Simulation of the Impact of SRT on Anaerobic Digestability of Ultrasonicated Hog Manure
Abstract
:1. Introduction
2. Experimental Section
2.1. Analytical methods
2.2. Protein measurement
2.3. Ultrasonication and anaerobic digestion set-up
| Parameter (mg/L) | Unsonicated manure (influent to the control digester) | Sonicated manure | |
|---|---|---|---|
| Manure before sonication | Manure after sonication (influent to the digester) | ||
| TSS | 15,119 ± 552 | 15,792 ± 680 | 13,916 ± 785 |
| VSS | 11,000 ± 526 | 11,496 ± 510 | 8816 ± 411 |
| TCOD | 26,638 ± 1829 | 28,000 ± 1540 | 28,333 ± 1471 |
| SCOD | 12,645 ± 1238 | 13,050 ± 1260 | 15,854 ± 1289 |
| Ammonia | 753 ± 30 | 824 ± 88 | 464 ± 71 |
| P-Protein | 2671 ± 87 | 2854 ± 210 | 2569 ± 183 |
| B-Protein | 675 ± 73 | 708 ± 62 | 623 ± 76 |
| S-Protein | 2613 ± 188 | 2920 ± 360 | 3416 ± 210 |
| TKN | 1779 ± 89 | 1879 ± 98 | 1800 ± 107 |
| STKN | 939 ± 108 | 939 ± 66 | 1054 ± 44 |
| VFA* | 1650 ± 187 | 1680 ± 308 | 1797 ± 257 |
2.4. Specific energy input
3. Results and Discussion
3.1. Ultrasonication of hog manure
3.2. Solids destruction
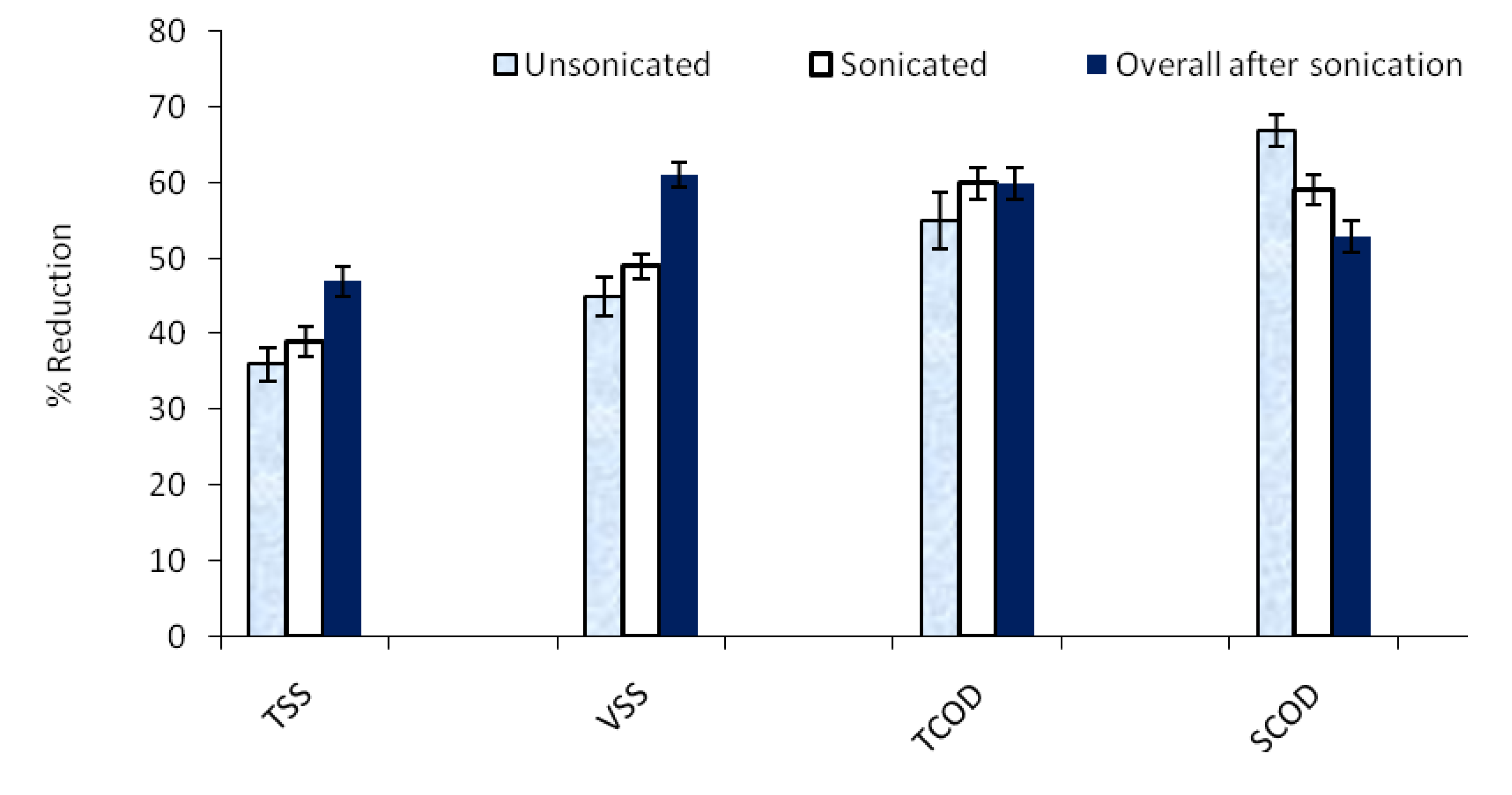
3.3. COD destruction
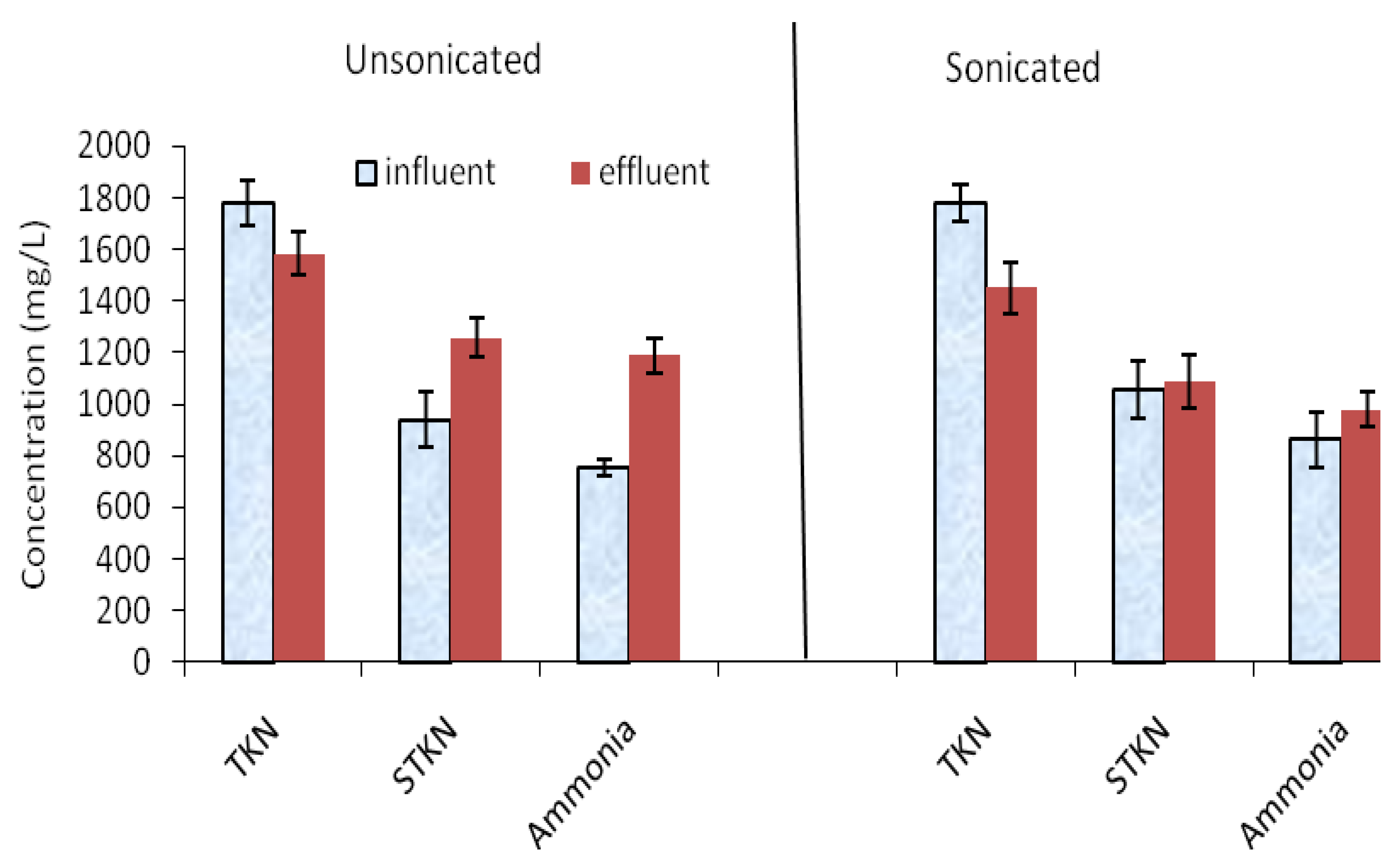
3.4. Nitrogen compounds and odorous contaminants

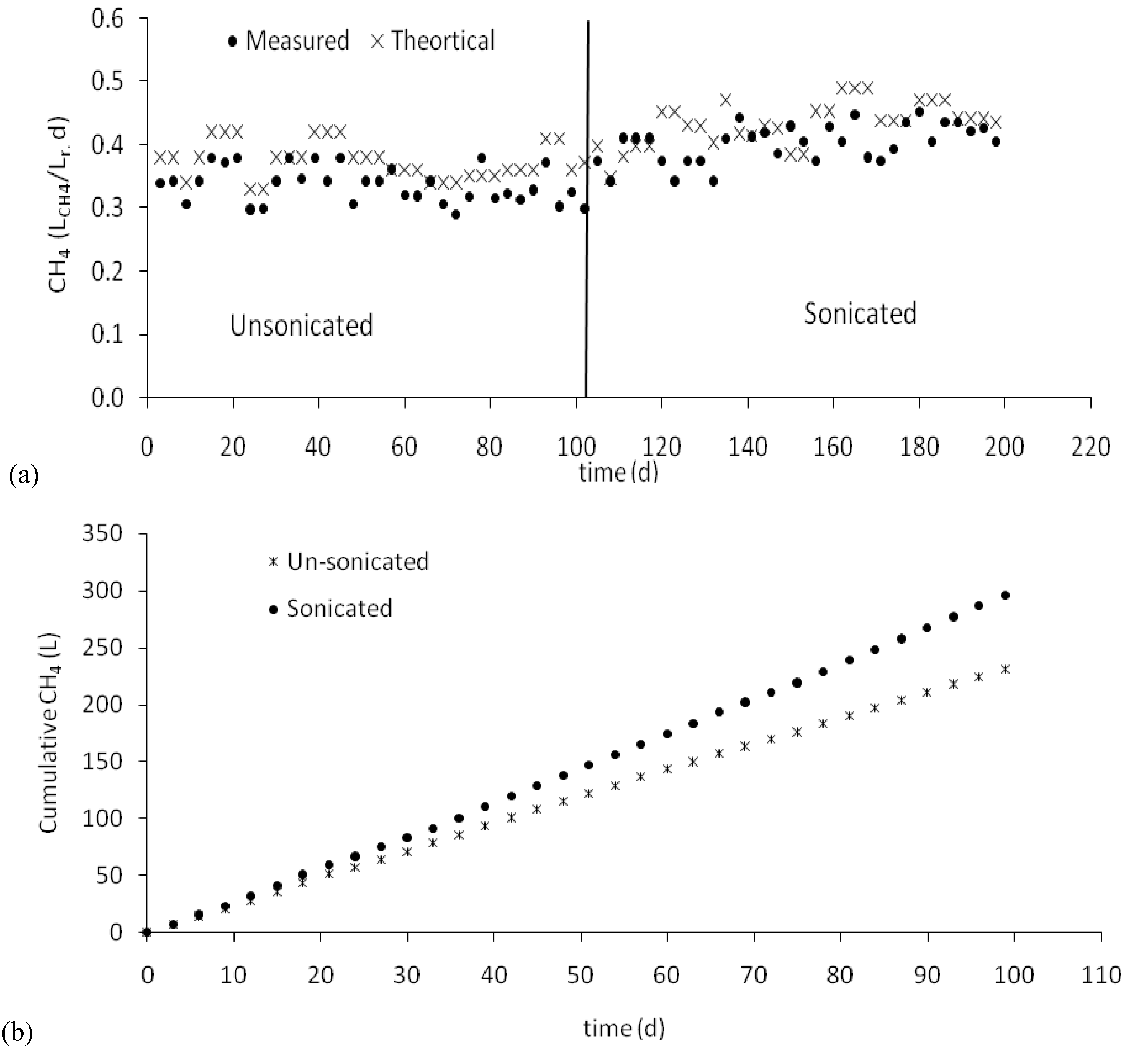
3.5. Biogas production
3.6. BioWin model
3.7. Economic analysis
| Unsonicated | Sonicated | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter (mg/L) | Measured | Actual model influent | Simulated effluent | Measured | Actual model influent | Simulated effluent | ||
| Influent | effluent | influent | effluent | |||||
| TSS | 15,119 ± 552 | 9618 ± 687 | 14,642 | 9556 | 15,792 ± 680 | 7432 ± 409 | 14,402 | 8019 |
| VSS | 11,000 ± 526 | 6050 ± 414 | 11,640 | 6510 | 11,496 ± 510 | 4489 ± 768 | 9360 | 4762 |
| TCOD | 26,638 ± 1829 | 11,890 ± 998 | 26,600 | 13,188 | 28,000 ± 1540 | 11,284 ± 978 | 27,600 | 12,301 |
| SCOD | 12,645 ± 1238 | 4188 ± 507 | 12,396 | 4340 | 13,050 ± 1260 | 6147 ± 462 | 16,250 | 6360 |
| Ammonia | 753 ± 30 | 1187 ± 65 | 846 | 1129 | 824 ± 88 | 980 ± 100 | 846 | 1201 |
| TKN | 1779 ± 89 | 1582 ± 184 | 1779 | 1468 | 1879 ± 98 | 1450 ± 164 | 1779 | 1453 |
| STKN | 939 ± 108 | 1257 ± 176 | 1404 | 1170 | 939 ± 66 | 1088 ± 71 | 1517 | 1240 |
| Acetic and propionic acids* | 1187 ± 123 | 140 ± 8 | 1187 | 139 | 843 ± 162 | 172 ± 14 | 843 | 138 |
| VSSdest (%) | 45 ± 2.5 | 44.1 | 51 ± 1.6 | 50.8 | ||||
| CH4** | 2.53 ± 0.21 | 2.6 | 3.0 ± 0.26 | 3.1 | ||||
| SRT (d) | Unsonicated manure | Sonicated manure | ||
|---|---|---|---|---|
| VSS destruction (%) | CH4 (L/d) | VSS destruction (%) | CH4 (L/d) | |
| 3 | 21 | 3.0 | 13.2 | 3.9 |
| 5 | 26 | 6.0 | 22 | 7.0 |
| 7.5 | 32.9 | 4.5 | 33.2 | 5.4 |
| 10 | 37.8 | 3.6 | 40.9 | 4.3 |
| 15 | 44.1 | 2.6 | 50.8 | 3.1 |
| 20 | 48 | 2.0 | 56.9 | 2.5 |
| 25 | 50.6 | 1.7 | 61.1 | 2.0 |
| 30 | 52.5 | 1.4 | 64.1 | 1.7 |
| SRT (d) | Unsonicated manure | Sonicated manure | Net* $ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Energy in | Energy out | Energy in | Energy out | ||||||
| Dewatering | Gas | dewatering | Gas | ||||||
| wt of sludge after treatment (ton) | $ for dewatering and transportation | CH4 (m3) | $ from CH4 | wt of sludge after treatment (ton) | $ for dewatering and transportation | CH4 (m3) | $ from CH4 | ||
| 3 | 0.87 | 218 | 80 | 22 | 0.80 | 199 | 112 | 31 | 18 |
| 5 | 0.83 | 207 | 267 | 75 | 0.74 | 186 | 335 | 94 | 31 |
| 7.5 | 0.76 | 191 | 300 | 84 | 0.68 | 169 | 383 | 107 | 36 |
| 10 | 0.72 | 180 | 320 | 90 | 0.63 | 157 | 411 | 115 | 39 |
| 15 | 0.66 | 165 | 347 | 97 | 0.57 | 141 | 445 | 125 | 42 |
| 20 | 0.63 | 156 | 356 | 100 | 0.53 | 132 | 467 | 131 | 46 |
| 25 | 0.60 | 150 | 378 | 106 | 0.50 | 125 | 482 | 135 | 44 |
| 30 | 0.58 | 146 | 373 | 105 | 0.48 | 121 | 492 | 138 | 49 |
Conclusions
- The overall TSS and VSS removal efficiencies of sonicated manure were higher than the unsonicated manure by 36% and 31%, respectively.
- There was no significant difference in TCOD removal efficiency for the sonicated and unsonicated manure during anaerobic digestion, while the SCOD removal efficiency in the digester receiving sonicated manure was lower than that receiving the unsonicated manure.
- There was no significant difference in particulate protein removal efficiency for the sonicated and unsonicated manure in the anaerobic digester, whereas the overall removal efficiency was slightly increased (by 10%) for sonicated manure.
- The overall removal efficiency of bound protein for sonicated manure was higher than the unsonicated manure by 17.5%.
- The concentration of H2S in the headspace of the bioreactor decreased from 988 ppm in the unsonicated manure digester to 566 ppm for sonicated manure digester, respectively.
- The effluent ammonia for digested unsonicated manure (1200 mg/L) was higher than that of sonicated manure (980 mg/L).
- The methane production rate increased from 0.34 LCH4/Lr.d for the unsonicated manure to 0.39 LCH4/Lr.d for the sonicated one.
- BioWin simulations indicated that at shorter SRTs, VSS destruction efficiencies for sonicated manure were less than the unsonicated manure despite higher methane production. However, interestingly the improvement in VSS destruction efficiencies during anaerobic digestion by sonication becomes apparent at SRTs around 15–30 days, which are commonly used SRTs for anaerobic digestion of biosolids in full scale.
- The net cost benefit of ultrasonication, calculated as the difference between the cost of methane output minus cost of energy input (only for ultrasonication) minus the cost of biosolids dewatering and disposal for the sonicated and unsonicated manure, increases sharply initially and stabilizes at $42–49/ton dry solids for SRTs of 15 to 30 days.
Nomenclature
| AD | Anaerobic digestion |
| B-Protein | Bound protein |
| LCH4 | Litre of CH4 |
| Lr | Litre of reactor |
| P | Ultrasonic power |
| P-Protein | Particulate protein |
| SCOD | Soluble chemical oxygen demand |
| SE | Specific energy input |
| S-Protein | Soluble protein |
| SRT | Solids retention time |
| SRTs | Solids retention times |
| STKN | Soluble total Kjeldahl nitrogen |
| t | Ultrasonic duration |
| TCOD | Total chemical oxygen demand |
| TKN | Total Kjeldahl nitrogen |
| TS | Total solids |
| TSS | Total suspended solids |
| V | Volume of sonicated manure |
| VFA | Volatile fatty acids |
| VSS | Volatile suspended solids |
| WAS | Waste-activated sludge |
Acknowledgements
References
- Van Velsen, A.F.M. Anaerobic digestion of swine waste. Neth. J. Agric. Sci. 1979, 27, 142–152. [Google Scholar]
- Andreadakis, A.D. Anaerobic digestion of swine waste. Water Sci. Technol. 1992, 25, 9–16. [Google Scholar]
- Lo, K.V.; Liao, P.H.; Gao, Y.C. Anaerobic treatment of swine wastewater using hybrid UASB reactors. Bioresour. Technol. 1994, 47, 153–157. [Google Scholar] [CrossRef]
- Sanchez, E.; Borja, R.; Weiland, P.; Travieso, L.; Martin, A. Effect of substrate concentration and temperature on the anaerobic digestion of piggery waste in a tropical climate. Proc. Biochem. 2001, 37, 483–489. [Google Scholar] [CrossRef]
- Pfeffer, J.T. Anaerobic digestion process. In Proceedings of the First Int. Symp. On Anaerobic Digestion, University College, Cardiff, Wales, UK; 1979. [Google Scholar]
- Eastman, J.A. Ferguson J.F. Solublisation of particulate organic carbon during the acid phase of anaerobic digestion. J. Wat. Poll. Cont. Fed. 1981, 53, 352–365. [Google Scholar]
- Parkin, G.F.; Owen, W.F. The fundamentals of anaerobic digestion of wastewater sludge. J. Environ. Engineer. 1986, 112, 867–920. [Google Scholar] [CrossRef]
- Wang, Q.; Noguchi, C.; Hara, Y.; Sharon, C.; Kakimoto, K.; Kato, Y. Studies on anaerobic digestion mechanism: Influence of pretreatment temperature on biodegradation of waste activated sludge. Environ. Technol. 1997, 18, 999–1008. [Google Scholar] [CrossRef]
- González-Fernández, C.; León-Cofreces, C.; García-Encina, P.A. Different pretreatments for increasing the anaerobic biodegradability in swine manure. Bioresour. Technol. 2008, 99, 8710–8714. [Google Scholar] [CrossRef] [PubMed]
- Tiehm, A.; Nickel, K.; Nies, U. The use of ultrasound to accelerate the anaerobic digestion of sewage sludge. Water Sci. Technol. 1997, 36, 121–128. [Google Scholar] [CrossRef]
- Wang, Q.; Kuninobu, M.; Kakimoto, K.; Ogawa, H.I.; Kato, Y. Upgrading of anaerobic digestion of waste activated biosolid by ultrasonic pretreatment. Bioresour. Technol. 1999, 68, 309–313. [Google Scholar] [CrossRef]
- Tiehm, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated biosolid disintegration for improving anaerobic stabilization. Water Res. 2001, 35, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Neyens, E.; Baeyens, J.; Dewil, R.; De Heyder, B. Advanced biosolid treatment affects extracellular polymeric substances to improve activated biosolid dewatering. J. Hazard. Mater. 2004, 106, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lawler, D.F.; Chung, Y.J.; Hwang, S.J.; Hull, B.A. Anaerobic digestion: Effects on particle size and dewaterability. J. Water Pollut. Control Fed. 1986, 58, 1107–1117. [Google Scholar]
- Quarmby, J.; Scott, J.R.; Mason, A.K.; Davies, G.; Parsons, S.A. The application of ultrasound as a pretreatment for anaerobic digestion. Environ. Technol. 1999, 20, 1155–1161. [Google Scholar] [CrossRef]
- Pérez-Elvira, S.I.; Nieto-Diez, P.; Fdz-Polanco, F. Sludge minimisation technologies. Review Environ. Sci. Bio-Technol. 2006, 5, 375–398. [Google Scholar] [CrossRef]
- Stukey, D.C.; McCarthy, P.L. Thermochemical pretreatment of nitrogenous materials to increase methane yield. Biotechnol. Bioeng. Symp. 1978, 8, 219–233. [Google Scholar]
- Lens, P.; Visser, A.; Janssen, A.; Pol, L.; Lettinga, G. Biotechnological treatment of sulfate-rich wastewaters. Critic. Review Environ. Sci. Technol. 1998, 28, 41–88. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th Edition ed; American Public Health Association: Washington DC, USA, 1998. [Google Scholar]
- Speece, R.E. Anaerobic Biotechnology for Industrial Waste Waters; Archae Press: Nashville, TN, USA, 1996. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurements with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Bougrier, C.; Carrère, H.; Delgenès, J.P. Solubilisation of waste-activated sludge by ultrasonic treatment. Chem. Eng. J. 2005, 106, 163–169. [Google Scholar] [CrossRef]
- Tiehm, A.; Nickel, K.; Nies, U. The use of ultrasound to accelerate the anaerobic digestion of sewage sludge. Water Sci. Technol. 1997, 36, 121–128. [Google Scholar] [CrossRef]
- Nickel, K.; Neis, U. Ultrasonic disintegration of biosolids for improved biodegradation. Ultrason. Sonochem. 2007, 14, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Braguglia, C.M.; Mininni, G.; Gianico, A. Is sonication effective to improve biogas production and solids reduction in excess sludge digestion? Water sci. Technol. 2008, 57, 479–483. [Google Scholar] [CrossRef] [PubMed]
- McDermott, B.L.; Chalmer, A.D.; Goodwin, J.A. Ultrasonication as a pre-treatment method for the enhancement of the psychrophilic anaerobic digestion of aquaculture effluents. Environ. Technol. 2001, 22, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Dimock, R.; Morgenroth, E. The influence of particle size on microbial hydrolysis of protein particles in activated sludge. Water Res. 2006, 40, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.; Glindemann, D.; Novak, J.T.; Murthy, S.N.; Gerwin, S.; Forbes, R. Standardized biosolid incubation, headspace odor measurement and odor production consumption cycles. In Proceedings of the Water Env. Federation and AWWA Odors and Air Emissions Conference, Bellevue, Washington, April 2004.
- Lens, P.N.L.; Kuenen, J.G. The biological sulfur cycle: New opportunities for environmental biotechnology. Water Sci. Technol. 2001, 44, 57–66. [Google Scholar] [PubMed]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, 2nd Edition ed; John Wiley & Sons: New York, NY, USA, 1981. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Elbeshbishy, E.; Nakevski, A.; Hafez, H.; Ray, M.; Nakhla, G. Simulation of the Impact of SRT on Anaerobic Digestability of Ultrasonicated Hog Manure. Energies 2010, 3, 974-988. https://doi.org/10.3390/en3050974
Elbeshbishy E, Nakevski A, Hafez H, Ray M, Nakhla G. Simulation of the Impact of SRT on Anaerobic Digestability of Ultrasonicated Hog Manure. Energies. 2010; 3(5):974-988. https://doi.org/10.3390/en3050974
Chicago/Turabian StyleElbeshbishy, Elsayed, Angel Nakevski, Hisham Hafez, Madhumita Ray, and George Nakhla. 2010. "Simulation of the Impact of SRT on Anaerobic Digestability of Ultrasonicated Hog Manure" Energies 3, no. 5: 974-988. https://doi.org/10.3390/en3050974
APA StyleElbeshbishy, E., Nakevski, A., Hafez, H., Ray, M., & Nakhla, G. (2010). Simulation of the Impact of SRT on Anaerobic Digestability of Ultrasonicated Hog Manure. Energies, 3(5), 974-988. https://doi.org/10.3390/en3050974




