Abstract
This paper provides an overview of recent studies on energy storage in bifunctional TiO2 composite materials under UV and visible light. The working mechanism, property improvements and applications of these bifunctional TiO2 composite systems are introduced, respectively. The latest results obtained in our laboratory, especially a new process for photoelectric conversion and energy storage in TiO2/Cu2O bilayer films under visible light, are also presented. Hopefully this review will stimulate more fundamental and applied research on this subject in the future.
1. Introduction
The economic growth in many parts of the World during the past decade was sustainable thanks to affordable energy prices. The dependence on oil and electricity has made energy an important component in our everyday life and the recent increases of oil and gas prices have prompted everyone to take a careful look at energy issue. In the 20th century, the population has quadrupled and our energy demand went up 16 times. The exponential energy demand is exhausting our fossil fuel supply at an alarming rate [1,2]. About 13 terawatts (TW) of energy is currently needed to sustain the lifestyle of 6.5 billion people worldwide. By 2050, it is estimated we will need an additional 10 TW of clean energy to maintain the current lifestyle. In order to meet the increasing energy demands in the near future, we will be forced to seek environmentally clean alternative energy resources [3,4]. Three major options are at our disposal to tackle the generation of 10 TW of clean energy in the coming years. These include carbon neutral energy (fossil fuel in conjunction with carbon sequestration), nuclear power, and various forms of renewable energy. Among these last options, solar energy stands out as the most viable choice to meet the energy demand. Despite the vast size of this resource, the energy produced from solar sources remains less than 0.01% of the total energy demand [5].
Nearly all energy consumed in our planet comes from the solar light, either in a roundabout way or directly in the form of heat and light radiation. The effective utilization of clean, safe, abundant and regenerated solar energy will lead to promising solutions. not only for energy issues due to the exhaustion of nature energy sources. but also for many problems caused by environmental pollution. Sunlight can be converted into other forms of energy by a variety of methods. Generally speaking, the first step to utilize solar light is conversion, by which we can use it in our own way. The second is storage, by which we can store excess energy in the day time and use it during the night or any other time when the visible light available is insufficient.
Photoelectric conversion is one of the most widely-used and well-developed techniques in the field of solar energy utilization today. The photovoltaic (PV) industry is booming, with an estimated growth rate in excess of 30% per year over the last decade [6]. Crystalline silicon solar cells, which are presently demonstrated to have energy conversion efficiencies close to 25%, account for the most important part of the world PV market [7,8]. Dye sensitized solar cells, which have already reached conversion efficiencies exceeding 11%, offer the prospect of the low cost fabrication without expensive and energy-intensive high temperature and high vacuum processes to facilitate market entry [9]. Thin film PV technologies based on inorganic materials are developing rapidly both in the laboratory and in industry [6]. They are also a formidable competitor aiming to boost their market share.
However, these traditional photovoltaic materials generally function as photoelectric or chemical energy conversion devices under irradiation. The excess energy produced by these devices has to be stored by energy storage systems such as electrochemical storage cells or supercapacitors [10]. The existence of the energy storage systems always results in an increase of expense and the consumption of extra energy. Now that traditional photovoltaic materials cannot play the role of energy storage and traditional energy storage systems do not have the ability of photoelectric conversion, their combination should be a new promising field in the development and utilization of solar energy.
TiO2 nanostructure hybrid materials are the focus of both fundamental and applied research [11]. Nanohybrid materials developed by the modification of pristine TiO2 with anions, cations, and metals are named second-generation TiO2 photocatalysts [12]. Electron transfer between a TiO2 substrate and the interfused material endows them with novel properties, such as absorption of visible light and enhanced light electricity conversion efficiency and improved activity in photocatalysis [13,14,15], which can be used in quantum dot solar cells [16,17] and gas sensors [18]. In recent years, functionalized TiO2 hybrid materials were also reported as energy storage materials.
In 2000, Zou’s group found that TiO2/carbon fiber electrodes prepared by laser deposition had dual functions of opto-electric conversion and electrochemical energy storage. Since 2001, a few Japanese research groups have developed several TiO2 composite systems with dual abilities of photoelectric conversion and energy storage (bifunctional composite system) and a few similar TiO2 composite systems were developed in recent years by other groups. This is a very new research field that has not yet been paid the deserved attention. Incidentally, the application for TiO2 composite system in solar energy has been overviewed extensively [9,19,20], but that for both photoelectric conversion and energy storage of solar energy has not yet been reviewed until now. So, according to the publications and the research carried out in our laboratory, a general overview of the subject of energy storage in TiO2 composite systems under solar light is presented here.
2. Survey of Bifunctional TiO2 Composite Materials
In 2000, TiO2 composite materials with energy storage abilities were first developed by Xinjing Zou’s group [21] who found that TiO2/carbon fiber electrodes functioned as a photo-rechargeable battery with the dual functions of opto-electric conversion and electrochemical energy storage. In 2001, Tatsuma et al. [22] developed a TiO2/WO3 photoelectrochemical anticorrosion system with energy storage ability. A TiO2 coating is coupled with a WO3 coating as an electron pool, in which the reductive energy can be stored. Snce then, TiO2/MoO3 [23], SrTiO3/WO3 [24], TiO2/phosphotungstic acid (PWA) [25], TiO2/Ni(OH)2 [26] and several other TiO2/WO3 [27,28,29] composite systems have also been developed by the groups of Tetsu Tatsuma and his collaborators. The energy can be stored either in reduced WO3, MoO3, PWA or in oxidized Ni(OH)2 under UV light. In 2003, Raghavan et al. [30] showed that SnO2 coupled with TiO2 can store reductive energy generated on UV illumination of TiO2, which enables the continued cathodic protection effect under dark conditions. In 2008, Yasomanee et al. [31] reported that TiO2/Cu2O composite films showed a multi-electron storage ability under UV-vis irradiation. It was noticed that the irradiation of ITO/TiO2/Cu2O led to the formation of trapped electrons and this stored energy resulted in H2 evolution from H2O, even in the dark. Additionally, Chien-Tsung Wang [32] demonstrated that V2O5 served as an energy storage material to accumulate photoelectrons generated by the TiO2 semiconductor, due to the relative energy levels of the conduction bands of V2O5 and TiO2. In 2009, Zhang’s [33], Zhao’s [34] and Tatsuma’s [35] groups reported that optical energy can be stored by TiO2 nanotube array/Ni(OH)2 composite electrodes, WO3/TiO2 nanohybrid materials and Au–TiO2/WO3 systems, respectively. It is worth mentioning that the Au–TiO2 composite system, which is based on plasmon resonance absorption of Au nanoparticles, can store reductive energy in WO3 under visible-light irradiation. Recently, our group found that visible-light energy can be stored by Ti3+ in TiO2/Cu2O bilayer film [36]. The appearance of visible-light induced energy storage system is a significant progress in this field and beneficial practical application since it can work directly under solar light.
3. Working Mechanism of Optical Energy Storage
The energy can be stored by reduced materials (such as WO3,MoO3 and PWA), oxidized ones (Ni(OH)2) or TiO2 itself. Zou’s group described the double functions of opto-electric conversion and electrochemical energy storage for TiO2/carbon fiber electrodes by the characterization of photo charge and discharge currents. The systematic research on the mechanism of optical energy storage was first done by Tatsuma’s group. Based on their and our own work, we here introduce three representative optical energy storage mechanisms.
3.1. Reductive Energy Storage in TiO2/WO3 (MoO3 or PWA) Composite System
Figure 1 shows the mechanism of reductive energy storage by TiO2/WO3 composite system [27]. In this system, an excited electron and a corresponding hole are generated on TiO2 under UV light:
TiO2 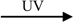 TiO2 * (e− + h+)
TiO2 * (e− + h+)
The electron may be transported through TiO2 (n-type semiconductor) to WO3, and if so, a cation should be intercalated into WO3. In pure water, only H+ is available as a cation:
WO3 + xe− + xH+  HxWO3
HxWO3
On the other hand, the hole should also be consumed at the TiO2 surface by H2O to generate chiefly O2 and H+. Thus, H+ is generated by TiO2 (Equation 3) and is consumed by WO3 (Equation 2):
2H2O + 4h+  O2 + 4H+
O2 + 4H+
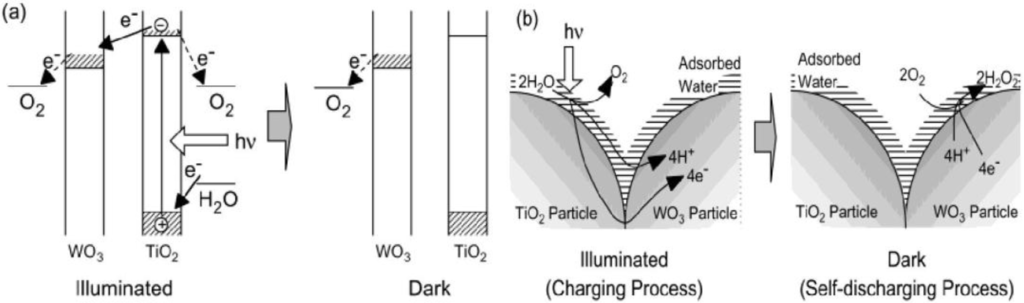
Figure 1.
(a) Mechanism of reductive energy storage of TiO2/WO3 composite system [28]. (b) Proposed models of electron and ion transfer in the charging and self-discharging processes of the TiO2/WO3 composite film in humid air [27].
The ITO electrode coated with the TiO2/WO3 composite film (type b, thick film, Figure 2a) was subjected to the 1 h UV irradiation in air to show the formation of HxWO3. As shown in Figure 2b, the behavior strongly depended on the relative humidity of the atmosphere. The film was well-colored in a humid atmosphere (relative humidity ≥25%), while it was not colored well in a dry atmosphere. The color of HxWO3 is blue. The reduction of WO3 (almost colorless) gave rise to the decrease in the reflectance shown in Figure 2b.
This can be explained in terms of ionic conductivity of the film surface. In the humid atmosphere, an adsorbed water layer should form on the film surface. This layer and the surface hydroxyl groups, of which dissociation should be facilitated by this water layer, should contribute to the ionic conduction. It is necessary for the photoelectrochemical reduction of WO3 (Figure 1b). To the contrary, in the dry atmosphere, the adsorbed water layer is almost absent so that the ionic conductivity should not be sufficient for the WO3 reduction.
As the excess electrons are accepted by WO3 and more HxWO3 is generated, the reductive energy can be stored by WO3 in pure water, humid air or solution. It can be seen that WO3 retains the reductive energy for a certain period even after the light is turned off (Figure 1a).
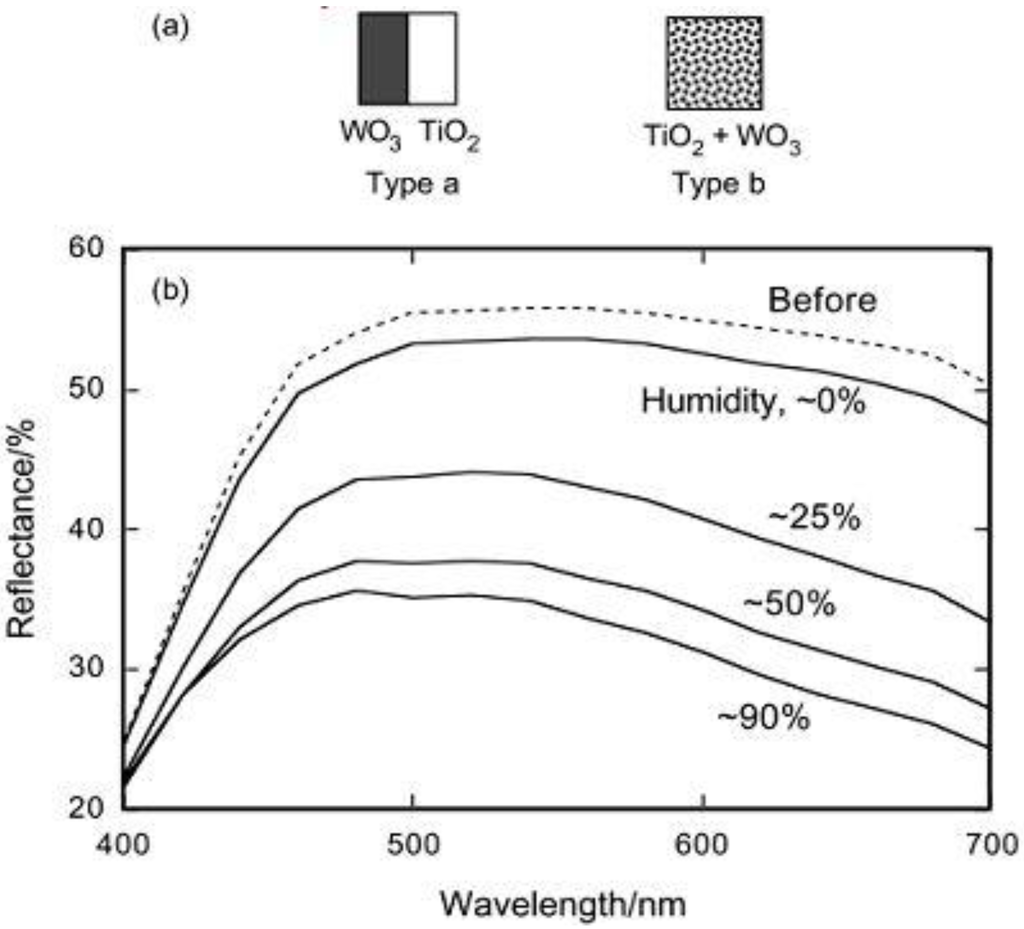
Figure 2.
(a) Schematic models for TiO2/WO3 separated films (type a) and TiO2/WO3 composite film (type b). (b) Changes in the reflectance of the TiO2/WO3 composite film (type b, thick film) after UV irradiation (22 mW cm−2, 20 min) at a relative humidity of about 0, 25, 50, and 90% [27]. Reproduced with permission from the ACS, ©2002.
3.2. Oxidized Energy Storage in TiO2/Ni(OH)2 Composite System
Two kinds of models for storing the oxidative energy of TiO2 were proposed, the p-n junction model and the mediation model (Figure 3) [26]. In the former, a redox-active p-type semiconductor is combined with TiO2 to form a p-n junction (Figure 3a).
Holes generated at the junction are separated from excited electrons and transported into the bulk of the p-type semiconductor for the oxidative energy storage. The electrical neutrality will be retained by the intercalation of the anions or the deintercalation of the cations so that oxidative energy will keep stable. In the mediation model (Figure 3b), an oxidant like O2, which is photocatalytically produced by the oxidation of water adsorbed on TiO2, diffuses and oxidizes a redox-active material.
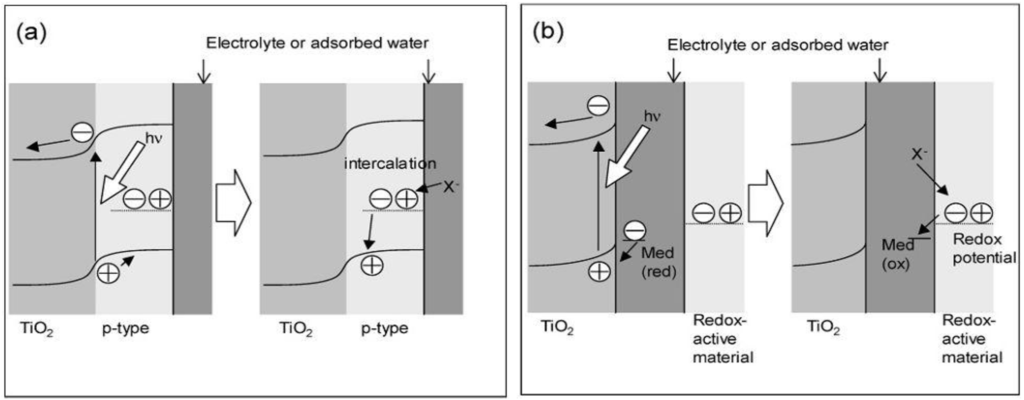
Figure 3.
Models for the oxidative energy storage photocatalysts. (a) p-n junction model and (b) mediation model [26].
Ni(OH)2 is reported to be a p-type semiconductor [37,38], and is known as a cathode active material of secondary batteries [39] with relatively positive redox potential (+0.5 to +0.6 V vs Ag|AgCl). Therefore, it is expected to be suitable for oxidative energy storage. After a TiO2/Ni(OH)2 bilayer film was irradiated with UV light (365 nm; 10 mW cm−2) in pH 10 buffer, the film turned to brown as did the electrochemically oxidized film. Actually, the visible absorption spectrum of the irradiated film looked like that of the electrochemically oxidized film. These results suggest that the irradiation gave rise to the oxidation of Ni(OH)2. However, a Ni(OH)2 film without TiO2 was not colored under UV-irradiation. Therefore, TiO2 should contribute to the photooxidation of Ni(OH)2. The brown-colored film could be turned back to colorless by electrochemical reduction. Namely, the photoelectrochemically stored oxidative energy could be taken out and used.
The photooxidation of Ni(OH)2 most probably proceeds as follows. Electrons are photoexcited from the valence band to the conduction band (e−CB) in TiO2 or at the TiO2/Ni(OH)2 junction (Equation 1). The correspondingly formed holes in the valence band (h+ VB) accept electrons from Ni(OH)2, either directly or indirectly (Equation 4):
Ni(OH)2 + xh+VB + xOH−  NiOx(OH)2−x + xH2O
NiOx(OH)2−x + xH2O
The photo-excited electrons should be consumed by an electron acceptor, most likely dissolved oxygen diffused to the TiO2 surface through the pores in the Ni(OH)2 layer because of a reaction such as Equations 5a or 5b:
O2 + e− CB  O2−
O2−
O2 + 2H+ + 2e− CB  H2O2
H2O2
At the current stage, it is uncertain whether the oxidative energy storage is based on the p-n junction model (Figure 1a) or the mediation model (Figure 1b). According to the literature [40], TiO2 is known to form a p-n junction with NiO. Ni(OH)2 is also reported to be a p-type semiconductor similar to NiO [30]. Hence, it is possible that a p-n junction forms at the TiO2/Ni(OH)2 interface. If the mediation model worked, the mediator might be, for example, the H2O2 generated by Equation 5b. However, a Ni(OH)2 film without TiO2 was not oxidized when it was exposed to 0.1–10 M H2O2 with or without UV light. Rather, oxidized Ni(OH)2 was reduced by H2O2 in the dark. Thus, the mediator, if any, is more likely to be an oxidant other than H2O2. If the energy storage proceeded according to the p-n junction model, the efficiency would be improved by increasing the junction area, for instance, by making the TiO2 layer porous. On the other hand, if the mediation model worked, the distance between Ni(OH)2 and TiO2 could be optimized so as to suppress the rereduction by the electrons on TiO2.
In all, although it is uncertain whether the oxidative energy storage is based on the p-n junction model or the mediation model, it is sure that the energy stored in Ni(OH)2 is oxidized energy, which is different from that in WO3 according to their color changes and results of photoelectrochemical experiments.
3.3. Multi-electrons Energy Storage in TiO2/Cu2O Composite System
Recently, we prepared a TiO2/Cu2O bilayer film on doped fluorine SnO2 (FTO) conducting glass according to literature [41,42]. Ti3+ in the TiO2/Cu2O bilayer film is demonstrated to store energy under visible light. It is well known that the band gap of TiO2 is about 3.2 eV and the conduction band of TiO2 is about –0.2 eV [43]. Cu2O is a semiconductor with one of the highest conduction bands. The band gap of Cu2O is about 2.0 eV and the potential of its conduction band is –1.4 eV [44].
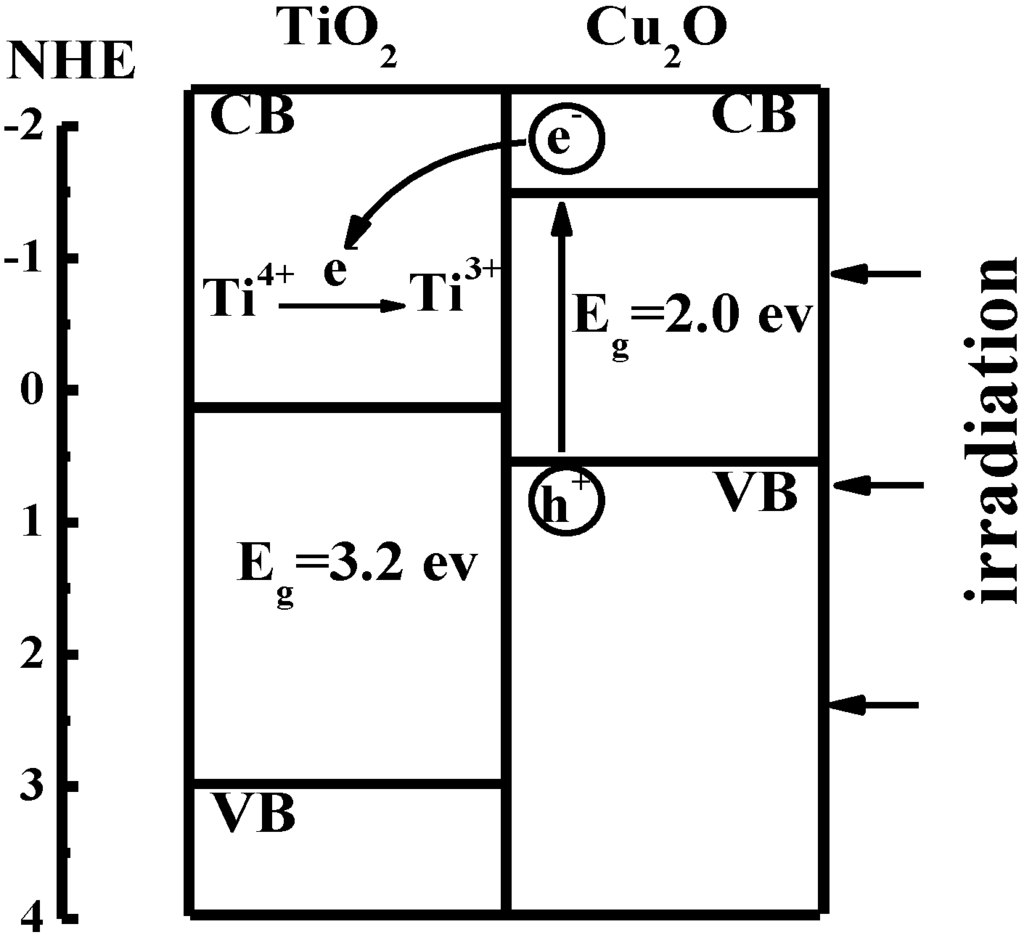
Figure 4.
Sketch of interfacial electron transfer in TiO2/Cu2O bilayer film [36].
As shown in Figure 4, the photogenerated electrons from the conduction band of Cu2O were captured by Ti4+ ions in TiO2 and Ti4+ ions were further reduced to Ti3+ ions. The Ti3+ ions have a long lifetime and bear the photogenerated electrons as a form of energy. The electron transfer process is shown in Equations 6 and 7:
Cu2O + hν (λ > 400 nm)  hvb+ + ecb−
hvb+ + ecb−
ecb− + Ti4+  Ti3+
Ti3+
The photovoltage measured as a function of time under visible-light irradiation for FTO/TiO2/Cu2O and FTO/Cu2O electrodes is shown in Figure 5. It can be seen that the potential for both of the two electrodes shifted positively under the same irradiation. The positive potential shift could be due to hole generated on Cu2O, which is one of p-type semiconductors. As TiO2 has no response to visible light, the photopotential of TiO2/Cu2O bilayer film almost results from Cu2O. So, just like Cu2O electrode, the potential shift of the TiO2/Cu2O electrode should be similar to that of Cu2O. This result is similar to the report in [32]. The net positive photovoltage results from the interfacial potential difference of electrostatic double layer formed between holes on the surface of Cu2O and SO42− layer in the electrolyte.
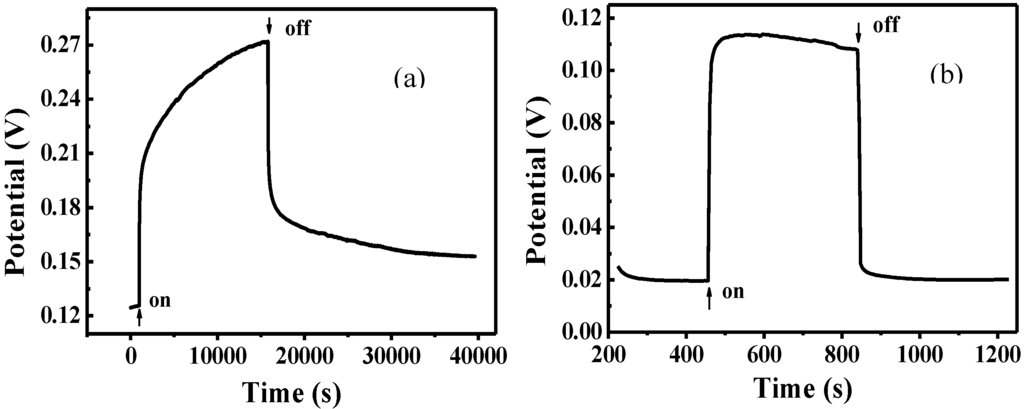
Figure 5.
Photovoltage measured as a function of time for FTO/TiO2/Cu2O (a) and FTO/Cu2O (b) electrodes [36].
Moreover, the potential for the FTO/Cu2O electrode jumped to the maximum instantly and remain unchanged. It indicates that the generation and recombination of e−-h+ pairs in Cu2O established a dynamic equilibrium. However, it took the FTO/TiO2/Cu2O electrode about four hours to reach its maximum. It indicates that there was a process for the accumulation of holes in FTO/TiO2/Cu2O electrode. Since photogenerated electrons and holes were produced in Cu2O under the irradiation, it can be proposed that there was a process for the accumulation of electrons in FTO/TiO2/Cu2O electrode. The photogenerated electrons in Cu2O have much energy due to the absorption of visible light. Thus, when these electrons are transferred to the conduct band of TiO2 and trapped by Ti4+, they are seen as Ti3+ and are stored as a form of energy in the bilayer film. The holes still stay in Cu2O. The longer the irradiation time, the more electrons were generated by Cu2O and injected into TiO2 and thereafter the more energy stored. This process did not stop until the potential of the TiO2/Cu2O electrode reached the maximum.
After the irradiation was removed, the potential for both of these electrodes shifted negatively. The potential for FTO/Cu2O electrode dropped instantly to the minimum, the original potential before the irradiation. It indicates that the photogenerated electrons and holes are recombined completely. However, it took FTO/TiO2/Cu2O electrode a long time to reach its minimum, which was still higher than the original potential before the irradiation. It indicates that there are still holes in FTO/TiO2/Cu2O electrode and the photogenerated electrons and holes may not be recombined completely because a little amount of electrons are still trapped by Ti3+.
Additionally, under the same irradiation, the potential increment for FTO/TiO2/Cu2O electrode was much higher than that for FTO/Cu2O electrode. It demonstrates that much more photogenerated holes and electrons were present in the bilayer film. The better photoelectric properties of the bilayer film than that of Cu2O film suggests that the bilayer films have improved abilities for charge separation and charge carrier lifetime, and can store energy.
UV-vis diffuse reflectance measurements were further carried out to identify the conversion of Ti4+ to Ti3+ in the bilayer film after the irradiation. As shown in Figure 6, the absorbance of the bilayer film became weaker in the short wavelength range (200 nm ≤ λ ≤ 350 nm) while becoming stronger in the long wavelength range (350 nm < λ ≤ 800 nm) after the irradiation. It is confirmed based on the above data that Ti3+ ions were produced in the bilayer film after the irradiation. Because Ti4+ has no response to visible light while Ti3+ does [45,46,47], the presence of Ti3+ ions leads to the weaker absorbance of bilayer film in the short wavelength range while stronger in the long wavelength range. It is found that the transparent TiO2 film turned blue under the irradiation. The phenomenon is similar to Yasomanee’s report [31] and also demonstrates the presence of Ti3+.
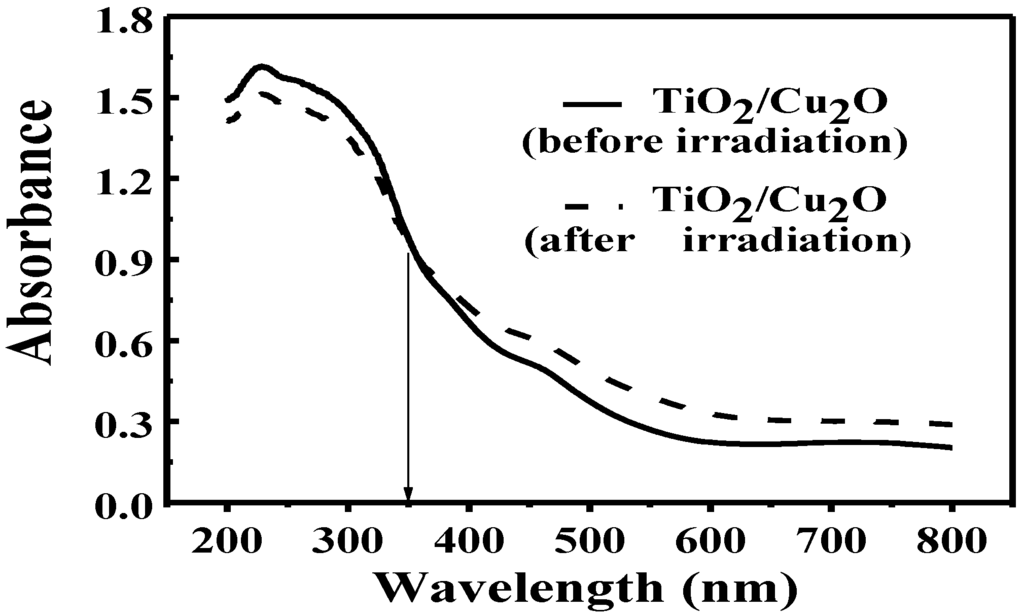
Figure 6.
UV-vis diffuse reflectance spectra of TiO2/Cu2O bilayer film before (solid line) and after irradiation (dashed line) [36].
As mentioned in the Introduction, similar work was also carried out by Yasomanee’s group. The main phenomena and results are in accord with each other. For example, as for TiO2/Cu2O composite (or bilayer) film, the color changes under irradiation, the photovoltage curves and hydrogen evolution reactions under irradiation or in dark are similar to each other. However, the explanations for the energy storage between us are different. Yasomanee proposed that electrons excited by UV–vis light are trapped at electron trapping centres of both Cu2O and TiO2. These electrons could release under dark and may eventually participate in the hydrogen evaluation reaction in dark. In our work, we demonstrate that Ti3+ ions in TiO2/Cu2O bilayer film formed under visible light showed energy storage ability.
4. Energy Storage Property Improvement in TiO2 Composite System
Since the first bifunctional TiO2 systems was developed by Xinjing Zou’s group, the work on the optimization and improvement of the systems has been carried out. Tatsuma et al made a remarkable achievement in this area. For instance, further efforts were taken to optimize the TiO2/WO3 system in order to obtain maximum quantum yield for the electron storage and maximum specific capacity (x in HxWO3) [29]. To attain the maximum energy storage ability of the TiO2/WO3 system in the gas phase, film composition (W/Ti mole ratio), UV light intensity and film thickness (film mass), were optimized in terms of apparent quantum yield (flux of stored electrons per flux of incident photons), which is an index of the photoelectrochemical charging rate, capacity (maximum charge stored) as well as the specific capacity (x in HxWO3). According to the experimental results, the maximal apparent quantum yield (~8%) was obtained at a W/Ti ratio = 0.5 and a film thickness ≥10 μm (film mass ≥24 mg cm−2). Although a lower light intensity results in a better apparent quantum yield, it leads to slower charging. The capacity was highest at W/Ti = 0.5 and increased with the film thickness. The specific capacity (~0.10) was almost independent of the parameters in the range examined. Considering all these results, it can be concluded that the optimum W/Ti ratio is 0.5 and the film should be thick. In the case where the electric conductivity of the film is important (e.g., photoelectrochemical protection of metals, in which electrons should be transferred from WO3 to the metals), the optimum thickness should be around 10 μm (film mass ≥24 mg cm−2).
To improve the applicabilities of the energy storage materials furthermore, MoO3, another energy storage materials that have different discharging potentials, capacities and charging–discharging was rated from those of WO3 [23].
It is known that MoO3 and WO3 undergo the following redox reactions:
where M = H, Li, Na, etc.
WO3 + xM+ + xe−  MxWO3
MxWO3
MoO3 + xM+ + xe−  MxMoO3
MxMoO3
As a comparison, MoO3 and WO3 films were electrochemically charged at −0.4, −0.5 or −0.6 V versus Ag|AgCl for 1 h, and discharged at 1 μA (cut-off potential = −0.1 V; Figure 7 and Figure 8). As the charging potential was shifted more negatively, both the charging and discharging capacities of WO3 increased.
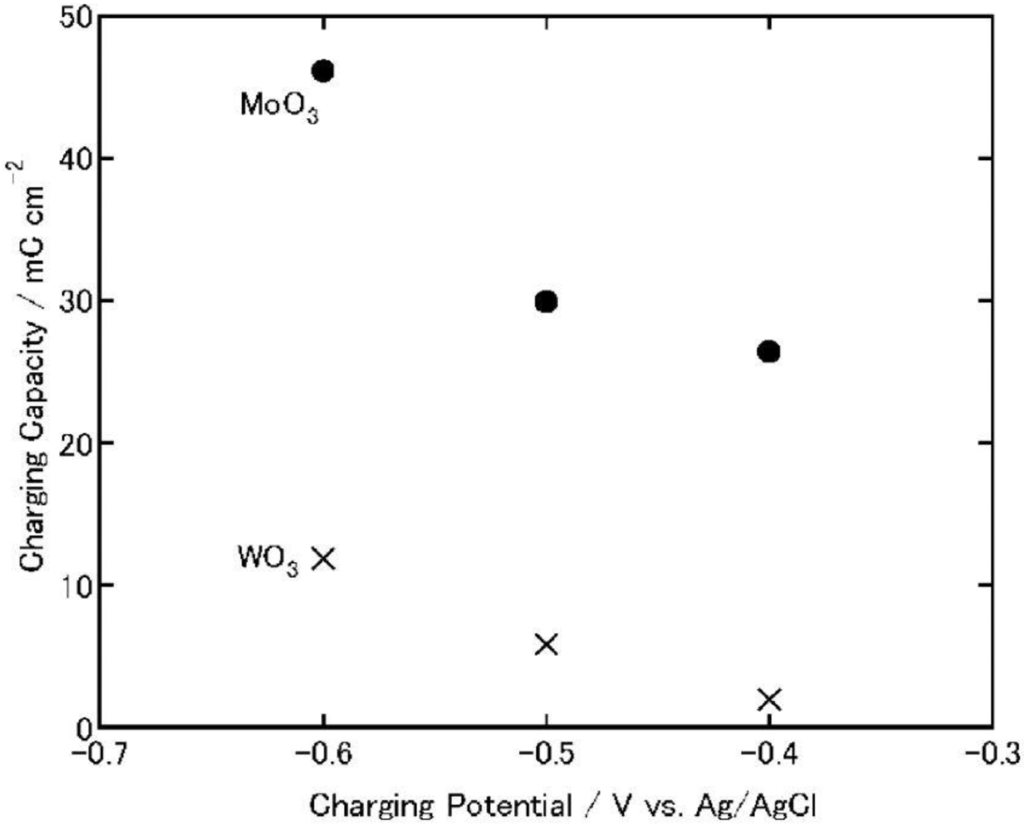
Figure 7.
Charging capacities of MoO3 and WO3 coatings on ITO electrodes when the coatings are charged electrochemically (1 h) in 3 wt. % NaCl aqueous solutions (pH 5) [23]. Reproduced with permission from Elsevier, © 2004.
On the other hand, in the case of MoO3, its discharging capacity decreased while its charging capacity increased. The discharging efficiency (discharging capacity/charging capacity) of WO3 increased constantly as the charging potential was shifted negatively (0.23, 0.46 and 0.66 at −0.4, −0.5 and −0.6 V, respectively). However, that of MoO3 decreased (0.12, 0.076 and 0.014 at −0.4, −0.5 and −0.6 V. respectively). After the discharging, the color of the WO3 was almost the same as that before charging while that of the MoO3 film was slightly blue, indicating that the film was still partially reduced. Thus, the low discharging efficiency of MoO3 may be explained in terms of slow reoxidation or high electrical resistance of MxMoO3. In addition, at more negative potentials, MoO3 might be partially reduced to irreversibly intercalated state or to metallic state, which cannot be reoxidized easily to the initial state. Moreover, the fast reoxidation of MoO3 by dissolved oxygen during the electrochemical charging may be responsible for the low efficiency.
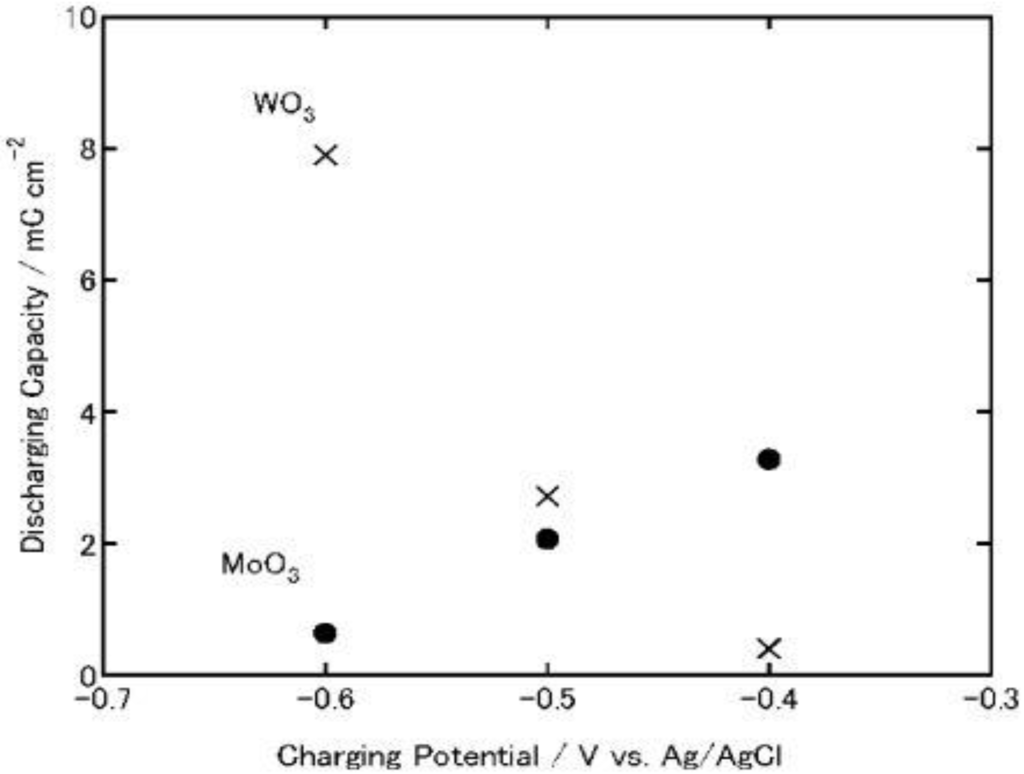
Figure 8.
Discharging capacities of MoO3 and WO3 coatings on ITO electrodes. The coatings were discharged electrochemically at 1μA (cut-off potential = −0.1V vs. Ag|AgCl) after electrochemical charging (Figure 3) in 3 wt. % NaCl aqueous solution (pH 5) [23]. Reproduced with permission from Elsevier, © 2004.
In either event, when the charging potential is −0.4 V, which is the photopotential of TiO2, the charging and discharging capacities of MoO3 are one order of magnitude larger than those of WO3. Therefore, MoO3 could be used as an energy storage material coupled with TiO2.
For a comparison, TiO2/MoO3 and TiO2/WO3 bilayer films were irradiated with UV light for 8 h in air at relative humidity of 80%, and then its open-circuit potential was monitored in a 3 wt. % NaCl aqueous solution, respectively. As a result, the self-discharging time of TiO2/MoO3 bilayer film was elongated to >1.5 h (Figure 8). However, the TiO2/WO3 bilayer film charged under the same conditions exhibited longer self-discharging time than TiO2/MoO3 film. Thus, TiO2/MoO3 composite film should be more suitable to be used as energy storage materials in air under humid conditions if the MoO3 overlayer is sufficiently thin and the film retains enough adsorbed water on the surface.
Although WO3 has a longer self-discharging time than MoO3, MoO3 exhibited larger charging and discharging capacities and greater ability for oxygen reduction than WO3. TiO2/MoO3 composite film can also be charged photoelectrochemically in an electrolyte or humid air. Therefore, TiO2/MoO3 composites may function as energy storage materials under humid conditions.
However, TiO2 photocatalyst has some limitations, which come from its own electronic structure. It can work only under UV illumination (wavelength < 380 nm). To overcome the limitation, visible-light responsive Au–TiO2 photocatalyst have been developed [35].
Figure 9a shows the model for visible-light responsive materials with reductive energy storage abilities. The photopotential of the TiO2-coated electrode without Au nanoparticles was ~0.0 V under visible light. The open-circuit potential of the Au-TiO2-coated electrode was ~+0.1 V vs. Ag|AgCl in the electrolyte, and the potential shifted to ~0.2 V under visible light (> 480 nm; ~600 mW cm−2). When the Au-TiO2 film is irradiated, some of the Au nanoparticles are excited by localized surface plasmon resonance (LSPR) and electrons are transferred to TiO2. When there is an energy storage material (WO3, MoO3, and PWA) combined with the Au-TiO2 film, the reductive energy will be stored. On the other hand, the positive charges left on the Au nanoparticles are consumed by the oxidation of ethanol in the electrolyte, probably to acetic acid.
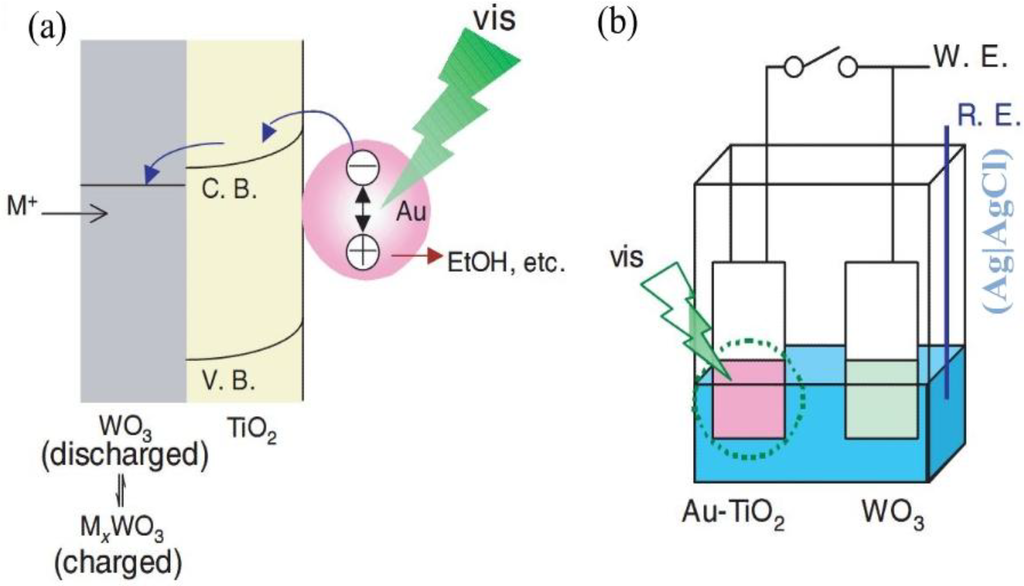
Figure 9.
(a) A model for visible-light responsive materials with reductive energy storage abilities. (b) Experimental setup for reductive energy storage in a WO3 film by a visible-light irradiated Au-TiO2 film [35]. Reproduced with permission from Elsevier, © 2008.
5. Application of Energy storage in TiO2 Composite System
5.1. Anticorrosion
Figure 10 illustrates the mechanism of a photoelectrochemical anticorrosion system with energy storage ability. Electrons in the valence band are excited to the conduction band under the irradiation with appropriate light. The excited electrons are injected to the metal so as to keep its potential more negative than the corrosion potential. The excess electrons are accepted by the electron pool (combined WO3) so that the reductive energy generated at the irradiated semiconductor can be stored. After the UV light is turned off, electrons stored in WO3 are injected into the metal so that it is still protected from corrosion. It is obvious that the WO3 functions in ths case as an energy storage device.
An anticorrosion experiment was carried out using for Type 304 stainless steel as a substrate. Two kinds of samples were prepared. One was coated with TiO2 and WO3 (single coating, area, 50% each), and the other was fully coated with TiO2 (see insets of Figure 11). As Figure 11 shows, the potential of the stainless steel plate fully coated with TiO2 (curve a) was about 0.4 V vs Ag|AgCl, while the stainless steel is not oxidized during UV irradiation. After the UV light was turned off, however, the potential was shifted in a few minutes to the corrosion potential of the stainless steel.
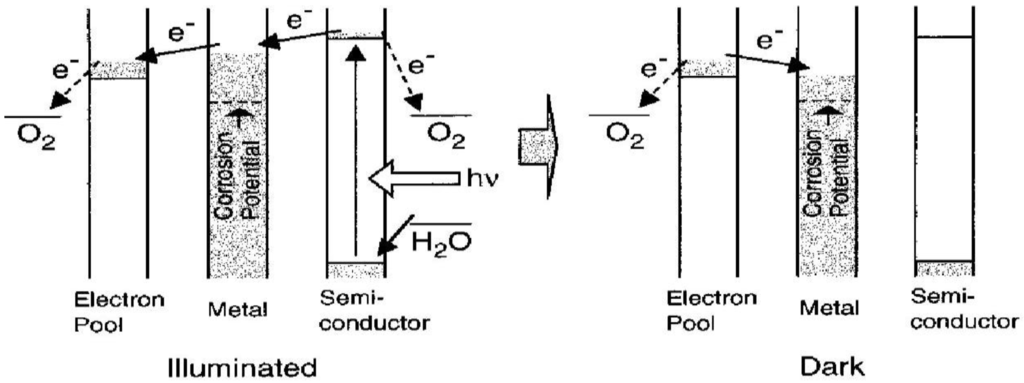
Figure 10.
Mechanism of a photoelectrochemical anticorrosion system with an energy storage ability [22].
The potential in the dark was very unstable, indicating that the stainless steel was corroded. In contrast, the potential of the stainless steel plate coated with TiO2 and WO3 (curve b) was more negative than the corrosion potential for a few hours, even after the light was turned off. Actually, the unstable potential behavior reflecting the corrosion of the substrate stainless steel was not observed for 4 h in dark. These results clearly indicate that the anticorrosion effect of the TiO2/WO3 system is retained after dark. Potential behavior of the ITO electrode coated with TiO2 and WO3 (single coating) (area, 50% each) was also examined for comparison. Even though the sample was prepared and tested in the same way as that for the coatings on the stainless steel, it took longer time to be discharged (>6 h). This difference is probably due to a difference in discharging processes. That is, a reduced WO3 coating on an ITO electrode is oxidized primarily by oxygen directly while that on a stainless steel plate should be oxidized also by oxygen through the stainless steel. This may be reasonable because stainless steel should catalyze the oxygen reduction more efficiently than ITO.
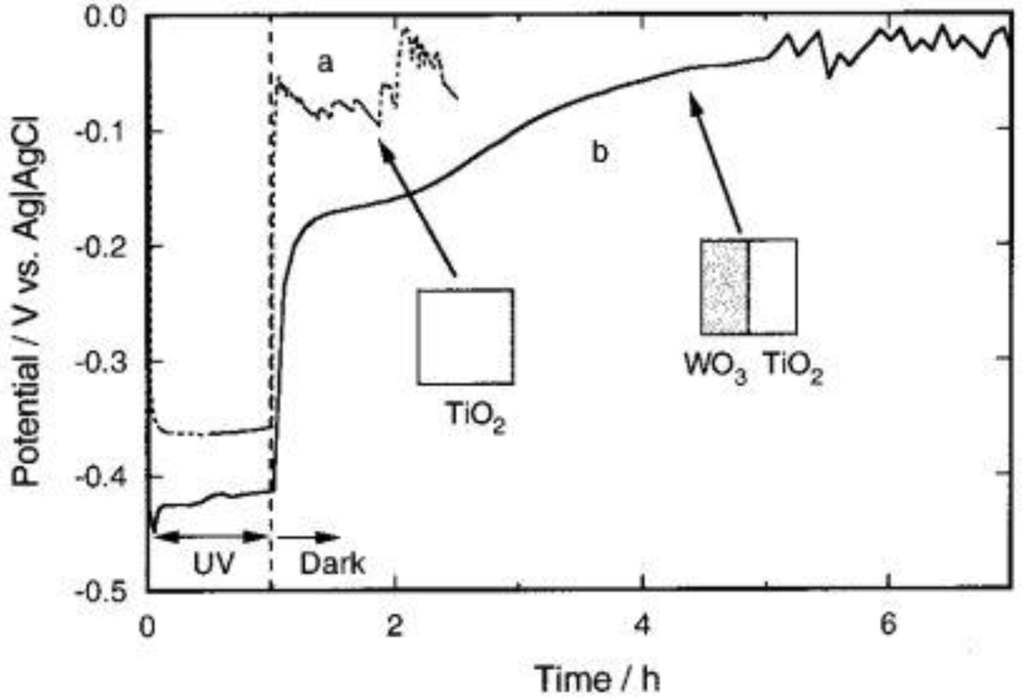
Figure 11.
Changes in the potential of a type 304 stainless steel (1.75 cm2) coated with TiO2 (a) and that coated with TiO2 and WO3 (single coating) (area, 50% each) (b) in an air-saturated 3 wt % NaCl solution, pH 5. TiO2 was irradiated with UV light for the first 1 h [22]. Reproduced with permission from the ACS, © 2001.
5.2. Bactericidal Effect
Bactericidal effects of the photoelectrochemicall charged and discharged TiO2/WO3 films were examined [28]. A 20 μL aliquot of an E. coli suspension was applied onto the film surface, and left for 6 h. The suspension was then transferred onto an agar culture medium and incubated. The number of colonies formed was summarized in the histogram (Figure 12). It is obvious that the survival ratio for the charged sample was lower than that for the discharged sample. The average number of colonies for the control experiment, in which the E. coli suspension was directly transferred to the agar culture medium, was 72. Thus, the average survival ratio for the charged samples is 25% while that for the discharged samples is 58%. Although the bactericidal effect is not very strong, multiplication of E. coli is suppressed at least in part by the charged TiO2/WO3 film. The moderate bactericidal effect may prevent bacteria from increasing during the night, and the survived bacteria may be killed by TiO2 due to its strong bactericidal effect in the next day.
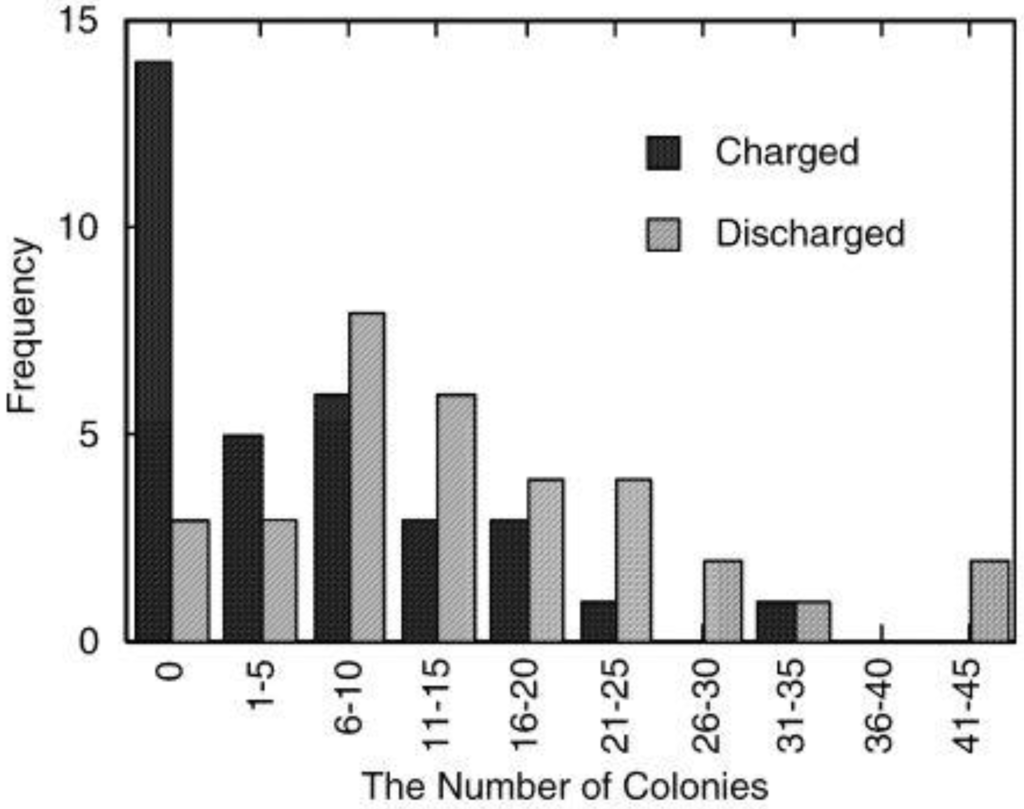
Figure 12.
Bactericidal effect of the photoelectrochemically charged (irradiated with UV light for 24 h) and pre-discharged TiO2/WO3 films. A 20 μL aliquot of E. coli suspension that contains ~70 cells was applied onto a film surface, and left for 6 h at room temperature, 100% relative humidity. The suspension was then transferred onto a deoxycholate agar culture medium and incubated for 24 h at 37 °C. The number of thus formed colonies was counted [28]. Reproduced with permission from Elsevier, © 2003.
The bactericidal effect should be based on the reductive energy stored in WO3. Possible reactions proceeding during the discharge of the charged WO3 are as follows:
O2 + H+ + e−  HO2
HO2
O2 + 2H+ + 2e−  H2O2
H2O2
O2 + 4H+ + 4e−  2H2O
2H2O
Incidentally, the equimolar amount of protons and electrons are supplied from the reduced WO3, HxWO3 (Equation 13):
HxWO3  WO3 + xe− + xH+
WO3 + xe− + xH+
Among the possible products, HO2· and H2O2 have bactericidal effects. Although the consumption of O2 could also be responsible for the bactericidal effect, the supply of O2 from ambient air should be much faster than the O2 consumption. Furthermore, HO2· and corresponding deprotonated anion O2−· are less stable than H2O2. Therefore, H2O2 generation from the charged TiO2/WO3 film was examined.
It is found that in the case of the charged film, the H2O2 concentration was 2 × 10−5 M while H2O2 was not detected for the pre-discharged film. Furthermore, the toxicity of H2O2 for E. coli was examined as well. The survival ratios of E. coli exposed to various concentrations of H2O2 for 6 h are summarized in Table 1. Because the average H2O2 concentration in water on the charged TiO2/WO3 film during the 6 h treatment may be as low as 1 × 10−5 M, the bactericidal effect of the same film in the dark can be explained mainly in terms of H2O2 generation.

Table 1.
Bactericidal effect of H2O2 solutions of various concentrations a [28] Reproduced with permission from Elsevier, © 2003.
| H2O2 concentration (M) | Survival ratio a (%) |
|---|---|
| 0 | 65 |
| 1 × 10−6 | 65 |
| 1 × 10−5 | 29 |
| 1 × 10−4 | 3 |
| 1 × 10−3 | 0 |
a E. coli was exposed to H2O2 solutions for 6 h.
5.3. Photochromism
When TiO2 composite systems were charged under the irradiation and discharged in the dark, the energy storage materials were involved in photoelectrochemical reaction and color changes correspondingly. In addition to the Tatsuma group’s reports [27], the photochromic effects of TiO2/WO3 composite systems were also described in [48,49].
Ni(OH)2 is known to be an anodic electrochromic material that changes color from colorless to brown upon oxidation [50,51]. The Ni(OH)2/TiO2 bilayer film was irradiated with UV light (365 nm; 10 mW cm−2) [26]. The film turned from colorless (Figure 13a) to brown (Figure 13b) while the electrochemically oxidized film did. However, a Ni(OH)2 film without TiO2 was electrochemically bleached rapidly and completely. As for the Ni(OH)2/TiO2 bilayer film, the bleaching was not completed but it was confirmed that the photooxidative coloration and electrochemical bleaching could be repeated at least 5 times. It appears that the TiO2/Ni(OH)2 bilayer film can potentially be used as a photochromic material.
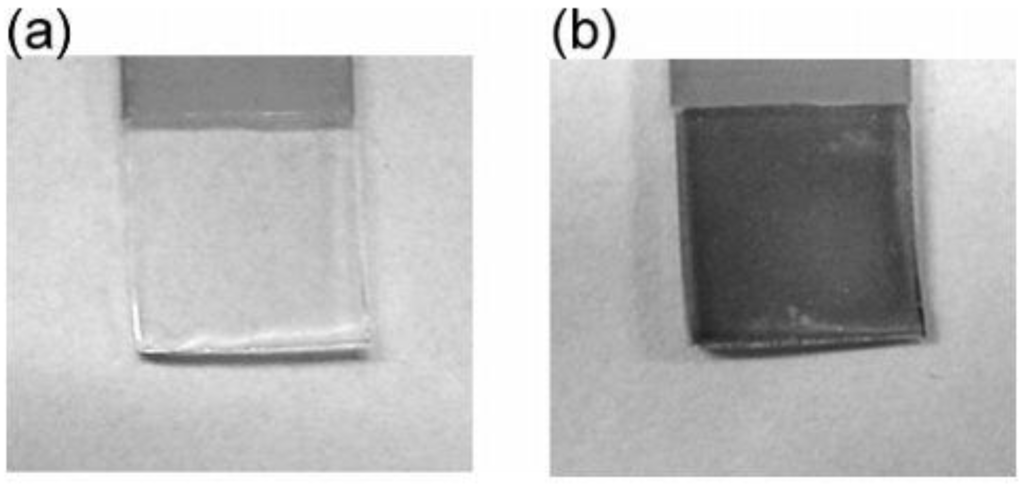
Figure 13.
TiO2/Ni(OH)2 film before (a) and after (b) UV irradiation for 2 h in NaHCO3/NaOH buffer (pH 10) [26]. Reproduced with permission from the ACS, ©2005.
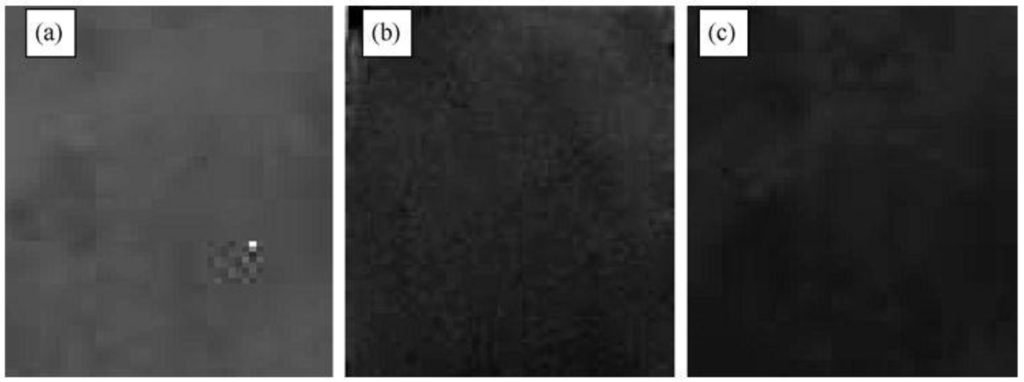
Figure 14.
Color changes of TiO2/Ni(OH)2 electrode (a) before irradiation, (b) after irradiation for 1 h and (c) after irradiation for 2 h [33]. Reproduced with permission from Elsevier, © 2009.
Figure 14 [33] shows the similar color changes of the TiO2/Ni(OH)2 electrode upon irradiation. Before the irradiation, the color was gray. After 1 h irradiation, it changed to brown. As the irradiation went on for longer times, it eventually changed to dark brown. The gray sample changed to dark brown because of the oxidization of Ni(OH)2 to NiOOH. The UV–vis absorbance increased as the electrode color becomes darker. The darkening degree of the electrode depended on the light intensity and exposure time. The larger the light intensity or the longer the exposure time, the darker it became.
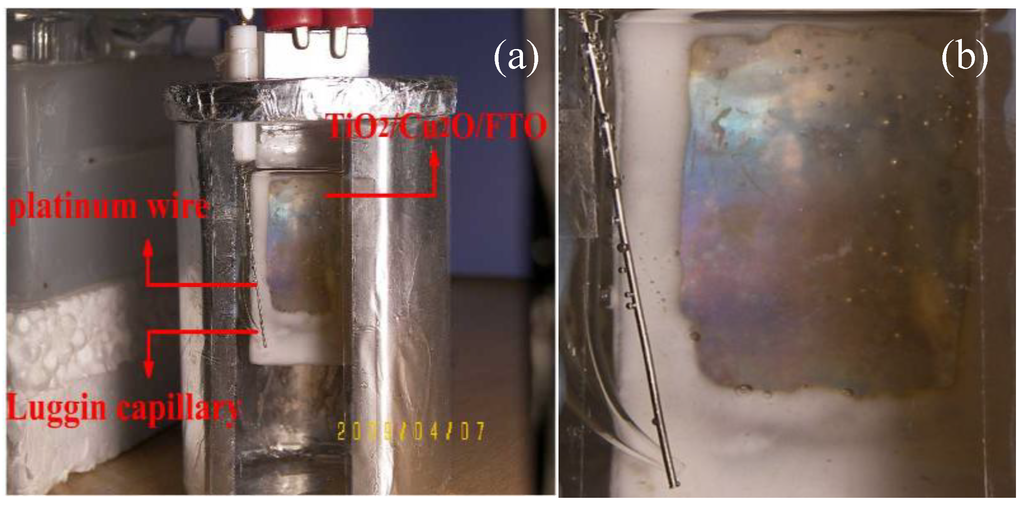
Figure 15.
Photograph of three electrode system in photoelectrochemical experiment with persistent visible irradiation for 6 h with (a) low magnification and (b) high magnification [36].
Figure 15 shows a photograph of TiO2/Cu2O bilayer film [36] in a photoelectrochemical experiment with visible-light irradiation for 6 h. It is found that the transparent TiO2 film turned blue under the irradiation. The phenomenon is similar to Yasomanee’s report [31]. The change of color demonstrates that Ti4+ in TiO2 was reduced to Ti3+ [52,53].
5.4. Photocatalytic H2 Evolution
As for the application of the energy stored in Ti3+, TiO2/Cu2O bilayer film was used to reduce H+ for the formation of H2 from water splitting. The TiO2/Cu2O bilayer film was immersed in aqueous Na2S and Na2SO3 solution and irradiated with visible light. Figure 16 shows the H2 yield as the function of time under and after the irradiation. It can also be found that H2 evolution was still noticeable after the irradiation stopped until the third hour. We believe that the electrons trapped in Ti3+ ions as stored energy leads to H2 evolution from H2O splitting in dark. Based on the mechanism of the energy storage in TiO2/Cu2O bilayer film proposed in Figure 5, the possible electrons transfer reactions are:
Cu2O + hλ (λ > 400 nm)  hvb+ + ecb
hvb+ + ecb
ecb−+ Ti4+  Ti3+
Ti3+
2Ti3+ + 2H+  2 Ti4+ + H2
2 Ti4+ + H2
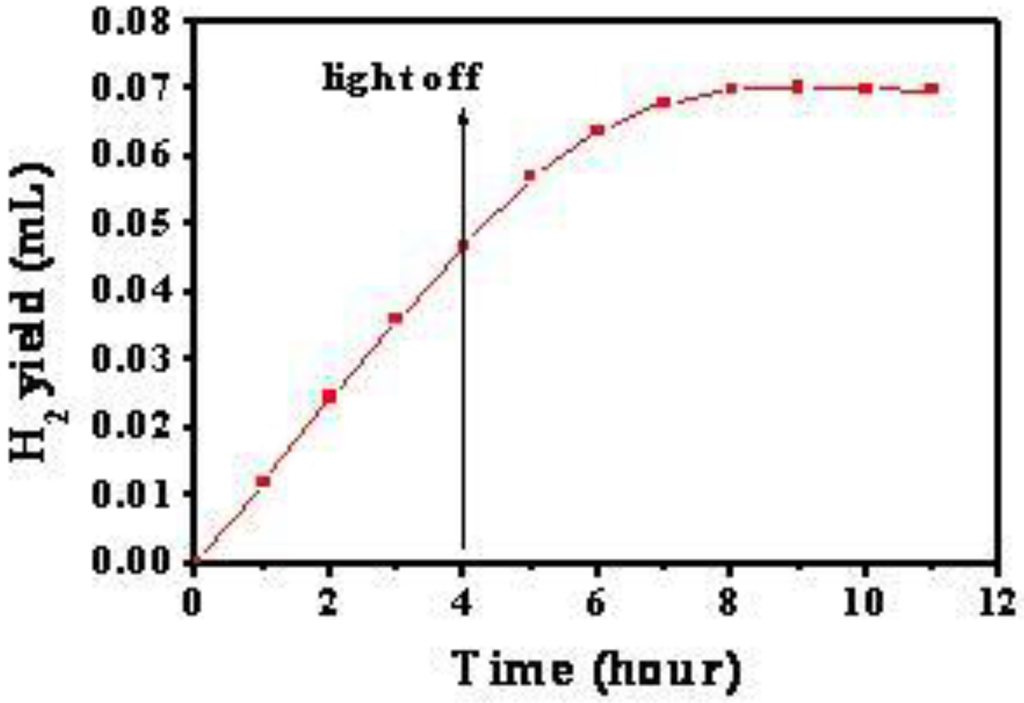
Figure 16.
Curve of H2 yield as the function of time under and after visible-light irradiation [36].
The amount of the electrons in Ti3+ ions as stored energy can be calculated approximately according to the H2 yield as follows:
where n (C cm−2) is the amount of electrons/cm2 trapped in Ti3+ ions in TiO2/Cu2O film, V is the volume of H2 produced in the dark, Q is the number of electrons per coulomb, S is the surface area of TiO2/Cu2O bilayer film, M and NA are the molar volume of a gas in standard conditions and Avogadro constant respectively. The calculated result indicates that more than 5 × 10−2 C cm−2 electrons were stored in TiO2/Cu2O film under 4 h visible-light irradiation.
6. Concluding Remarks
The progress made on the energy storage in bifunctional modified TiO2 systems under UV light and visible light is summarized. Substantially, this research has the advantage of the combination of the dual functions of opto-electric conversion and energy storage together in a single system. Moreover, cheap composite thin films based on TiO2 and other metal oxide semiconductor may utilize solar energy and take the place of accumulators for energy storage in some special applications. However, it can be seen from the current literatures on this subject is still in its infancy, although it is full of hope. We believe this subject warrants more extended and in-depth study in the future, comparable to the efforts that have been taken on the separate areas of photoelectric conversion and solar energy storage, respectively [54,55,56,57,58].
We may need to continue this study focusing on two aspects. On one hand, the working mechanism of the reported systems has yet not been understood fully and needs further study. In addition, some of these results reviewed here may turn out to be of mere fundamental interest and relevant for the a particular environment, application or problem. Some might be helpful for understanding the properties of the modified TiO2 energy storage materials [59,60,61,62]. On the other hand, a concerted effort is needed to screen potential useful bifunctional systems and find ways to design relevant devices for practical applications, which may begin with material preparation. Also, nanostructured architectures should be considered to be synthesized for the composition of the bifunctional TiO2 composite material for the improvement of opto-electric conversion and energy storage property.
Anyway, although research has only just begun to explore the science and engineering applications of this remarkable material platform, we do hope this review can promote more fundamental and applied lines of research on this subject in the coming future.
Acknowledgements
We acknowledge the financial supports from National Natural Science Foundation of China (Grant Nos 20973070 and 90510012), National Basic Research Program of China (No. 2009CB939704), the Key Project of Chinese Ministry of Education (No. 109116) and the Programme of Introducing Talents of Discipline to Universities (No. B08033).
References
- Weisz, P.B. Basic choices and constraints on long-term energy supplies. Phys. Today. 2004, 57, 47–52. [Google Scholar] [CrossRef]
- Bartlett, A.A. Sustained availability: A management program for nonrenewable resources. Am. J. Phys. 1986, 54, 398–402. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature. 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Crabtree, G.W.; Nozik, A.J.; Wasielewski, M.R.; Alivisatos, A.P. Basic Energy Sciences Report on Basic Research Needs for Solar Energy Utilization; Office of Science, U.S. Department of Energy: Washington, DC, USA, 2005. Available online: http://www.sc.doe.gov/bes/reports/files/SEU_rpt.pdf (accessed on 19 August 2009).
- Kamat, P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Aberle, G.A. Thin-film solar cells. Thin Solid Films 2009, 517, 4706–4710. [Google Scholar] [CrossRef]
- Green, M.A.; Zhao, J.; Wang, A.; Wenham, S.R. Progress and outlook for high-efficiency crystalline silicon solar cells. Sol. Energy Mater. Sol. Cells 2001, 65, 9–16. [Google Scholar] [CrossRef]
- Nij, J.F.; Szlufcik, J.; Poortmans, J.; Sivoththaman, S.; Mertens, R.P. Advanced cost-effective crystalline silicon solar cell technologies. Sol. Energy Mater. Sol. Cells 2001, 65, 249–259. [Google Scholar] [CrossRef]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Ilincaa, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Tachikaw, T.; Fujitsuka, M.; Majima, T. Mechanistic insight into the tio2 photocatalytic reactions: Design of new photocatalysts. J. Phys. Chem. C. 2007, 111, 5259–5275. [Google Scholar] [CrossRef]
- Serpone, N. Is the band gap of pristine tio2 narrowed by anion- and cation-doping of titanium dioxide in second-generation photocatalysts? J. Phys. Chem. B. 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Hirakaw, T.; Kamat, V.P. Charge separation and catalytic activity of Ag@TiO2 core-shell composite clusters under uv-irradiation. J. Am. Chem. Soc. 2005, 127, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Wolf, E.E.; Kamat, V.P. Catalysis with TiO2/Gold nanocomposites. Effect of metal particle size on the fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ma, W.; Chen, C.; Zhao, J.; Shuai, Z. Efficient degradation of toxic organic pollutants with Ni2O3/TiO2-xBx under visible irradiation. J. Am. Chem. Soc. 2004, 126, 4782–4783. [Google Scholar] [CrossRef] [PubMed]
- István, R.; Kuno, M.; Kamat, V.P. Size-dependent electron injection from excited cdse quantum dots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 4136–4137. [Google Scholar] [CrossRef] [PubMed]
- Robel, I.; Subramanian, V.; Kuno, M.; Kamat, V.P. Quantum dot solar cells. Harvesting light energy with CdSe. J. Am. Chem. Soc. 2006, 128, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Trinchi, A.; Li, Y.X.; Wlodarski, W.; Kaciulis, S.; Pandolfic, L.; Viticoli, S.; Comini, E.; Sberveglieri, G. Investigation of sol–gel prepared CeO2–TiO2 thin films for oxygen gas sensing. Sens. Actuat. B. 2003, 95, 145–150. [Google Scholar] [CrossRef]
- Mor, K.G.; Varghese, K.O.; Paulose, M.; Shankar, K.; Grimes, A.C. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energ. Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Zou, X.J.; Maesako, N.; Nomiyama, T.; Horie, Y.; Miyazaki, T. Photo-rechargeable battery with TiO2/carbon fiber electrodes prepared by laser deposition. Sol. Energy Mater. Sol. Cells 2000, 62, 133–142. [Google Scholar] [CrossRef]
- Tatsuma, T.; Saitoh, S.; Ohko, Y.; Fujishima, A. TiO2-WO3 photoelectrochemical anticorrosion system with an energy storage ability. Chem. Mater. 2001, 13, 2838–2842. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ngaotrakanwiwat, P.; Tatsuma, T. Energy storage TiO2–MoO3 photocatalysts. Electrochim. Acta 2004, 49, 2025–2029. [Google Scholar] [CrossRef]
- Ohko, Y.; Saitoh, S.; Tatsuma, T.; Fujishima, A. SrTiO3-WO3 photocatalysis systems with an energy storage ability. Electrochemistry 2002, 70, 460–462. [Google Scholar]
- Ngaotrakanwiwat, P.; Saitoh, S.; Ohko, Y.; Tatsuma, T.; Fujishim, A. TiO2-phosphotungstic acid photocatalysis systems with an energy storage ability. J. Electrochem. Soc. 2003, 150, A1405–A1407. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tatsuma, T. Oxidative energy storage ability of a TiO2-Ni(OH)2 bilayer photocatalyst. Langmuir 2005, 21, 12357–12361. [Google Scholar] [CrossRef] [PubMed]
- Tatsuma, T.; Saitoh, S.; Ngaotrakanwiwat, P.; Ohko, Y.; Fujishima, A. Energy storage of TiO2-WO3 photocatalysis systems in the gas phase. Langmuir 2002, 18, 7777–7779. [Google Scholar] [CrossRef]
- Tatsuma, T.; Taked, S.; Saitoh, S.; Ohko, Y.; Fujishima, A. Bactericidal effect of an energy storage TiO2–WO3 photocatalyst in dark. Electrochem. Commun. 2003, 5, 793–796. [Google Scholar] [CrossRef]
- Ngaotrakanwiwat, P.; Tatsuma, T. Optimization of energy storage TiO2–WO3 photocatalysts and further modification with phosphotungstic acid. J. Electroanal. Chem. 2004, 573, 263–269. [Google Scholar] [CrossRef]
- Subasri, R.; Shinohara, T. Investigations on SnO2–TiO2 composite photoelectrodes for corrosion protection. Electrochem. Commun. 2003, 5, 897–902. [Google Scholar] [CrossRef]
- Yasomanee, J.P.; Bandara, J. Multi-electron storage of photoenergy using Cu2O–TiO2 thin film photocatalyst. Sol. Energy Mater. Sol. Cells 2008, 92, 348–352. [Google Scholar] [CrossRef]
- Wang, C.T.; Huang, H.H. Photo-chargeable titanium/vanadium oxide composites. J. Non-Cryst. Solid. 2008, 354, 3336–3342. [Google Scholar] [CrossRef]
- Zhang, W.K.; Wang, L.; Huang, H.; Gan, Y.P.; Wang, C.T.; Tao, X.Y. Light energy storage and photoelectrochemical behavior of the titanate nanotube array/Ni(OH)2 electrode. Electrochim. Acta 2009, 54, 4760–4763. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.C.; Yu, C.L.; Ma, W.H.; Zhao, J.C. Photoinduced electron storage in WO3/TiO2 nanohybrid material in the presence of oxygen and postirradiated reduction of heavy metal ions. J. Phys. Chem. C 2009, 113, 13160–13165. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tatsuma, T. Visible light-induced photocatalysts with reductive energy storage abilities. Electrochem. Commun. 2008, 10, 1404–1407. [Google Scholar] [CrossRef]
- Xiong, L.B.; Ouyang, M.L.; Yan, L.L.; Li, J.L.; Qiu, M.Q.; Yu, Y. Visible-light energy storage by Ti3+ in TiO2/Cu2O bilayer film. Chem. Lett. 2009, 38, 1154–1155. [Google Scholar] [CrossRef]
- Zhao, C.J.; Gu, Y.; Chen, H.; Jiang, Z.Y. Thermal effects on the measurement of photocurrent at anodic films on nickel electrodes. Electrochim. Acta 2002, 47, 1801–1809. [Google Scholar] [CrossRef]
- Carpenter, M.K.; Corrigan, D.A. Photoelectrochemistry of nickel hydroxide thin films. J. Electrochem. Soc. 1989, 136, 1021–1026. [Google Scholar] [CrossRef]
- Madou, M.J.; McKubre, M.C.H. Impedance measurements and photoeffects on Ni electrodes. J. Electrochem. Soc. 1983, 130, 1056–1061. [Google Scholar] [CrossRef]
- Uekawa, N.; Suzuki, T.; Ozeki, S.; Kaneko, K. Rectification effect by a p-n junctioned oxide film. Langmuir 1992, 8, 1–3. [Google Scholar] [CrossRef]
- Jing, L.Q.; Sun, X.J.; Cai, W.M.; Xu, Z.L.; Du, Y.G.; Fu, H.G. The preparation and characterization of nanoparticle TiO2/Ti films and their photocatalytic activity. J. Phys. Chem. Solids. 2003, 64, 615–623. [Google Scholar] [CrossRef]
- Nair, M.T.S.; Guerrero, L.; Arenas, O.L.; Nair, P.K. Chemically deposited copper oxide thin films: Structural, optical and electrical characteristics. Appl. Surf. Sci. 1999, 150, 143–151. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Li, J.L.; Chen, Z.G.; Tang, Y.W.; Yu, Y. Preparation of Fenton reagent with H2O2 generated by solar light-illuminated nano-Cu2O/MWNTs composites. Appl. Catal. A. Gen. 2005, 299, 292–297. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Kameneva, O.; Alexandrov, A.; Bityurin, N.; Chhor, K.; Kanaev, A. Chemical activity of photoinduced Ti3+ centers in titanium oxide gels. J. Phys. Chem. B. 2006, 110, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.I.; Kameneva, O.; Rozes, L.; Sanchez, C.; Bityurin, N.; Kanaev, A. Extinction of photo-induced Ti3+ centres in titanium oxide gels and gel-based oxo-PHEMA hybrids. Chem. Phys. Lett. 2006, 429, 523–527. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Kameneva, O.; Alexandrov, A.; Bityurin, N.; Marteau, P.; Chhor, K.; Sanchez, C.; Kanaev, A. Light-induced charge separation and storage in titanium oxide gels. Phys. Rev. E 2005, 71, 0214031–0214037. [Google Scholar] [CrossRef]
- He, T.; Ma, Y.; Cao, Y.A.; Hu, X.L.; Liu, H.M.; Zhang, G.J.; Yang, W.S.; Yao, A.N. Photochromism of WO3 colloids combined with TiO2 nanoparticles. J. Phys. Chem. B 2002, 106, 12670–12676. [Google Scholar] [CrossRef]
- Bechinger, C.; Ferrere, S.; Zaban, A.; Sprague, J.; Gregg, B.A. Photoelectrochromic windows and displays. Nature 1996, 383, 608–610. [Google Scholar] [CrossRef]
- Porqueras, I.; Bertran, E. Electrochromic behaviour of nickel oxide thin films deposited by thermal evaporation. Thin Solid Films 2001, 398–399, 41–44. [Google Scholar] [CrossRef]
- Ragan, D.D.; Svedlindh, P.; Graqvis, C.G. Electrochromic Ni oxide films studied by magnetic measurements. Sol. Energy Mater. Sol. Cells 1998, 54, 247–254. [Google Scholar] [CrossRef]
- Bityurin, N.; Kuznetsov, A.I.; Kanaev, A. Kinetics of UV-induced darkening of titanium-oxide gels. Appl. Surf. Sci. 2005, 248, 86–90. [Google Scholar] [CrossRef]
- Bityurin, N.; Znaidi, L.; Kanaev, A. Laser-induced absorption in titanium oxide based gels. Chem. Phys. Lett. 2003, 374, 95–99. [Google Scholar] [CrossRef]
- Richard, E.; Nocera, D.G. Preface: Overview of the forum on solar and renewable energy. Inorg. Chem. 2005, 44, 6799–6801. [Google Scholar] [CrossRef] [PubMed]
- Kazempour, S.J.; Moghaddam, M.P.; Haghifam, M.R.; Yousefi, G.R. Electric energy storage systems in a market-based economy:Comparison of emerging and traditional technologies. Renew. Energ. 2009, 34, 2630–2639. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Wittwer, V. Materials for solar energy conversion: An overview. Sol. Energy Mater. Sol. Cells 1998, 54, 39–48. [Google Scholar] [CrossRef]
- Baker, J. New technology and possible advances in energy storage. Energ. Policy 2008, 36, 4368–4373. [Google Scholar] [CrossRef]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energ. Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]
- Miyasakaa, T.; Murakami, T.N. The photocapacitor: An efficient self-charging capacitor for direct storage of solar energy. Appl. Phys. Lett. 2004, 85, 3932–3934. [Google Scholar] [CrossRef]
- Dhanabalan, A.; Dos Santos, D.S.; Mendonca, C.R., Jr.; Misoguti, L.; Balogh, D.T.; Giacometti, J.A.; Zilio, S.C.; Oliveira, O.N., Jr. Optical storage in mixed Langmuir-Blodgett (LB) films of disperse Red-19 isophorone polyurethane and cadmium stearate. Langmuir 1999, 15, 4560–4564. [Google Scholar] [CrossRef]
- Xie, Y.B.; Huang, C.J.; Zhou, L.M.; Liu, Y.; Huang, H.T. Supercapacitor application of nickel oxide–titania nanocomposites. Compos. Sci. Technol. 2009, 69, 2108–2114. [Google Scholar] [CrossRef]
- Hirakawa, T.; Kamat, P.V. Photoinduced electron storage and surface plasmon modulation in Ag@TiO2 Clusters. Langmuir 2004, 20, 5645–5647. [Google Scholar] [CrossRef] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).