Principles and Materials Aspects of Direct Alkaline Alcohol Fuel Cells
Abstract
:1. Introduction
- ▪
- Alcohol crossover from the anode to the cathode
- ▪
- The relatively low activity and complex reaction mechanism of most alcohols
2. Principles and Mechanisms of a Direct Alkaline Alcohol Fuel Cell (DAAFC)
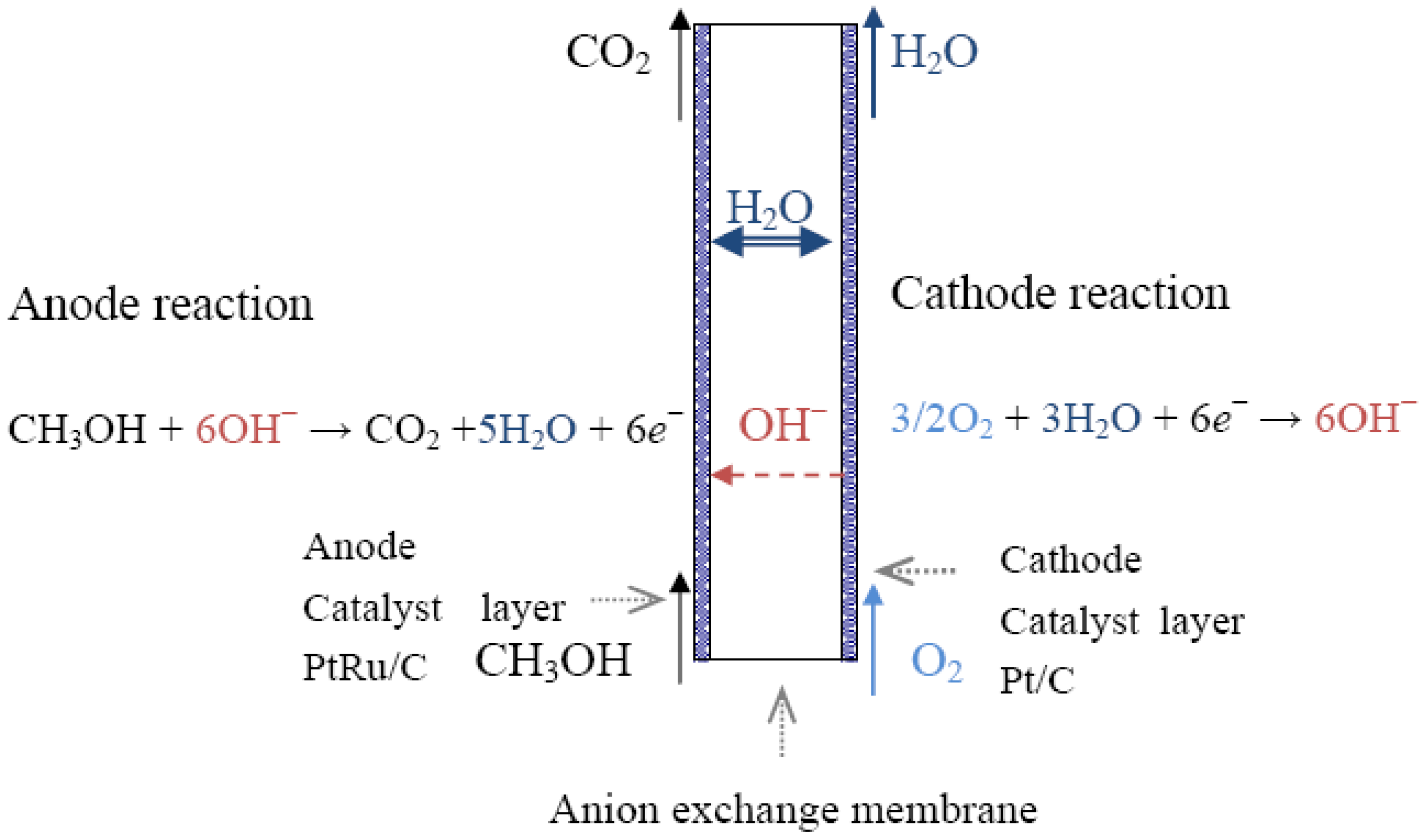
2.1. Alcohol Oxidation
Pt + (CH3OH)sol→Pt-(CH3OH)ads
Pt-(CH3OH)ads + OH−→Pt-(CH3O)ads + H2O + e−
Pt-(CH3O)ads + OH−→Pt-(CH2O)ads + H2O + e−
Pt-(CH2O)ads + OH−→Pt-(CHO)ads + H2O + e−
Pt-(CHO)ads + OH−→Pt-(CO)ads + H2O + e−
Pt-(CHO)ads + Pt-(OH)ads + 2OH−→2Pt + CO2 + 2H2O + 2e−
Pt-(CHO)ads + Pt-(OH)ads + OH−→Pt + Pt-(COOH)ads + H2O + e−
Pt-(CO)ads + Pt-(OH)ads + OH−→2Pt + CO2 + H2O + e−
Pt-(CO)ads + Pt-(OH)ads↔Pt + Pt-(COOH)ads
Pt-(COOH)ads + OH−→Pt-(OH)ads + HCOO−
Pt-(COOH)ads + Pt-(OH)ads→2Pt +CO2 + H2O
2. M + CH3CH2OH→M-(CH3 CH2OH)ads
3. M-(CH3CH2OH)ads + 3OH−→M-(CH3CO)ads + 3H2O + 3e−
4. M-(CH3CO)ads + M-OHads→M-CH3COOH + M
5. M-CH3COOH + OH−→M + CH3COO− + H2O
M = Au or Pd.
| Fuel | Anode Reactions | E° (V/SHE) | Energy density (Wh/kg) |
|---|---|---|---|
| Methanol CH3OH | CH3OH + 6OH−→CO2 + 5H2O + 6e− | −0.81 | 6100 |
| Ethanol CH3CH2OH | CH3CH2OH +2OH−→CH3CHO + 2H2O + 2e− CH3CH2OH + 4OH−→CH3COOH + 3H2O + 4e− CH3CH2OH + 12 OH−→2CO2 +9 H2O+12e− | −0.77 | 8030 |
| iso-Propanol CH3CHOHCH3 | CH3CHOHCH3 + 2OH−→CH3COCH3 + 2H2O + 2e− CH3COCH3 + 16OH−→3CO2 + 11H2O + 16e− | −0.67 | 8600 |
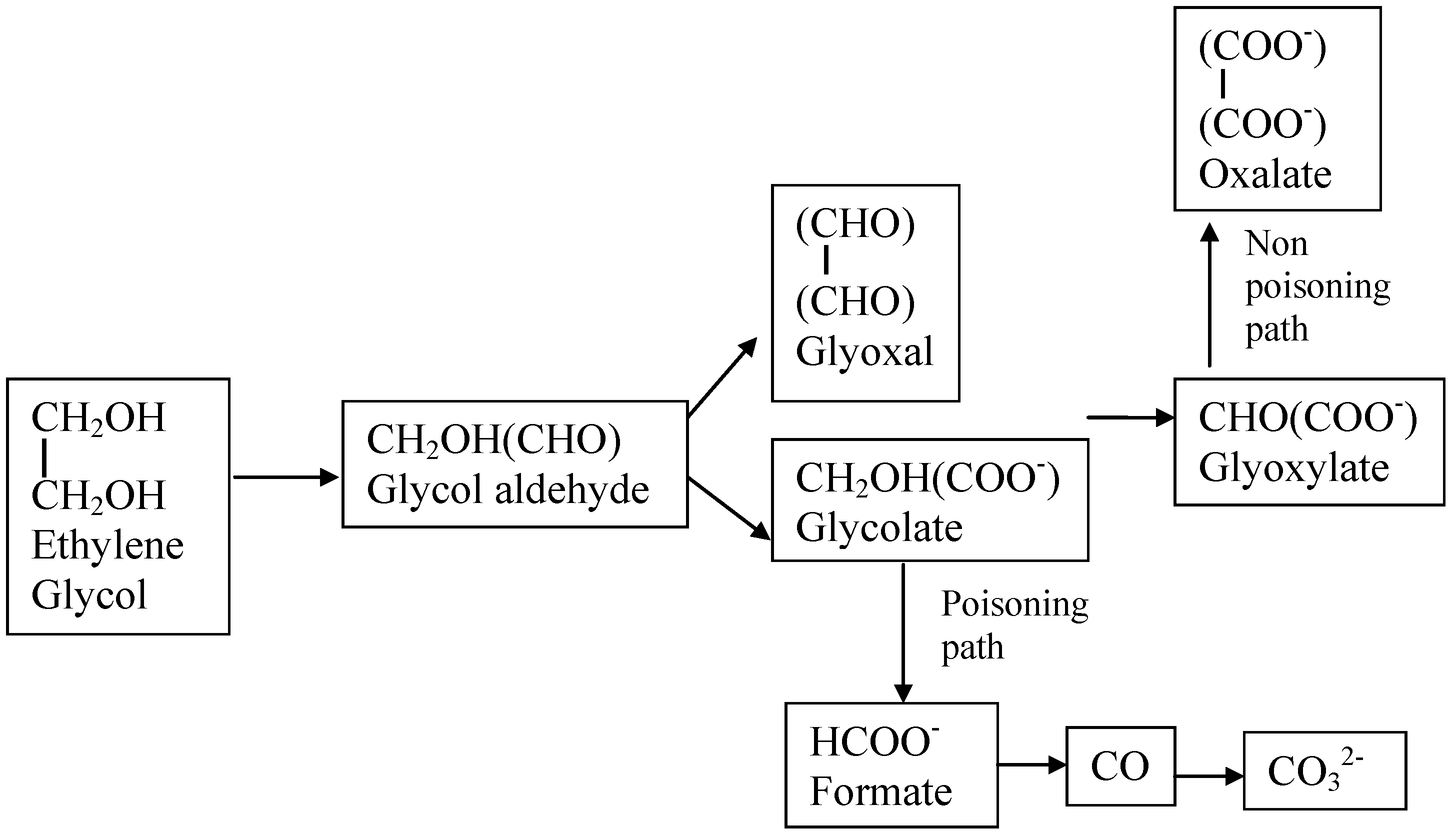
| Ethylene glycol (CH2OH)2 | (CH2OH)2 + 14OH−→2CO32− + 10H2O + 10e− or (CH2OH)2 + 10OH−→(CO2)22− + 8H2O + 8e− | −0.72 | 5200 |
| Glycerol HOCH2CHOHCH2OH | HOCH2CHOHCH2OH+ 20OH−→3CO32− + 14H2O + 14e− or HOCH2CHOHCH2OH + 12OH−→(COO−-COH-COO−) + 10H2O + 10e− | −0.69 | 5000 |
2.2. Oxygen Reduction

O2− + H2O→HO2− + OH
OH + e− ↔ OH−
3. Catalysts for DAAFCs
3.1. Alcohol Oxidation Catalysts
3.1.1. Precious Metal Catalysts
| Catalyst | OCV/V | Power density/mWcm−2 |
|---|---|---|
| Pt/C | 0.66 | 19 |
| PtBi/C | 0.83 | 22 |
| PtPdBi/C | 0.81 | 28 |
3.1.2. Non-Precious Metal Catalysts
3.2. Catalysts for Oxygen Reduction
3.2.1. Precious Metal ORR Catalysts
3.2.2. Non-Precious ORR Catalysts
4. Electrolyte and Membrane Electrolyte for DAAFC
4.1. Cation Exchange Membranes
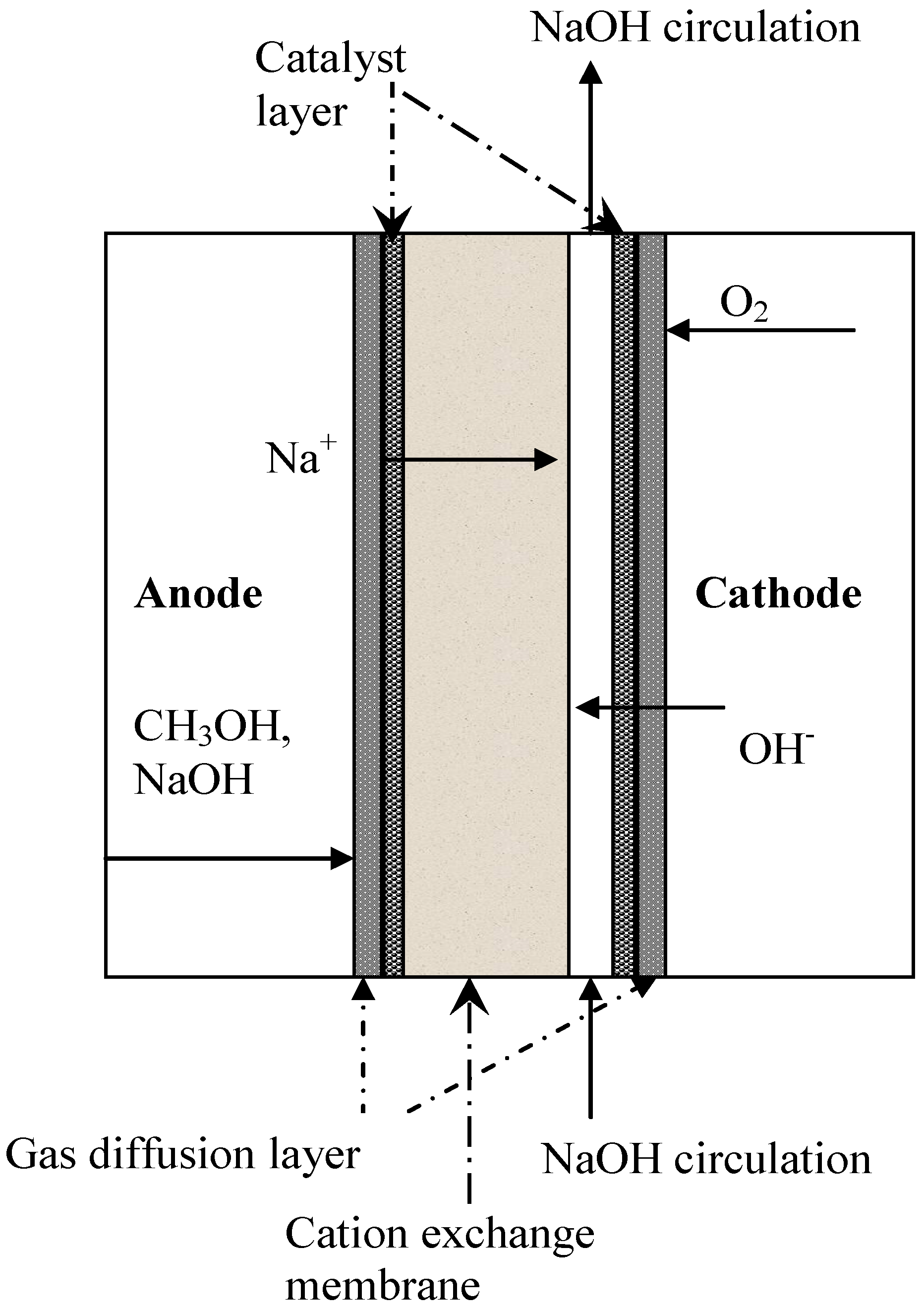
4.2. Anion Exchange Membranes (AEM)
| Membrane | MORGANE®-ADP |
| Material | Cross-linked fluorinated polymer |
| Exchange group | Quaternary ammonium |
| Thickness/μm fully humidified | 150–160 |
| Resistance (in 0.6 M NaCl)/Ω.cm2 | 1.5–4.5 |
| Resistance (in 1 M NaOH)/Ω | 0.5 |
| Maximum operational temperature/°C | 55 |
| Working pH | 0-10 |
| Membrane | AHA | A201 | A901 |
|---|---|---|---|
| Type | Strong basic anion permeable | ||
| Electric Resistance (0.5 N NaCl)/Ω.cm2 | 4.1 | 0.30 | |
| Burst strength/MPa | ≥0.90 | 0.4 | 0.2 |
| Exchange group | Tetraalkyl ammonium groups with polyolefin backbone chain | Quaternary ammonium | Quaternary ammonium |
| Thickness/µm | 240 | 28 | 10 |
| Ion exchange capacity/mmol g-1 | 1.15–1.25 | 1.7 | 1.7 |
| OH- conductivity/mS cm-2 | 29 | 11.4 |
5. Performance of DAAFC
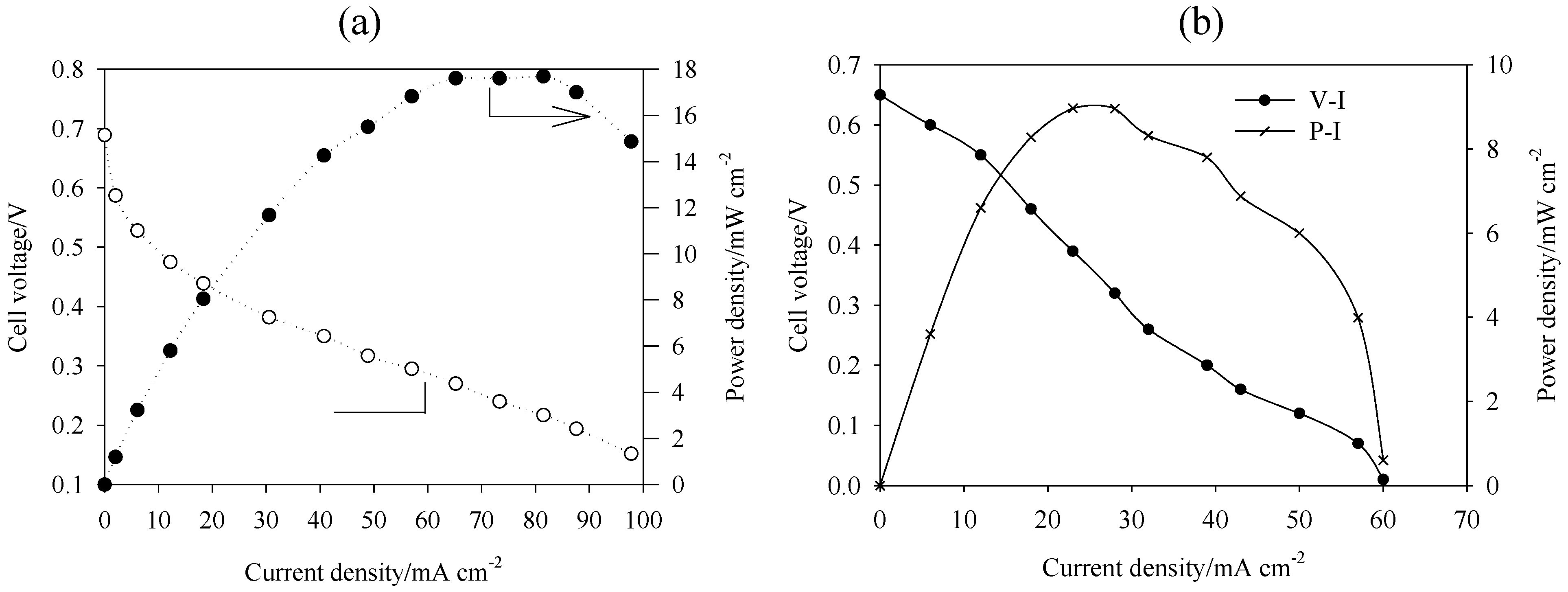
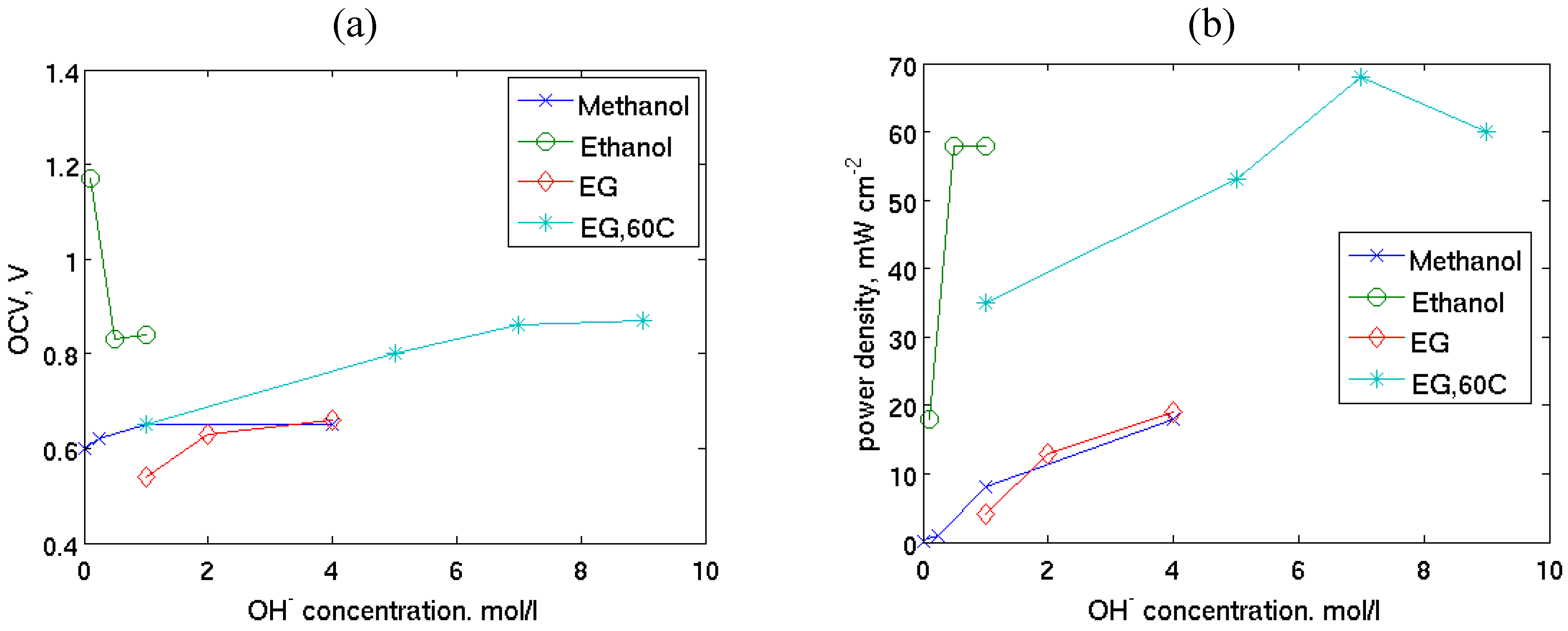
| Fuel/oxidant | Catalysts | Electrolyte/membrane | T/°C | OCV/V | Imax/mA cm−2 | Pmax/mW cm−2 | Ref. | |
| Anode | Cathode | |||||||
| DMAFC w/o alkaline | ||||||||
| 2 M methanol/O2 no backpressure no backpressure 2.5 bar back pressure 2.5 bar back pressure | Pt 4 mg cm−2 | Pt 4 mg cm−2 | Quaternised radiation-grafted ETFE AAEM | 50 60 60 80 | 0.46 0.52 0.58 0.63 | 22 34 34 68 | 1.5 2.4 4.2 8.5 | [123] |
| 1 M methanol/O2 | Pt/Ru 1 mg cm−2 | Pt/C Pd/C . 0.5 mg cm−2 | Tokuyama A201 | 80 | 0.57 0.64 | 34 34 | 2.6 2.6 | [57] |
| 2 M methanol/air | Pt/Ru 1 mg cm−2 | Pt 1 mg cm−2 | ADP | 30 40 50 60 | 0.6 0.62 0.65 0.65 | 32 43 57 60 | 5.9 6.9 7.6 9.0 | [102] |
| 16% methanol in N2/air | PtRu | Pt | PVA+10M KOH PVA+10M KOH/Ni-LDH | 40 | 0.9 | 80 100 | 22 35 | [135] |
| Fuel/oxidant | Catalysts | Electrolyte/membrane | T/°C | OCV/V | Imax/mA cm−2 | Pmax/mW cm−2 | Ref. | |
| DMAFC w/alkaline | ||||||||
| 1 M methanol/O2 no NaOH 0.25 M NaOH 1 M NaOH 4 M NaOH (2 M methanol) | Pt/C 2 mg cm−2 | Pt/C 2 mg cm−2 | ADP | Room T | 0.48 0.52 0.6 0.6 | 3 10 52 110 | 0.2 1 8 18 | [125] |
| 2 M methanol, 1 M NaOH/air | Pt/C 2 mg cm−2 | Pt/C 2 mg cm−2 | ADP | 60 | 0.7 | 100 | 18 | [21] |
| 2 M methanol, 1 M NaOH/ air | Pt/C 2 mg cm−2 | Pt/C 2 mg cm−2 | Nafion | 60 | 0.80 | 23 | 4.5 | [101] |
| 7 M methanol, 1 M KOH/ air (passive) | PtRu 4 mg cm−2 | Pt/C 1 mg cm−2 | Tokuyama | Room T | 0.71 | 58 | 12.8 | [126] |
| 2 M methanol, 2 M KOH/ O2 | PtRu/C 2 mg cm−2 | Pt/C 1 mg cm−2 | PBI/KOH | 90 | 1.0 | 105 | 30 | [116] |
| 2 M methanol, 2 M KOH/ O2 | PtRu/C 2 mg cm−2 | Pt/C 1 mg cm−2 | PBI/KOH | 75 90 | 0.92 0.98 | 150 190 | 49 61 | [117] |
| 1 M methanol, 1 M KOH/saturated O2 in 1 N H2SO4 | PtRu 2 mg cm−2 | Pt 2 mg cm−2 | laminar flow- based micro fuel cells | Room T | 1.4 | 50 | 12 | [125] |
| 4 M methanol, 4 M KOH/ air | Pt/Ru 4 mg cm−2 | MnO2/C 4 mg cm−2 | QPVA/Al2O3 | Room T | 0.88 | 153 | 36 | [122] |
| DAAFC w/ alkaline | ||||||||
| 1 M KOH /humidified O2 methanol ethylene glycol glycerol erythritol xylitol | Pt/C 1 mg cm−2 | Ag/C 1 mg cm−2 or PtRu/C 4 mg cm−2 | AHA | 50 | 0.80 | 46 65 55 42 29 | 6.0 9.0 6.8 5.5 4.0 | [13] |
| 1 M ethanol, KOH/ humidified O2 0.1 M KOH 0.5 M KOH 1.0 M KOH | PtRu 3 mg cm−2 | Pt 3 mg cm−2 | AHA | Room T | 1.17 0.83 0.84 | 100 350 400 | 18 58 58 | [34] |
| 4 M KOH/air 2 M methanol 2 M ethanol 2 M isopropanol | Pt/Ru 3.6 mg cm−2 | MnO2/C | PVA/TiO2 composite membrane | Room T | 0.80 | 33.6 35.7 21.5 | 9.3 8.0 5.5 | [121] |
| 2 M glycerol, 4 M NaOH/ O2 | Pt/C Pd/C Au/C AuPd/C | Pt/C 2 mg cm−2 | ADP | Room T | 0.68 0.59 0.60 0.49 | 20 16 7.5 3.2 | 4.2 2.4 1.0 0.3 | [58] |
| 2 M EG, 4 M NaOH/ O2 | Pt PtBi PtPdBi 2 mg cm−2 | Pt 2 mg cm−2 | ADP | 20 | 0.66 0.83 0.81 | 132 132 120 | 19 22 28 | [51] |
| 2 M EG, NaOH/O2 1 M NaOH 2 M NaOH 4 M NaOH | Pt 2 mg cm−2 | Pt 2 mg cm−2 | ADP | 20 | 0.54 0.63 0.66 | 42 102 132 | 4 13 19 | [51] |
| Fuel/oxidant | Catalysts | Electrolyte/membrane | T/°C | OCV/V | Imax/mA cm−2 | Pmax/mW cm−2 | Ref. | |
| 2 M EG, 4 M NaOH /O2 | Pt/C 2 mg cm−2 | Pt/C 2 mg cm−2 | ADP | Room T | 0.65 | 130 | 18 | [125] |
| 0.5 M ethanol, air, 2 M KOH | PtRu/C 1.8 mg cm−2 | Pt/C 2 mg cm−2 | Porous separator | Room T | 1.0 | 110 | 30 | [128] |
| DAAFC with non Pt catalyst, with alkaline | ||||||||
| 2 M KOH/ O2 10 wt% methanol 10 wt% ethanol 5 wt% glycerol | Pd/MWCNT 1 mg cm−2 | Fe-Co HypermecTM K-14 | Tokuyama A-210 | 80 | 0.89 0.87 0.87 | 530 350 550 | 95 74 79 | [61] |
| 2 M KOH/O2 10 wt% methanol 10 wt% ethanol 5 wt% glycerol | PdNiZn/C 1 mg cm−2 | Fe-Co HypermecTM K-14 | Tokuyama A-210 | 80 | 0.76 0.88 0.81 | 610 800 540 | 120 165 119 | [61] |
| 2 M methanol, 0.05 M H2O2, 0.2M KOH | Ni(OH)2 | Ag2O | - | Room T | 0.11 | 1 | 0.03 | [127] |
| 10 wt% ethanol, 2 M KOH / O2 | PdNiZn/C 1 mg cm−2 | Fe-Co HypermecTM K-14 | Tokuyama A-210 | 25 40 60 80 | 0.65 0.68 0.74 0.82 | 400 440 550 590 | 65 80 120 160 | [61] |
| 1 M ethanol, 1 M KOH/O2 | HypermecTM 2 mg cm−2 | HypermecTM 1 mg cm−2 | Tokuyama | 30 40 50 60 | 0.66 0.72 0.76 0.91 | 170 200 230 270 | 12 17 22 30 | [98] |
| 2 M ethanol, 3 M KOH/air | RuV/C 4.5 mg cm−2 | TMPhP/C 9mg cm−2 | PBI/KOH | 80 | 0.93 | 278 | 125 | [118] |
| 1 M EG, KOH /O2 1 M KOH 5 M KOH 7 M KOH 9 M KOH | PdNi 2 mg cm−2 | HypermecTM 1 mg cm−2 | Tokuyama | 60 | 0.65 0.8 0.86 0.87 | 320 350 390 370 | 35 53 67 60 | [59] |
6. Summary and Outlook
- (1)
- Metal oxides as the promoter for catalysts.Some metal oxides, in particular CeO2, Mn3O4 and SnO2, have multiple oxidation states and can store and release oxygen [132]. These metal oxides can be used as promoters to improve catalyst activity and fuel cell performance.
- (2)
- Transition metal macrocycle based oxygen reduction catalysts with improved activity and selectivity.These catalysts have shown highly selective catalytic activity for oxygen reduction in the presence of methanol and CO in direct methanol fuel cells (DMFC) and hydrogen fuel cells [133,134]. Metal macrocyclic catalysts are stable in neutral and alkaline media. This suggests their application will be more feasible for DAAFCs.
- (3)
- Novel anion exchange membranes with improved stability and ionic conductivity.One of the key issues in the development of DAAFCs is the membrane electrolyte. There is still no AEM equivalent to Nafion for solid polymer electrolyte alkaline fuel cells, even though significant progress has been made in the last decade. Further investigation on poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) based membranes, alkaline doped AEMs and composite AEMs might be possible to produce a novel AEM with improved properties suitable for DAAFC applications.
Acknowledgement
References
- Bockris, J.O.; Conway, B.E.; White, R.E. Modern Aspects of Electrochemistry; Plenum Press: New York, NY, USA, 2001; Volume 34, p. 200. [Google Scholar]
- Zhang, X.Y.; Lu, W.; Da, J.Y.; Wang, H.T.; Zhao, D.Y.; Webley, P.A. Porous platinum nanowire arrays for direct ethanol fuel cell applications. Chem. Commun. 2009, 8, 195–197. [Google Scholar] [CrossRef]
- Varela, F.J.R.; Savadogo, O. Ethanol-tolerant Pt-alloy cathodes for direct ethanol fuel cell (DEFC) applications. Asia-Pac. J. Chem. Eng. 2009, 4, 17–24. [Google Scholar] [CrossRef]
- Neto, A.O.; Linardi, M.; dos Anjos, D.M.; Tremiliosi, G.; Spinace, E.V. Electro-oxidation of ethanol on PtSn/CeO2-C electrocatalyst. J. Appl. Electrochem. 2009, 39, 1153–1156. [Google Scholar] [CrossRef]
- Ling, J.; Longtin, G.; Savadogo, O. Comparison of ethanol and methanol crossover through different MEA components and structures by cyclic voltammetry. Asia-Pac. J. Chem. Eng. 2009, 4, 25–32. [Google Scholar] [CrossRef]
- Xu, C.W.; Cheng, L.Q.; Shen, P.K.; Liu, Y.L. Methanol and ethanol electrooxidation on Pt and Pd supported on carbon microspheres in alkaline media. Electrochem. Commun. 2007, 9, 997–1001. [Google Scholar] [CrossRef]
- Antolini, E. Catalysts for direct ethanol fuel cells. J. Power Source. 2007, 170, 1–12. [Google Scholar] [CrossRef]
- Qi, Z.G.; Kaufman, A. Performance of 2-propanol in direct-oxidation fuel cells. J. Power Source. 2002, 112, 121–129. [Google Scholar] [CrossRef]
- Qi, Z.G.; Hollett, M.; Attia, A.; Kaufman, A. Low temperature direct 2-propanol fuel cells. Electrochem. Solid State Lett. 2002, 5, A129–A130. [Google Scholar] [CrossRef]
- Chetty, R.; Scott, K. Catalysed titanium mesh electrodes for ethylene glycol fuel cells. J. Appl. Electrochem. 2007, 37, 1077–1084. [Google Scholar] [CrossRef]
- Neto, A.O.; Linardi, M.; Spinace, E.V. Electro-oxidation of ethylene glycol on PtSn/C and PtSnNi/C electrocatalysts. Ionics 2006, 12, 309–313. [Google Scholar] [CrossRef]
- Neto, A.O.; Vasconcelos, T.R.R.; Da Silva, R.; Linardi, M.; Spinace, E.V. Electro-oxidation of ethylene glycol on PtRu/C and PtSn/C electrocatalysts prepared by alcohol-reduction process. J. Appl. Electrochem. 2005, 35, 193–198. [Google Scholar] [CrossRef]
- Matsuoka, K.; Iriyama, Y.; Abe, T.; Matsuoka, M.; Ogumi, Z. Alkaline direct alcohol fuel cells using an anion exchange membrane. J. Power Source. 2005, 150, 27–31. [Google Scholar] [CrossRef]
- Arechederra, R.L.; Treu, B.L.; Minteer, S.D. Development of glycerol/O-2 biofuel cell. J. Power Source. 2007, 173, 156–161. [Google Scholar] [CrossRef]
- Gulzow, E. Alkaline fuel cells: A critical view. J. Power Source. 1996, 61, 99–104. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Hu, L.; Zhuang, L.; Lu, J.; Xu, B. A feasibility analysis for alkaline membrane direct methanol fuel cell: thermodynamic disadvantages versus kinetic advantages. Electrochem. Commun. 2003, 5, 662–666. [Google Scholar] [CrossRef]
- Adzic, R.R.; Avramovivic, M.L.; Tripkovic, A.V. Structural Effects in Electrocatalysis—Oxidation of Formaldehyde on Gold and Platinum Single-Crystal Electrodes in Alkaline-Solution. Electrochim. Acta 1984, 29, 1353–1357. [Google Scholar] [CrossRef]
- Chen, Y.G.; Zhuang, L.; Lu, J.T. Non-Pt anode catalysts for alkaline direct alcohol fuel cells. Chin. J. Catal. 2007, 28, 870–874. [Google Scholar] [CrossRef]
- Taraszewska, J.; Roslonek, G. Electrocatalytic oxidation of methanol on a glassy carbon electrode modified by nickel hydroxide formed by ex situ chemical precipitation. J. Electroanal. Chem. 1994, 364, 209–213. [Google Scholar] [CrossRef]
- Miyazaki, K.; Sugimura, N.; Matsuoka, K.; Iriyama, Y.; Abe, T.; Matsuoka, M.; Ogumi, Z. Perovskite-type oxides La1-xSrxMnO3 for cathode catalysts in direct ethylene glycol alkaline fuel cells. J. Power Source. 2008, 178, 683–686. [Google Scholar] [CrossRef]
- Yu, E.H.; Scott, K. Direct methanol alkaline fuel cell with catalysed metal mesh anodes. Electrochem. Commun. 2004, 6, 361–365. [Google Scholar] [CrossRef]
- Beden, B.; Leger, J.M.; Lamy, C. Modern Aspects of Electrochemistry; Bockris, J.O’M., Conway, B.E., White, R.E., Eds.; Plenum Press: New York, NY, USA, 1992; Volume 22, p. 97. [Google Scholar]
- Bagotzky, V.S.; Vassilyev, Y.B. Mechanism of electro-oxidation of methanol on the platinum electrode. Electrochim. Acta 1967, 12, 1323–1343. [Google Scholar] [CrossRef]
- Beden, B.; Kadirgan, F.; Lamy, C.; Leger, J.M. Oxidation of methanol on a platinum electrode in alkaline medium: Effect of metal ad-atoms on the electrocatalytic activity. J. Electroanal. Chem. 1982, 142, 171–190. [Google Scholar] [CrossRef]
- Lamy, C.; Leger, J.M.; Clavilier, J. Structural effects in the electrooxidation of methanol in alkaline medium: Comparison of platinum single crystal and polycrystalline electrodes. J. Electroanal. Chem. 1982, 135, 321–328. [Google Scholar] [CrossRef]
- Morallón, E.; Vázquez, J.L.; Aldaz, A. Electrochemical behaviour of basal single crystal Pt electrodes in alkaline medium. J. Electroanal. Chem. 1990, 288, 217–228. [Google Scholar] [CrossRef]
- Tripkovic, A.V.; Popovic, K.D.; Momcilovic, J.D.; Draic, D.M. Kinetic and mechanistic study of methanol oxidation on a Pt(111) surface in alkaline media. J. Electroanal. Chem. 1996, 418, 9–20. [Google Scholar] [CrossRef]
- Tripkovic, A.V.; Popovic, K.D.; Momcilovic, J.D.; Drazic, D.M. Kinetic and mechanistic study of methanol oxidation on a Pt(100) surface in alkaline media. J. Electroanal. Chem. 1998, 448, 173–181. [Google Scholar] [CrossRef]
- Tripkovic, A.V.; Popovic, K.Ð.; Momcilovic, J.D.; Drazic, D.M. Kinetic and mechanistic study of methanol oxidation on a Pt(110) surface in alkaline media. Electrochim. Acta 1998, 44, 1135–1145. [Google Scholar] [CrossRef]
- Tremiliosi-Filho, G.; Gonzalez, E.R.; Motheo, A.J.; Belgsir, E.M.; Léger, J.M.; Lamy, C. Electro-oxidation of ethanol on gold: Analysis of the reaction products and mechanism. J. Electroanal. Chem. 1998, 444, 31–39. [Google Scholar] [CrossRef]
- De Lima, R.B.; Varela, H. Catalytic oxidation of ethanol on gold electrode in alkaline media. Gold Bull. 2008, 41, 15–22. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Law, H.M.; Nguyen, H.T.; Kristian, N.; Wang, S.; Chan, S.H.; Wang, X. Enhancement effect of Ag for Pd/C towards the ethanol electro-oxidation in alkaline media. Appl. Catal. B Environ. 2009, 91, 507–515. [Google Scholar] [CrossRef]
- Fujiwara, N.; Siroma, Z.; Yamazaki, S.-I.; Ioroi, T.; Senoh, H.; Yasuda, K. Direct ethanol fuel cells using an anion exchange membrane. J. Power Source. 2008, 185, 621–626. [Google Scholar] [CrossRef]
- Fang, X.; Wang, L.Q.; Shen, P.K.; Cui, G.F.; Bianchini, C. An in situ Fourier transform infrared spectroelectrochemical study on ethanol electrooxidation on Pd in alkaline solution. J. Power Source. 2010, 195, 1375–1378. [Google Scholar] [CrossRef]
- Cui, G.F.; Song, S.Q.; Shen, P.K.; Kowal, A.; Bianchini, C. First-Principles Considerations on Catalytic Activity of Pd toward Ethanol Oxidation. J. Phys. Chem. C 2009, 113, 15639–15642. [Google Scholar] [CrossRef]
- Matsuoka, K.; Iriyama, Y.; Abe, T.; Matsuoka, M.; Ogumi, Z. Electro-oxidation of methanol and ethylene glycol on platinum in alkaline solution: Poisoning effects and product analysis. Electrochim. Acta 2005, 51, 1085–1090. [Google Scholar] [CrossRef]
- Roquet, L.; Belgsir, E.M.; Léger, J.M.; Lamy, C. Kinetics and mechanisms of the electrocatalytic oxidation of glycerol as investigated by chromatographic analysis of the reaction products: Potential and pH effects. Electrochim. Acta 1994, 39, 2387–2394. [Google Scholar] [CrossRef]
- Schell, M.; Xu, Y.; Zdraveski, Z. Mechanism for the Electrocatalyzed Oxidation of Glycerol Deduced from an Analysis of Chemical Instabilities. J. Phys. Chem. 1996, 100, 18962–18969. [Google Scholar] [CrossRef]
- Damjanovic, A.; Genshaw, M.A.; Bockris, J.O.M. The Mechanism of Oxygen Reduction at Platinum in Alkaline Solutions with Special Reference to H[sub 2]O[sub 2]. J. Electrochem. Soc. 1967, 114, 1107–1112. [Google Scholar] [CrossRef]
- Damjanovic, A.; Sepa, D.B.; Vojnovic, M.V. New Evidence Supports the Proposed Mechanism for O2 Reduction at Oxide Free Platinum-Electrodes. Electrochim. Acta 1979, 24, 887–889. [Google Scholar] [CrossRef]
- Hsueh, K.L.; Gonzalez, E.R.; Srinivasan, S.; Chin, D.T. Effects of Phosphoric Acid Concentration on Oxygen Reduction Kinetics at Platinum. J. Electrochem. Soc. 1984, 131, 823–828. [Google Scholar] [CrossRef]
- Damjanovic, A.; Brusic, V. Electrode kinetics of oxygen reduction on oxide-free platinum electrodes. Electrochim. Acta 1967, 12, 615–628. [Google Scholar] [CrossRef]
- Damjanovic, A.; Genshaw, M.A.; Bockris, J.O.M. The Role of Hydrogen Peroxide in Oxygen Reduction at Platinum in H2SO4 Solution. J. Electrochem. Soc. 1967, 114, 466–472. [Google Scholar] [CrossRef]
- Beden, B.; Kadirgan, F.; Kahyaoglu, A.; Lamy, C. Electrocatalytic oxidation of ethylene glycol in alkaline medium on paltinum-gold alloy electrodes modified by underpotential deposition of lead adatoms. J. Electroanal. Chem. 1982, 135, 329–334. [Google Scholar] [CrossRef]
- Bi, Y.P.; Lu, G.X. Control growth of uniform platinum nanotubes and their catalytic properties for methanol electrooxidation. Electrochem. Commun. 2009, 11, 45–49. [Google Scholar] [CrossRef]
- Lović, J. The kinetics and mechanism of methanol oxidation on Pt and PtRu catalysts in alkaline and acid media. J. Serb. Chem. Soc. 2007, 72, 709–712. [Google Scholar] [CrossRef]
- Tripkovic, A.V.; Popovic, K.D.; Grgur, B.N.; Blizanac, B.; Ross, P.N.; Markovic, N.M. Methanol electrooxidation on supported Pt and PtRu catalysts in acid and alkaline solutions. Electrochim. Acta 2002, 47, 3707–3714. [Google Scholar] [CrossRef]
- Verma, A.; Basu, S. Direct alkaline fuel cell for multiple liquid fuels: Anode electrode studies. J. Power Source. 2007, 174, 180–185. [Google Scholar] [CrossRef]
- Bai, Y.X.; Wu, J.J.; Xi, J.Y.; Wang, J.S.; Zhu, W.T.; Chen, L.Q.; Qiu, X.P. Electrochemical oxidation of ethanol on Pt-ZrO2/C catalyst. Electrochem. Commun. 2005, 7, 1087–1090. [Google Scholar] [CrossRef]
- Demarconnay, L.; Brimaud, S.; Coutanceau, C.; Leger, J.M. Ethylene glycol electrooxidation in alkaline medium at multi-metallic Pt based catalysts. J. Electroanal. Chem. 2007, 601, 169–180. [Google Scholar] [CrossRef]
- Zheng, H.T.; Li, Y.L.; Chen, S.X.; Shen, P.K. Effect of support on the activity of Pd electrocatalyst for ethanol oxidation. J. Power Source. 2006, 163, 371–375. [Google Scholar] [CrossRef]
- Xu, C.W.; Tian, Z.Q.; Chen, Z.T.; Jiang, S.P. Pd/C promoted by Au for 2-propanol electrooxidation in alkaline media. Electrochem. Commun. 2008, 10, 246–249. [Google Scholar] [CrossRef]
- Ye, J.; Liu, J.; Xu, C.; Jiang, S.P.; Tong, Y. Electrooxidation of 2-propanol on Pt, Pd and Au in alkaline medium. Electrochem. Commun. 2007, 9, 2760–2763. [Google Scholar] [CrossRef]
- Liu, J.; Ye, J.; Xu, C.; Jiang, S.P.; Tong, Y. Electro-oxidation of methanol, 1-propanol and 2-propanol on Pt and Pd in alkaline medium. J. Power Source. 2008, 177, 67–70. [Google Scholar] [CrossRef]
- Xu, C.; Shen, P.K.; Liu, Y. Ethanol electrooxidation on Pt/C and Pd/C catalysts promoted with oxide. J. Power Source. 2007, 164, 527–531. [Google Scholar] [CrossRef]
- Bunazawa, H.; Yamazaki, Y. Ultrasonic synthesis and evaluation of non-platinum catalysts for alkaline direct methanol fuel cells. J. Power Source. 2009, 190, 210–215. [Google Scholar] [CrossRef]
- Lamy, C.; Coutanceau, C.; Leger, J.-M. Electrocatalytic Oxidation of Glycerol in a Solid Alkaline Membrane Fuel Cell (SAMFC). In Proceedings of ECS 216th Meeting, Vienna, Austria, 4–9 October, 2009.
- An, L.; Zhao, T.S.; Shen, S.Y.; Wu, Q.X.; Chen, R. Performance of a direct ethylene glycol fuel cell with an anion-exchange membrane. Int. J. Hydrogen Energ. 2010, 35, 4329–4335. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bianchini, C.; Filippi, J.; OberhauserIal, W.; Marchionni, A.; Vizza, F.; Psaro, R.; Sordelli, L.; Foresti, M.L.; Innocenti, M. Ethanol Oxidation on Electrocatalysts Obtained by Spontaneous Deposition of Palladium onto Nickel-Zinc Materials. Chemsuschem 2009, 2, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Bambagioni, V.; Bianchini, C.; Marchionni, A.; Filippi, J.; Vizza, F.; Teddy, J.; Serp, P.; Zhiani, M. Pd and Pt-Ru anode electrocatalysts supported on multi-walled carbon nanotubes and their use in passive and active direct alcohol fuel cells with an anion-exchange membrane (alcohol = methanol, ethanol, glycerol). J. Power Source. 2009, 190, 241–251. [Google Scholar] [CrossRef]
- Singh, R.N.; Singh, A. Anindita Electrocatalytic activity of binary and ternary composite films of Pd, MWCNT and Ni, Part II: Methanol electrooxidation in 1 M KOH. Int. J. Hydrogen Energ. 2009, 34, 2052–2057. [Google Scholar] [CrossRef]
- Xu, C.W.; Tian, Z.Q.; Shen, P.K.; Jiang, S.P. Oxide (CeO2, NiO,Co(3)O(4)and Mn3O4)-promoted Pd/C electrocatalysts for alcohol electrooxidation in alkaline media. Electrochim. Acta 2008, 53, 2610–2618. [Google Scholar] [CrossRef]
- Borkowska, Z.; Tymosiak-Zielinska, A.; Nowakowski, R. High catalytic activity of chemically activated gold electrodes towards electro-oxidation of methanol. Electrochim. Acta 2004, 49, 2613–2621. [Google Scholar] [CrossRef]
- Borkowska, Z.; Tymosiak-Zielinska, A.; Shul, G. Electrooxidation of methanol on polycrystalline and single crystal gold electrodes. Electrochim. Acta 2004, 49, 1209–1220. [Google Scholar] [CrossRef]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The oxidation of organic compounds at a nickel anode in alkaline solution. J. Electroanal. Chem. 1971, 31, 39–49. [Google Scholar] [CrossRef]
- Van Effen, R.M.; Evans, D.H. A study of aldehyde oxidation at glassy carbon, mercury, copper, silver, gold and nickel anodes. J. Electroanal. Chem. 1979, 103, 383–397. [Google Scholar] [CrossRef]
- Motheo, A.J.; Machado, S.A.S.; Rabelo, F.J.B.; Santos, J.R., Jr. Electrochemical study of ethanol oxidation on nickel in alkaline media. J. Braz. Chem. Soc. 1994, 5, 161–165. [Google Scholar] [CrossRef]
- Abdel Rahim, M.A.; Abdel Hameed, R.M.; Khalil, M.W. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Source. 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Khalil, M.W.; Abdel Rahim, M.A.; Zimmer, A.; Hassan, H.B.; Abdel Hameed, R.M. Nickel impregnated silicalite-1 as an electro-catalyst for methanol oxidation. J. Power Source. 2005, 144, 35–41. [Google Scholar] [CrossRef]
- Kumar, K.S.; Haridoss, P.; Seshadri, S.K. Synthesis and characterization of electrodeposited Ni-Pdalloy electrodes for methanol oxidation. Surf. Coat. Tech. 2008, 202, 1764–1770. [Google Scholar] [CrossRef]
- Shobba, T.; Mayanna, S.M.; Sequeira, C.A.C. Preparation and characterization of Co-W alloys as anode materials for methanol fuel cells. J. Power Source. 2002, 108, 261–264. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, D.B.; Chen, S.L.; Huang, Y.; Cheng, X. Preparation and Electrocatalytic Properties of Fe, Co, Ni-Polymer-C Complex Catalysts for Ethanol Electro-oxidation. Acta Chim. Sin. 2009, 67, 917–922. [Google Scholar]
- Raghuveer, V.; Viswanathan, B. Can La2-xSrxCuO4 be used as anodes for direct methanol fuel cells? Fuel 2002, 81, 2191–2197. [Google Scholar] [CrossRef]
- Raghuveer, V.; Ravindranathan Thampi, K.; Xanthopoulos, N.; Mathieu, H.J.; Viswanathan, B. Rare earth cuprates as electrocatalysts for methanol oxidation. Solid State Ionics 2001, 140, 263–274. [Google Scholar] [CrossRef]
- Yu, H.-C.; Fung, K.-Z.; Guo, T.-C.; Chang, W.-L. Syntheses of perovskite oxides nanoparticles La1-xSrxMO3-[delta] (M = Co and Cu) as anode electrocatalyst for direct methanol fuel cell. Electrochim. Acta 2004, 50, 811–816. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, T.; Singh, A.; Anindita; Mishra, D.; Tiwari, S.K. Perovskite-type La2-xSrxNiO4 (0 ≤ x ≤ 1) as active anode materials for methanol oxidation in alkaline solutions. Electrochim. Acta 2008, 53, 2322–2330. [Google Scholar] [CrossRef]
- Tsivadze, A.; Tarasevich, M.; Bogdanovskaya, V.; Ehrenburg, M. Platinum-free nanosize electrocatalysts for glycerol oxidation. Dokl. Chem. 2008, 419, 54–56. [Google Scholar] [CrossRef]
- Yu, E.Y.; Scott, K.; Reeve, R.W. Electrochemical Reduction of Oxygen on Carbon Supported Pt and Pt/Ru Fuel Cell Electrodes in Alkaline Solutions. Fuel Cells 2003, 3, 169–176. [Google Scholar] [CrossRef]
- Gamburzev, S.; Petrov, K.; Appleby, A.J. Silver-carbon electrocatalyst for air cathodes in alkaline fuel cells. J. Appl. Electrochem. 2002, 32, 805–809. [Google Scholar] [CrossRef]
- Kostowskyj, M.A.; Gilliam, R.J.; Kirk, D.W.; Thorpe, S.J. Silver nanowire catalysts for alkaline fuel cells. Int. J. Hydrogen Energ. 2008, 33, 5773–5778. [Google Scholar] [CrossRef]
- Meng, H.; Wu, M.; Hu, X.X.; Nie, M.; Wei, Z.D.; Shen, P.K. Selective cathode catalysts for mixed-reactant alkaline alcohol fuel cells. Fuel Cells 2006, 6, 447–450. [Google Scholar] [CrossRef]
- Jiang, L.; Hsu, A.; Chu, D.; Chen, R. Oxygen Reduction Reaction on Carbon Supported Pt and Pd in Alkaline Solutions. J. Electrochem. Soc. 2009, 156, B370–B376. [Google Scholar] [CrossRef]
- Kim, J.; Momma, T.; Osaka, T. Cell performance of Pd-Sn catalyst in passive direct methanol alkaline fuel cell using anion exchange membrane. J. Power Source. 2009, 189, 999–1002. [Google Scholar] [CrossRef]
- Klápste, B.; Vondrák, J.; Velická, J. MnOx/C composites as electrode materials II. Reduction of oxygen on bifunctional catalysts based on manganese oxides. Electrochim. Acta 2002, 47, 2365–2369. [Google Scholar] [CrossRef]
- Tachibana, K.; Matsuki, K. Development of in situ a.c. impedance measurement system under constant-current conditions and its application to galvanostatic discharge of electrolytic manganese dioxide in alkaline solution. J. Power Source. 1998, 74, 29–33. [Google Scholar] [CrossRef]
- Mao, L.; Sotomura, T.; Nakatsu, K.; Koshiba, N.; Zhang, D.; Ohsaka, T. Electrochemical Characterization of Catalytic Activities of Manganese Oxides to Oxygen Reduction in Alkaline Aqueous Solution. J. Electrochem. Soc. 2002, 149, A504–A507. [Google Scholar] [CrossRef]
- Elzing, A.; van der Putten, A.; Visscher, W.; Barendrecht, E. The cathodic reduction of oxygen at cobalt phthalocyanine: Influence of electrode preparation on electrocatalysis. J. Electroanal. Chem. 1986, 200, 313–322. [Google Scholar] [CrossRef]
- Kiros, Y.; Schwartz, S. Pyrolyzed macrocycles on high surface area carbons for the reduction of oxygen in alkaline fuel cells. J. Power Source. 1991, 36, 547–555. [Google Scholar] [CrossRef]
- Sarangapani, S.; Lessner, P.; Manoukian, M.; Giner, J. Non-noble electrocatalysts for alkaline fuel cells. J. Power Source. 1990, 29, 437–442. [Google Scholar] [CrossRef]
- Kiros, Y.; Lindström, O.; Kaimakis, T. Cobalt and cobalt-based macrocycle blacks as oxygen-reduction catalysts in alkaline fuel cells. J. Power Source. 1993, 45, 219–227. [Google Scholar] [CrossRef]
- Van Den Brink, F.; Visscher, W.; Barendrecht, E. Electrocatalysis of cathodic oxygen reduction by metal phthalocyanines: Part I. Introduction, cobalt phthalocyanine as electrocatalyst: experimental part. J. Electroanal. Chem. 1983, 157, 283–304. [Google Scholar] [CrossRef]
- Van Den Brink, F.; Visscher, W.; Barendrecht, E. Electrocatalysis of cathodic oxygen reduction by metal phthalocyanines: Part II. Cobalt phthalocyanine as electrocatalyst: A mechanism of oxygen reduction. J. Electroanal. Chem. 1983, 157, 305–318. [Google Scholar] [CrossRef]
- Van den Brink, F.; Visscher, W.; Barendrecht, E. Electrocatalysis of cathodic oxygen reduction by metal phthalocyanines: Part IV. Iron phthalocyanine as electrocatalyst: Mechanism. J. Electroanal. Chem. 1984, 175, 279–289. [Google Scholar] [CrossRef]
- Van Den Brink, F.; Visscher, W.; Barendrecht, E. Electrocatalysis of cathodic oxygen reduction by metal phthalocyanines: Part III. Iron phthalocyanine as electrocatalyst: Experimental part. J. Electroanal. Chem. 1984, 172, 301–325. [Google Scholar] [CrossRef]
- Bianchini, C.; Bambagioni, V.; Filippi, J.; Marchionni, A.; Vizza, F.; Bert, P.; Tampucci, A. Selective oxidation of ethanol to acetic acid in highly efficient polymer electrolyte membrane-direct ethanol fuel cells. Electrochem. Commun. 2009, 11, 1077–1080. [Google Scholar] [CrossRef]
- Zhiani, M.; Gasteiger, H.A.; Piana, M.; Catanorchi, S.; Bert, P. Comparative Study between Pt/C and Low Cost Cathode Nano-Particle Catalyst in Alkalien Direct Ethanol Fuel Cell (DEFC). In Proceedings of ECS 216th Meeting, Vienna, Austria, 4–9 October 2009.
- Li, Y.S.; Zhao, T.S.; Liang, Z.X. Performance of alkaline electrolyte-membrane-based direct ethanol fuel cells. J. Power Source. 2009, 187, 387–392. [Google Scholar] [CrossRef]
- Yu, E.H.; Scott, K. Development of direct methanol alkaline fuel cells using anion exchange membranes. J. Power Source. 2004, 137, 248–256. [Google Scholar] [CrossRef]
- Yu, E.H.; Scott, K. Direct methanol alkaline fuel cells with catalysed anion exchange membrane electrodes. J. Appl. Electrochem. 2005, 35, 91–96. [Google Scholar] [CrossRef]
- Yu, E.H.; Scott, K.; Reeve, R.W. Application of sodium conducting membranes in direct methanol alkaline fuel cells. J. Appl. Electrochem. 2006, 36, 25–32. [Google Scholar] [CrossRef]
- Baldauf, M.; Preidel, W. Status of the development of a direct methanol fuel cell. J. Power Source. 1999, 84, 161–166. [Google Scholar] [CrossRef]
- Scott, K.; Yu, E.; Vlachogiannopoulos, G.; Shivare, M.; Duteanu, N. Performance of a direct methanol alkaline membrane fuel cell. J. Power Source. 2008, 175, 452–457. [Google Scholar] [CrossRef]
- Yanagi, H.; Fukuta, K. Anion Exchange Membrane and Ionomer for Alkaline Membrane Fuel Cells (AMFCs). ECS Trans. 2008, 16, 257–262. [Google Scholar]
- Danks, T.N.; Slade, R.C.T.; Varcoe, J.R. Comparison of PVDF- and FEP-based radiation-grafted alkaline anion-exchange membranes for use in low temperature portable DMFCs. J. Mater. Chem. 2002, 12, 3371–3373. [Google Scholar] [CrossRef]
- Danks, T.N.; Slade, R.C.T.; Varcoe, J.R. Alkaline anion-exchange radiation-grafted membranes for possible electrochemical application in fuel cells. J. Mater. Chem. 2003, 13, 712–721. [Google Scholar] [CrossRef]
- Kang, J.J.; Li, W.Y.; Lin, Y.A.; Li, X.P.; Xiao, X.R.; Fang, S.B. Synthesis and ionic conductivity of a polysiloxane containing quaternary ammonium groups. Polym. Adv. Tech. 2004, 15, 61–64. [Google Scholar] [CrossRef]
- Yi, F.; Yang, X.P.; Li, Y.J.; Fang, S.B. Synthesis and ion conductivity of poly(oxyethylene) methacrylates containing a quaternary ammonium group. Polym. Adv. Tech. 1999, 10, 473–475. [Google Scholar] [CrossRef]
- Fang, J.; Shen, P.K. Quaternized poly(phthalazinon ether sulfone ketone) membrane for anion exchange membrane fuel cells. J. Membrane Sci. 2006, 285, 317–322. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.X. Sulfonated polyethersulfone Cardo membranes for direct methanol fuel cell. J. Membrane Sci. 2005, 246, 167–172. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, Y.H.; Liao, B.; Cong, G.M. Synthesis and characterization of aminated poly(2,6-dimethyl-1,4-phenylene oxide). J. Appl. Polym. Sci. 1996, 61, 1111–1115. [Google Scholar] [CrossRef]
- Wu, L.; Xu, T.W.; Wu, D.; Zheng, X. Preparation and characterization of CPPO/BPPO blend membranes for potential application in alkaline direct methanol fuel cell. J. Membrane Sci. 2008, 310, 577–585. [Google Scholar] [CrossRef]
- Wu, L.; Xu, T.W. Improving anion exchange membranes for DMAFCs by inter-crosslinking CPPO/BPPO blends. J. Membrane Sci. 2008, 322, 286–292. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.X. Quaternized polyethersulfone Cardo anion exchange membranes for direct methanol alkaline fuel cells. J. Membrane Sci. 2005, 262, 1–4. [Google Scholar] [CrossRef]
- Xing, B.; Savadogo, O. Hydrogen/oxygen polymer electrolyte membrane fuel cells (PEMFCs) based on alkaline-doped polybenzimidazole (PBI). Electrochem. Commun. 2000, 2, 697–702. [Google Scholar] [CrossRef]
- Hou, H.Y.; Sun, G.Q.; He, R.H.; Sun, B.Y.; Jin, W.; Liu, H.; Xin, Q. Alkali doped polybenzimidazole membrane for alkaline direct methanol fuel cell. Int. J. Hydrogen Energ. 2008, 33, 7172–7176. [Google Scholar]
- Hou, H.Y.; Sun, G.Q.; He, R.H.; Wu, Z.M.; Sun, B.Y. Alkali doped polybenzimidazole membrane for high performance alkaline direct ethanol fuel cell. J. Power Source. 2008, 182, 95–99. [Google Scholar] [CrossRef]
- Modestov, A.D.; Tarasevich, M.R.; Leykin, A.Y.; Filimonov, V.Y. MEA for alkaline direct ethanol fuel cell with alkali doped PBI membrane and non-platinum electrodes. J. Power Source. 2009, 188, 502–506. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.J.; Chien, W.C. Development of alkaline direct methanol fuel cells based on crosslinked PVA polymer membranes. J. Power Source. 2006, 162, 21–29. [Google Scholar] [CrossRef]
- Yang, C.C.; Lee, Y.J.; Chiu, S.J.; Lee, K.T.; Chien, W.C.; Lin, C.T.; Huang, C.A. Preparation of a PVA/HAP composite polymer membrane for a direct ethanol fuel cell (DEFC). J. Appl. Electrochem. 2008, 38, 1329–1337. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.J.; Lee, K.T.; Chien, W.C.; Lin, C.T.; Huang, C.A. Study of poly(vinyl alcohol)/titanium oxide composite polymer membranes and their application on alkaline direct alcohol fuel cell. J. Power Source. 2008, 184, 44–51. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chiu, S.-J.; Chien, W.-C.; Chiu, S.-S. Quaternized poly(vinyl alcohol)/alumina composite polymer membranes for alkaline direct methanol fuel cells. J. Power Source. 2010, 195, 2212–2219. [Google Scholar] [CrossRef]
- Cairns, E.J.; Bartosik, D.C. A Methanol Fuel Cell with an Invariant Alkaline Electrolyte. J. Electrochem. Soc. 1964, 111, 1205–1210. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Slade, R.C.T. An electron-beam-grafted ETFE alkaline anion-exchange membrane in metal-cation-free solid-state alkaline fuel cells. Electrochem. Commun. 2006, 8, 839–843. [Google Scholar] [CrossRef]
- Coutanceau, C.; Demarconnay, L.; Lamy, C.; Léger, J.M. Development of electrocatalysts for solid alkaline fuel cell (SAFC). J. Power Source. 2006, 156, 14–19. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.-K.; Hwang, K.-T.; Lee, J.-Y. Performance of air-breathing direct methanol fuel cell with anion-exchange membrane. Int. J. Hydrogen Energ. 2010, 35, 768–773. [Google Scholar] [CrossRef]
- Bidault, F.; Kucernak, A. A novel cathode for alkaline fuel cells based on a porous silver membrane. J. Power Source. 2010, 195, 2549–2556. [Google Scholar] [CrossRef]
- Choban, E.R.; Spendelow, J.S.; Gancs, L.; Wieckowski, A.; Kenis, P.J.A. Membraneless laminar flow-based micro fuel cells operating in alkaline, acidic, and acidic/alkaline media. Electrochim. Acta 2005, 50, 5390–5398. [Google Scholar] [CrossRef]
- Priestnall, M.A.; Kotzeva, V.P.; Fish, D.J.; Nilsson, E.M. Compact mixed-reactant fuel cells. J. Power Source. 2002, 106, 21–30. [Google Scholar] [CrossRef]
- Sung, W.; Choi, J.-W. A membraneless microscale fuel cell using non-noble catalysts in alkaline solution. J. Power Source. 2007, 172, 198–208. [Google Scholar] [CrossRef]
- Zeng, R.; Shen, P.K. Selective membrane electrode assemblies for bipolar plate-free mixed-reactant fuel cells. J. Power Source. 2007, 170, 286–290. [Google Scholar] [CrossRef]
- Shen, P.K.; Xu, C. Alcohol oxidation on nanocrystalline oxide Pd/C promoted electrocatalysts. Electrochem. Commun. 2006, 8, 184–188. [Google Scholar] [CrossRef]
- Faubert, G.; Cote, R.; Guay, D.; Dodelet, J.P.; Denes, G.; Bertrand, P. Iron catalysts prepared by high-temperature pyrolysis of tetraphenylporphyrins adsorbed on carbon black for oxygen reduction in polymer electrolyte fuel cells. Electrochim. Acta 1998, 43, 341–353. [Google Scholar] [CrossRef]
- Scott, K.; Shukla, A.K.; Jackson, C.L.; Meuleman, W.R.A. A mixed-reactants solid-polymer-electrolyte direct methanol fuel cell. J. Power Source. 2004, 126, 67–75. [Google Scholar] [CrossRef]
- Ganley, C.J.; Karikari, N.K.; Raghavan, D. Performance enhancement of alkaline direct methanol fuel cells by Ni/Al layered double hydroxides. J. Fuel Cell Sci. Tech. 2010, 7, 031019. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, E.H.; Krewer, U.; Scott, K. Principles and Materials Aspects of Direct Alkaline Alcohol Fuel Cells. Energies 2010, 3, 1499-1528. https://doi.org/10.3390/en3081499
Yu EH, Krewer U, Scott K. Principles and Materials Aspects of Direct Alkaline Alcohol Fuel Cells. Energies. 2010; 3(8):1499-1528. https://doi.org/10.3390/en3081499
Chicago/Turabian StyleYu, Eileen Hao, Ulrike Krewer, and Keith Scott. 2010. "Principles and Materials Aspects of Direct Alkaline Alcohol Fuel Cells" Energies 3, no. 8: 1499-1528. https://doi.org/10.3390/en3081499
APA StyleYu, E. H., Krewer, U., & Scott, K. (2010). Principles and Materials Aspects of Direct Alkaline Alcohol Fuel Cells. Energies, 3(8), 1499-1528. https://doi.org/10.3390/en3081499





