Abstract
In biodiesel production, the vegetable oil used as raw material for transesterification should be free of water and free fatty acids (FFAs), which may consume catalyst and reduce catalyst efficiency. In this work biodiesel was prepared from acidified oils (AO) through a supercritical methanol route, in which the esterification of FFAs and transesterification of glyceride with methanol occurred simultaneously. The effects of the mass ratio of methanol to AO, the operation temperature as well as the water content on the FFAs conversion and glycerol yield were investigated. The results indicated that the FFAs conversion for esterification under the condition of 1:1 methanol/oil ratio, 310 °C and 15 min reaction time reached 98.7%, and the glycerol yield for transesterification under 0.25:1 methanol/oil ratio, 290 °C and 20 min reaction time reached 63.5% respectively.
1. Introduction
Biodiesel, a mixture of mono-alkyl esters of long-chain fatty acids, is an alternative diesel fuel derived from vegetable and animal oils/fats by transesterification and esterification with short-chain alcohols (e.g., methanol or ethanol) [1]. It has many advantages such as environmental friendliness, biodegradability, clean and renewability, low toxicity, high security of use and storage, adaptability to presently used engines, and good blending ability with petroleum-based diesel fuels [2,3]. Compared with the petroleum-based diesel fuels the use of biodiesel and biodiesel blends in an unmodified diesel engine reduces the particulate matter, hydrocarbons and carbon monoxide emissions, but slightly increases nitrogen oxides emissions [4].
Several vegetable oils have been evaluated as the raw material for biodiesel production, including soybean oil, rape oil, palm oil, sunflower oil, cotton-seed oil, and waste oils/fats [5]. By transesterification, the triglycerides of fatty acids in vegetable oil react with linear monohydroxy alcohols in the presence of catalyst to form biodiesel. The alcohol may be methanol, ethanol or butanol, while the catalyst may be basic or acidic [6,7,8]. In China, biodiesel is mainly produced from waste vegetable oils or animal fats called acidified oils (AO), generated by acidification of soapstock in the vegetable oil refining process. The use of AO in production may lead to significant decrease in the cost of biodiesel. However, a principal limitation to the commercial utilization of AO in biodiesel industry is that it contains a large amount of free fatty acids (FFAs) with acid values ranging from dozens to more than one hundred [3,4]. Therefore, the AO cannot be directly used to prepare biodiesel by alkaline-catalyzed transestrification because in the presence of an alkali catalyst the large amount of FFAs may result in the formation of soap.
In addition, the methyl ester cannot be separated from the mixture owing to the emulsification of soap in the reaction system [9,10,11]. Hence, the content of fatty acids must be kept below 0.5%. This means that the AO must be refined to remove fatty acids prior to use [12]. The FFAs in AO are often firstly esterified by acid catalysts and then is transesterified by base catalysts. The alcohol and catalyst also have to satisfy rigorous specifications, i.e., the total water content must be 0.1–0.3 wt% or less [13]. This is because the presence of water in the feedstock promotes the hydrolysis of alkyl esters to FFAs and soap formation. In fact, commercial base-catalyzed processes often employ an additional step, acid-catalyzed pre-esterification, to remove the excess FFAs to avoid soap formation [14,15]. Current biodiesel production technology using toxic, corrosive homogeneous base catalysts (e.g., NaOH, KOH or NaOCH3) or acid catalysts (e.g., H2SO4, H3PO4, p-toluenesulfonic acid), which involve the processes of neutralization and separation [16]. The use of liquid acids for esterification causes some problems such as the difficulty in recovery of catalyst after the reaction and in the associated wastewater [17,18,19]. In addition, the higher methanol to oil molar ratio and reaction temperature need to be employed for acid-base catalysed transesterification.
Supercritical technology is a suitable alternative for biodiesel production from a technical and environmental point of view [20,21,22]. In its supercritical state of methanol (239 °C and 8.1 MPa), methanol’s solubility parameter and dielectric constant decreases significantly and the methanol thus becomes more like a non-polar substance. This would increase the solubility of oil in methanol and enable the transesterification reaction to be complete very quickly [23,24,25,26]. No catalyst is needed for the supercritical methanol method, thus the corrosion and environmental problems associated with liquid catalysts can be avoided [25]. Besides, lower production costs can also be expected because the product separation is easy. Furthermore, the method is more tolerant of water and FFAs than the conventional base catalysed transesterification route [27,28,29].
In this study, biodiesel was produced from AO using the non-catalytic supercritical methanol method. The esterification of FFAs and the transesterification of glyceride with methanol occur simultaneously [30,31]. The effects of methanol to AO ratio, operation temperature and time as well as water content on the esterification and transesterification were investigated.
2. Results and Discussion
2.1. Effect of Methanol/AO Ratio on the Esterification
In order to optimize the reaction conditions, the effects of different mass ratios of methanol to AO, i.e., 0.125:1, 0.25:1, 0.5:1, 1:1, 1.5:1 and 2:1, on the esterification were investigated at 310 °C with the reaction time ranging from 5 to 30 min. The results are presented in Figure 1. As can be seen, the results showed that the methanol/AO ratio had a key effect on the esterification [32,33].
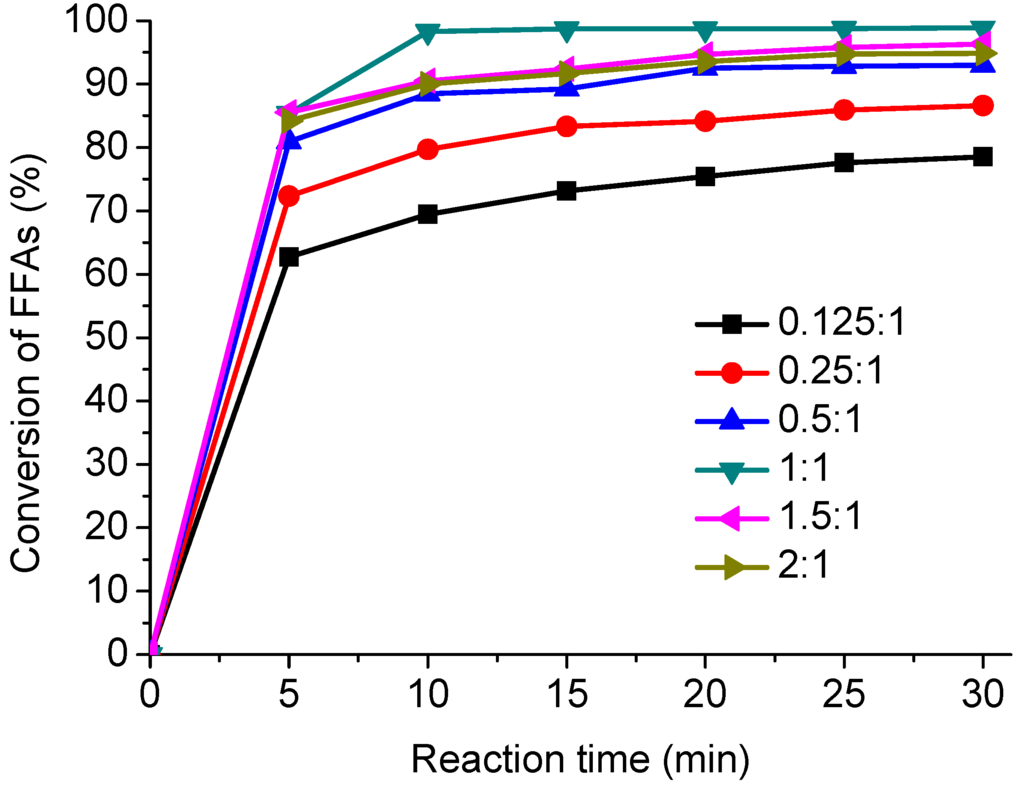
Figure 1.
Effect of methanol/AO mass ratio on the esterification.
Generally, the esterification was performed in the presence of an excess of methanol to favor the forward reactions since the reaction is reversible. The conversion of FFAs increased rapidly from 78.5% to 98.9% as the methanol/AO mass ratio rose from 0.125:1 to 1:1 in 30 min. However, the conversion of FFAs decreased slightly, from 98.9% to 94.9%, when the methanol to AO mass ratio was further increased from 1:1 to 1.5:1 and 2:1. Therefore, the optimum methanol/AO mass ratio in this system was determined to be 1:1. The excess methanol used in the reaction can be collected by distillation and reused. In general the conclusion of most researchers is that conversion is highest at the highest methanol to oil ratio [34].
According to the curves in Figure 1, the results demonstrated that the FFAs conversion increased with the reaction time in a given methanol/AO ratio. At the beginning of reaction, the conversion of FFAs increased rapidly with reaction time. As the reaction proceeded, the forward reaction rate reduced while the reverse reaction rate increased.
2.2. Effect of Temperature on the Esterification
The effects of temperature on the esterification of AO were investigated at 250, 270, 290, 310, 330 and 350 °C with fixed methanol/AO ratio of 1:1 and the reaction time ranging from 5 to 30 min. Figure 2 shows an example of the relationship between the reaction time and the temperature. It was observed that increasing the reaction temperature had a favorable influence on the degree of esterification.
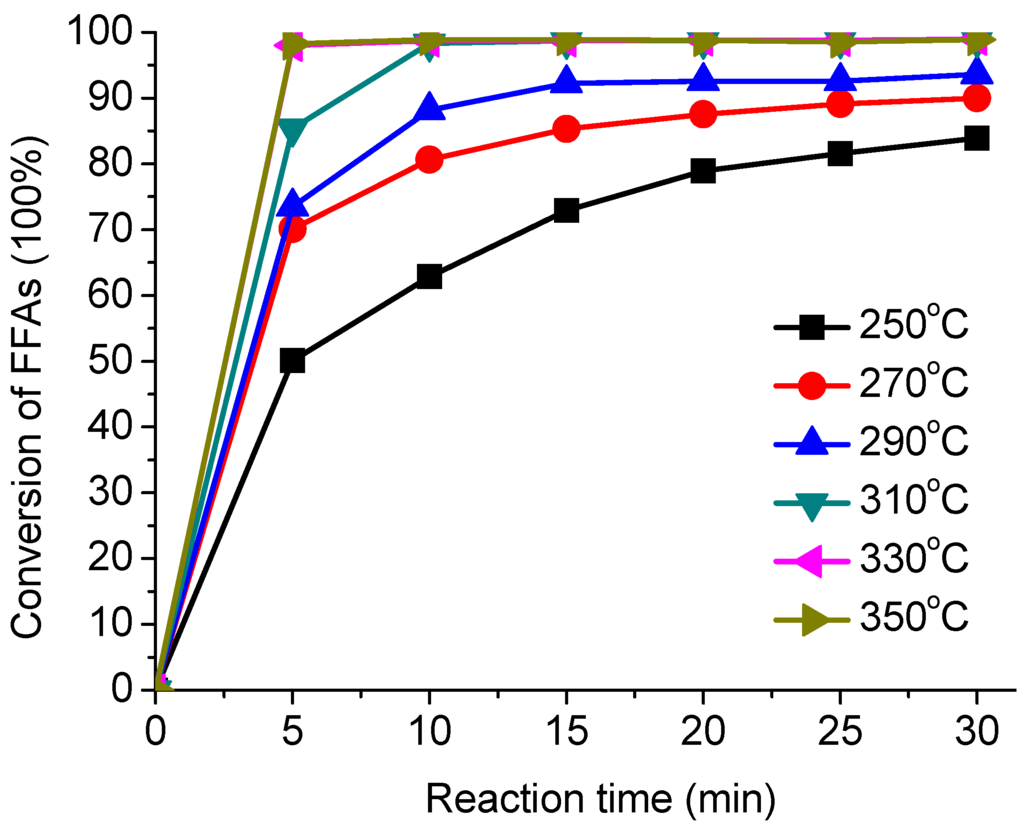
Figure 2.
Effect of different temperature on the esterification.
The conversion of FFAs increased rapidly with temperature. When the reaction temperature was increased to 310 °C, the reaction occurred at the highest conversion of 98.9%. A further increase in temperature seems to be unnecessary as the conversion of FFAs does not change any more. The reaction rate rises with the promotion of the molecule motion and mass transfer rate as the temperature increases. As shown in Figure 2, the conversion of FFAs increases with the reaction time at a certain reaction temperature. The reaction time to reach the equilibrium depends on the reaction temperature.
2.3. Effect of Methanol/AO Ratio on the Transesterification
The transesterification reaction of AO in supercritical methanol follows the same mechanism as that in liquid methanol in the catalysed transesterification method. The transesterification reaction may be expressed as:
Triglycerides + 3 Methanol ↔ Glycerin + 3 Methyl esters
This is a reversible reaction with 1 mol triglycerides reacting with 3 mol methanol. In this work, the methanol used was in excess to shift the reaction equilibrium to the right side so as to produce more methyl ester product [26]. Although the methanol was excessive, it could be easily recovered by refluxing the product mixtures.
The methanol/AO ratio also is one of the most important variables affecting the glycerin yield. In order to study the effects of methanol/AO ratio on the transesterification, different methanol/AO ratios as 0.125:1, 0.25:1, 0.5:1, 1:1, 1.5:1 and 2:1 were used with a fixed temperature of 310 °C and reaction times ranging from 5 to 30 min. The results are shown in Figure 3. As can be seen, the yield of glycerin increased rapidly from 47.1% to 64.4% as the methanol/AO ratio rose from 0.125:1 to 0.5:1 in 30 min. But the yield decreased from 64.4% to 53.3% when the methanol/AO ratio was further increased from 0.5:1 to 2:1. This might be due to the thermodynamic equilibrium limitation and the difficulties in separating excessive methanol from methyl esters and glycerol. The studies done by Tan et al. and Song et al. also gave similar final conversions with the supercritical methanol method [20,35].
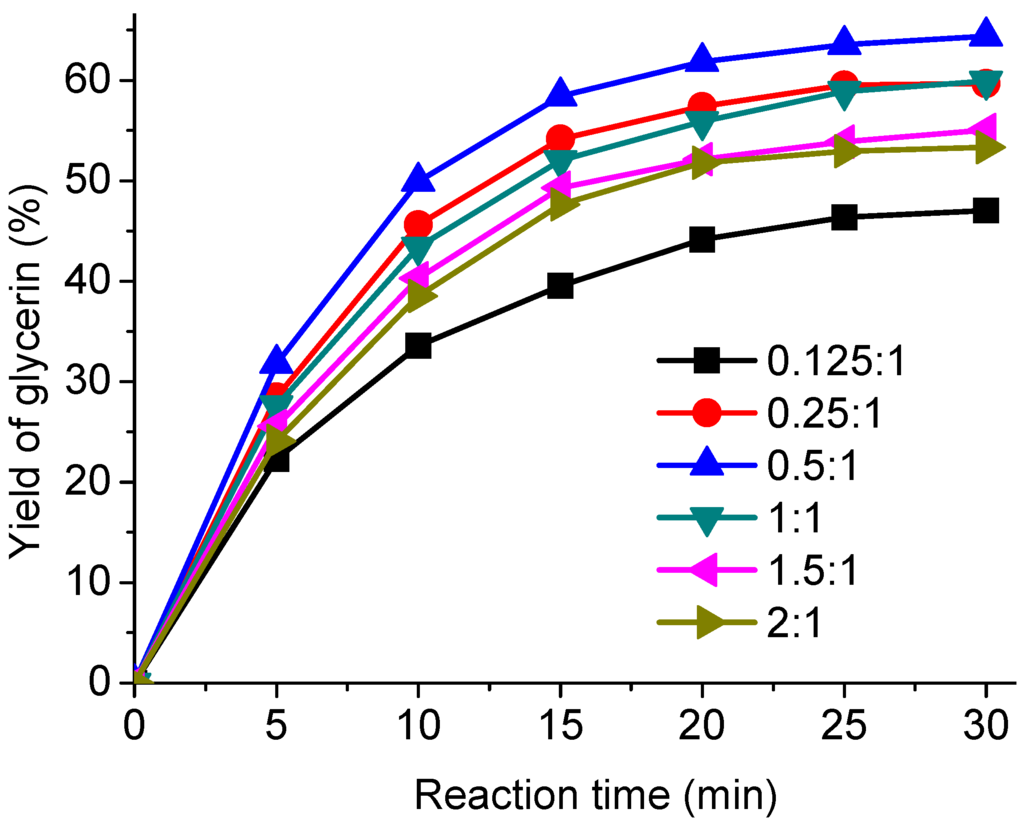
Figure 3.
Effect of methanol to AO mass ratio on the transesterification.
2.4. Effect of Temperature on the Transesterification
The effects of temperature on the AO transesterification were investigated using 250, 270, 290, 310, 330 and 350 °C with a fixed methanol/AO ratio 1:1 and a 30 min reaction time. The results are shown in Figure 4. It can be seen that the transesterification was greatly affected by the temperature. The yield of glycerin increased with the reaction temperature in a range of 250–290 °C. According to the current literature on non-catalytic transesterification reactions under high temperature and pressure conditions, the solubility could decrease mass-transfer limitations [21,22]. However, the yield of glycerin decreased as the reaction temperature rose from 310 to 330 °C, and especially decreased rapidly at 350 °C. The possible reason is that the glycerin was decomposed under such harsh conditions. For the effect of reaction temperature, Saka and Kusdiana [26] and Song et al. [35] also reported an increase in the FAME yield with reaction temperature provided the reaction temperature is below 380 °C. However, in this study, the conversions of reaction were evaluated using the yield of glycerin instead of FAME by gas chromatography. The temperature above 310 °C is not suitable for transesterification reaction and glycerin tends to decompose. Hence, the operating temperature for the supercritical methanol method cannot exceed 310 °C to avoid unnecessary decomposition.
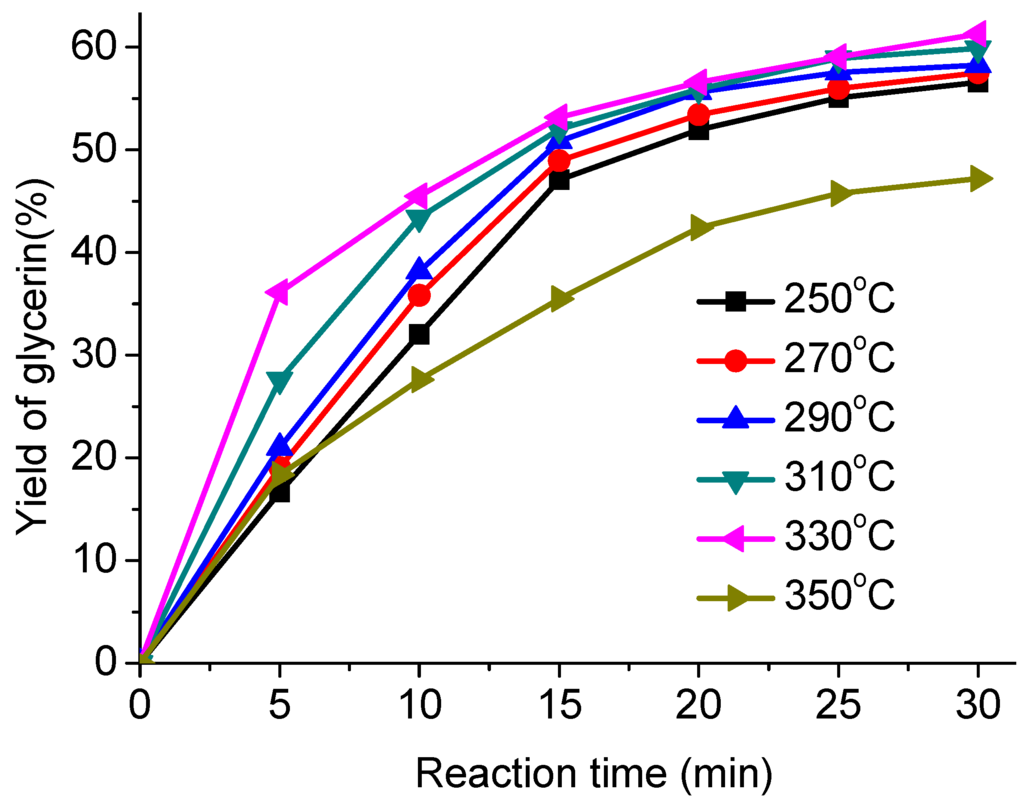
Figure 4.
Effect of different temperature on the transesterification.
2.5. Effect of Water Content on the Esterification
The effects of water contents on the esterification were shown in Figure 5. In this experiment, water is deliberately introduced into the reaction system with varied percentage from 5% to 50%, respectively. The conversion of FFAs decreased with increasing the water content, which was consistent with the literature results [27].
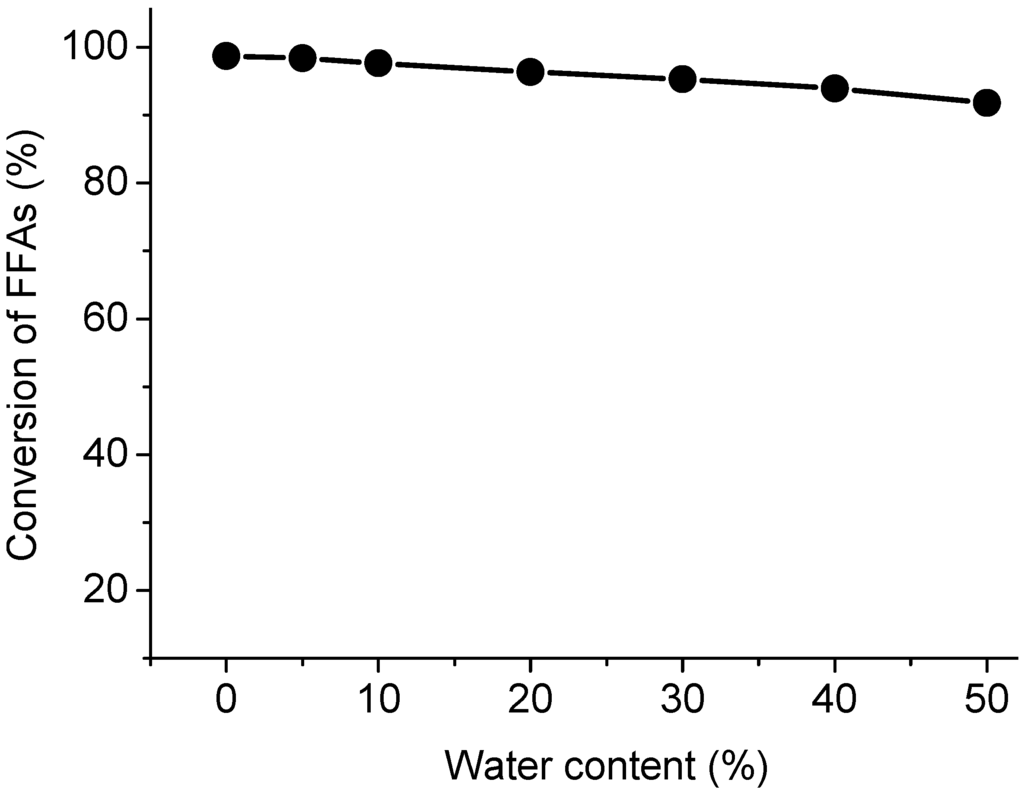
Figure 5.
Effect of water content on the esterification.
The conversion of FFAs was slightly reduced when the water content was increased. The reasons for this methyl ester decrease have not been fully elucidated yet. However, one of the possible reasons is that the methyl ester formed may be further hydrolyzed to FFAs at high temperature [27]. On the other hand, the water lowered concentrations of methanol, leading to the conversion of FFAs declined. In the hybrid membrane catalytic esterification reaction system, the conversion of FFAs decreased rapidly with increasing water content [36]. The water in the methyl esterification of fatty acids produced a similar effect for both the acid-catalyzed and supercritical methanol methods. Although the FFAs reaction was still influenced due to the presence of water, the supercritical methanol method demonstrated higher water-tolerance than the conventional acid catalysis route. In the supercritical state, methanol acts as an acid catalyst to enhance the transesterification and methyl esterification reactions [24,26]. It was observed that the supercritical condition had a favorable influence on the esterification.
2.6. Effects of Water Content on the Transesterification
Water content in acidified oil is a major concern because it can adversely affect the yield of biodiesel. Hence, in this experiment, water was deliberately introduced into the reaction mixtures with varied percentage from 5% to 50% for supercritical methanol method. As shown in Figure 6, the yield of glycerin in supercritical methanol decreases steadily with increasing the amount of water content in acidified oil. The similar result of transesterification of triglycerides with methanol was reported by Kusdiana and Saka [27]. In supercritical methanol reaction, the presence of water in the reaction mixture induces the hydrolysis of triglycerides, which produces FFAs and glycerol, and subsequently leads to the formation of FAME and water by esterification with FFAs and methanol. These phenomena can be well explained by a two step process as shown in Equations (2) and (3), which are hydrolysis and esterification reactions, respectively [37].
Glyceride + Water → Free Fatty Acid + Glycerin
Free Fatty Acid + Methanol → Fatty Acid Methyl Ester + Water
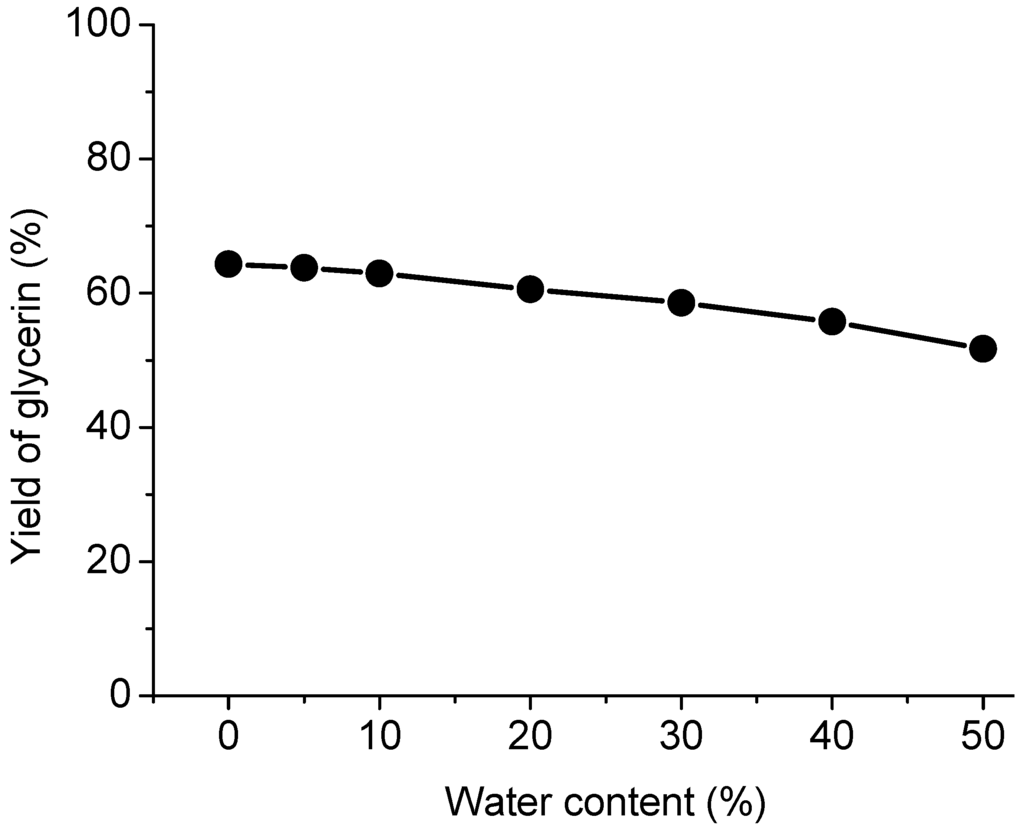
Figure 6.
Effect of water content on the transesterification.
The hydrolysis reaction rate is much greater than that of transesterification. Meanwhile, the methanol concentration decreased due to the presence of water. Combined with the two effects, it represented a slight decline in the yield of glycerol. Comparatively, the supercritical methanol treatment has been shown to have higher tolerance towards water content in oils/fats compared with the heterogeneous catalyst process. This shows the versatility of supercritical methanol method in utilizing for a wide range of feedstock, including those low-quality oils/fats.
3. Experimental Section
3.1. Materials
The acidified oil used in this study, with an acid value of 155.65 mg KOH/g AO, was supplied by Hubei Haolin Bioenergy Company, China. Acidified oil was filtered to remove impurities and distilled to remove water by using rotary evaporator. No water was detected by a Karl Fisher water assay with a sensitivity of 500 ppm (KF-1A, Shanghai Precision and Scientific Instrument Co., Ltd., Shanghai, China). Methanol, ethanol, potassium hydroxide and other chemicals were reagent grade and used without any further purification.
The supercritical reaction was performed in a stainless steel batch reactor (100 cm3) with a mechanical stirrer in which the pressure and temperature were monitored over time. The reactor was heated by an external heater and cooled by a water bath, with the power adjusted using the DLW-A temperature controller (Zhuhai Delai Environmental Science and Technology Co. Ltd, Guangdong, China). The reactor was made of American Society for Testing and Materials (ASTM)-316 SS which can endure strong acid and alkaline chemicals in addition to possessing high tensile strength. The temperature in the reactor was measured with an iron-constantan thermocouple and controlled within ±5 °C range while the pressure was measured with a manometer and controlled within ±0.25 MPa.
3.2. Reaction Procedure
The acidified oil and liquid methanol weighted in a given ratio were premixed and poured into the stainless reactor. The temperature and pressure were controlled above the critical values of methanol, i.e., 239 °C and 8.1 MPa, respectively. The reaction was operated for a specific duration ranging from 5 to 30 min. The reaction vessel was then immersed quickly into a tin bath preheated at the settled reaction temperature, and retained for an interval for supercritical treatments of methanol, from 250 to 350 °C. The pressure of the reaction system was controlled at 25 MPa using high-pressure nitrogen. It was, subsequently, moved into a water bath to decrease the reactor temperature and to stop the reaction. After reaching room temperature and atmospheric pressure, the product was collected and washed with methanol. The unreacted methanol and the water generated in esterification were removed with a vacuum evaporator. The mixture was settled to separate the upper biodiesel layer and the lower glycerin aqueous layer in a separation funnel. The upper biodiesel layer was washed to remove the residual glycerin. The washed solution together with the glycerin solution was retained to test the glycerol yield. The acidic value of the biodiesel was measured after trace water and methanol were removed by vacuum evaporation. After drying with anhydrous sodium sulfate, biodiesel samples were obtained. The effects of water on the conversion of FFAs in esterification of fatty acids and the yield of glycerin in transesterification of triglycerides were investigated at 310 °C with the methanol/AO mass ratio of 1:1.
3.3. Determination of Acid Value and FFAs Conversion
The acid value of samples was determined by a standard titration method following China Standard—GB/T5530–2005 (Animal and Vegetable Fats and Oils-Determination of Acid Value and Acidity). Briefly the steps were as follows: sample (0.25 g) was added into an amount of neutralized ethanol and was fully dissolved by heating with phenolphthalein (0.5 mL) as indicator. The sample was then titrated by 0.1 M KOH solution. The acid value was calculated using the following Equation (4):
where S (mg KOH/g AO) is the acid value; c (mol/L) is the concentration of the KOH used for titration; V (mL) is the volume of KOH employed for titration; m (g) is the weight of the sample taken to be analyzed.
The conversion of FFAs is defined as the change of acid value before and after reaction of the oil with respect to acid value of the initial oil. It can be determined from the following Equation (5):
where S0 refers to initial acid value and Si refers to the acid value at some reaction time, respectively.
3.4. Determination of the Glycerin Yields
The glycerin yield of the sample was determined by a standard titration method following China Standard—GB/T 13216.6-91 (Test Methods for Glycerines-Determination of Glycerol Content). Cold gycerin was oxidized by sodium periodate in a strong acid medium. The formic acid generated by reaction was indicated by pH meter and titrated with sodium hydroxide standard solution. This reaction was described in the following Equation (6):
CH2OH-CHOH-CH2OH + 2NaIO4 → HCOOH + 2HCHO + 2NaIO3 + H2O
The glycerin contents was described as percentage by mass and calculated according to Equation (7):
where the δ (%) is the glycerin content; V2 (mL) is the volume of the KOH employed for titration; V1 (mL) is the initial volume of the KOH employed for titration; m (g) is the weight of the sample taken to be analyzed; c (mol·L−1) is the concentration of the KOH used for titration; 0.0921 (g·mmol−1) is the molar quantity of glycerin.
According to the total mass of glycerin aqueous solution, the amounts of glycerin were calculated in accordance with Equation (8):
where I refers to actual yield of glycerin and M refers to weight of glycerin aqueous solution.
Therefore, the glycerin yields were calculated in accordance with Equation (9):
where β (%) refers to glycerin yield; I (g) refers to actual yield of glycerin and I0 (g) refers to theoretical yield of glycerin.
4. Conclusions
In supercritical methanol, the esterification and glyceride transesterification of FFAs with methanol occurred simultaneously. The presence of water has little effect on the yield, as complete conversions were always achieved, regardless of the content of water. In the conventional transesterification of fats/vegetable oils for biodiesel production, FFAs and water always generate negative effects, since the presence of FFAs and water causes soap formation which consumes catalyst and reduces catalyst effectiveness, resulting in low conversions. The non-catalytic supercritical methanol transesterification technology has been shown to be superior to conventional catalytic reactions in terms of cost and energy consumption. It has huge potential to be explored and developed in the near future in order to fulfill the escalating demand of renewable energy sources in the world.
The optimum conditions for the esterification were as follows: methanol-oil mass ratio 1:1, reaction temperature 310 °C, reaction time 15 min. Under these optimal conditions, the conversion of FFAs was 98.7%. The optimal conditions for transesterification were as follows: methanol-oil mass ratio 0.5:1, reaction temperature 330 °C and reaction time 25 min. Under these optimal conditions, the yield of glycerin was 63.5%.
Acknowledgments
The authors gratefully acknowledge the financial support by National High Technology Research and Development Program of China (“863” Program, Grant No. 2009AA03Z223), National and Tianjin Natural Science Foundation of China (Grant No. 20676100, 08JCYBJC26400 and 08JCZDJC24000). The authors also appreciate Hubei Haolin Bioenergy Ltd., China for kindly offering the acidic oil.
References
- Ma, F.R.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Narasimharao, K.; Lee, A.; Wilson, K. Catalysts in Production of Biodiesel: A Review. J. Biobased Mater. Bioenergy 2007, 1, 19–30. [Google Scholar]
- Koh, M.Y.; Ghazi, T.I.M. A review of biodiesel production from Jatropha curcas L. oil. Renew. Sustain. Energy Rev. 2011, 15, 2240–2251. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C.; Islam, M.R. Waste cooking oil as an alternate feedstock for biodiesel production. Energies 2008, 1, 3–18. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Antolín, G.; Tinant, F.V.; Briceňo, Y.; Castaňo, V.; Pérez, C.; Ramírez, A.I. Optimization of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 2002, 83, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Al-Widyan, M.I.; Al-Shyoukh, A.O. Experimental evaluation of the transesterification of waste palm oil into biodiesel. Bioresour. Technol. 2002, 85, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.R.; Clements, L.D.; Hanna, M.A. The effect of mixing on transesterification of beef tallow. Bioresour. Technol. 1999, 69, 289–293. [Google Scholar] [CrossRef]
- Freedman, B.; Pryde, E.H.; Mounts, T.L. Variables affecting the yields of fatty esters from transesterified vegetable oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Liu, K.S. Preparation of fatty acid methyl esters for gas-chromatographic analysis of lipids in biological materials. J. Am. Oil Chem. Soc. 1994, 71, 1179–1187. [Google Scholar] [CrossRef]
- Mittelbach, M. Diesel fuel derived from vegetable oils, VI: Specifications and quality control of biodiesel. Bioresour. Technol. 1996, 56, 7–11. [Google Scholar] [CrossRef]
- Ma, F.R.; Clements, L.D.; Hanna, M.A. The effects of catalyst, free fatty acids, and water on transesterification of beef tallow. Trans. ASABE 1998, 41, 1261–1264. [Google Scholar] [CrossRef]
- Haas, M.J. The interplay between feedstock quality and esterification technology in biodiesel production. Lipid Technol. 2004, 16, 7–11. [Google Scholar]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Berrios, M.; Siles, J.; Martín, M.A.; Martín, A. A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil. Fuel 2007, 86, 2383–2388. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Errazu, A.F. Esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides. Biomass Bioenergy 2008, 32, 892–895. [Google Scholar] [CrossRef]
- Tanabe, K.; Hölderich, W.F. Industrial application of solid acid-base catalysts. Appl. Catal. A 1999, 181, 399–434. [Google Scholar] [CrossRef]
- Tashtoush, G.M.; AI-Widyan, M.I.; AI-Jarrah, M.M. Experimental study on evaluation and optimization of conversion of waste animal fat into biodiesel. Energy Convers. Manag. 2004, 45, 2697–2711. [Google Scholar] [CrossRef]
- Wilson, K.; Clark, J.H. Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl. Chem. 2000, 72, 1313–1319. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Production of FAME by palm oil transesterification via supercritical methanol technology. Biomass Bioenergy 2009, 33, 1096–1099. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Supercritical ethanol technology for the production of biodiesel: Process optimization studies. J. Supercrit. Fluids 2009, 49, 286–292. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from vegetable oils via transesterification in supercritical methanol. Energy Convers. Manag. 2002, 43, 2349–2356. [Google Scholar] [CrossRef]
- Noureddini, H.; Harkey, D.W.; Gutsman, M.R. A continuous process for the glycerolysis of soybean oil. J. Am. Oil Chem. Soc. 2004, 81, 203–207. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Madras, G.; Kolluru, C.; Kumar, R. Synthesis of biodiesel in supercritical fluids. Fuel 2004, 83, 2029–2033. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Kusidiana, D.; Saka, S. Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour. Technol. 2004, 91, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Sawangkeaw, R.; Bunyakiata, K.; Ngamprasertsith, S. A review of laboratory-scale research on lipid conversion to biodiesel with supercritical methanol (2001–2009). J. Supercrit. Fluids 2010, 55, 1–13. [Google Scholar] [CrossRef]
- Boer, K.D.; Bahri, P.A. Supercritical methanol for fatty acid methyl ester production: A review. Biomass Bioenergy 2011, 35, 983–991. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Methyl esterification of free fatty acids of rapeseed oil as treated in supercritical methanol. Chem. Eng. Jpn. 2001, 34, 383–387. [Google Scholar] [CrossRef]
- Demirbas, A. Diesel fuel from vegetable oil via transesterification and soap pyrolysis. Energy Sources 2002, 24, 835–841. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from sunflower oil in supercritical methanol with calcium oxide. Energy Convers. Manag. 2007, 48, 937–941. [Google Scholar] [CrossRef]
- He, H.Y.; Wang, T.; Zhu, S.L. Continuous production of biodiesel fuel from vegetable oil using supercritical methanol process. Fuel 2007, 86, 442–447. [Google Scholar] [CrossRef]
- Minami, E.; Saka, S. Kinetics of hydrolysis and methyl esterification for biodiesel production in two-step supercritical methanol process. Fuel 2006, 85, 2479–2483. [Google Scholar] [CrossRef]
- Song, E.S.; Lim, J.W.; Lee, H.S.; Lee, Y.W. Transesterification of RBD palm oil using supercritical methanol. J. Supercrit. Fluids 2008, 44, 356–363. [Google Scholar] [CrossRef]
- Ding, J.C.; He, B.Q.; Li, J.X. Cation Ion-Exchange Resin/Polyethersulfone Hybrid Catalytic Membrane for Biodiesel Production. J. Biobased Mater. Bioenergy 2011, 5, 82–91. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Effects of free fatty acids, water content and co-solvent on biodiesel production by supercritical methanol reaction. J. Supercrit. Fluids 2010, 53, 88–91. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).