Experimental Investigations of Extracted Rapeseed Combustion Emissions in a Small Scale Stationary Fluidized Bed Combustor
Abstract
:1. Introduction
| Supply and Source of Material | Potential (million tons) | Oil equivalent (million barrels) | Fraction (%) |
|---|---|---|---|
| Natural forest | 6.842 | 2.40 | 27.36 |
| Artificial forest | 3.718 | 1.32 | 14.87 |
| Forestry residues | 3.850 | 1.35 | 15.39 |
| Distributed tree | 6.550 | 2.12 | 26.19 |
| Industry and fruit-tree | 2.400 | 0.84 | 9.59 |
| Waste wood | 1.649 | 0.58 | 6.59 |
| Total woody biomass | 25.01 | 8.61 | 100.0 |
| Straw, wheat | 33.52 | 7.30 | 62.69 |
| Rice husk | 6.50 | 2.16 | 12.17 |
| Bagasse | 4.45 | 0.82 | 8.32 |
| Other wastes | 9.00 | 1.80 | 16.83 |
| Total agricultural biomass | 53.47 | 12.08 | 100.0 |
| Source of agricultural residues | Conventional fuels | ||||
|---|---|---|---|---|---|
| Fuels1) | NCV (MJ/kg)1) | $USD Cent / MJ2) | Fuels1) | NCV (MJ/kg) 1) | $USD Cent / MJ 2) |
| Rice husk | 14.4 | 0.014-0.021 | Anthracite | 31.4 | 0.255 |
| Rice straw | 14.6-15.0 | 0.027-0.033 | Brown coal | 11.3 | 0.442 |
| Maize haulm | 14.7 | Free of cost | Peat | 28.5 | 0.246 |
| Maize-cob | 15.4 | Free of cost | Diesel | 35.0 | 0.623 |
| Coffee husk | 16.6 | Free of cost | Natural gas | 40.0 | 9.100 |
| Bagasse | 8.2-16.2 | Free of cost | |||
| Wood and waste wood | 10.9-16.6 | 0.102-0.110 | |||
| Residues from kapok | 11.9 | Free of cost | |||
| Coconut shells | 17.9 | Free of cost | |||
| Sawdust | 18.5-19.0 | Free of cost | |||
| Residues of cashew nut | 24.0-25.0 | Free of cost | |||
2. Results and Discussion
(The gypsum is carried out of the reactor and collected in the cyclone with the fuel ash).
(The HCl is bonded by the additive process)
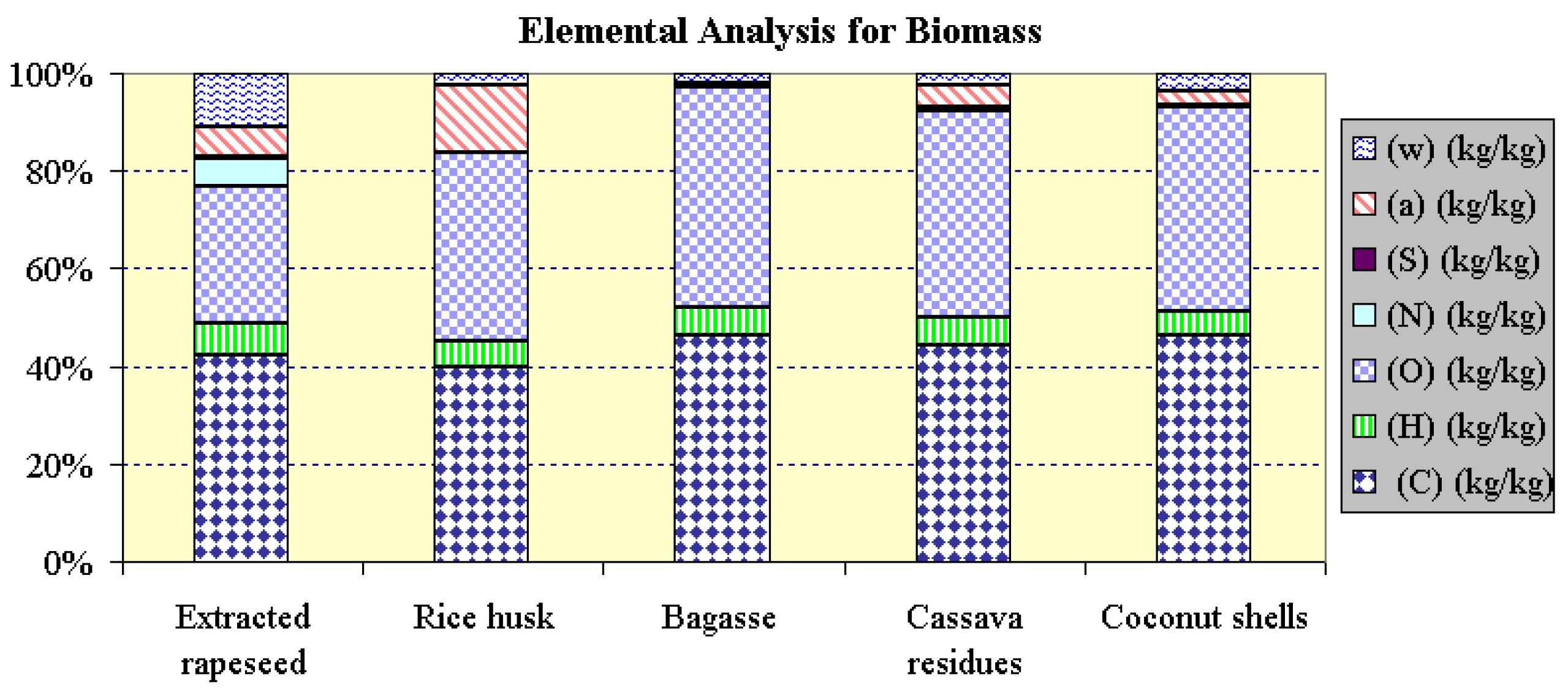
| Fuel | ER 1) | Rice husk 2) | Bagasse 2) | Cassava residue 2) | Coconut shells 2) | |
|---|---|---|---|---|---|---|
| Components | unit | Value | ||||
| General analysis | ||||||
| Fixed carbon | % mass* | 17.20 | 16.21 | 2.67 | 6.87 | 6.69 |
| Volatile substances | % mass* | 76.54 | 69.87 | 96.59 | 88.63 | 90.31 |
| Ash | % mass* | 6.26 | 13.92 | 0.74 | 4.50 | 3.00 |
| Elemental analysis | ||||||
| C | kg/kg | 0.4235 | 0.3979 | 0.4638 | 0.4434 | 0.4622 |
| H | kg/kg | 0.0636 | 0.0523 | 0.0576 | 0.0576 | 0.0520 |
| O (as difference) | kg/kg | 0.2828 | 0.3863 | 0.4519 | 0.4237 | 0.4163 |
| N | kg/kg | 0.0542 | 0.0013 | - | 0.0065 | 0.0026 |
| S | kg/kg | 0.0040 | - | - | - | - |
| Ash | kg/kg | 0.0626 | 0.1392 | 0.0074 | 0.0450 | 0.0300 |
| Moisture | kg/kg | 0.1094 | 0.0230 | 0.0193 | 0.0238 | 0.0369 |
| NCV | kJ/kg | 15 983 | 15 196 | 16 686 | 15 942 | 17 408 |
| Cl | mg/kg | 244 | 1020 | 260 | 1970 | 1670 |
| Ca | mg/kg | 59000 | 4596 | 60296 | 166000 | 59700 |
| K | mg/kg | 35000 | 13500 | 78200 | 237000 | 180000 |
| Mg | mg/kg | 38700 | 2209 | 59900 | 29700 | 15600 |
| P | mg/kg | 86200 | 8649 | 70600 | 124000 | 14700 |
| Cr | mg/kg | 1 | 4 | 188 | 5 | 17 |
| Zn | mg/kg | 513 | 72 | 1239 | 1359 | 160 |
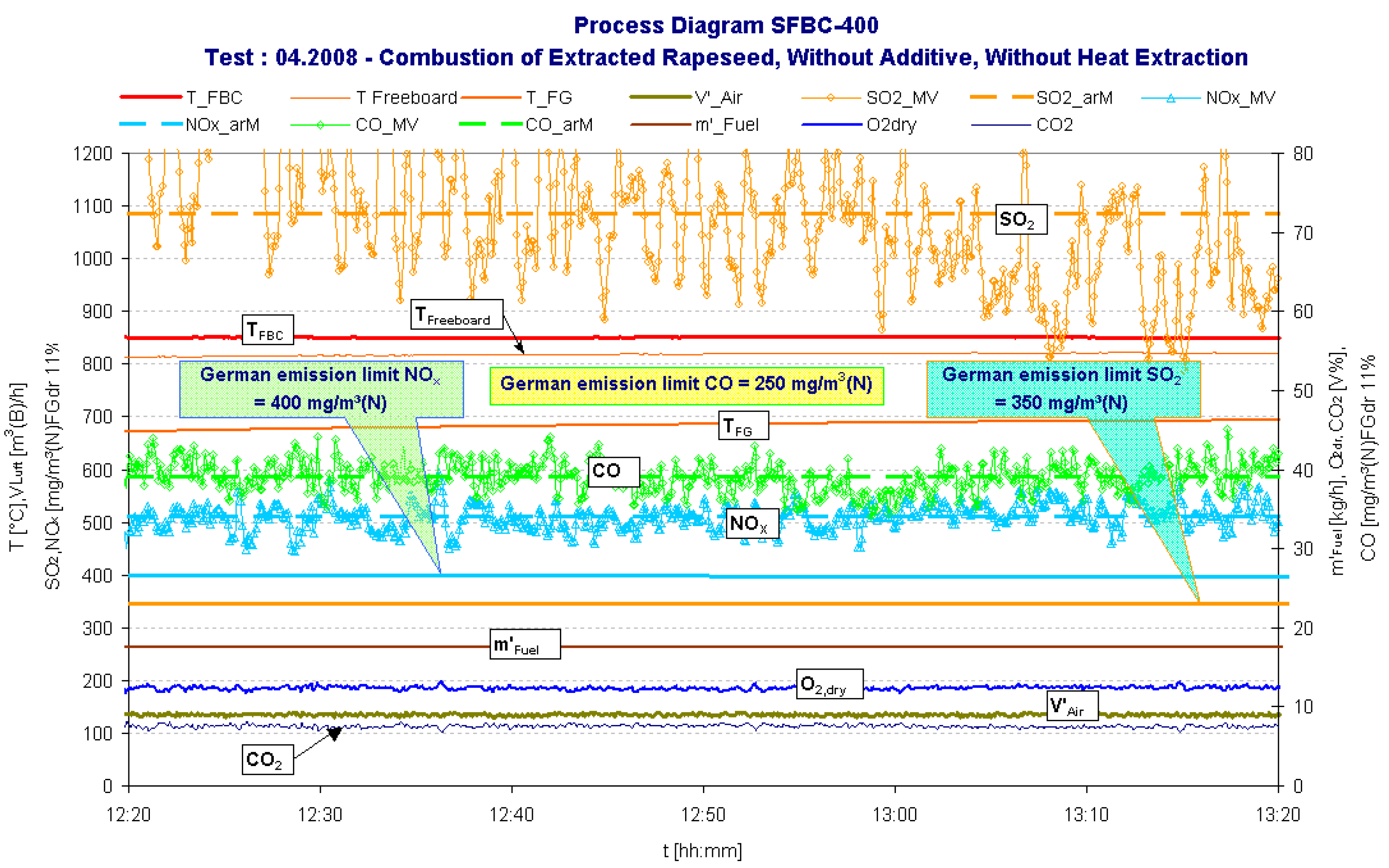
| Date | yy.mm | 08.04 | |
| Measurement start | hh:mm | 12:20 | |
| Measurement end | hh:mm | 13:20 | |
| Duration | min | 60 | |
| Operating Parameters | |||
| Name | Symbol | Unit | value |
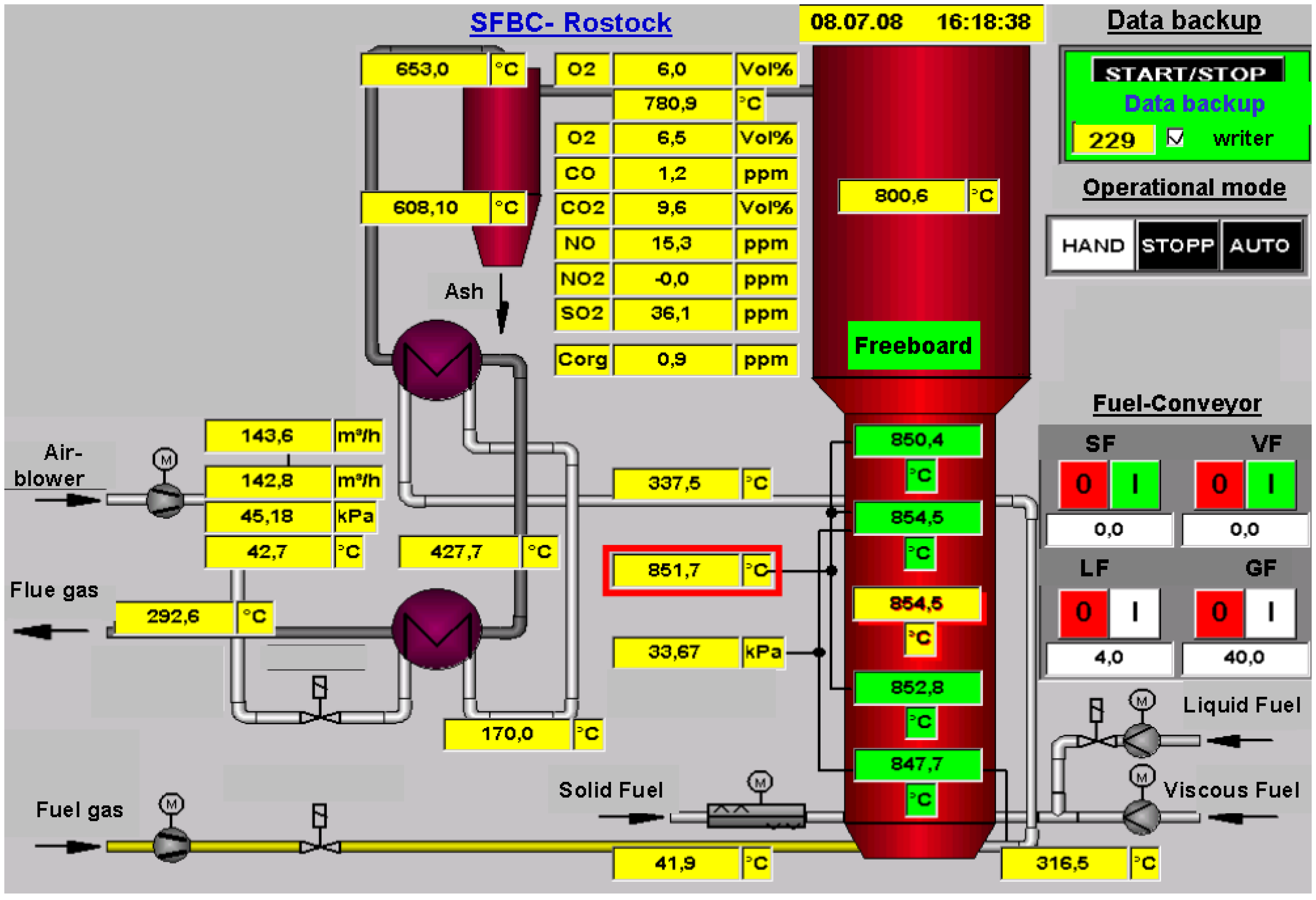
| Height of daybed | hBed | m | 0.95 |
| Mass of bed | mBed | kg | 247.5 |
| Volume of combustion air | V’Air | m3/h | 135 |
| Fuel flow rate | m’Fuel | kg/h | 17.5 |
| Fuel capacity | Q’Fuel | kWth | 88 |
| Combustion capacity | Q’Combustion | kWth | 90 |
| Rated output (flue gas) | Q’Flue gas | kWth | 53 |
| Fluidized bed temperature | TFB | °C | 850.6 |
| Freeboard temperature | TFreeboard | °C | 818.8 |
| Temperature of combustion air | Tcombustion air | °C | 40.9 |
| Humid oxygen | O2 humid | Vol. % | 10.8 |
| Dry oxygen | O2 dry | Vol. % | 12.4 |
| Emissions (with regard to standard 11 % O2 content in flue gas) | |||
| Carbon dioxide (1) | CO2 | Vol. % | 7.5 |
| Maximum Carbon dioxide (2) | CO2 max | Vol. % | 18.4 |
| Total organic carbon | Corg | ppm | - |
| Carbon monoxide | CO | mg/m³(N) | 39 |
| Nitrogen oxides | NOx | mg/m³(N) | 511 |
| Sulphur dioxide | SO2 | mg/m³(N) | 1087 |
| German emission limit (TA-Luft 2002) | |||
| Carbon monoxide | CO | mg/m³(N) | 250 |
| Nitrogen oxides | NOx | mg/m³(N) | 400 |
| Sulphur dioxide | SO2 | mg/m³(N) | 350 |
| Emissions as measured (influenced by dry oxygen content) | |||
| Unit | Value*) | value*) | |
| Dry oxygen (O2 dry) | Vol. % | 10,7 | 13,2 |
| Carbon monoxide (CO) | mg/m³(N) | 34,8 | 44,2 |
| Nitrogen oxides (NOx) | mg/m³(N) | 446 | 594 |
| Sulphur dioxide (SO2) | mg/m³(N) | 786 | 1582 |
- The relative high NOx concentration appears because of the relatively high oxygen concentration during the combustion process and especially high concentration of nitrogen in fuel (approx. 5.4%). It can be minimized if the oxygen concentration is reduced by higher fuel mass rate, e.g. by heat-extraction from the bed or by recirculation of “cold” cleaned flue gases.The value of N2O however was not measured because it is expected to be very very low for solid biomass fuel combustion [12].
- The high SO2 content in the flue gas is the result of the high ER feedstock sulphur content of 0.4%, which is around four times as high as most other agricultural biomass residues.
- Interestingly the CO-emission concentrations were remarkably low in comparison to the German emission limit, especially during the combustion of extracted rapeseed. This indicates a clean burn out in the SFBC combustion chamber.
- The ash-melting-problem (ash sintering can cause agglomeration of the bed material leading to severe slagging and fouling of the SFBC reactor and finally termination the bed fluidization) can be solved by decreasing the bed temperature below the critical limit. The critical temperature limit depends on the sintering temperature of the biomass ash, and is often below 850° C for residual agricultural biomass fuels. The ER test run (Figure 2) shows that a clean combustion in the SFB reactor is possible at temperatures below 850° C. This can be verified by the very low concentrations of carbon monoxide (CO) and the sum parameter Corg (gaseous hydrocarbons) measured during the test run (Figure 2 and Table 4). Thus a steady-state and stable combustion with a complete fuel conversion can be achieved in the combustions chamber at suitable temperatures for residual biomass combustion.
- Turbulence (good mixing of the feedstock with the bed material)
- Temperature (low, so low thermal NOx, and suitable for biomass, but still complete combustion)
- Time (high residence time at appropriate temperature window to ensure complete combustion)
| Properties | Value (mg/kg ash) | Properties | Value (mg/kg ash) | Properties | Value (mg/kg ash) |
|---|---|---|---|---|---|
| K | 41,200 | Al | 902 | Cu | 68 |
| P | 54,000 | Fe | 8,410 | Ni | 380 |
| Mg | 27,800 | Mn | 520 | Pb | 360 |
| Ca | 71,496 | Cd | <1 | Zn | 240 |
| Na | 945 | Cr | 365 | Sr | 144 |
| Ti | 35 |
- the fluidized bed combustion temperature will be similar,
- the combustion air flow rate will be similar and
- the concentration of dry oxygen in flue gas (air/fuel ratio) will be similar.
- the amount of heat available for energetic processes will also be similar and
- the NOx emission is expected to be significantly lower.
- the SO2 emissions are expected to be a lot lower and within the TA-Luft 2002 emissions limits and
- the CO- emission values will be similar.
3. Conclusions
4. Materials and Methods
4.1. The Properties of Experimental Materials


4.2. Presentation of the Experimental Plant
Acknowledgments
Nomenclature
| $USD Cent | US-Dollar Cent |
| CET | Chair of Environmental Technology |
| CHP | Combined Heat and Power |
| ER | Extracted Rapeseed |
| kW | Kilowatt (s) |
| kWth | Kilowatt thermal |
| MJ | Megajoule |
| NCV | Net Calorific Value |
| SFBC | Stationary Fluidized Bed Combustor |
| TA-Luft 2002 | Technische Anleitung zur Reinhaltung der Luft, 2002 |
References
- Tung, N.D.; Steinbrecht, D.; Beu, J.; Backhaus, E. Experimental Study on Hemp Residues Combustion in a small Scale stationary Fluidized Bed Combustor. Agricultural Engineering International: the CIGR Ejournal. 2008, X, Manuscript EE 08 006. 1–11. [Google Scholar]
- Nguyen, T.D.; Steinbrecht, D. Modeling a Combined Heat and Power Cogeneration System in Vietnam with a Fluidized Bed Combustor Burning Biomass. Agricultural Engineering International: the CIGR Ejournal. 2008, X, Manuscript EE 08 008. 1–22. [Google Scholar]
- Nguyen, Q.K. Problems of biomass energy development of Vietnam; The report at the conference of developing firm energy in Vietnam: Hanoi/Vietnam, 2006; pp. 1–8. Available online: http://www.vids.org.vn.
- Renewable Energy in Asia: the Vietnam report. An overview of the energy systems, renewable energy options, initiatives, actors and opportunities in Vietnam, August 2005; Australian Business Council for Sustainable Energy: 3rd Floor, 60 Leicester Street, Carlton Victoria 3053, 2005; pp. 1–18. Available online: www.bcse.org.au.
- (IET)-Institute of Environmental Technology 3 Science Park Drive. PSB Science Park Annex Singapore 118223. EC-ASEAN, Energy Facility Program, “New and Renewable Energy Opportunities for Electricity Generation in Vietnam”, This report was produced in conjunction with the Technology Partnership for New & Renewable Energy (NRE). Ho Chi Minh City Vietnam, 4 & 5 March 2004; pp. 1–31. Available online: www.riet.org.
- England, S.B.; Kammen, D.M. Energy resources and development in Vietnam. Annu. Rev. Energy Environ. 1993, 18, 137–167. Available online: www.annualreviews.org/aronline. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Tran, Q.C. Potential of Distributed Power Generation from Biomass Residues in Vietnam-Status and Prospect. In Electricity Supply Industry in Transition and Prospect for Asia; Hanoi-Vietnam, 14–16 January 2004; pp. 28–29. [Google Scholar]
- Pham, K.T. The quantitative research practicability of using sun energy, small hydroelectricity and biomass in industrial scale in Vietnam; the report of scientific technological summarizing, institute of energy, institute of energy, 7/2005, Hanoi-Vietnam; 2005; pp. 1–12. [Google Scholar]
- Pham, V.L.; Thang, H.V. Report of the studied investigation “Study the technological solution in using renewable energy to serve the production, process of agricultural, silvicultural, aquatic products and life”; Branch theme: KC-07.04.04, appertaining national level theme KC-07.04; scientific technological ministry: Vietnam, 2003; pp. 1–15. [Google Scholar]
- General Statistical Office. 2006. Vietnam Statistical Yearbook. August 2006. [Google Scholar]
- Steinbrecht, D. Abschnitt 1, Technische Verbrennung, Emissionen, Verfahrenstechnische Berechnungen mit Verbrennungsabgasen; Univ. Rostock: Deutschland, 2008. [Google Scholar]
- Steinbrecht, D. Experimentelle Untersuchungen zur SWSF - Verbrennung von Reststoffen der Biodiesel-Produktion; Internationales Wirbelschicht - Treffen 2008, Göteborg - Wien - Hamburg - Rostock - München - Graz - Essen an der TU Graz, 1. -3. Mai 2008.
- TA Luft. 2002. Erste Allgemeine Verwaltungsvorschrift zum Bundes-Immissionsschutzgesetz (Technische Anleitung zur Reinhaltung der Luft). Stand: 24.06.2002. Bei: http://www.bmu.de (February 2008).
- Steinbrecht, D. Abschnitt 2, Wirbelschichtfeuerungen, Schwerpunkt Stationäre Wirbelschicht-feuerungen. Univ. Rostock: Deutschland, 2008. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dinh Tung, N.; Steinbrecht, D.; Vincent, T. Experimental Investigations of Extracted Rapeseed Combustion Emissions in a Small Scale Stationary Fluidized Bed Combustor. Energies 2009, 2, 57-70. https://doi.org/10.3390/en20100057
Dinh Tung N, Steinbrecht D, Vincent T. Experimental Investigations of Extracted Rapeseed Combustion Emissions in a Small Scale Stationary Fluidized Bed Combustor. Energies. 2009; 2(1):57-70. https://doi.org/10.3390/en20100057
Chicago/Turabian StyleDinh Tung, Nguyen, Dieter Steinbrecht, and Tristan Vincent. 2009. "Experimental Investigations of Extracted Rapeseed Combustion Emissions in a Small Scale Stationary Fluidized Bed Combustor" Energies 2, no. 1: 57-70. https://doi.org/10.3390/en20100057
APA StyleDinh Tung, N., Steinbrecht, D., & Vincent, T. (2009). Experimental Investigations of Extracted Rapeseed Combustion Emissions in a Small Scale Stationary Fluidized Bed Combustor. Energies, 2(1), 57-70. https://doi.org/10.3390/en20100057





