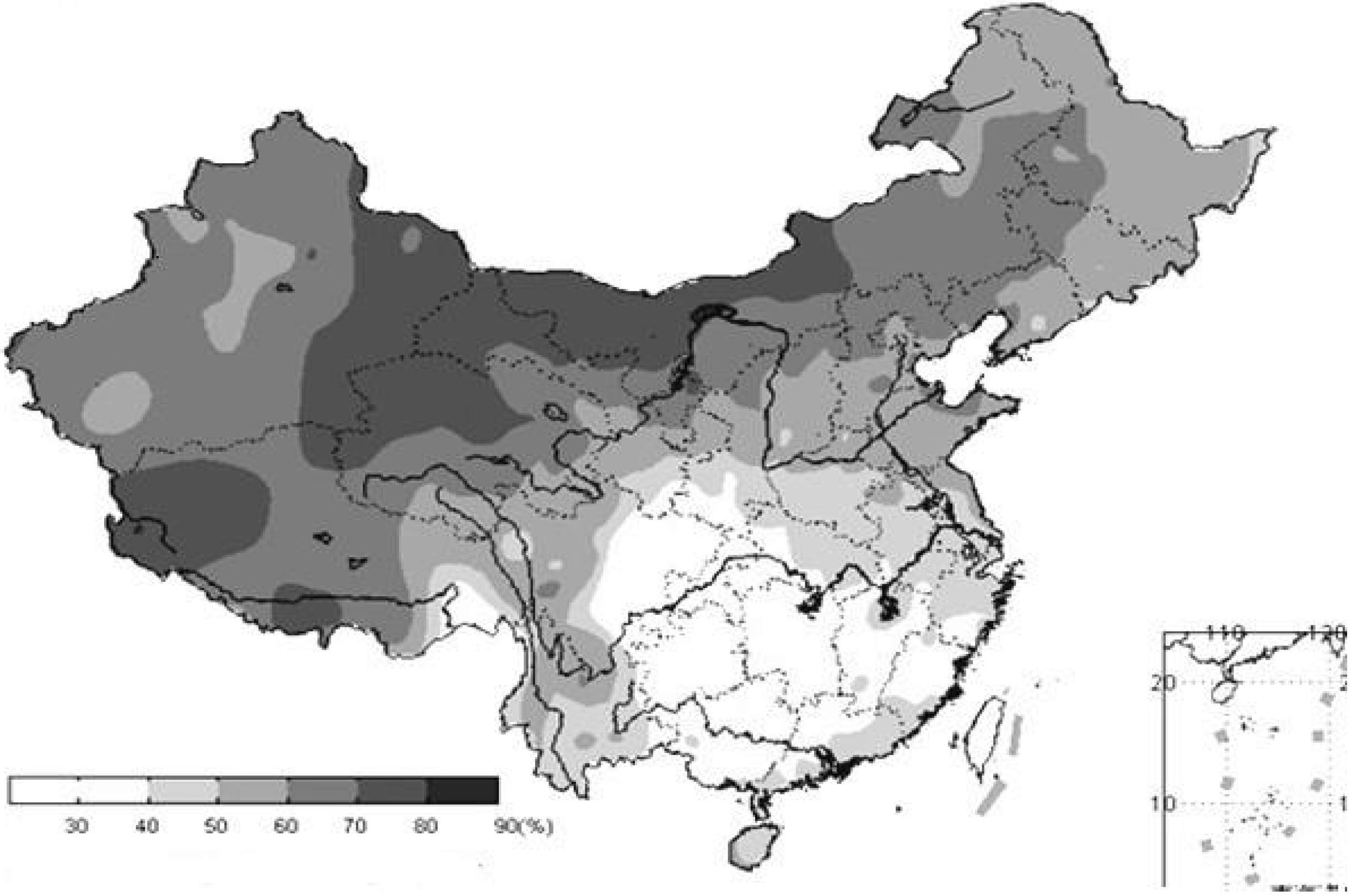
3.2. Sunlight
| Species | Suitable Light Intensities (μmol m−2 s−1) | Saturation Light Intensities (μmol m−2 s−1) | L/D (h) | References |
|---|---|---|---|---|
| Spirulina platensis | 10~630 | ~270 | 6:5~8:3, 24:0 | [40] |
| Botryococcus braunii | 400~1600 | ~800 | ~14:10 | [41] |
| Nannochloropsis oculata | 90~126 | – | 12:12 | [42] |
| Haematococcus pluvialis | 70~90 | ~200 | ~12:12 | [43] |
| Dunaliella salina | 50~90 | ~220 | ~12:12 | [44] |
| Chlorella pyrenoidosa | 120~160 | ~150 | ~10:14 | [45] |
| Nitzschia closterium | 70~160 | – | ~24:0, ~12:12 | [46] |
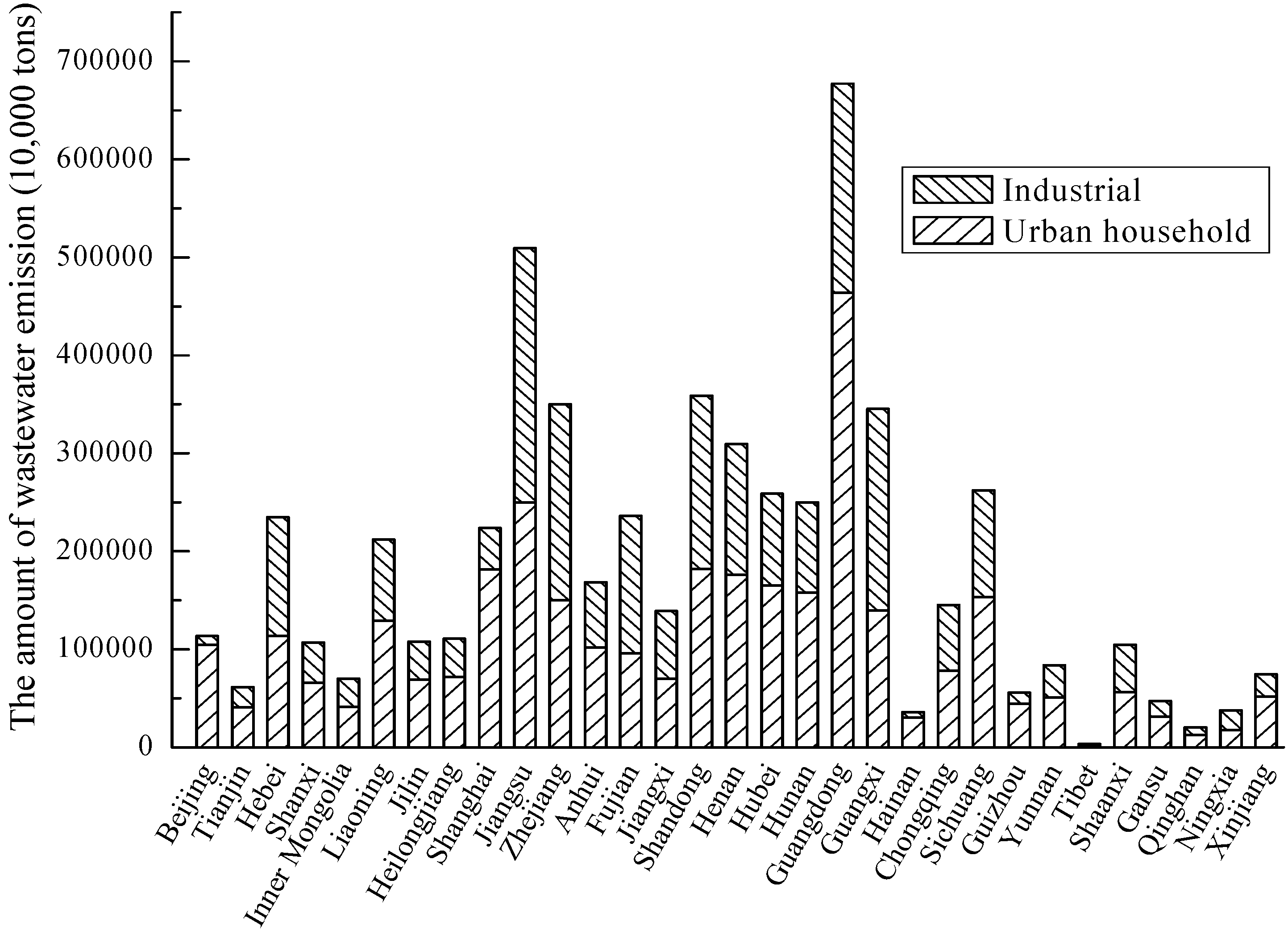
3.3. Water
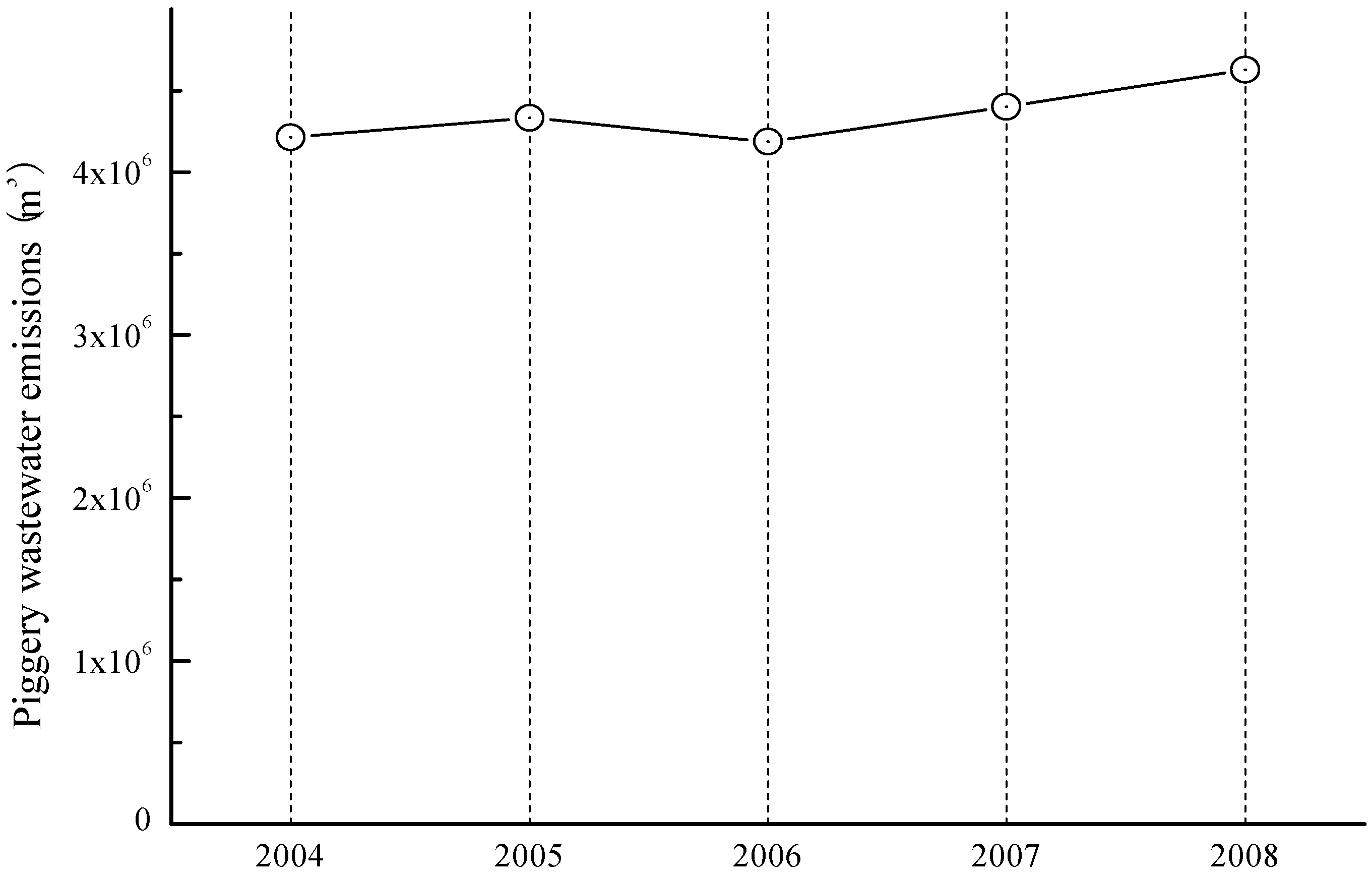
| Species | Type of Sewage | Process | References |
|---|---|---|---|
| Scenedesmus sp. | Urban wastewater from secondary treatment or Synthetic | Batch; Semicontinuous | [49,50] |
| Spirulina (Arthrospira) sp. | Pig wastewater anaerobic effluents | Outdoor raceways | [51] |
| Chlamydomonas reinhardtii | Different stages from Metropolitan Wastewater Treatment Plant | Batch; Biocoil photobioreactor | [52] |
| Chlorella sp. | Dairy manure | Attached | [53] |
| Scenedesmus obliquus | Olive-oil mill;urban wastewater | Photobioreactor; Semicontinuous; Batch | [54,55,56] |
| Chlamydomonas globosa; Chlorella minutissima and Scenedesmus bijuga | Untreated carpet industry | Raceway ponds; Vertical tank reactors and polybags | [57] |
| Chlorella vulgaris | Municipal; Synthetic; Steel-making facility; Distillery | Immobilized; Photobioreactor; Batch; Microalgae pond | [56,58,59,60,61,62] |
| Chlorella sorokinia | Municipal | Immobilized | [63] |
| Phaeodactylum tricornutum, Oscillatoria sp. | Primary sewage and seawater | Corrugated raceways | [64] |
| Scenedesmus rubescens | Synthetic; municipal | Immobilization | [59] |
| Senedesmus dimorphu | Analogue wastewater | Bio-coil reactor conical flask | [65] |
| Monoraphidium | Tertiary treatment of urban wastewater | [66] | |
| Aphanothece microscopica | Refinery industry | Photobioreactor | [67] |
| Botryococcus braunii | Livestock wastewater | Batch | [68] |
3.4. Land
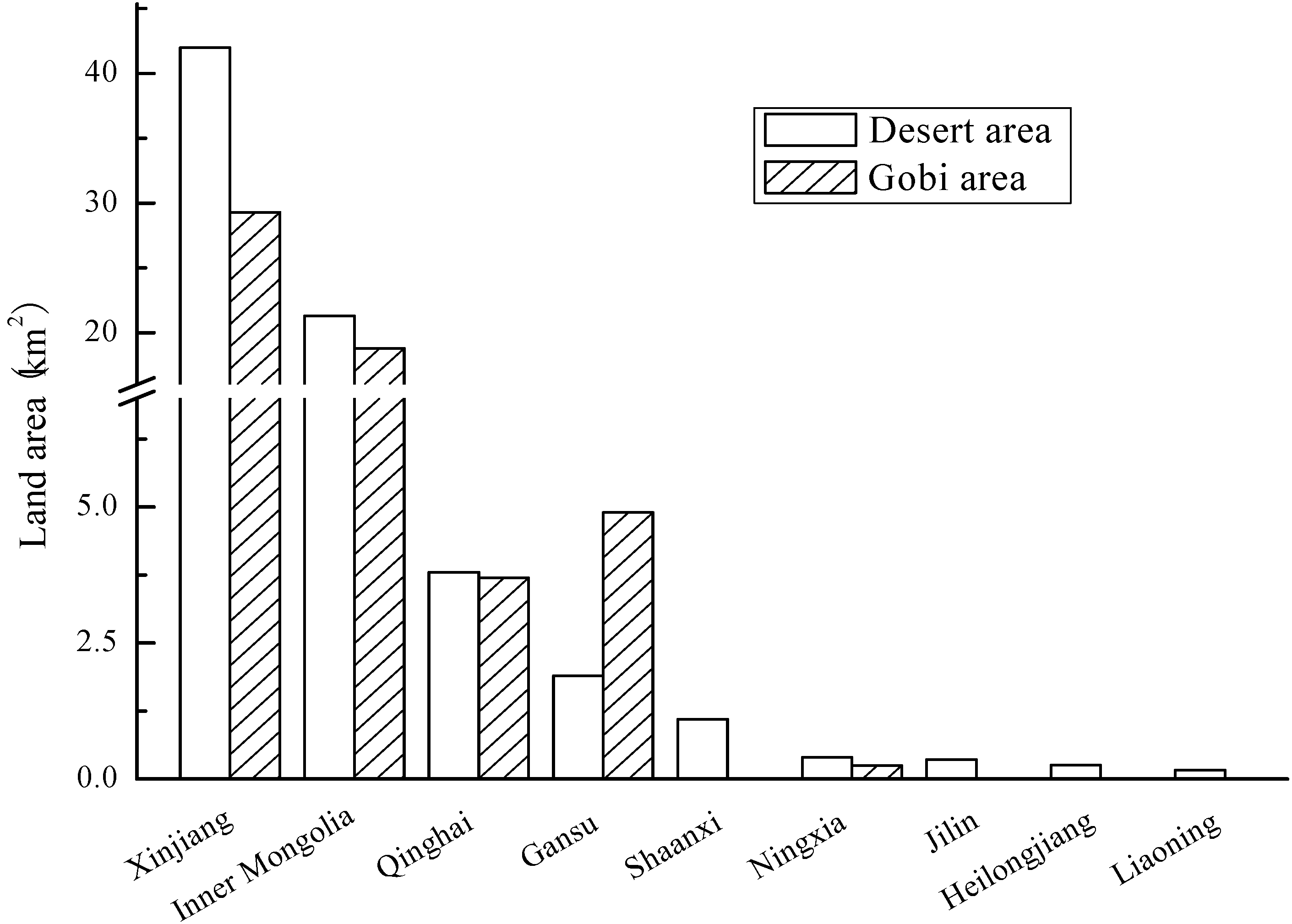
4. Microalgae R&D Efforts in China
5. Conclusions
Acknowledgments
References
- The National Renewable Energy Laboratory (NREL). Jet Fuel from Microalgal Lipids; NREL: Golden, CO, USA, July 2006. Available online: http://www.nrel.gov/docs/fy06osti/40352.pdf (accessed on 16 August 2011).
- Demirbas, A.; Fatih Demirbas, M. Algae Energy: Algae as a New Source of Biodiesel; Springer-Verlag: London, UK, 2010. [Google Scholar]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae; NREL Report No. TP-580-24190; NREL: Golden, CO, USA, July 1998. [Google Scholar]
- Tian, Y. Leaving the Sunlight-Thought and Practice on the Microalgae Photoreactor; Popular Science Press: Beijing, China, 2008. [Google Scholar]
- Zhang, W. China’s Biodiversity: A Country Study; China Environmental Science Press: Beijing, China, 1998. [Google Scholar]
- Liu, R. Checklist of Marine Biota of China Seas; Science Press: Beijing, China, 2008. [Google Scholar]
- Nelson, D.M.; Treguer, P. Production and dissolution of biogenic silica in the ocean: Revisedglobal estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar]
- Liu, X. The Report of Conservation of Chinese Bio-diversity Resources; Science Press: Beijing, China, 2004. [Google Scholar]
- Kumar, A.; Ergas, S.; Yuan, X. Enhanced CO2 fixation and biofuel production via microalgae: Recent developments and future directions. Trends Biotechnol. 2010, 28, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 69–274. [Google Scholar]
- Lee, Y. Microalgal mass culture systems and methods: Their limitation and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Huntley, M.E.; Redalje, D.G. CO2 mitigation and renewable oil from photosynthetic microbes: A new appraisal. Mitig. Adapt. Strateg. Global Change 2007, 12, 573–608. [Google Scholar] [CrossRef]
- Pushparaj, B.; Pelosi, M.R.; Tredici, E. An integrated culture system for outdoor production of microalgae and cyanobacteria. J. Appl. Phycol. 1997, 9, 113–119. [Google Scholar] [CrossRef]
- Kanazawa, Z.; Fujita, C.; Yuhara, T.; Sasa, T. Mass culture of unicellular algae using the open pond circulation method. J. Gen. Appl. Microbiol. 1958, 4, 135–139. [Google Scholar] [CrossRef]
- Doucha, J.; Straka, F.; Karel, L. Utilization of flue gas for cultivation of microalgae (Chlorella sp.) in an outdoor open thin layer photobioreactor. J. Appl. Phycol. 2005, 17, 403–412. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A. The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J. Appl. Phycol. 2006, 18, 703–712. [Google Scholar] [CrossRef]
- Garcia, G.M.; Moreno, J.; Canavate, J.P.; Anguis, V.; Prieto, A. Conditions for open-air outdoor culture of Dunaliella salina in southern Spain. J. Appl. Phycol. 2003, 15, 177–184. [Google Scholar] [CrossRef]
- Olguin, E.J.; Galicia, S.; do Merca, G.; Perez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical condition. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Jimenez, C.; Cossio, B.R.; Labella, D.; Niell, F.X. The feasibility of industrial production of Spirulina (Arthrospira) in southern Spain. Aquaculture 2003, 217, 179–190. [Google Scholar] [CrossRef]
- Richmond, A. Spirulina, Micro-Algal Biotechnology; Cambridge University Press: Cambridge, UK, 1988; pp. 85–121. [Google Scholar]
- Moreno, J.; Vargas, A.; Rodriguez, H.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of a nitrogen-fixing marine cyanobacterium, Anabaena sp. ATCC 33047. Biomol. Eng. 2003, 20, 191–197. [Google Scholar]
- Laws, E.A.; Terry, K.L.; Wickman, J.; Chalup, M. A simple algal production system designed to utilise the flashing light effect. Biotechnol. Bioeng. 1983, 25, 2319–2335. [Google Scholar] [CrossRef] [PubMed]
- Negoro, M.; Shioji, N.; Miyamoto, K.; Miura, Y. Growth of microalgae in high CO2 gas and effects of SOx and NOx. Appl. Biochem. Biotechnol. 1991, 28/29, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Li, X.; Liu, Y.; Zhang, Y. Preliminary study on CO2 industrial point sources and their distribution in China. Chin. J. Rock Mech. Eng. 2006, 25, 2918–2923. [Google Scholar]
- Bilanovic, D.; Andargatchew, A.; Kroeger, T.; Shelef, G. Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations–Response surface methodology analysis. Energy Convers. Manag. 2009, 50, 262–267. [Google Scholar] [CrossRef]
- Yoo, C.; Jun, S.; Lee, J.; Ahn, C.; Oh, H. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ikenouchi, M. The biological CO2 fixation and utilization project by rite 2. Energy Convers. Manag. 1997, 38, S493–S497. [Google Scholar] [CrossRef]
- Sydney, E.B.; Sturm, W.; de Carvalho, J.C. Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol. 2010, 101, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Fulke, A.B.; Mudliar, S.N.; Yadav, R.; Shekh, A.; Srinivasan, N.; Rishiram, R.; Krishnamurthi, K.; Devi, S.S.; Chakrabarti, T. Bio-mitigation of CO2, calcite formation and simultaneous biodiesel precursors production using Chlorella sp. Bioresour. Technol. 2010, 101, 8473–8476. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Oh Kyeong, K.; Kim, Y.S. Optimization of the influential factors for the improvement of CO2 utilization efficiency and CO2 mass transfer rate. J. Ind. Eng. Chem. 2009, 15, 471–475. [Google Scholar] [CrossRef]
- Kurano, N.; Ikemoto, H.; Miyashita, H.; Hasegawa, T.; Hata, H.; Miyachi, S. Fixation and utilization of carbon dioxide by microalgal photosynthesis. Energy Convers. Manag. 1995, 36, 689–692. [Google Scholar] [CrossRef]
- Kurano, N.; Ikemoto, H.; Miyashita, H. Fixation and utilization of carbon dioxide by microalgal photosynthesis. Energy Convers. Manag. 1995, 36, 689–692. [Google Scholar] [CrossRef]
- Kurano, N.; Sasaki, T.; Miyachi, S. Advances in chemical conversions for mitigating carbon dioxide. Stud. Surf. Sci. Catal. 1998, 114, 55–63. [Google Scholar]
- Morais, M.G.; Costa, J.A.V. Isolation and selection ofmicroalgae fromcoal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers. Manag. 2007, 48, 2169–2173. [Google Scholar] [CrossRef]
- De Morais, M.G.; Costa, J.A. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Lopesa, E.; Revahb, S.; Hernández, S. Development of operational strategies to remove carbon dioxide in photobioreactors. Chem. Eng. J. 2009, 135, 120–126. [Google Scholar] [CrossRef]
- Chae, S.R.; Hwang, E.J.; Shin, H.S. Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photo-bioreactor. Bioresour. Technol. 2006, 97, 322–329. [Google Scholar] [CrossRef]
- National Algal Biofuels Technology Roadmap; U.S. Department of Energy: Washington, DC, USA, 2010.
- Chen, F. Microalgae Biotechnology; China Light Industry Press: Beijing, China, 1999. [Google Scholar]
- Wang, B.; Mao, H.; Jiao, Z.; Zheng, Y. The analysis of cultivating Spirulina on agro-meteorological conditions in chenghai lake. Yunnan Geogr. Environ. Res. 2009, 6, 25–28. [Google Scholar]
- Yin, D.; Geng, Y.; Mei, H. The effects of several environmental factors on the photosynthesis of Botryococcus braunii. J. Wuhan Bot. Res. 2008, 26, 64–69. [Google Scholar]
- Jiang, X. Effects of temperatures, light intensities and nitrogen concentrations on the growth and fatty acid compositions of Nannochloropsis oculata. Mar. Sci. 2002, 8, 9–13. [Google Scholar]
- Lu, K.; Jiang, X.; Zhai, X. Effects of illumination on growth of Haematococcus pluvialis. Hebei Fish. 2002, 6, 6–8. [Google Scholar]
- Lin, G. Study on the conditions of high density cultivation of Dunaliella salina. Shandong Fish. 2007, 9, 41–44. [Google Scholar]
- Wen, G.; Liang, W.; Li, Z. Studies on the ecological factors of four microalgae populations in mixed-culture. Prog. Fish. Sci. 2009, 30, 142–148. [Google Scholar]
- Shi, J.; Pan, K. Effects of different light intensities on growth and biochemical composition of Nitzschia closterium f. minutissima and Isochrysis galbana Parke 8701. J. Fish. Sci. China 2004, 11, 121–128. [Google Scholar]
- Li, W.; Wang, R. Water Resources Ecosystem Management in China-Challenges and Opportunities. In Proceedings of the Ecosystem Service and Sustainable Watershed Management in North China International Conference, Beijing, China, 23–25 August 2000.
- National Bureau of Statistics of China. China Statistical Yearbook; China Statistics Press: Beijing, China, 2004–2009. [Google Scholar]
- Li, X.; Hu, H.; Yang, J. Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol. 2010, 27, 59–63. [Google Scholar] [CrossRef]
- Voltolina, D.; Gómez-villa, H.; Correa, G. Biomass production and nutrient removal in semicontinuous cultures of Scenedesmus sp. (chlorophyceae) in artificial wastewater, under a simulated day-night cycle. Vie Milieu 2004, 54, 21–25. [Google Scholar]
- Olguín, E.J. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Kong, Q.; Li, L. Culture of Microalgae Chlamydomonas reinhardtii in wastewater for biomass feedstock production. Appl. Biochem. Biotechnol. 2010, 160, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.; Wen, Z. Development of an attached microalgal growth system for biofuel production. Appl. Microbiol. Biotechnol. 2010, 5, 525–534. [Google Scholar]
- Hodaifa, G. Daily doses of light in relation to the growth of Scenedesmus obliquus in diluted three-phase olivemill wastewater. J. Chem.Technol. Biotechnol. 2009, 84, 1550–1558. [Google Scholar] [CrossRef]
- Voltolina, D. Nitrogen removal and recycling by Scenedesmus obliquus in semicontinuous cultures using artificial wastewater and a simulated light and temperature cycle. Bioresour. Technol. 2005, 96, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.E.; Sancheze, S.; Jimenez, J.M. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Claxton, R. Biomass and bioenergy production potential of microalgae consortium in open and closed bioreactors using untreated carpet industry effluent as growth medium. Bioresour. Technol. 2010, 101, 6751–6760. [Google Scholar] [CrossRef] [PubMed]
- De-Bashana, L.E.; Moreno, M.; Hernandez, J.P. Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense. Water Res. 2002, 36, 2941–2948. [Google Scholar]
- Shi, J. Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: An experimental study. J. Appl. Phycol. 2007, 19, 417–423. [Google Scholar] [CrossRef]
- Kapdan, I. K.; Aslan, S. Application of the Stover-Kincannon kinetic model to nitrogen removal by Chlorella vulgaris in a continuously operated immobilized photobioreactor system. J. Chem. Technol. Biotechnol. 2008, 83, 998–1005. [Google Scholar] [CrossRef]
- Yun, Y.; Sun, B. Carbon dioxide fixation by algal cultivation using wastewater nutrients. J. Chem. Technol. Biotechnol. 1997, 69, 451–455. [Google Scholar] [CrossRef]
- Travieso, L.; Benítez, F.; Sánchez, E. Performance of a laboratory-scale microalgae pond for secondary treatment of distillerywastewaters. Chem. Biochem. Eng. 2008, 22, 467–473. [Google Scholar]
- De-Bashan, L.E.; Hernandez, J.-P.; Morey, T. Microalgae growth-promoting bacteria as “helpers” for microalgae: A novel approach for removing ammonium and phosphorus from municipal wastewater. Water Res. 2004, 38, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Rupert, J.; Paul, J.; Valerie Smith, J. Wastewater nutrient removal by marine microalgae grown on a corrugated raceway. Water Res. 1997, 31, 1701–1707. [Google Scholar] [CrossRef]
- Liu, Y.; Ruan, R.; Kong, Q. Mass culture of high oil content microalgae on wastewater and power plant flue gases. Chin. J. Bioprocess Eng. 2008, 3, 29–33. [Google Scholar]
- Larsdotter, K.; Jansen, J. Phosphorus removal from wastewater by microalgae in Sweden a year round perspective. Environ. Technol. 2010, 31, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Lopes, E. Biotransformations of carbon dioxide in photobioreactors. Energy Convers. Manag. 2010, 51, 894–900. [Google Scholar] [CrossRef]
- Shen, Y.; Yuan, W.; Pei, Z.; Mao, E. Culture of microalga Botryococcus in livestock wastewater. Trans. ASABE 2008, 51, 1395–1400. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A; Colosi, M.; White, L.M. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Ling, F. The trend of microalgae industry in China. Ecol. Econ. 2004, 2, 58–61. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huo, S.; Dong, R.; Wang, Z.; Pang, C.; Yuan, Z.; Zhu, S.; Chen, L. Available Resources for Algal Biofuel Development in China. Energies 2011, 4, 1321-1335. https://doi.org/10.3390/en4091321
Huo S, Dong R, Wang Z, Pang C, Yuan Z, Zhu S, Chen L. Available Resources for Algal Biofuel Development in China. Energies. 2011; 4(9):1321-1335. https://doi.org/10.3390/en4091321
Chicago/Turabian StyleHuo, Shuhao, Renjie Dong, Zhongming Wang, Changle Pang, Zhenhong Yuan, Shunni Zhu, and Li Chen. 2011. "Available Resources for Algal Biofuel Development in China" Energies 4, no. 9: 1321-1335. https://doi.org/10.3390/en4091321
APA StyleHuo, S., Dong, R., Wang, Z., Pang, C., Yuan, Z., Zhu, S., & Chen, L. (2011). Available Resources for Algal Biofuel Development in China. Energies, 4(9), 1321-1335. https://doi.org/10.3390/en4091321





