1. Introduction
To date, organic and organic-inorganic photovoltaics (PVs) (third generation solar cells) continue to attract great attention from the PV community, due to their promising features such as low fabrication cost, flexibility and light weight. Third generation solar cells follows the second generation (thin film inorganics such as amorphous silicon (a-Si), cadmium telluride (CdTe), and copper indium gallium selenide (CIGS)) and first generation (semiconducting, crystalline) PVs. The class of organic PVs includes devices with flat and bulk heterojunction between the various types of conjugated polymers, small molecules, fullerene derivatives, and carbon nanotubes [
1,
2,
3]. Despite substantial progress in solar cell architecture, design and rational choice of the donor-acceptor materials over the past two decades, organic PVs are still unable to overcome the 6–7% barrier of conversion efficiency [
4,
5]. In terms of the low conversion efficiency, there are currently at least four major fundamental aspects making organic PVs vulnerable for commercial applications as compared with conventional semiconducting solar cells: (i) low carrier mobility, (ii) lack of absorption in the red/NIR part of spectrum, (iii) poor environmental stability, and (iv) excitonic character of photocarrier generation. If the first three above-mentioned statements are obvious, the fourth requires some explanation.
The source of photocarriers in organics is small radius excitons generated as a result of photon absorption. Excitons in organics have a binding energy in the range 200–400 meV [
6,
7], which is considerably higher than the binding exciton energy for semiconductor materials ~2–40 meV [
8]. Thermal energy at room temperature is not sufficient (~25 meV) for exciton dissociation to hole and electron in the bulk medium. Therefore, exciton dissociation should occur at the interface between the donor and acceptor materials with proper HOMO and LUMO energy levels. The mechanism of exciton dissociation is not completely understood, however the major factor governing the charge separation at the interface is the charge transfer process between the donor and acceptor components. After charge separation, holes and electrons move to the opposite electrodes owing to drift (induced by the electric field at the interface) and diffusion (gradient of the charge concentration from the interface to the surface). In the case of a flat heterojunction, the diffusion length of the singlet excitons (several tens nanometers) should be comparable with the thickness of at least of one active layer (donor or acceptor) to minimize energy loss, otherwise not all excitons are able to reach the interface. However, at such small thickness, the absorbed energy is very low since the penetration length of incident light for organics is typically more than 100 nm. The possible solution could be formation of bulk heterojunctions (blend of donor and acceptor materials) with separation scale factor comparable with the excitonic diffusion lengths and relatively thick films to absorb more light energy. Nevertheless, an increase of the film thickness should result in a longer photocarrier pathway to the external electrodes, which reduces the conversion efficiency owing to carriers trapping and lower carrier mobility.
An alternative to pure organic PVs is the class of organic-inorganic hybrid solar cells [
9,
10,
11,
12], where heterojunction is formed between inorganic semiconductors and organic compounds (small molecules, oligomers, polymers, carbon nanotubes). An advantage of hybrid PVs over organics PVs lies in the high carrier mobility of the semiconductor and the light absorption at longer wavelengths than for organic compounds. On the other hand, the existence of the organic component allows hybrid solar cells to be superior over conventional semiconducting PVs in terms of cost efficiency, scalable wet processing, the variety of organic materials (mismatch between inorganic components can be minimized or prevented), light weight, and flexibility. Moreover, the recent progress in advanced semiconducting nanostructures in combination with organic nanomaterials [e.g., fullerenes and carbon nanotubes (CNTs)] opens new opportunities to overcome the 10% barrier of conversion efficiency for hybrid solar cells in the near future. Note, that band engineering can be a useful instrument in the design of the hybrid solar cell architecture, however it is not as straightforward as for semiconducting PVs. For example, the chemical functionalizing of the organic component (introduction of reducing or oxidizing groups in the chemical structure) is capable of significantly affecting the band gap energy and position of the Fermi level for conducting polymers and small molecules. Even physical doping (e.g., by simple exposure of polymer film upon iodine vapors) can strongly increase the polymer conductivity and reduce its transparency, thus altering the polymer optoelectronic properties. Thus, the PV performance of hybrid solar cell can be readily optimized by tuning the band structure of organic component (work function, band gap, Fermi level) for the best matching with the corresponding band structure of the inorganic counterpart.
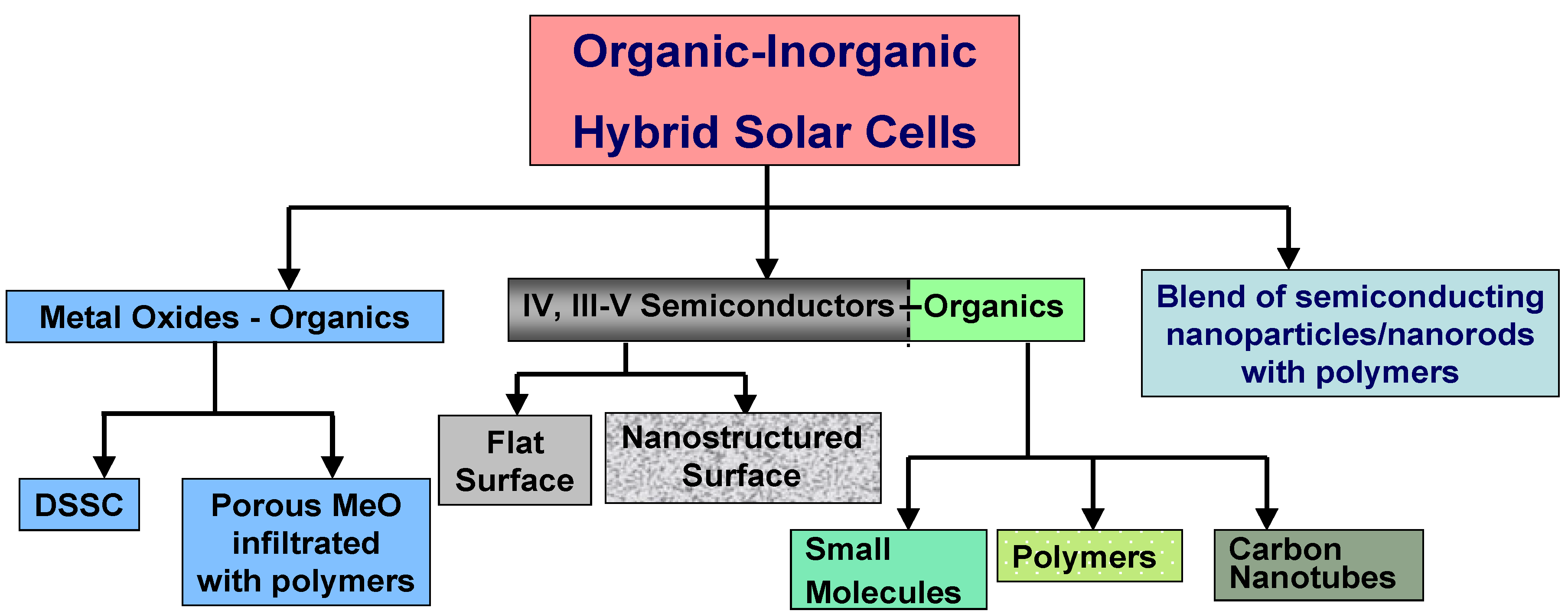
Figure 1 systemizes the types of hybrid PVs depending on the nature of organic and inorganic component and the morphology of the devices. This review is focused on hybrid solar cells based on Group IV (Si) and Group III-V (mostly GaAs) semiconductors forming the heterojunction with various organic components. However, for generality purposes, we will briefly describe other types of hybrid PVs.
Figure 1.
Classification of hybrid solar cells.
Figure 1.
Classification of hybrid solar cells.
Dye-sensitized solar cells (DSSCs) [
9,
11] is probably the most well studied hybrid solar cells. DSSC is composed of nanoporous metal oxide (usually TiO
2) infiltrated with sensitized dye molecules (ruthenium based “N
3” dye) and liquid electrolyte. Photon absorption by the dye results in fast electron transfer to the metal oxide followed by electron transport to the electrode (SnO
2). Dye regeneration occurs through the electron transfer from the liquid electrolyte. DSSC possess the highest conversion efficiency (up to 11%) among organic and hybrid PVs [
9,
13]. The major factor contributing to the enhanced efficiency is the highly mobile ions in the liquid electrolyte which rapidly compensates the Coulomb interaction between photogenerated electron-hole pairs and thus minimizing the recombination process at the interface. At the same time, the liquid electrolyte is the serious drawback of DSSCs, making them problematic for future technology transition. Attempts to employ solid electrolytes were not very successful and so far the highest reported conversion efficiency for solid DSSC is only 4.5% [
14].
Another class of hybrid PVs as shown in
Figure 1 is similar to DSSC because of the same nanoporous metal-oxide inorganic matrixes (e.g., TiO
2, ZnO
2) incorporated in the device architecture. However, instead of employing dye or electrolyte, conjugated polymers (e.g., polythiophene derivatives [
10,
12], or thiophene-fluorene copolymers [
10,
15]) function as the organic counterpart. It was previously envisioned that such organic-inorganic system should be very efficient for charge separation and carrier transport due to the large interfacial area between the nanoporous metal oxide and the infiltrated polymer, as well as the relatively high carrier mobility in polythiophenes (~0.1 cm
2 V
−1 s
−1 [
16]). Electron mobility in metal oxide semiconductors is significantly higher (e.g., ~440 cm
2V
−1s
−1 for ZnO [
17]) than in organic systems. However, reported conversion efficiency to date for these hybrid PVs are relatively low (~0.1–0.6%), which could be associated with the high recombination rate at the interface (in contrast to DSSC) and the twisted conformation of polymers inside the nanopores, leading to the reduction of hole mobility [
10,
12,
18]. An interesting theoretical model describing the factors limiting the efficiency of organic solar cells (hybrid metal-oxide/organic PVs were included) was reported by Nelson
et al. [
19]. Note, that for metal oxide based hybrid PVs, only the dye or the polymer component contributes to the photoconversion process while the inorganic component provides the interface for charge separation and photocarrier transportation. Because of the wide band gap (TiO
2 ~ 3.1 eV; ZnO ~ 3.4 eV), metal oxide semiconductors cannot absorb solar light at lower spectral energy.
Hybrid PVs based on inorganic nanoparticles (mostly Group II-VI) blended with conjugated polymers (
Figure 1) have been considered as a promising nanocomposite photosensitive materials for the past decade [
20]. High charge mobility in semiconducting colloidal nano-objects, contribution to the solar light absorption (as distinct from the metal oxides based solar cells), bulk heterojunction and easy wet processing could provide advantages over other hybrid and organic PVs. Nevertheless, their conversion efficiency remains low in the range of 1–3%. The main reasons likely are the electrically inactive network of colloidal nanoparticles/nanorods and the high interfacial recombination rate.
3. Hybrid Devices Composed from Nanostructured Semiconductors (Si, GaAs, InP, Ge) and Organics
Recent progress in the nanostructuring of semiconductors opens a new opportunity for improvements in the performance of hybrid solar cells. First of all, the quantum confinement dramatically changes the opto-electronic properties of nanostructured semiconductor leading to the tunable band gap, enhancement of the light absorption, and altered electrical conductivity depending on the characteristic size at nanoscale environment. Such circumstance provides an additional parameter (nanoscale tunability) for optimal compatibility between the organic and the inorganic components in order to form heterojunctions with advanced PV characteristics. The morphology of the heterojunction is also important as such devices have increased interfacial area and reduced average distance from one point to an interface. As the separation of charge carriers occurs at interfaces through exciton dissociation, such structures enhance it, and thus lead to an increase in the photocurrent.
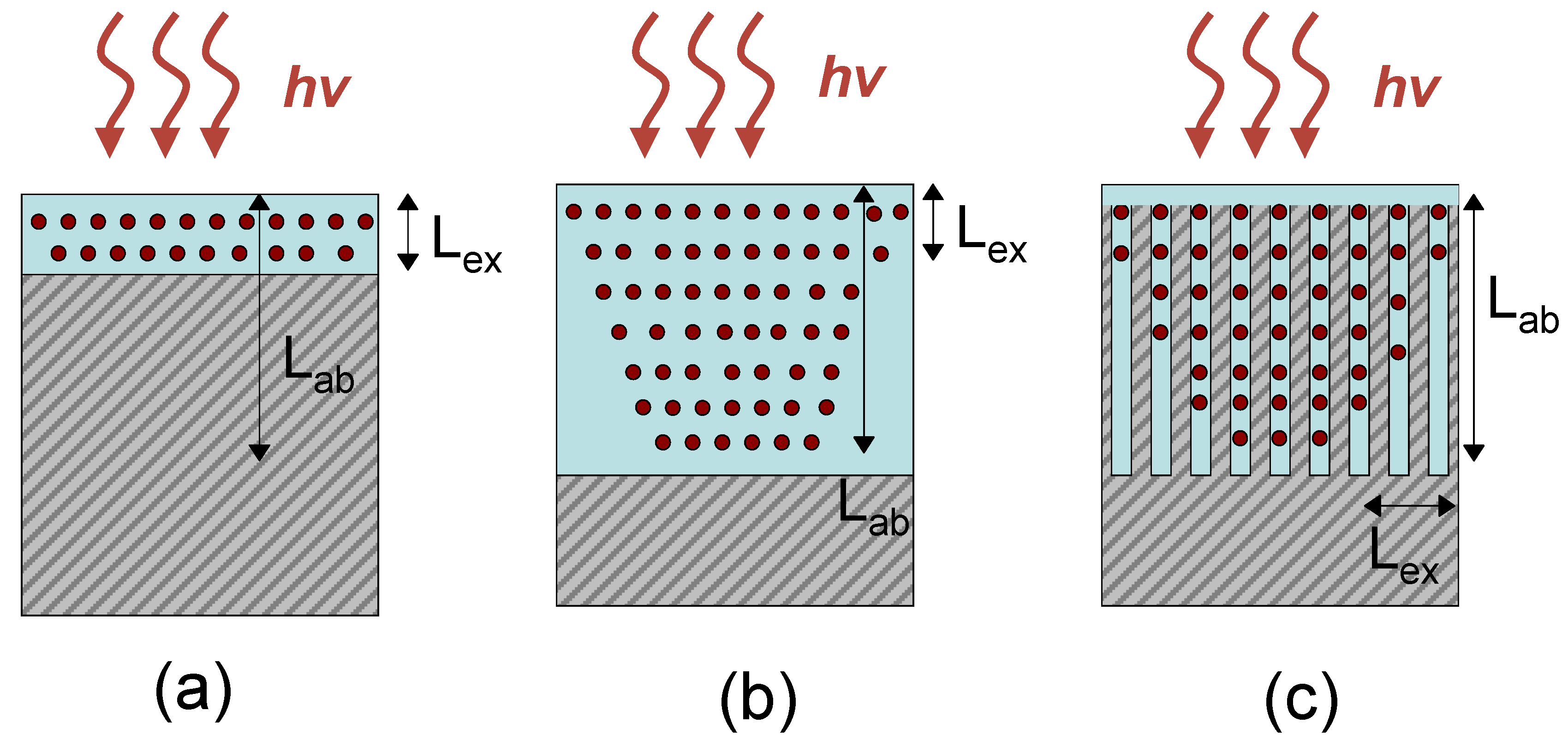
Figure 3 presents the schematic demonstrating potential advantages of nanostructured organic-inorganic interface over flat heterojunction. In terms of the efficiency of the organic layer in the case of a flat heterojunction, the figure of merit can be formulated as F ~ L
ex/L
ab, where L
ex is the exciton diffusion length and L
ab = 1/k is the light penetration length (k is the absorption coefficient). Thus, thin organic layer with thickness of d ~ L
ex and d < L
ab, provides efficient charge separations (all excitons reach the interface) but low absorbance (
Figure 3a). In contrary, relatively thick organic layer (L
ab ~ d > L
ex) absorbs more light energy, but only a fraction of the excitons are capable of dissociating at the interface (
Figure 3b). In the case of nanostructured semiconductor infiltrated with organics, the characteristic length (average distance between pillars or pore diameter) can be comparable with the exciton diffusion length, while the nanostructured layer can be thick enough to absorb substantial light energy (
Figure 3c). However, it should be noted that the large interface increases interfacial recombination. As a rule, for many polymers and small molecules, L
ex (several tens nm for singlet excitons) is lower than L
ab of several hundreds nm. Therefore, an optimal design of nanostructured semiconductor could comprise of axial nanoobjects such as pillars or pores (better ordered) with an average distance ~5–20 nm and length of 500–1,000 nm or longer.
Figure 3.
Exciton (dots) generation in the hybrid structures with flat (a,b) and nanostructured heterojunction (c).
Figure 3.
Exciton (dots) generation in the hybrid structures with flat (a,b) and nanostructured heterojunction (c).
3.1. Nanowires and nanopillars
Recently, a new trend in hybrid photovoltaics emerges as a result of intensive efforts in the field of nanowires/nanopilars growth from Group IV, III-V semiconductors. These nanostructures provides a large interfacial surface in contact with the organic materials and can be easily fabricated by various techniques such as chemical vapor deposition (CVD) [
43], laser ablation [
41], molecular beam epitaxy (MBE) [
42], dry etching [
24]. Hybrid solar cells based on Si [
39,
40,
41], GaAs [
42], InP [
43], Ge [
44] nanowires (NW) and polymers (mostly P3HT) has been reported in literature for the last 2–3 years.
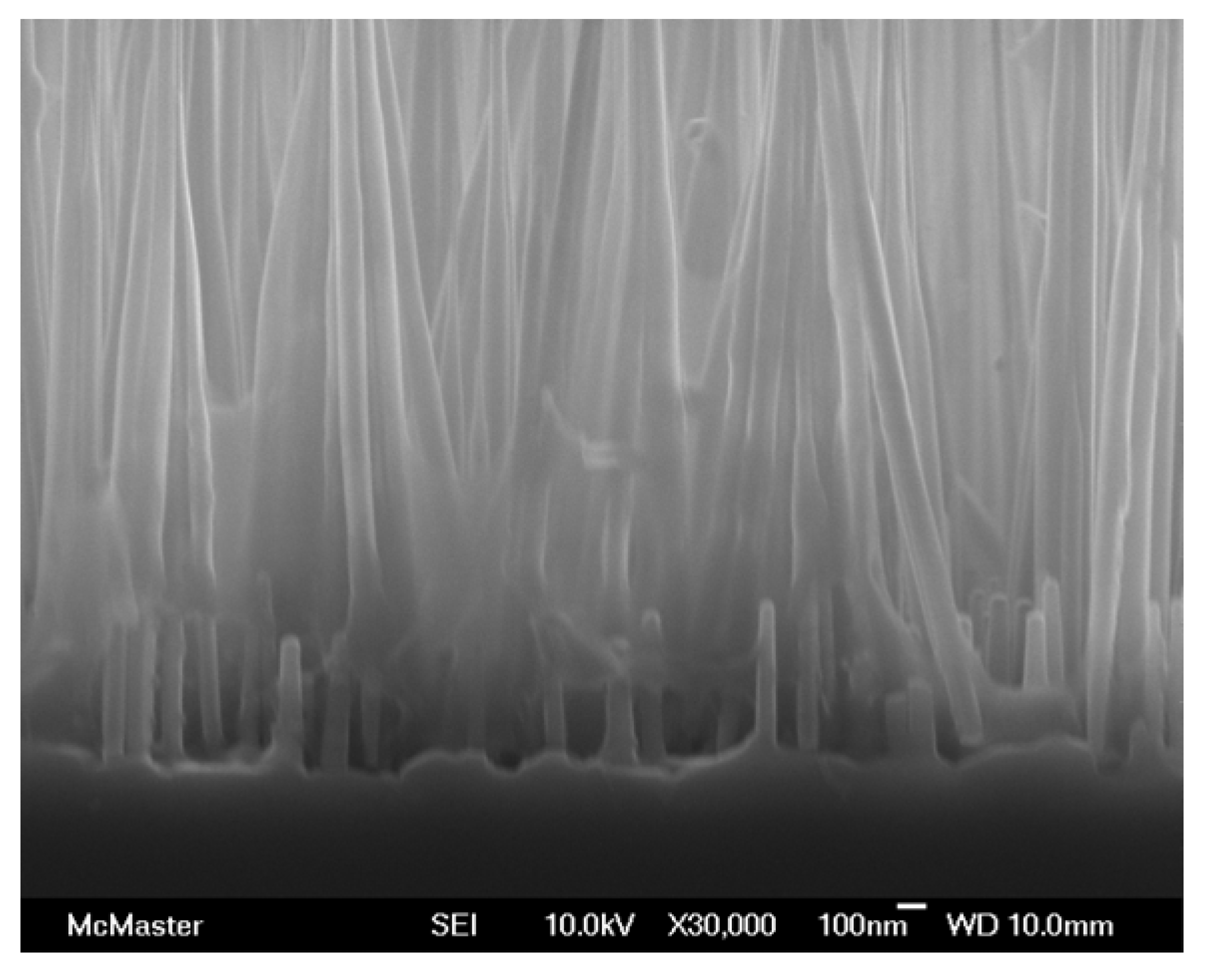
Figure 4 shows the cross-sectional SEM image of ordered GaAs nanowires grown on
n-type GaAs substrate after infiltration with P3HT polymer [
42]. The conversion efficiency of the cell with ITO/P3HT-NW/GaAs/Me structure can be varied from <0.1% to 1.04% (2.6 sun) depending on the P3HT solvent composition and the nanowire etching time (prior to deposition to remove the native oxide). The control devices (nanowires without P3HT and P3HT without nanowires) demonstrated negligible PV response, which is indicative of the importance of the nanostructured interface in the device architecture.
Figure 4.
Cross-sectional image of GaAs nanowires infiltrated with P3HT [
42]. Reproduced with permission from the Institute of Physics and IOP Publishing Limited, ©2009.
Figure 4.
Cross-sectional image of GaAs nanowires infiltrated with P3HT [
42]. Reproduced with permission from the Institute of Physics and IOP Publishing Limited, ©2009.
PV response of Si nanowires-P3HT interface has been investigated in study [
39]. The NWs of smaller sizes (3–5 nm) resulted in higher open-circuit voltage and higher short circuit current than nanowires with larger diameters (10–20 nm) [
39]. The authors suggested that a widening of the band gap for smaller NWs decreases the conduction band offset between the Si NW and the P3HT leading to reduced voltage loss during exciton dissociation. The highest conversion efficiency was 1.15% (1 sun) for Si NWs with diameters of 3–5 nm and 35 wt% ratio of NW to P3HT. In study [
41], the Si NWs were fabricated by etching process. Then, the NWs were pressed into P3HT layer followed by removal of the wafer. The reported conversion efficiency of such device was 1.93%. In both studies [
39,
41], the Si NWs definitely contributed in the photoconversion process by light harvesting in the NIR range, where the polymer cannot absorb light energy.
A novel approach in the design of hybrid solar cells has been described in Ref. [
43], where InP nanowires were directly grown on ITO substrate. It was shown that Ohmic contacts were formed between the NWs and the ITO layer, which is a substantial achievement compared to NW/polymer blend sandwiched between two electrodes. In this case, the NWs provide a direct pathway for electron transport and collection at the ITO electrode. PV response was improved by the passivation of the InP surface with sulfur, which reduces nonradiactive recombination. However, the conversion efficiency was low (<0.1%), likely because of the thick non-uniform P3HT (4–6 μm) layer prepared by drop casting. At such thicknesses, charge transport through the polymer network cannot supply enough holes to the external electrode due to low hole mobility and hole trapping ( L
ab ~ d > L
ex,
Figure 3b).
Nanostructured surface of amorphous Si (a-Si), prepared by nanosphere lithography and reactive ion etching, resulted in an improvement of the PV response for ITO/nanostructured a-Si/P3HT/Ag device [
24]. Such nanostructure can be presented as interconnected array of nanopillars with average pore sizes of 30–40 nm. The conversion efficiency was increased up to 0.3% as compared with flat heterojunctions (0.16%) [
23]. The higher pillar height leads to enhancement of the short circuit current and is likely associated with an increase of the surface interface between P3HT and a-Si.
3.2. Nanoporous wet etched structures
An alternative approach to nanowire growth is the use of nanoporous semiconductors fabricated by electrochemical etching process (mostly porous Si, however porous GaAs and other III-V semiconductors were also investigated [
45]). The electrochemical etching of Si is a well known technique for preparation of various porous structures with pore sizes from ~5–50 nm (mesoporous) to several hundreds nanometers (macroporous) [
46]. There are only a few reports about hybrid cells based on mesoporous Si infiltrated with small molecules [
47,
48,
49], however this fabrication method is simple (wet processing as distinct from nanowire growth) and can obtain conversion efficiencies up to ~3% [
48], exceeding those of nanowire based hybrid PVs [
39,
40,
41,
42,
43,
44].
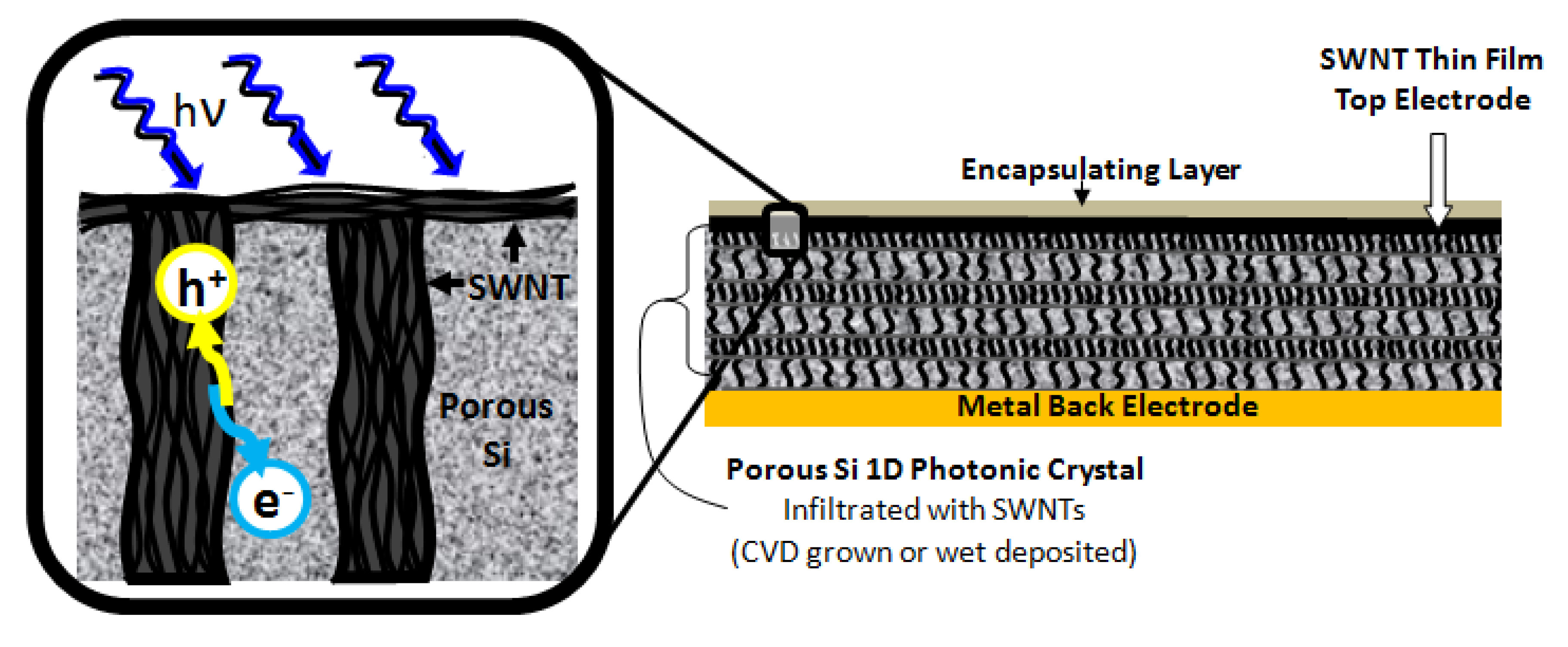
In study [
47], mesoporous
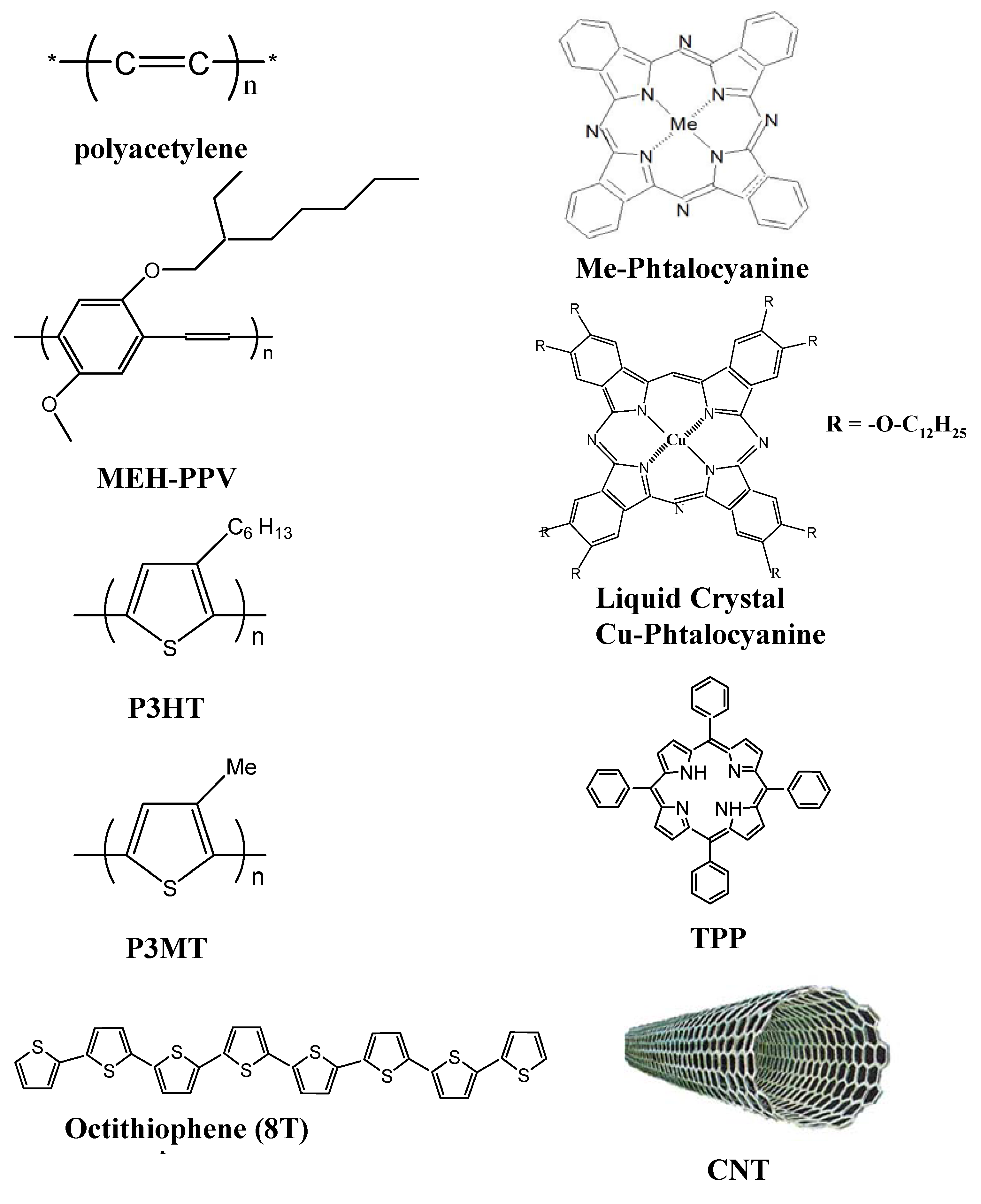
n-type Si (PSi) was infiltrated with CuPC and its derivative discotic liquid crystal CuPC (CuPCL) (
Figure 2). The choice of CuPCL was motivated by the possibility of increasing the hole mobility along the quasi-1D columns, similar to LC perylene derivatives [
31]. It was shown by A. Komolov
et al. [
50] that electron transfers from CuPC film to
n-Si (100) substrates extends a positively charged layer into the CuPC film up to 10 nm. Porous Si has several important functions in hybrid solar cells. It serves as light absorber, antireflection coating and as nanostructured material for electron transfer and transport. The CuPC provides the light absorption, charge separation at the interface with PSi and hole transport medium.
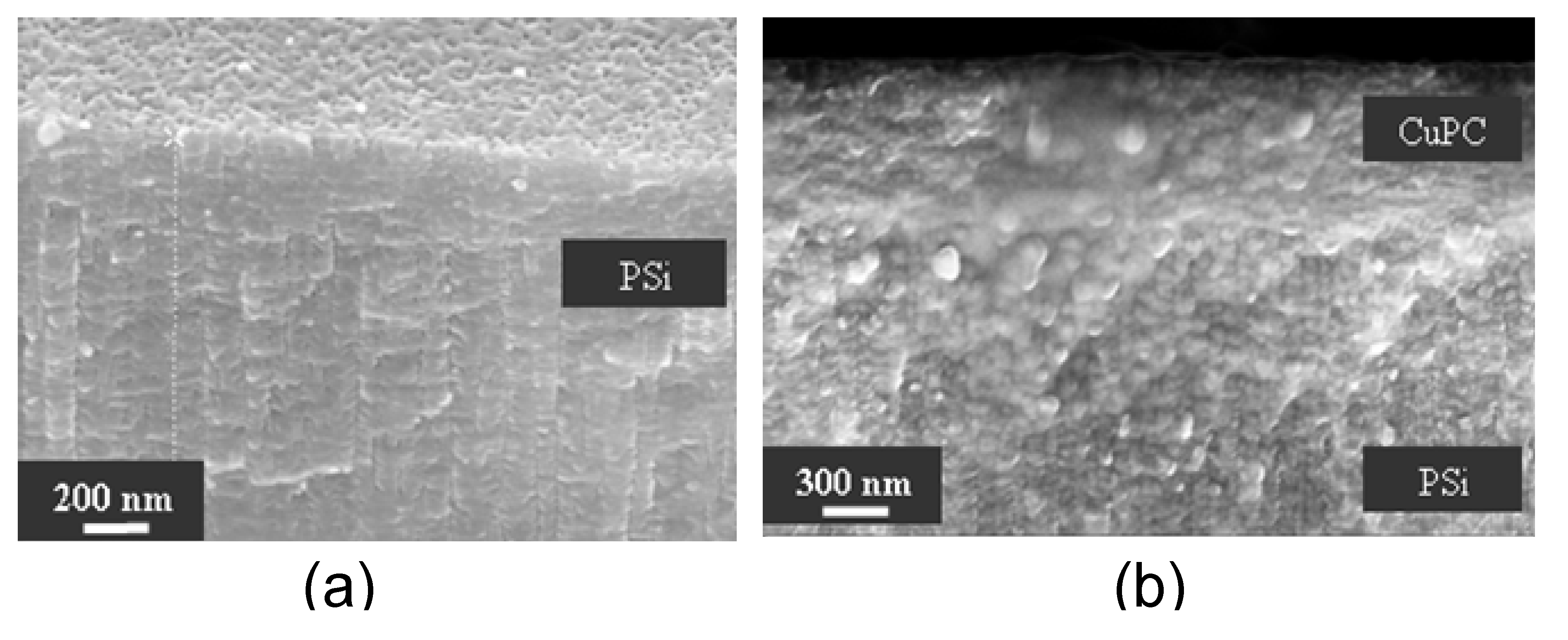
Figure 5 shows the SEM image of PSi prior to and after infiltration with CuPC.
Contrary to anticipations, CuPCL based cells demonstrated significantly lower conversion efficiency, ~0.01% (comparable with the control device—the same cell without organic components) than solar cells infiltrated with CuPC with efficiencies up to 3%. Such striking differences can be explained in terms of the interfacial area between the organic and the inorganic components. Presumably, the electron transfer from CuPCL to Si is slow enough or is nonexistent due to relatively long alkyloxy chains (
Figure 2), which could prevent close contact of the CuPCL molecular core with PSi. Thus, the critical issue becomes an average distance between a CuPC core to the PSi surface and LC organization cannot compensate the low photoinduced electron transfer caused by the long alkyloxy chains.
Figure 5.
(a) SEM image of Si surface and cross-section after electrochemical etching: 15% HF/ethanol solution, 20 s, 50 mA/cm
2, the porous layer depth is 1,400 nm. (b) The same sample after infiltration with CuPC/H
2SO
4 (cross-section only) [
48]. Reproduced with permission from SPIE, ©2005.
Figure 5.
(a) SEM image of Si surface and cross-section after electrochemical etching: 15% HF/ethanol solution, 20 s, 50 mA/cm
2, the porous layer depth is 1,400 nm. (b) The same sample after infiltration with CuPC/H
2SO
4 (cross-section only) [
48]. Reproduced with permission from SPIE, ©2005.
Figure 6.
(a)
I-V characteristics of the ITO/PSi-CuPC/Si/Cr/Au cells with different thicknesses of PSi layer at white light illumination (33 mW/cm
2): Circles: PSi layer thickness is 1.8 µm: I
sc = 7.40 mA/cm
2, V
oc = 0.237 V, FF = 29%, η = 1.6%; Squares: PSi layer depth is 12 µm (I
sc = 7.56 mA/cm
2, V
oc = 0.325 V, FF = 40%, η = 3%. (b) The dependence of reflectivity of PSi layer with porosity 35% on its thickness at 650 nm [
48]. Reproduced with permission from SPIE, ©2005.
Figure 6.
(a)
I-V characteristics of the ITO/PSi-CuPC/Si/Cr/Au cells with different thicknesses of PSi layer at white light illumination (33 mW/cm
2): Circles: PSi layer thickness is 1.8 µm: I
sc = 7.40 mA/cm
2, V
oc = 0.237 V, FF = 29%, η = 1.6%; Squares: PSi layer depth is 12 µm (I
sc = 7.56 mA/cm
2, V
oc = 0.325 V, FF = 40%, η = 3%. (b) The dependence of reflectivity of PSi layer with porosity 35% on its thickness at 650 nm [
48]. Reproduced with permission from SPIE, ©2005.
In addition, the effect of porosity and layer thickness on PV performance was investigated [
48]. The
I-V characteristics are shown in
Figure 6a for two devices with different thickness of PSi layer. The conversion efficiency was enhanced from 1.6% to 3% with an increase of the pore depth from 1.8 µm to 12 µm. At the same time, the reflectivity drops from 80 % to 30%, measured at 650 nm (
Figure 6b). Thus, an increase of the conversion efficiency is most likely a result of reduced reflectivity of the deeper porous layer. An efficient penetration depth of CuPC is about 200 nm according to XPS data, which is indicative of potential for the following improvements of PV properties using deep and uniform filling.
Two PSi samples with similar reflectance spectra but with different porosity (35% and 75%) were employed to fabricate PV devices. It was found that the sample with lower porosity (35%) exhibits higher conversion efficiency by an order of magnitude than the sample with higher porosity (75%). Hence, the Si porosity plays a key role in the carrier transport, affecting the device performance [
48]. According to study [
51], as crystalline Si becomes porous, conductivity decreases because of the strong dependence of the diffusion coefficient on the porosity value. When the porosity reaches 57%, the conductivity of the PSi is dramatically reduced. This implies that the conductivity in PSi with porosity higher then 57% is determined by a hopping mechanism between crystallites. Thus, an ideal structure of the PSi layer for PV applications could be a low porous layer with sufficient thickness of ~10 μm and higher.
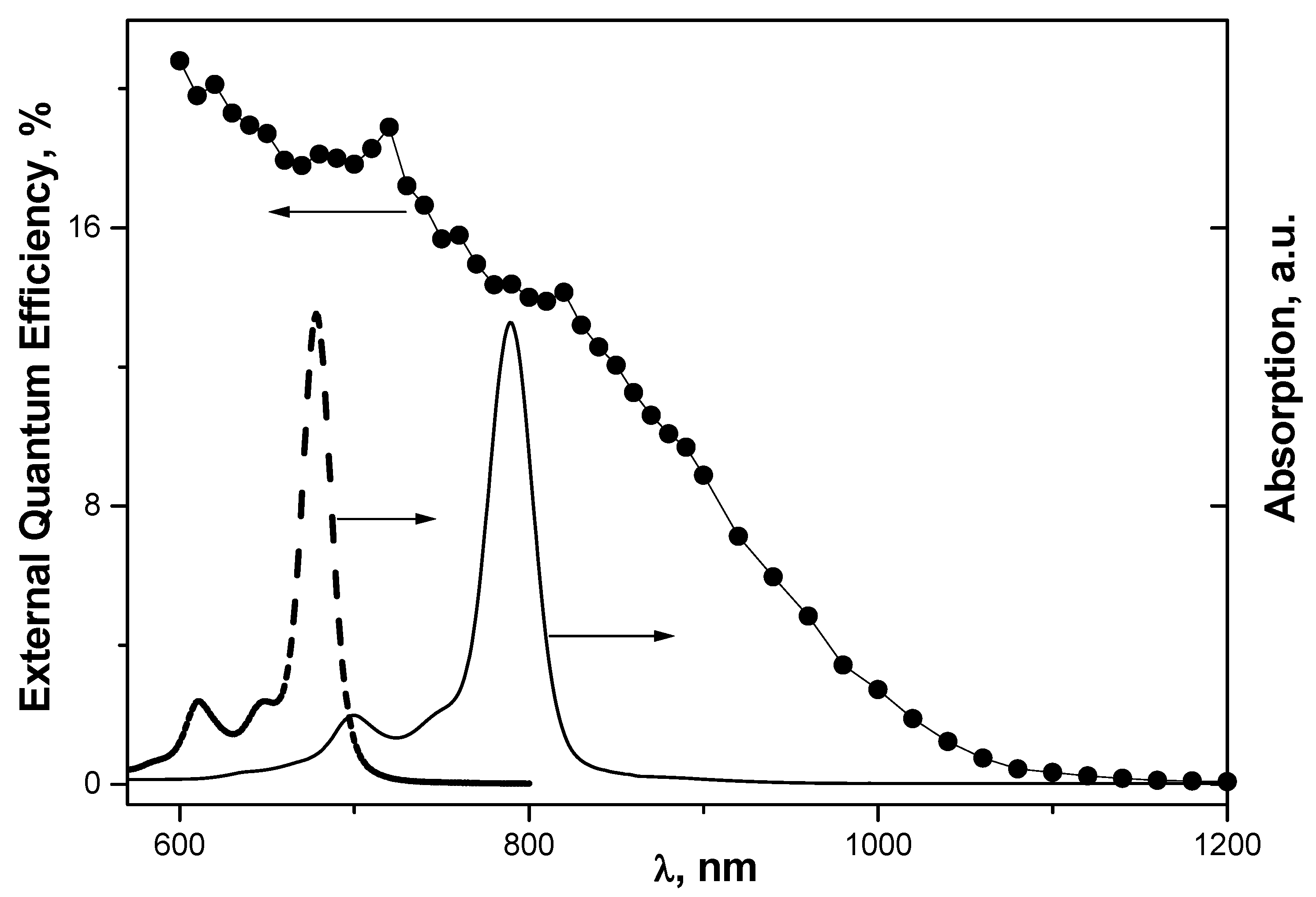
Figure 7.
External quantum efficiency of the Au/Psi-CuPC/Si/Al cell. Absorption spectra of CuPC in concentrated H
2SO
4 solution (solid line) and CuPC1 in chloroform solution (dashed line). Concentration is 10
−5 M. Shift of CuPC spectrum with respect to CuPC1 spectrum is the result of CuPC protonation in H
2SO
4 solution. Reprinted with permission from [
47]. Copyright [2004], American Institute of Physics.
Figure 7.
External quantum efficiency of the Au/Psi-CuPC/Si/Al cell. Absorption spectra of CuPC in concentrated H
2SO
4 solution (solid line) and CuPC1 in chloroform solution (dashed line). Concentration is 10
−5 M. Shift of CuPC spectrum with respect to CuPC1 spectrum is the result of CuPC protonation in H
2SO
4 solution. Reprinted with permission from [
47]. Copyright [2004], American Institute of Physics.
The contribution of CuPC to PV effect can be seen in the spectral dependence of external conversion efficiency, which exhibits two peaks (720 and 820 nm) matching the absorption bands of the protonated CuPC form in H
2SO
4 solution (
Figure 7). The long wavelength edge of the spectrum corresponds to the band gap of Si (1.1 eV) but not porous Si (~2 eV). Hence, the absorbance of the porous layer is low or the porosity in the
n-type Si is not sufficient to change the band gap.
The similar dependence of the conversion efficiency on the porosity layer has been observed in study [
49], where the device with porosity of 24% demonstrated an efficiency of 1.2% in contrast with the 0.91% value for the device with porosity of 62%. The authors suggested that such effect is associated with the increased distance between CuPC and PSi surface for highly porous structure. However, a more plausible explanation is related to the abrupt conductivity reduction for porosities more than 57% [
51].
4. Solar Cells Based on Silicon and Carbon Nanotubes
Despite significant achievements for the past decade, hybrid PVs from Group IV, III-V semiconductors and organics (polymer and small molecules) still remain promising objects for future research. Their conversion efficiency so far does not exceed the level of 3–4%, which is not sufficient for any commercial applications. This situation however can soon be dramatically changed, as a result of recent research related to PV properties of CNTs—Si heterojunction [
52,
53,
54,
55,
56].
It is known that the major drawback of hybrid solar cells is insufficient PV quality of the organic component, which is attributed to the low charge mobility, lack of absorbance in the red/NIR spectral range, and chemical instability. On the other hand, the obvious advantages of organics are wet processings, light weight and flexibility.
Remarkably, CNTs possess the above-mentioned advantage and are free from the mentioned deficiency. Indeed, the unique optoelectronic properties of CNTs [
57] make them a favorite candidate compared to other organic materials for incorporating in the structure of hybrid solar cells. First, CNT charge mobility can be up to 10
5 cm
2V
−1s
−1 for individual nanotubes [
58] and ~60 cm
2V
−1s
−1 for CNT films [
59], which is much higher than the hole mobility in P3HT polymer (one of the best organic conductor). Second, CNTs has a high light absorbance in the red and NIR spectrum (absorbance coefficient of 10
−4–10
−5 cm
−1). Third, they exhibit an exceptional environmental stability. Fourth, carbon nanotubes can be deposited easily to the semiconductor surface by wet processing techniques (coating, spraying, and printing). Finally, CNTs can be easily doped or functionalized, thus tuning the Fermi level to the favorable position when forming heterojunction with semiconductors [
60,
61,
62].
The photoconductive response of films prepared from single walled carbon nanotube (SWNT) under laser pulse excitation was first reported by Fujiwara
et al. [
63]. Subsequently, photocurrent response of SWNT film under continuous wave illumination were reported by Levitsky
et al. (SWNT films) [
64] and for individual SWNT bundle by Freitag
et al. [
65]. It is important that the combination of CNTs of varying diameters and chiralities, and correspondingly different band gap (the band gap is inversely proportional to diameters) makes it theoretically possible to obtain a continuous photoresponse over a broad spectral range covering the red and NIR part of the solar spectrum.
For the past decade, applications of CNTs in organic solar cells were mostly focused as nanoscale fillers for polymer films [
66,
67,
68,
69,
70,
71]. The donor-acceptor configuration, where the high aspect ratio and large surface area of nanotubes incorporated in these polymers was believed to be favorable for higher exciton dissociation and increased charge carrier transport. However, the power conversion efficiency for these structures remains relatively low (<1%) possibly due to the low carrier mobility in polymers and poor dispersion of CNTs in polymer matrix. Another way to employ CNTs in solar cell design is the use of CNTs as a transparent conductive coating to replace metal oxides electrodes (e.g., ITO) for organic PVs [
62,
73]. Nevertheless, little progress has been achieved in this direction as the conductivity of the CNT network is still lower than the conductivity of ITO at the same transparency level in the visible spectral range.
Very recently several groups reported the hybrid solar cells based on heterojunction of CNTs and
n-type Si [
52,
53,
54,
55,
56] where CNTs function as active photosensing component being involved in the light absorption, charge separation and charge transport. Surprisingly, only for the past two years, the conversion efficiency of such devices exceed the level of 7% [
54] and are very promising for even higher conversion efficiency with special CNTs treatments and device optimizations.
Figure 8.
SEM images (a–d) of SWNT films deposited on a
n-type Si by spraying technique from supernatant and sediment fractions. Cross-sectional view of (a) supernatant and (b) sediment. Top view of (c) supernatant, and (d) sediment. Diameter distribution histogram of SWNT bundles for (e) supernatant and (f) sediment. From the SEM images and histogram, sediment sample shows thicker and longer SWNT bundles compared to supernatant [
56]. Reproduced with permission from Institute of Physics and IOP Publishing Limited, ©2009.
Figure 8.
SEM images (a–d) of SWNT films deposited on a
n-type Si by spraying technique from supernatant and sediment fractions. Cross-sectional view of (a) supernatant and (b) sediment. Top view of (c) supernatant, and (d) sediment. Diameter distribution histogram of SWNT bundles for (e) supernatant and (f) sediment. From the SEM images and histogram, sediment sample shows thicker and longer SWNT bundles compared to supernatant [
56]. Reproduced with permission from Institute of Physics and IOP Publishing Limited, ©2009.
The hybrid
nSi/CNTs solar cells are comprised of a thin layer of nanotube films, deposited either via air-brush spraying of nanotube suspension [
52,
53,
56] or lift-off wet deposition process [
54,
55] on
n-type silicon wafers. The morphology of these films is a network of nanotube bundles (consisting of several tens of individual nanotubes) with highly porous structure (
Figure 8c,d). Because semiconducting single walled nanotubes (SWNTs) and double walled nanotubes (DWNTs) are naturally
p-type conductors, when interfaced with
n-type Si, they form
p-n heterojunctions.
I-V measurements of
nSi/SWNT heterojunction [
56] exhibit typical solar cell characteristics under dark and under light illumination (
Figure 9a). Such rectifying behavior can be explained by the schematic energy band diagram based on Anderson model as shown in
Figure 9b. As shown in the band diagram, a large built in electric field is formed at the interface. The source of photocarriers in SWNTs is excitons with a binding energy ~0.4 eV [
74], which are dissociated at the interface due to the built-in field. It is likely that the electrons then tunnel to the Si layer and holes are transported through the SWNT network to the external electrode. Photogenerated free carries in Si can also be separated at the interface followed by photohole injection from Si to SWNTs.
Figure 9.
(a) Current-Voltage (
I-V) plot of sediment and supernatant SWNT/
nSi devices under dark and light illumination. (b) Schematic energy band diagram of SWNT/
nSi heterojunction based on the Anderson model. Electron affinity,
χ and conduction band offset,
ΔEc for SWNT and
nSi are shown in the energy band diagram [
56]. Reproduced with permission from Institute of Physics and IOP Publishing Limited, ©2009.
Figure 9.
(a) Current-Voltage (
I-V) plot of sediment and supernatant SWNT/
nSi devices under dark and light illumination. (b) Schematic energy band diagram of SWNT/
nSi heterojunction based on the Anderson model. Electron affinity,
χ and conduction band offset,
ΔEc for SWNT and
nSi are shown in the energy band diagram [
56]. Reproduced with permission from Institute of Physics and IOP Publishing Limited, ©2009.
The role of SWNTs in photoconversion efficiency of the hybrid cell was not clearly understood in studies [
52,
53,
55] as no photocurrent action spectra were recorded. The first measurement of photocurrent spectra showed maximum quantum efficiency peak at 1.2 eV, which is close to the energy gap of Si (1.12 eV) [
54], however a low resolution did not allow to identify the possible band responsible for DWNT absorption and photoconversion. Later, Levitsky
et al. [
56] demonstrated a matching photocurrent band of the
nSi/SWNT device to the first transition band of the NIR absorption spectra of the SWNT film (
Figure 10). Thus, the CNTs in the hybrid nSi/CNT heterojunction solar cells contribute to the photo conversion process not only as a charge separator/transporter/collector but also as a light absorber. This is an important fact, distinguishing between a heterojunction solar cell with two active light absorbing components and a Schottky cell, where the metal component is not capable of absorbing photons.
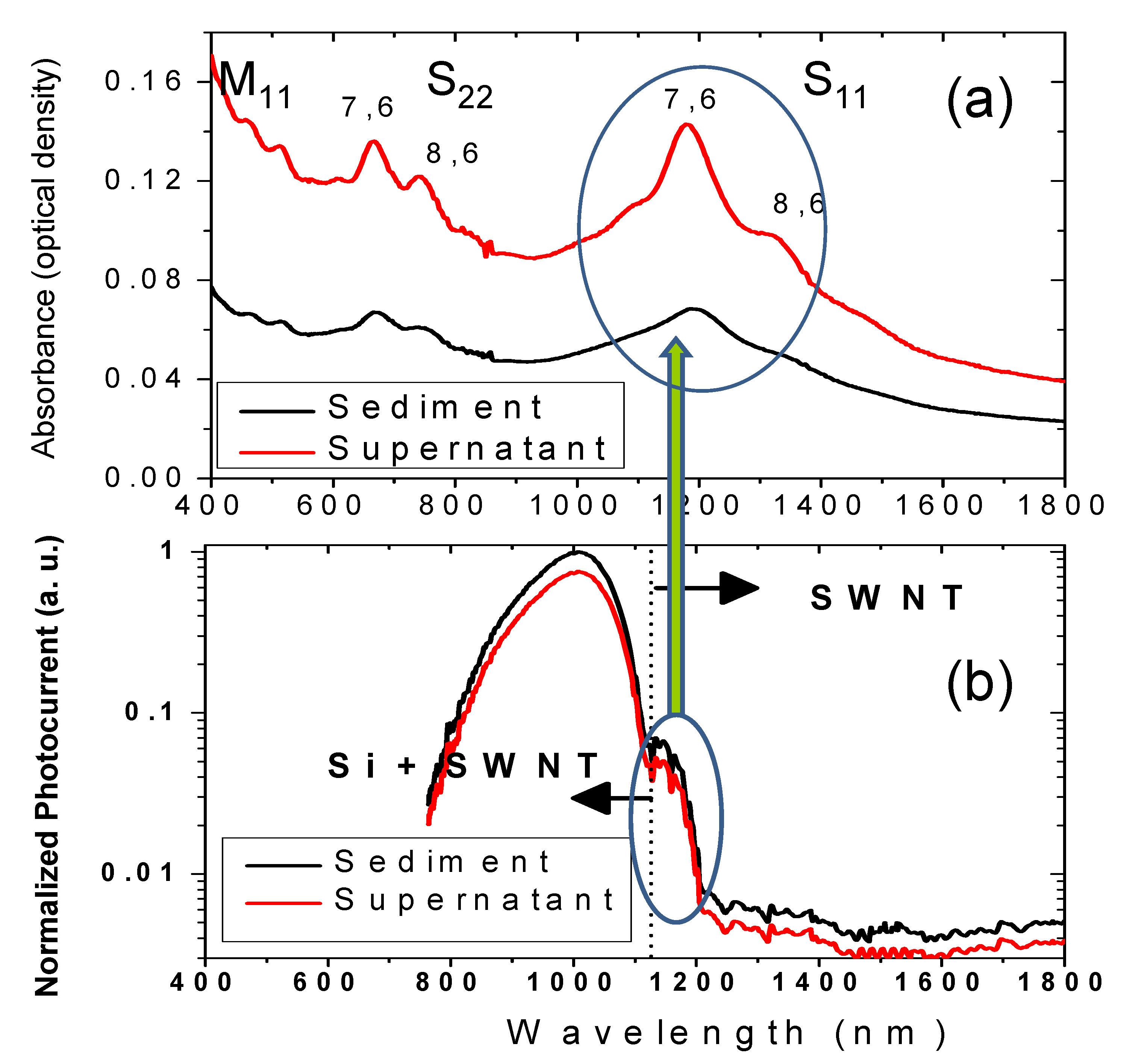
Figure 10.
(a) UV-Vis NIR spectrum of sediment and supernatant fraction from centrifugation process of SWNT films on glass with sheet resistance of 1 kΩ/square. M
11, S
22, and S
11 represents the band-gap transitions in metallic and semiconducting SWNTs. (b) Normalized photocurrent spectra (area and intensity of light source) of the solar cell devices (supernatant and sediment) showing a current peak band (due to SWNT) matching the S
11 band [
56]. Reproduced with permission from Institute of Physics and IOP Publishing Limited, ©2009.
Figure 10.
(a) UV-Vis NIR spectrum of sediment and supernatant fraction from centrifugation process of SWNT films on glass with sheet resistance of 1 kΩ/square. M
11, S
22, and S
11 represents the band-gap transitions in metallic and semiconducting SWNTs. (b) Normalized photocurrent spectra (area and intensity of light source) of the solar cell devices (supernatant and sediment) showing a current peak band (due to SWNT) matching the S
11 band [
56]. Reproduced with permission from Institute of Physics and IOP Publishing Limited, ©2009.
Although, the conversion efficiency of
nSi/CNT solar cells is still lower than that of conventional Si cells, there are several methods currently explored to increase the PV performance through CNT doping, functionalizing and separation. SWNT chemical modification by thionyl chloride allowed to increase the conversion efficiency by more than 50% reaching the value above 4% [
53]. Another approach, using high speed centrifugation, has been applied to separate SWNTs on high (sediment) and low (supernatant) conductive fractions [
56].
Figure 8 shows the SEM images and diameter distribution for SWNT film on Si surface deposited by spraying from sediment (SD) and supernatant (ST) fractions. SD based cells demonstrated an efficiency (~1.7 %) higher by a factor of ~8 as compared with ST cells (~0.2%) and by a factor of 2 as compared with sonication only based solar cells (~0.8%), where SWNT were sprayed without centrifugation [
56].
All of the above promising findings allow us to believe that PV performance of hybrid nSi/CNT solar cells could be further substantially improved to overcome the 10% threshold of conversion efficiency needed for commercial applications.