Optimization of the Dilute Acid Hydrolyzator for Cellulose-to-Bioethanol Saccharification
Abstract
:1. Introduction
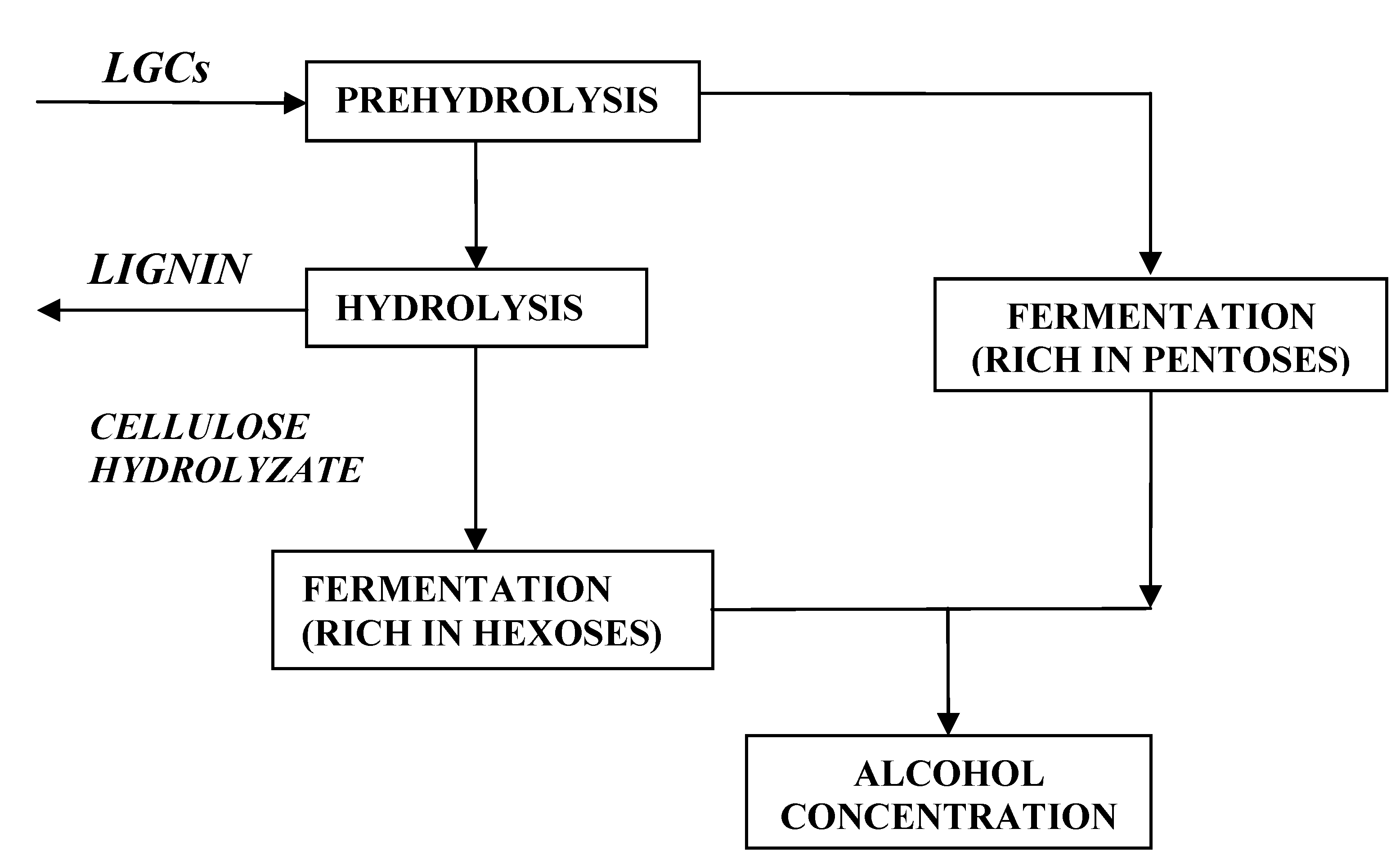
2. Lignocellolosics-to-Bioethanol
- Enzymatic hydrolysis
- Concentrated acid hydrolysis
- Dilute acid hydrolysis [7]
- it allows the production of useful byproducts such as xylitol and arabitol
- it increases the cellulose breakdown during hydrolysis and consequently the sugar yield
- it is more economical than the concentrated acid reaction because it requires cheaper equipment
3. Methodology
3.1. Kinetics of the Dilute Acid Hydrolysis
3.1.1. Kinetics of the Acid Cellulose Hydrolysis
| i: =1 for the production reaction, =2 for the degradation reaction |
| C: acid concentration (%w/w) |
| T: temperature (°Κ) |
| E1, E2: activation energy of the production and degradation of glucose |
| n1, n2: exponential acid parameters |
| A1, A2: pre-exponential parameters (min−1) |
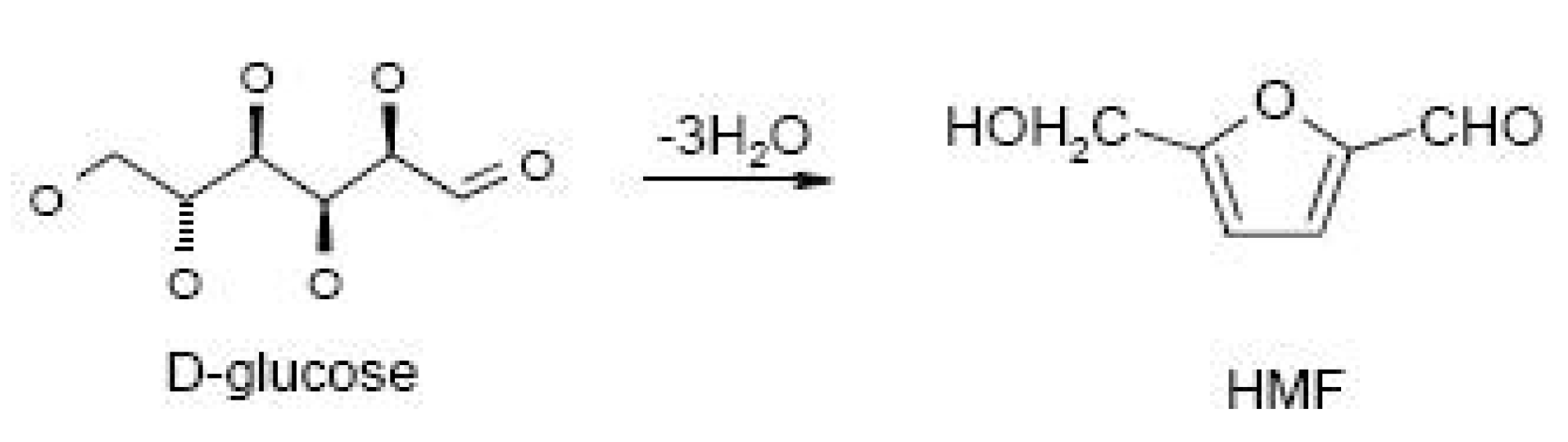
3.1.2. Kinetics of the Hemicellulose Acid Hydrolysis
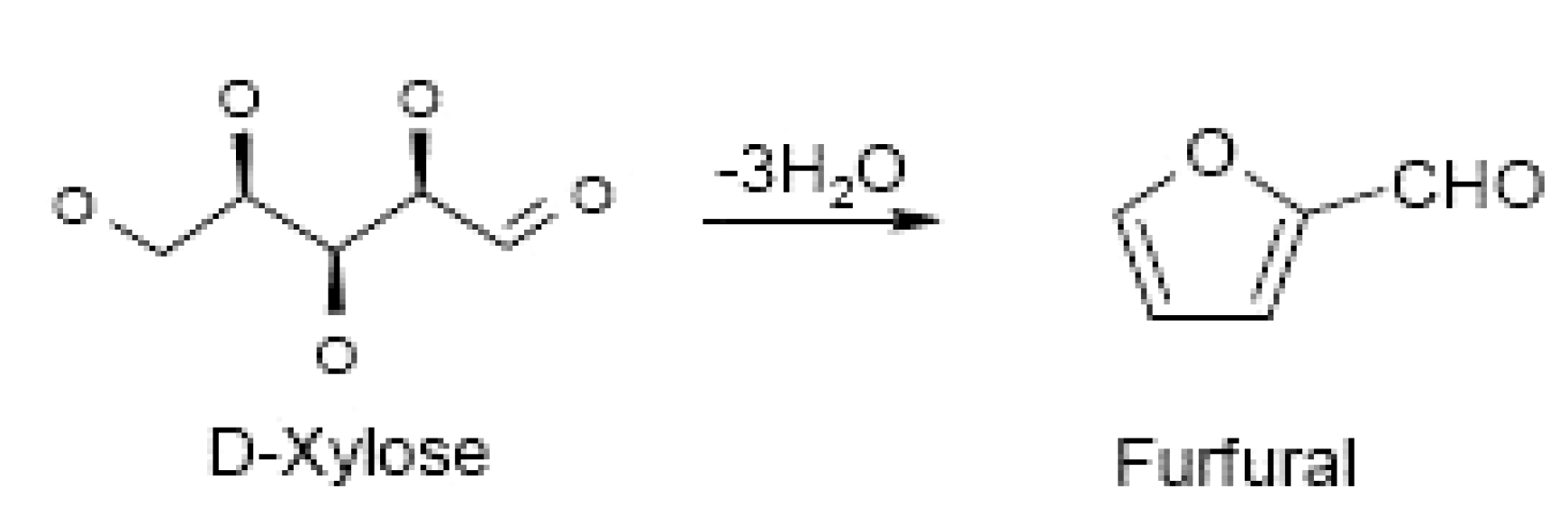
3.1.3. Further Assumptions for Hydrolysis Simulation
3.2. The Role of Degradation Products during Fermentation
3.2.1. The Role of Furfural
3.2.2. The Role of HMF
3.3. Sugar Production in Reactors
3.3.1. Concentrations in BR
| Ni: the number of moles |
| ni: the stoichiometric coefficient of i |
3.3.2. Concentrations in CSTR
| Ni: number of moles |
| ni: the stoichiometric coefficient of i |
| Fi: the input rhythm of i moles |
3.4. Selection of LGC
| R = 8.314 × 10−3 kJ/(mol K) T (°K)i = 1, 2 | |||||||
| xylan → xylose | xylose → F | ||||||
| LGC | A1 (min−1) | n1 | E1 (kJ/mol) | A2 (min−1) | n2 | E2 (kJ/mol) | Source |
| Corn stover | 3.68 × 1020 | 0 | 171.6 | 1.95 × 1014 | 0 | 133.9 | [18] |
| Wheat straw | 2.025 × 1020 | 1.55 | 167.0 | 1.52 × 1015 | 2.00 | 141.0 | [17] |
| Hardwood | 6.23 × 1013 | 1.17 | 116.43 | 2.33 × 1012 | 0.688 | 113.51 | [33,34,35] |
| glucan → glucose | glucose → HMF | ||||||
| LGC | A1 (min−1) | n1 | E1 (kJ/mol) | A2 (min−1) | n2 | E2 (kJ/mol) | Source |
| Corn stover | 2.71 × 1019 | 2.74 | 189.6 | 2.01 × 1014 | 1.86 | 137.3 | [14] |
| Wheat straw | 1.68 × 1019 | 0.7 | 190.37 | 2.21 × 1014 | 0.68 | 150.62 | [13] |
| Hardwood | 2.85 × 1013 | 1.2 | 133.05 | 2.75 × 1012 | 1.17 | 124.68 | [36] |
| lignocellulosics | Cellulose (%) | Hemicelluloses (%) | Lignin (%) |
|---|---|---|---|
| Hardwood | 40–55 | 24–40 | 18–25 |
| Corn stover | 45 | 35 | 15 |
| Wheat straw | 30 | 50 | 15 |
3.5. Cases Studied
| LGC material | Reactor type | Temperature (°C) | Acid concentration (% w/w) |
|---|---|---|---|
| Corn stover | BR | 120–230 per 10 | 0.5–2.5 per 0.5 |
| Corn stover | CSTR | 110–230 per 10 | 0.5–2.5 per 0.5 |
| Hardwood | BR | 100–230 per 10 | 0.5–2.5 per 0.5 |
| Hardwood | CSTR | 100–230 per 10 | 0.5–2.5 per 0.5 |
| Wheat straw | BR | 100–240 per 10 | 0.5–2.0 per 0.5 |
| Wheat straw | CSTR | 100–240 per 10 | 0.5–2.0 per 0.5 |
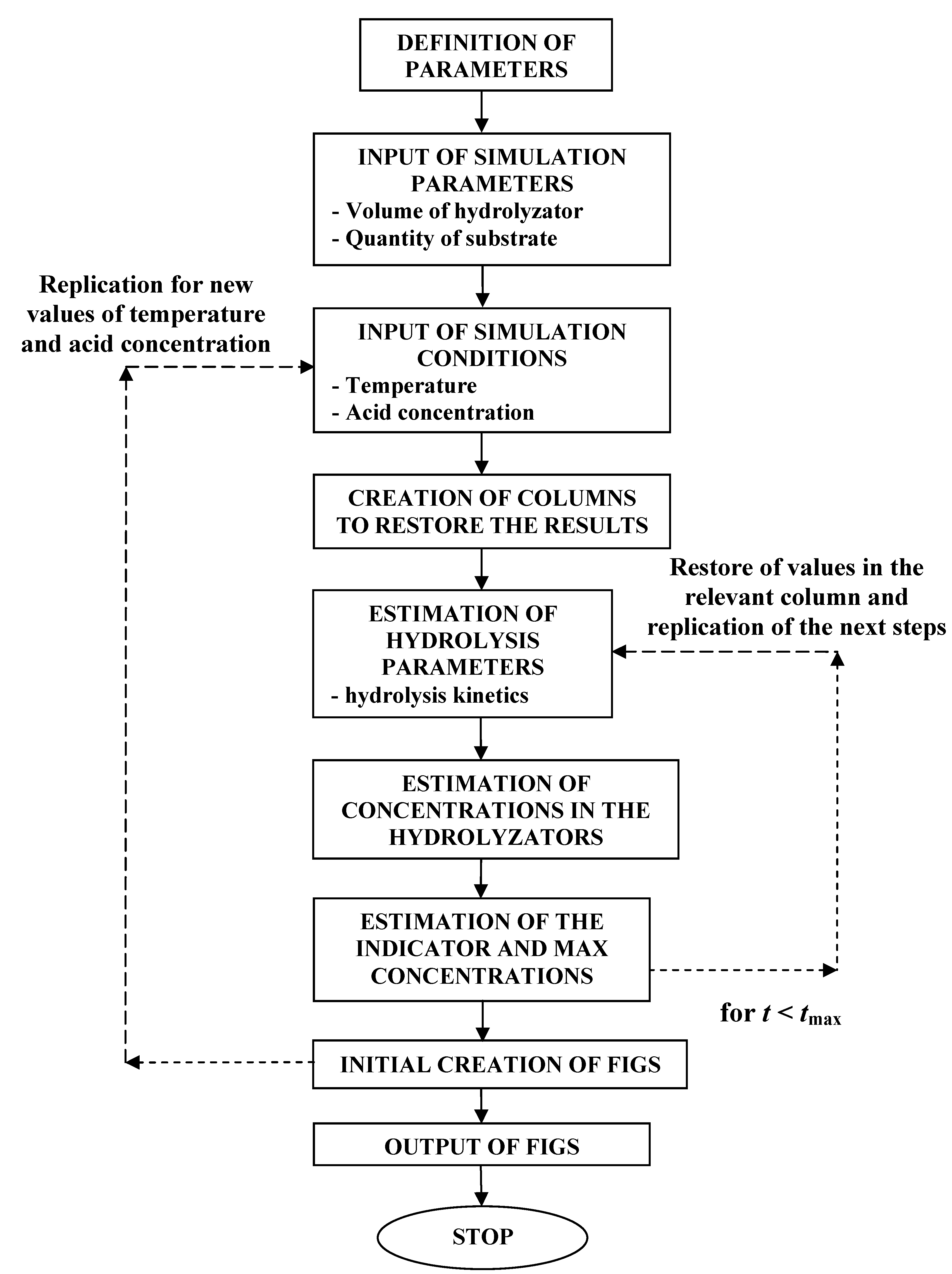
3.6. Simulation Method
- volume
- initial concentration of the LGC
- hydrolysis temperature
- H2SO4 concentration
- raw biomass material (substrate)
4. Results
4.1. General Comments
4.2. Comments on Corn Stover Hydrolysis
4.2.1. Hydrolysis in BR
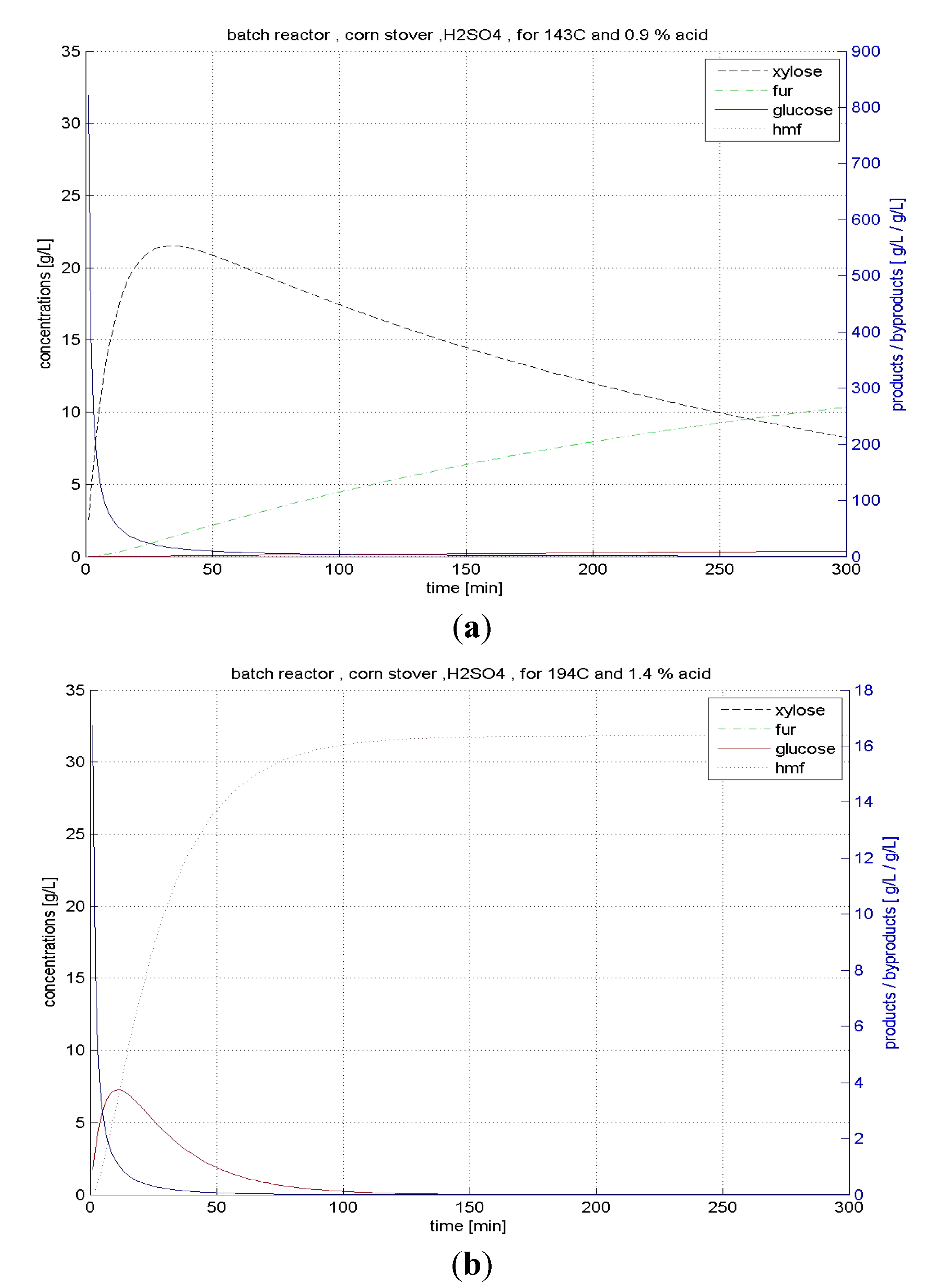
4.2.2. CSTR Hydrolysis
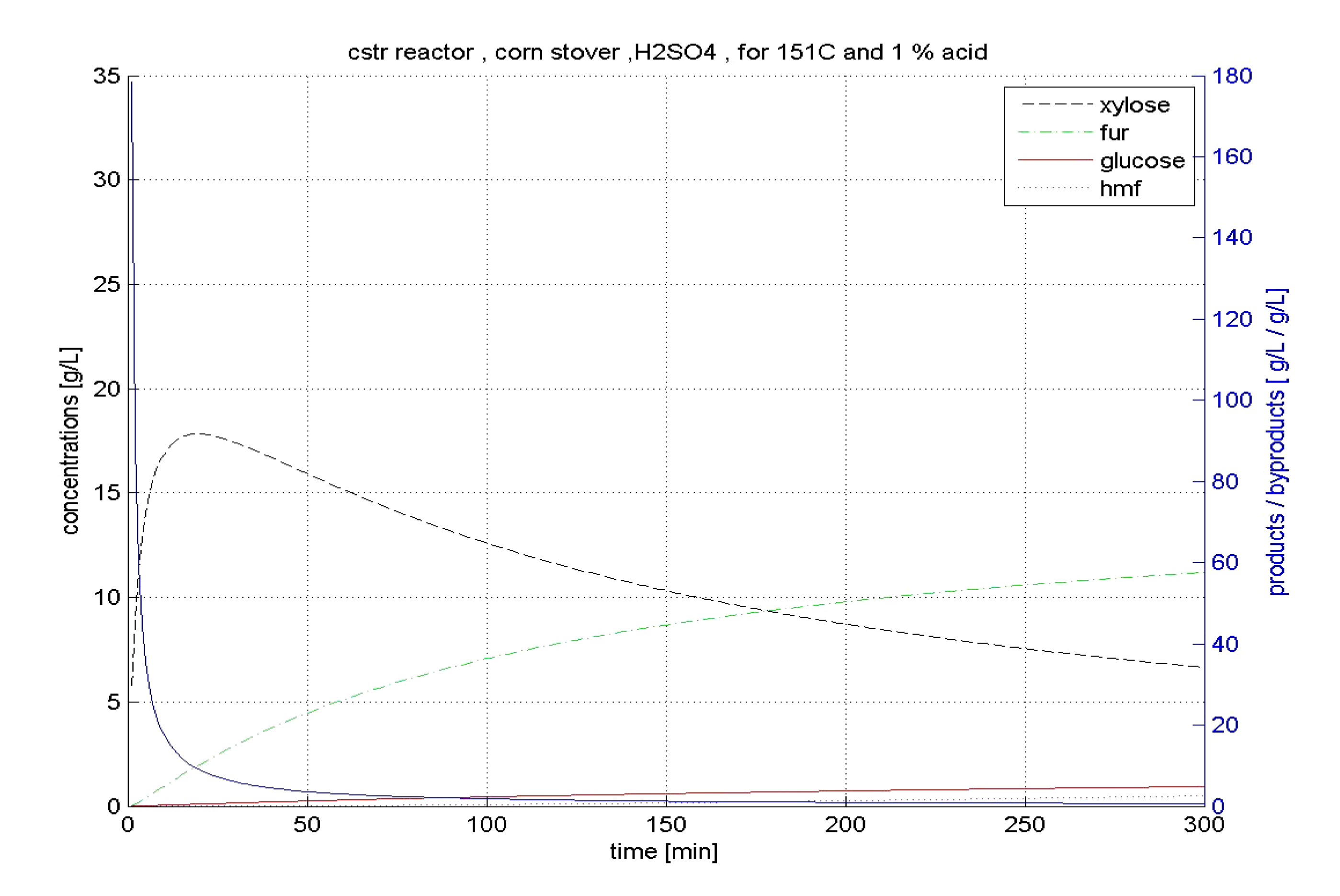
4.3. Comments on Hardwood Hydrolysis
4.3.1. BR Hydrolysis
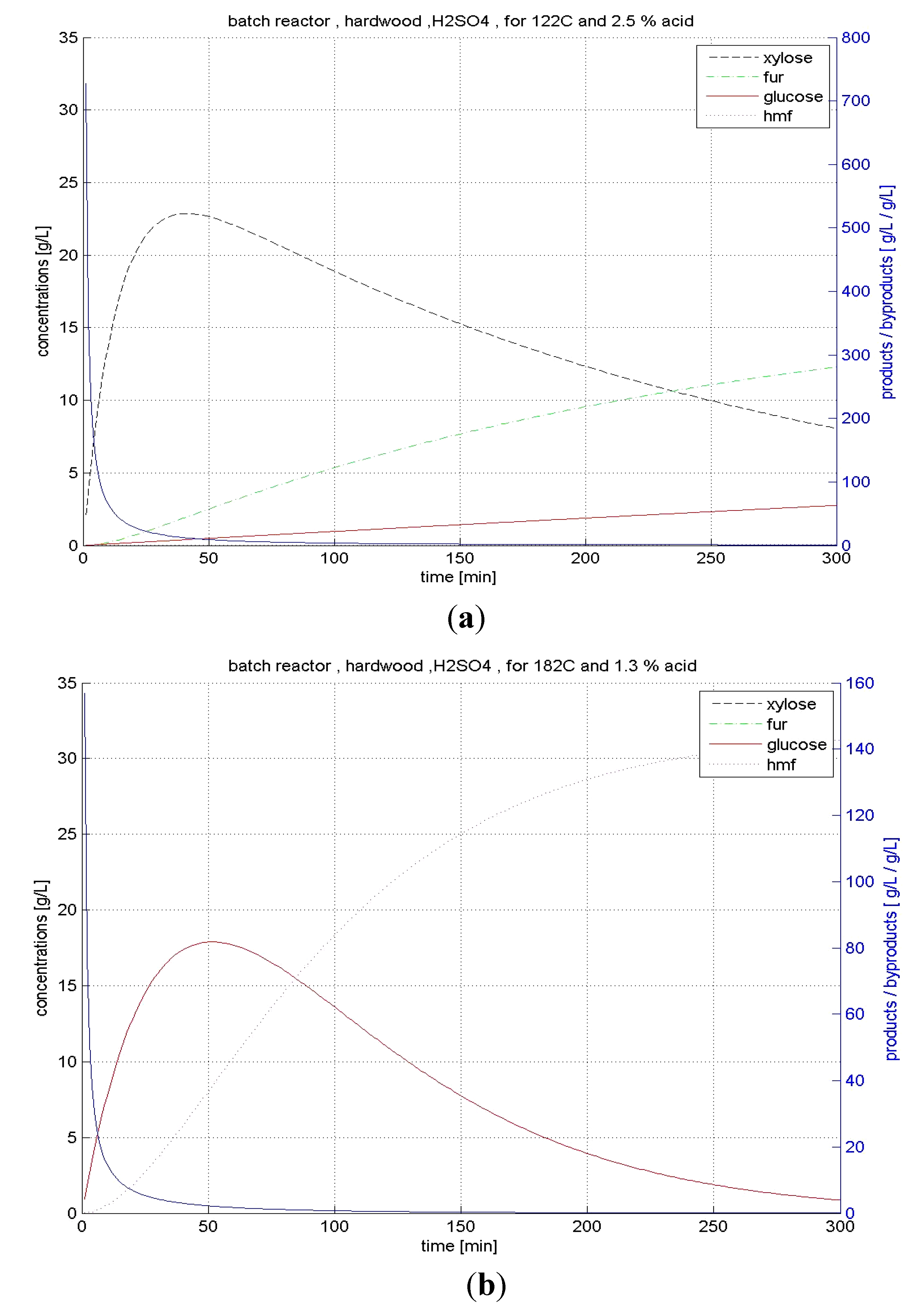
4.3.2. CSTR Hydrolysis

4.4. Comments on Wheat Straw Hydrolysis
4.4.1. BR Hydrolysis
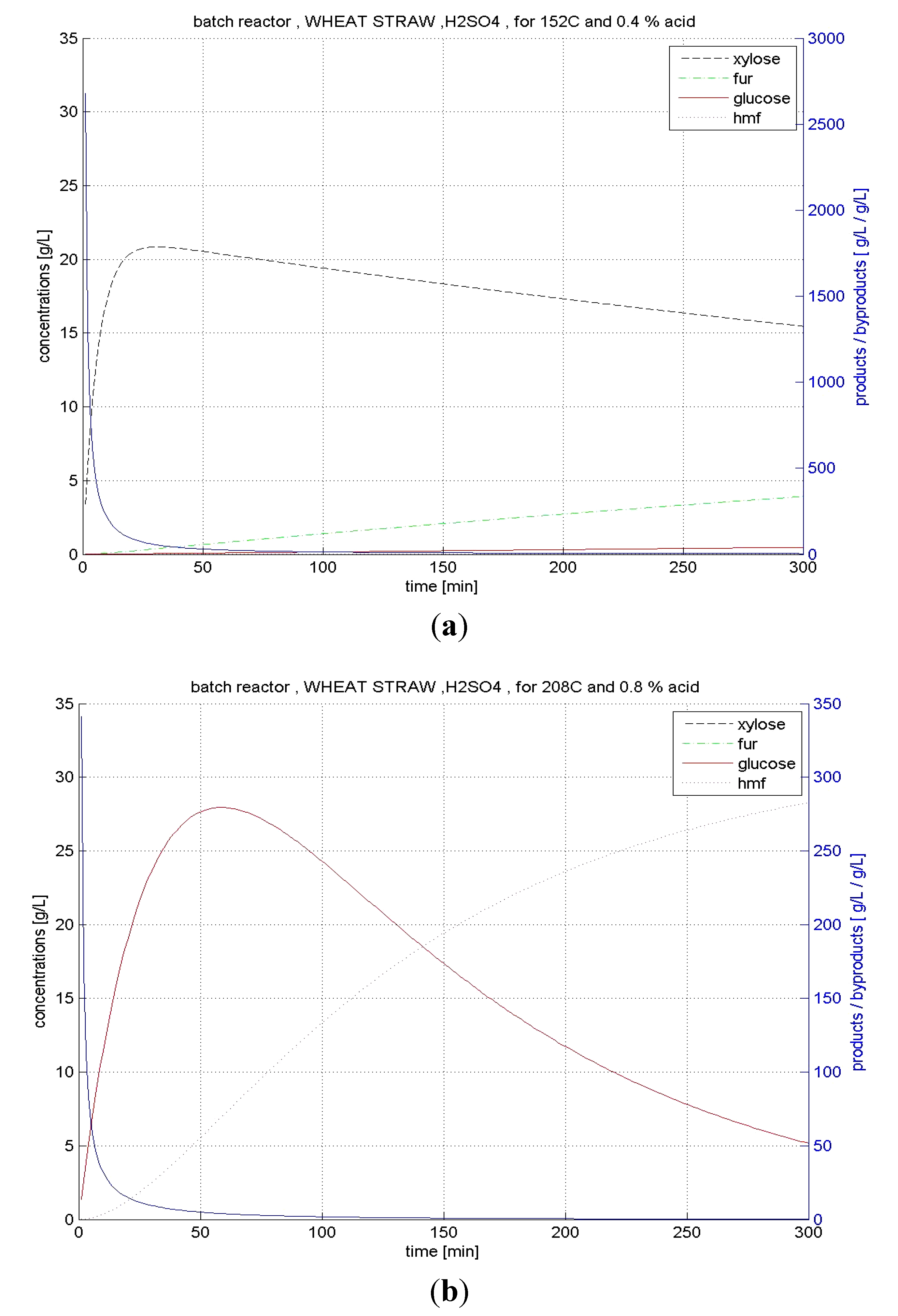
4.4.2. CSTR Hydrolysis
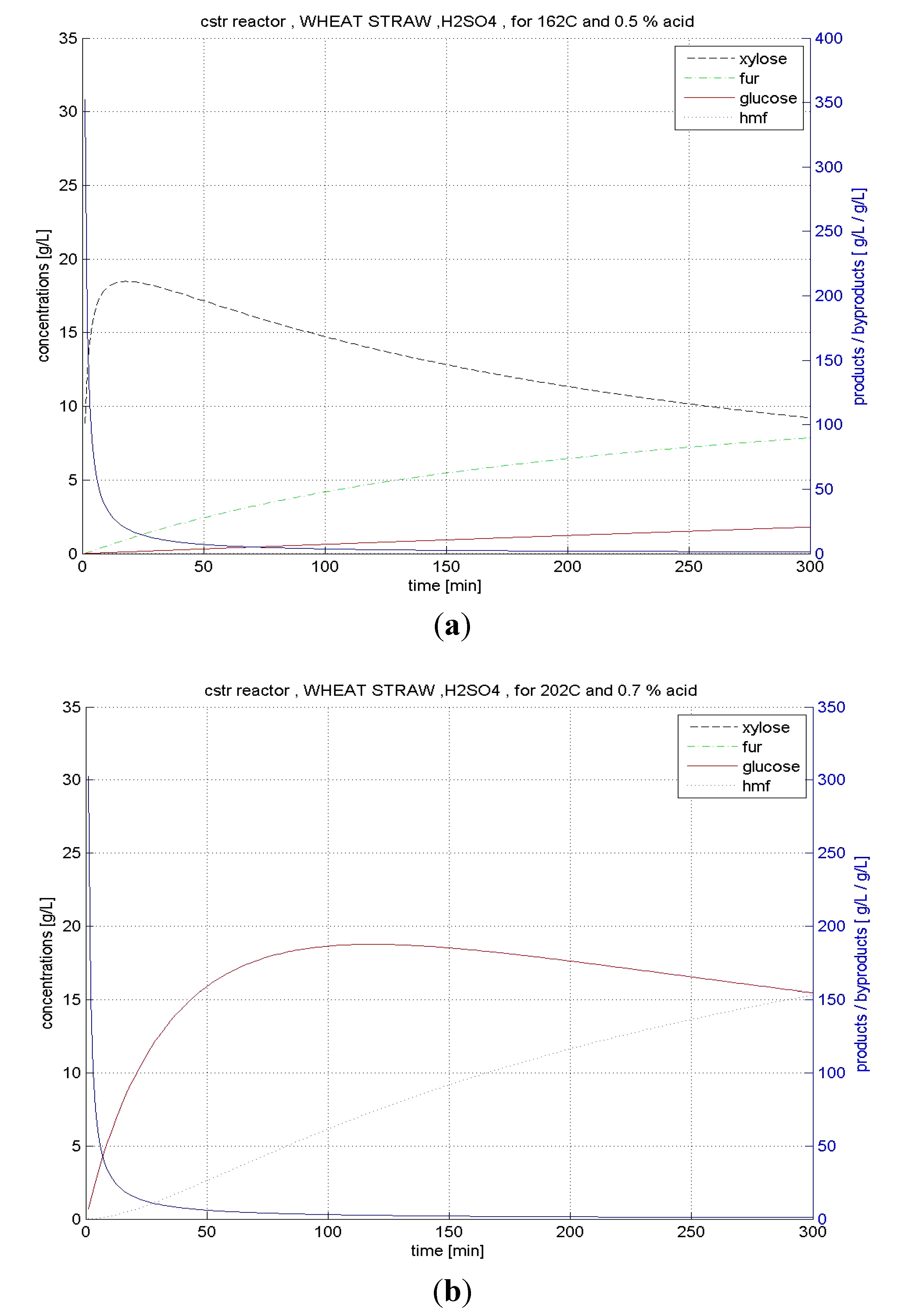
4.5. Overall Evaluation of Raw Materials and Reactors
| LGC | Reactor | Sugar | Τ (°C) | ac (% w/w) | Yield (%) | Indicator |
|---|---|---|---|---|---|---|
| Corn stover | BR | xylose | 143 | 0.9 | 87.9 | 18.66 |
| glucose | 194 | 1.4 | 14.8 | 1.95 | ||
| CSTR | xylose | 151 | 1.0 | 72.4 | 11.92 | |
| glucose | 185 | 1.6 | 9.2 | 1.35 | ||
| Hard- wood | BR | xylose | 122 | 2.5 | 83.1 | 14.66 |
| glucose | 198 | 0.6 | 39.8 | 2.67 | ||
| CSTR | xylose | 129 | 2.0 | 60.6 | 10.84 | |
| glucose | 187 | 1.3 | 23.6 | 3.25 | ||
| Wheat straw | BR | xylose | 152 | 0.4 | 96.1 | 70.82 |
| glucose | 208 | 0.8 | 55.9 | 7.83 | ||
| CSTR | xylose | 162 | 0.5 | 85.3 | 23.58 | |
| glucose | 202 | 0.7 | 36.2 | 5.04 |
5. Conclusions
Abbreviations:
| A1, A2 | pre-exponential parameters (min−1) |
| BR | batch reactor |
| C | acid concentration (%w/w) |
| CS | Cerevisae Sacharomycae |
| CSTR | Continuous Stirred Tank Reactor |
| E1, E2 | activation energy of the production and degradation of glucose |
| G | Glucose concentration |
| HMF | 5-hydroxymethylfurfural |
| LGCs | lignocellulosics |
| n1, n2 | exponential acid parameters |
| T | temperature (°Κ) |
References
- Mielenz, J.R. Ethanol production from biomass: technology and commercialization status. Curr. Opin. Microbiol. 2001, 4, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kondili, E.M.; Kaldellis, J.K. Biofuel implementation in East Europe: Current status and future prospects. Renew. Sustain. Energy Rev. 2007, 11, 2137–2151. [Google Scholar] [CrossRef]
- Lal, R.; Pimentel, M. Soil erosion: A carbon sink or source? Science 2008, 319, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Patzek, T. Green plants, fossil fuels, and now biofuels. Editorial: BioScience 2006, 56, 875. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Corn and cellulosic ethanol cause major problems. Energies 2008, 1, 35–37. [Google Scholar] [CrossRef]
- Tsoutsos, T.; Bethanis, D.; Gekas, V. Simulation of Fermentable Sugar Production from Lignocellulosics to Fuel Bioethanol. In Proceedings of the 15th European Biomass Conference and Exhibition, Berlin, Germany, 7–11 May 2007.
- Sidiras, D.; Koukios, E. Simulation of acid-catalysed organosolv fractionation of wheat straw. Bioresour. Technol. 2004, 94, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Stinson, J.M. Energy from Biomass; Technical Insights, Inc.: New York, NY, USA, 1983. [Google Scholar]
- Tsoutsos, T.D.; Koukios, E.G. Modeling and comparison of major types of dilute acid hydrolysis reactors for cellulose saccharification. Cellul. Chem. Technol. 1990, 24, 713–725. [Google Scholar]
- Taherzadeh, M.J.; Gustafsson, L.; Niklasson, C.; Lidén, G. Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999, 87, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Mamma, D.; Koullas, D.; Fountoukidis, G.; Kekos, D.; Macris, B.J.; Koukios, E. Bioethanol from sweet sorghum: Simultaneous saccharification and fermentation of carbohydrates by a mixed microbial culture. Process Biochem. 1996, 31, 377–381. [Google Scholar] [CrossRef]
- Saeman, J.F. Kinetics of wood saccharification: Hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind. Eng. Chem. 1945, 37, 43–52. [Google Scholar] [CrossRef]
- Tsoutsos, T.D.; Koukios, E.G. Selection of a reaction system for cellulose saccharification: A global approach. Chem. Biochem. Eng. Quart. 1991, 3, 151–156. [Google Scholar]
- Maloney, M.T. An engineering analysis of the production of xylose by dilute-acid hydrolysis of hardwood hemicellulose. Biotechnol. Prog. 1986, 2, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.P.; Lee, Y.Y.; McCaskey, T.A. Liquid Fuel and Chemical Production from Cellulosic Biomass—Hemicellulose Recovery and Pentose Utilization in a Biomass Processing Complex. In Proceedings of the 3rd Annual Biomass System Conference (DE-AS051), Golden, CO, USA, 1979; pp. 255–264.
- Sidiras, D.; Batzias, F.; Schroeder, E.; Ranjan, R.; Tsapatsis, M. Dye adsorption on autohydrolyzed pine sawdust in batch and fixed-bed systems. Chem. Eng. J. 2011, 171, 883–896. [Google Scholar] [CrossRef]
- McPharland, J.J.; Grethlein, H.E.; Converse, A.O. Kinetics of acid hydrolysis of corn stover. Sol. Energy 1982, 28, 55–63. [Google Scholar] [CrossRef]
- Ranganathan, D.G.; MacDonald, D.G.; Bakhshi, N.N. Kinetic studies of wheat straw hydrolysis using sulphuric acid. Can. J. Chem. Eng. 1985, 63, 840–844. [Google Scholar] [CrossRef]
- Bhandari, N.; Macdonald, D.G.; Bakhshi, N.N. Kinetic studies of corn stover saccharification using sulphuric acid. Biotechnol. Bioeng. 1984, 26, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, R.; Ramırez, J.A.; Garrote, G.; Vazquez, M. Kinetic study of the acid hydrolysis of sugar cane bagasse. J. Food Eng. 2002, 55, 309–318. [Google Scholar] [CrossRef]
- Aguilar, R.; Ramırez, J.A.; Garrote, G.; Vazquez, M. Hydrolysis of sugar cane bagasse using nitric acid: a kinetic assessment. J. Food Eng. 2004, 61, 143–152. [Google Scholar] [CrossRef]
- Maloney, M.T.; Chapman, T.W.; Baker, A.J. Dilute acid hydrolysis of paper birch: kinetic studies of xylan and acetyl-group hydrolysis. Biotechnol. Bioeng. 1985, 27, 335–361. [Google Scholar]
- Harris, J.F.; Baker, A.J.; Conner, A.H.; Jeffries, T.W.; Minor, J.L.; Pettersen, R.C.; Scott, R.W.; Springer, E.L.; Wegner, T.H.; Zerbe, J.I. Two-Stage Dilute Acid Hydrolysis of Wood: An Investigation of Fundamentals; Gen. Tech. Rep. FLP-45; Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 1985. [Google Scholar]
- Veeraraghavan, S.; Chambers, R.P.; Myles, M.Y.; Lee, A. Kinetic Model and Reactor Development in Hemicellulose Hydrolysis. In Proceedings of the AIChE National Meeting, Orlando, FL, USA, 1982.
- Carrasco, E.; Roy, C. Kinetic study of dilute-acid prehydrolysis of xylan-containing biomass. Wood Sci. Technol. 1992, 26, 189–208. [Google Scholar]
- California Energy Commission. Appendices evaluation of biomass-to-ethanol fuel potential in California. Available online: http://www.energy.ca.gov/reports/1999-12-22_500-99-022.html (accessed on 19 February 2011).
- Boyer, L.J.; Vega, J.L.; Klasson, K.T.; Klausen, E.C.; Gaddy, J.L. The effects of furfural on ethanol production by S. cerevisiae in Batch culture. Biomass Bioenergy 1992, 3, 41–48. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Dien, B.S.; Berhow, M.A.; Kurtzman, C.P.; Gorsich, S.W. Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical. J. Ind. Microbiol. Biotechnol. 2004, 31, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.; Hahn-Hiigerdal, B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzym. Microb. Technol. 1996, 18, 312–331. [Google Scholar] [CrossRef]
- Banerjee, N.; Bhatnagar, R.; Viswanathan, L. Inhibition of glycolysis by furfural in Saccharomyces cerevisiae. Eur. J. Appl. Microbiol. Biotechnol. 1981, 11, 226–228. [Google Scholar] [CrossRef]
- Sanchez, B.; Bautista, J. Effects of furfural and 5-hydroxymethylfurfural on the fermentation of Saccharomyces cerevisiae and biomass production from Candida guilliermondii. Enzym. Microb. Technol. 1988, 10, 315–318. [Google Scholar] [CrossRef]
- Azhar, A.F.; Bery, M.K.; Colcord, A.R.; Roberts, R.S.; Corbitt, G.V. Factors affecting alcohol fermentation of wood acid hydrolyzate. Biotechnol. Bioeng. Symp. 1981, 11, 293–300. [Google Scholar]
- Vamvuka, D.; Tsoutsos, T. Energy exploitation of biomass residues in the island of Crete. Energy Explor. Exploit. 2002, 20, 113–120. [Google Scholar] [CrossRef]
- Kwarteng, I.K. Kinetics of the Acid Hydrolysis of Hardwood in a Continuous Plugflow Reactor. Ph.D. Thesis, Thayer School of Engineering, Dartmouth College, Hanover, Germany, 1983. [Google Scholar]
- Converse, A.O.; Kwarteng, I.K.; Grethlein, H.E.; Ooshima, H. Kinetics of thermochemical pretreatment of lignocellulosic materials. Appl. Biochem. Biotechnol. 1989, 20/21, 63–78. [Google Scholar] [CrossRef]
- Lee, W.G.; Lee, J.S.; Shin, C.S.; Park, S.C.; Chang, H.N.; Chang, Y.K. Ethanol production using concentrated oak wood hydrolyzates and methods to detoxify. Appl. Biochem. Biotechnol. 1999, 13, 547–559. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- MathWorks. MATLAB Programming Tips, Version 6. 2002. e-book. Available online: http://www.caspur.it/risorse/softappl/doc/matlab_help/pdf_doc/matlab/programming_tips.pdf (accessed on 19 February 2011).
- MathWorks. Using MATLAB Graphics, Version 6. 2002. e-book. Available online: http://courses.washington.edu/css485/graphg.pdf (accessed on 19 February 2011).
- Abasaeed, A.E.; Lee, Y.Y. Effect of transient heat transfer and particle size on acid hydrolysis of hardwood cellulose. Bioresour. Technol. 1990, 35, 15–21. [Google Scholar] [CrossRef]
- Jimenez, L.; Ferrer, J.L. Saccharification of wheat straw for the production of alcohols. Process Biochem. 1991, 26, 153–156. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsoutsos, T.; Bethanis, D. Optimization of the Dilute Acid Hydrolyzator for Cellulose-to-Bioethanol Saccharification. Energies 2011, 4, 1601-1623. https://doi.org/10.3390/en4101601
Tsoutsos T, Bethanis D. Optimization of the Dilute Acid Hydrolyzator for Cellulose-to-Bioethanol Saccharification. Energies. 2011; 4(10):1601-1623. https://doi.org/10.3390/en4101601
Chicago/Turabian StyleTsoutsos, Theocharis, and Dimitris Bethanis. 2011. "Optimization of the Dilute Acid Hydrolyzator for Cellulose-to-Bioethanol Saccharification" Energies 4, no. 10: 1601-1623. https://doi.org/10.3390/en4101601
APA StyleTsoutsos, T., & Bethanis, D. (2011). Optimization of the Dilute Acid Hydrolyzator for Cellulose-to-Bioethanol Saccharification. Energies, 4(10), 1601-1623. https://doi.org/10.3390/en4101601






