Advancements in Inorganic Hole-Transport Materials for Perovskite Solar Cells: A Comparative Review
Abstract
1. Introduction
Role of Hole-Transport Materials (HTMs) in Perovskite Solar Cells
2. Comparative Analysis of Inorganic HTMs for Perovskite Solar Cells
2.1. Key Inorganic HTMs
2.2. HTMs for Specific Device Architectures
2.2.1. HTMs for Normal (n–i–p) Architectures
2.2.2. HTMs for Inverted (p–i–n) Architectures
2.2.3. Current Trends in HTMs–Device Integration
2.2.4. Tabular Comparisons: HTM–Device Architecture Matching
2.2.5. Tabular Comparisons: Deposition Techniques and Scalability
2.3. Deposition Techniques for Synthesizing Inorganic HTMs
3. Recent Literature and Comparative Contextualization
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| spiro-OMeTAD | 2,2′,7,7′-tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9′-spirobifluorene |
| PCE | Power conversion efficiency |
| PSCs | Perovskite solar cells |
| PV | Photovoltaic |
| ETM | Electron transport layer |
| HTM | Hole-transport layer |
| TCO | Transparent conductive oxides |
References
- International Energy Agency (IEA). World Energy Outlook 2023. 2023. Available online: https://www.iea.org/reports/world-energy-outlook-2023 (accessed on 7 February 2024).
- REN21. Renewables 2023 Global Status Report. 2023. Available online: https://www.ren21.net/reports/global-status-report/ (accessed on 2 February 2024).
- Sovacool, B.K.; Hook, A.; Martiskainen, M.; Brock, A.; Turnheim, B. The decarbonisation divide: Contextualizing landscapes of low-carbon exploitation and toxicity in Africa. Glob. Environ. Change 2020, 60, 102028. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (Version 58). Prog. Photovolt. 2021, 29, 657. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Zhang, K.; Li, W.; Zuo, C.; Odunmbaku, G.O.; Chen, J.; Chen, C.; Zhang, L.; Li, R.; et al. Major strategies for improving the performance of perovskite solar cells. IEnergy 2023, 2, 172–199. [Google Scholar] [CrossRef]
- Haegel, N.M.; Margolis, R.; Buonassisi, T.; Feldman, D.; Froitzheim, A.; Garabedian, R.; Green, M.; Glunz, S.; Henning, H.M.; Holder, B.; et al. Terawatt-scale photovoltaics: Trajectories and challenges. Science 2017, 356, 141–143. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory (NREL). Best Research-Cell Efficiency Chart. 2025. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 2 February 2025).
- Yoshikawa, K.; Kawasaki, H.; Yoshida, W.; Irie, T.; Konishi, K.; Nakano, K.; Uto, T.; Adachi, D.; Kanematsu, M.; Uzu, H.; et al. Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26%. Nat. Energy 2017, 2, 17032. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Morales Vilches, A.B.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic perovskite/silicon tandem solar cell with> 29% efficiency by enhanced hole extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef]
- Tao, S.; Schmidt, I.; Brocks, G.; Jiang, J.; Tranca, I.; Meerholz, K.; Olthof, S. Absolute energy level positions in tin-and lead-based halide perovskites. Nat. Commun. 2019, 10, 2560. [Google Scholar] [CrossRef]
- Nazar, M.; Younis, B.; Akbar, H.; Ahmad, S.; Rustam, R.; Akram, I.; Munir, F. Advancing Perovskite Solar Cells: Addressing Stability, Scalability, and Environmental Challenges. Dialogue Soc. Sci. Rev. (DSSR) 2024, 2, 395–422. [Google Scholar]
- De Wolf, S.; Aydin, E. Tandems have the power. Science 2023, 381, 30–31. [Google Scholar] [CrossRef]
- Shi, T.; Lin, S.; Fang, M.; Kong, D.; Yuan, Y.; Gao, Y.; Yang, B.; Han, H.; Zhou, C. Low-temperature fabrication of carbon-electrode based, hole-conductor-free and mesoscopic perovskite solar cells with power conversion efficiency> 12% and storage-stability> 220 days. Appl. Phys. Lett. 2020, 117, 163501. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, C.; Wang, H.; Xu, R.; Li, J.; Fu, G.; Wang, K.; Xiao, M. Enhancing Performance of NiOx-Based Inverted Perovskite Solar Cells: Advances in Buried Interface Material Modification Strategy. Small 2025, 21, 2411630. [Google Scholar] [CrossRef]
- Li, M.; Li, F.; Gong, J.; Zhang, T.; Gao, F.; Zhang, W.H.; Liu, M. Advances in Tin (II)-based perovskite solar cells: From material physics to device performance. Small Struct. 2022, 3, 2100102. [Google Scholar] [CrossRef]
- Hou, Y.; Du, X.; Scheiner, S.; McMeekin, D.P.; Wang, Z.; Li, N.; Killian, M.S.; Chen, H.; Richter, M.; Levchuk, I.; et al. A generic interface to reduce the efficiency-stability-cost gap of perovskite solar cells. Science 2017, 358, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Shin, S.S.; Seo, J. Toward efficient perovskite solar cells: Progress, strategies, and perspectives. ACS Energy Lett. 2022, 7, 2084–2091. [Google Scholar] [CrossRef]
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Tress, W.; Domanski, K.; Carlsen, B.; Agarwalla, A.; Alharbi, E.A.; Graetzel, M.; Hagfeldt, A. Performance of perovskite solar cells under simulated temperature-illumination real-world operating conditions. Nat. Energy 2019, 4, 568–574. [Google Scholar] [CrossRef]
- Li, N.; Niu, X.; Chen, Q.; Zhou, H. Towards commercialization: The operational stability of perovskite solar cells. Chem. Soc. Rev. 2020, 49, 8235–8286. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, R.; Wang, X.; Liu, C.; Ahmed, Y.; Huang, Z.; Zhang, Z.; Li, H.; Zhang, M.; Gao, Y.; et al. Oxidation-resistant all-perovskite tandem solar cells in substrate configuration. Nat. Commun. 2023, 14, 1819. [Google Scholar] [CrossRef]
- Ma, G.; Tan, Q.; Li, Z.; Xiu, J.; Wang, J.; Cheng, T.; He, D.; Sun, Q.; Ma, X.; Lamberti, F.; et al. Magnetron sputtered nickel oxide with suppressed interfacial defect states for efficient inverted perovskite solar cells. J. Energy Chem. 2025, 100, 348–355. [Google Scholar] [CrossRef]
- Feng, Z.; Lee, M.; Tian, R.; Patterson, R.; Wang, Y.; Qian, C.; Sun, K.; Liu, Z.; Yun, J.S.; Xu, M.; et al. Doped-NiOx Seed Layer on Textured Substrates for Low-Loss Contacts in Perovskite Solar Cells. Adv. Energy Mater. 2024, 2405016. [Google Scholar] [CrossRef]
- Caliò, L.; Salado, M.; Kazim, S.; Ahmad, S. Innovative Materials for the Development of Stable and Efficient Perovskite Solar Cells. In World Scientific Reference of Hybrid Materials: Volume 2: Devices from Hybrid and Organic Materials; World Scientific: Singapore, 2019; pp. 187–212. [Google Scholar]
- Singh, R.; Singh, P.K.; Bhattacharya, B.; Rhee, H.W. Review of current progress in inorganic hole-transport materials for perovskite solar cells. Appl. Mater. Today 2019, 14, 175–200. [Google Scholar] [CrossRef]
- Dussouillez, M.; Moon, S.J.; Mensi, M.; Wolff, C.M.; Liu, Y.; Yum, J.H.; Kamino, B.A.; Walter, A.; Sahli, F.; Lauber, L.; et al. Understanding and Mitigating the Degradation of Perovskite Solar Cells Based on a Nickel Oxide Hole Transport Material during Damp Heat Testing. ACS Appl. Mater. Interfaces 2023, 15, 27941–27951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, C.; Yao, J.; Choy, W.C. Dopant-free NiOx nanocrystals: A low-cost and stable hole transport material for commercializing perovskite optoelectronics. Angew. Chem. Int. Ed. 2023, 62, e202219307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Zhang, S.; Lin, Z.; Han, M.; Liu, X.; Chen, J.; Du, W.; Rahim, G.; Zhou, Y.; et al. A spiro-type self-assembled hole transporting monolayer for highly efficient and stable inverted perovskite solar cells and modules. Energy Environ. Sci. 2025, 18, 468–477. [Google Scholar] [CrossRef]
- Huang, Z.; Li, L.; Wu, T.; Xue, T.; Sun, W.; Pan, Q.; Wang, H.; Xie, H.; Chi, J.; Han, T.; et al. Wearable perovskite solar cells by aligned liquid crystal elastomers. Nat. Commun. 2023, 14, 1204. [Google Scholar] [CrossRef]
- Yin, X.; Song, Z.; Li, Z.; Tang, W. Toward ideal hole transport materials: A review on recent progress in dopant-free hole transport materials for fabricating efficient and stable perovskite solar cells. Energy Environ. Sci. 2020, 13, 4057–4086. [Google Scholar] [CrossRef]
- Shariatinia, Z. Recent progress in development of diverse kinds of hole transport materials for the perovskite solar cells: A review. Renew. Sustain. Energy Rev. 2020, 119, 109608. [Google Scholar] [CrossRef]
- Malinauskas, T.; Tomkute-Luksiene, D.; Sens, R.; Daskeviciene, M.; Send, R.; Wonneberger, H.; Jankauskas, V.; Bruder, I.; Getautis, V. Enhancing thermal stability and lifetime of solid-state dye-sensitized solar cells via molecular engineering of the hole-transporting material spiro-OMeTAD. ACS Appl. Mater. Interfaces 2015, 7, 11107–11116. [Google Scholar] [CrossRef]
- Jamshidi, M.; Gardner, J.M. Copper (I) iodide thin films: Deposition methods and hole-transporting performance. Molecules 2024, 29, 1723. [Google Scholar] [CrossRef]
- Christians, J.A.; Fung, R.C.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Nandi, P.; In, Y.; Shin, H. Practical and Thermal Atomic Layer Deposition of NiO as Hole-Transporting Layers for Inverted Perovskite Solar Cells. Solar RRL 2024, 8, 2300858. [Google Scholar] [CrossRef]
- Ameen, S.; Rub, M.A.; Kosa, S.A.; Alamry, K.A.; Akhtar, M.S.; Shin, H.S.; Seo, H.K.; Asiri, A.M.; Nazeeruddin, M.K. Perovskite solar cells: Influence of hole transporting materials on power conversion efficiency. ChemSusChem 2016, 9, 10–27. [Google Scholar] [CrossRef]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Grätzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shao, B.; Shen, Z.; You, S.; Yin, J.; Wehbe, N.; Wang, L.; Song, X.; Abulikemu, M.; Basaheeh, A.; et al. In situ energetics modulation enables high-efficiency and stable inverted perovskite solar cells. Nat. Photonics 2025, 19, 28–35. [Google Scholar] [CrossRef]
- Chen, J.; Cai, X.; Yang, D.; Song, D.; Wang, J.; Jiang, J.; Ma, A.; Lv, S.; Hu, M.Z.; Ni, C. Recent progress in stabilizing hybrid perovskites for solar cell applications. J. Power Sources 2017, 355, 98–133. [Google Scholar] [CrossRef]
- Luo, D.; Su, R.; Zhang, W.; Gong, Q.; Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2020, 5, 44–60. [Google Scholar] [CrossRef]
- Xie, F.; Mei, D.; Qiu, L.; Su, Z.; Chen, L.; Liu, Y.; Du, P. High-efficiency fiber-shaped perovskite solar cells with TiO2/SnO2 double-electron transport layer materials. J. Electron. Mater. 2023, 52, 4626–4633. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, F.; Qu, Z.; Yu, S.; Shen, T.; Deng, H.X.; Chu, X.; Peng, X.; Yuan, Y.; Zhang, X.; et al. Inactive (PbI2) 2RbCl stabilizes perovskite films for efficient solar cells. Science 2022, 377, 531–534. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhu, K. Rapid advances enabling high-performance inverted perovskite solar cells. Nat. Rev. Mater. 2024, 9, 399–419. [Google Scholar] [CrossRef]
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J.; et al. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3-V voltage loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Q.; Liu, G.; Chen, Y.; Guo, Z.; Li, N.; Niu, X.; Qiu, Z.; Zhou, W.; Huang, Z.; et al. Improved fatigue behaviour of perovskite solar cells with an interfacial starch–polyiodide buffer layer. Nat. Photonics 2023, 17, 1066–1073. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Ren, Z.; Li, Y.; Zhang, Y.; Kong, F.C.; Liu, T.; Shi, X.; Dou, Y.; Wang, L.; et al. Enhanced interface adhesion with a polymeric hole transporter enabling high-performance air-processed perovskite solar cells. Energy Environ. Sci. 2025, 18, 1366–1374. [Google Scholar] [CrossRef]
- Li, G.; Ren, Z. Multiple ligands-tailored SnO2 quantum dots endow in situ dual-interface binding for high efficiency and low energy loss perovskite photovoltaics. In Organic, Hybrid, and Perovskite Photovoltaics XXIII; SPIE: San Francisco, CA, USA, 2022; p. PC122090D. [Google Scholar]
- Kim, G.; Min, H.; Lee, K.S.; Lee, D.Y.; Yoon, S.M.; Seok, S.I. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 2020, 370, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, R.; Zeng, H.; Zhao, Y.; Liu, X.; You, S.; Li, M.; Luo, L.; Lira-Cantu, M.; Li, L.; et al. Improved performance and stability of perovskite solar modules by interface modulating with graphene oxide crosslinked CsPbBr 3 quantum dots. Energy Environ. Sci. 2022, 15, 244–253. [Google Scholar] [CrossRef]
- Bai, Y.; Hao, M.; Ding, S.; Chen, P.; Wang, L. Surface chemistry engineering of perovskite quantum dots: Strategies, applications, and perspectives. Adv. Mater. 2022, 34, 2105958. [Google Scholar] [CrossRef]
- Akin, S.; Bauer, M.; Hertel, D.; Meerholz, K.; Zakeeruddin, S.M.; Graetzel, M.; Bäuerle, P.; Dar, M.I. Robust Nonspiro-Based Hole Conductors for High-Efficiency Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2205729. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, B.; Shi, L.; Zhou, S.; Xu, J.; Liu, Z.; Yun, J.S.; Choi, E.; Zhang, M.; Lv, Y.; et al. Perovskite solar cells based on spiro-OMeTAD stabilized with an alkylthiol additive. Nat. Photonics 2023, 17, 96–105. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Q.; Zhang, C.; Cheng, C.; Wang, K. Tailoring the interface by a multifunctional amphiphilic molecule enabled 24.84%-efficiency and stable perovskite solar cells. Nano Energy 2023, 118, 109003. [Google Scholar] [CrossRef]
- Sajid, S.; Alzahmi, S.; Salem, I.B.; Park, J.; Obaidat, I.M. Inorganic hole transport materials in perovskite solar cells are catching up. Mater. Today Energy 2023, 37, 101378. [Google Scholar] [CrossRef]
- Huang, D.; Xiang, H.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in nanostructured inorganic hole-transporting materials for perovskite solar cells. Nanomaterials 2022, 12, 2592. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Park, N.G. Inorganic hole transporting materials for stable and high efficiency perovskite solar cells. J. Phys. Chem. C 2018, 122, 14039–14063. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L. Inorganic hole-transporting materials for perovskite solar cells. Small Methods 2018, 2, 1700280. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, Z.; Su, J.; Hu, Z.; Chang, J.; Hao, Y. Recent progress of inorganic hole transport materials for efficient and stable perovskite solar cells. Nano Select 2021, 2, 1055–1080. [Google Scholar] [CrossRef]
- Park, H.H. Efficient and stable perovskite solar cells based on inorganic hole transport materials. Nanomaterials 2021, 12, 112. [Google Scholar] [CrossRef]
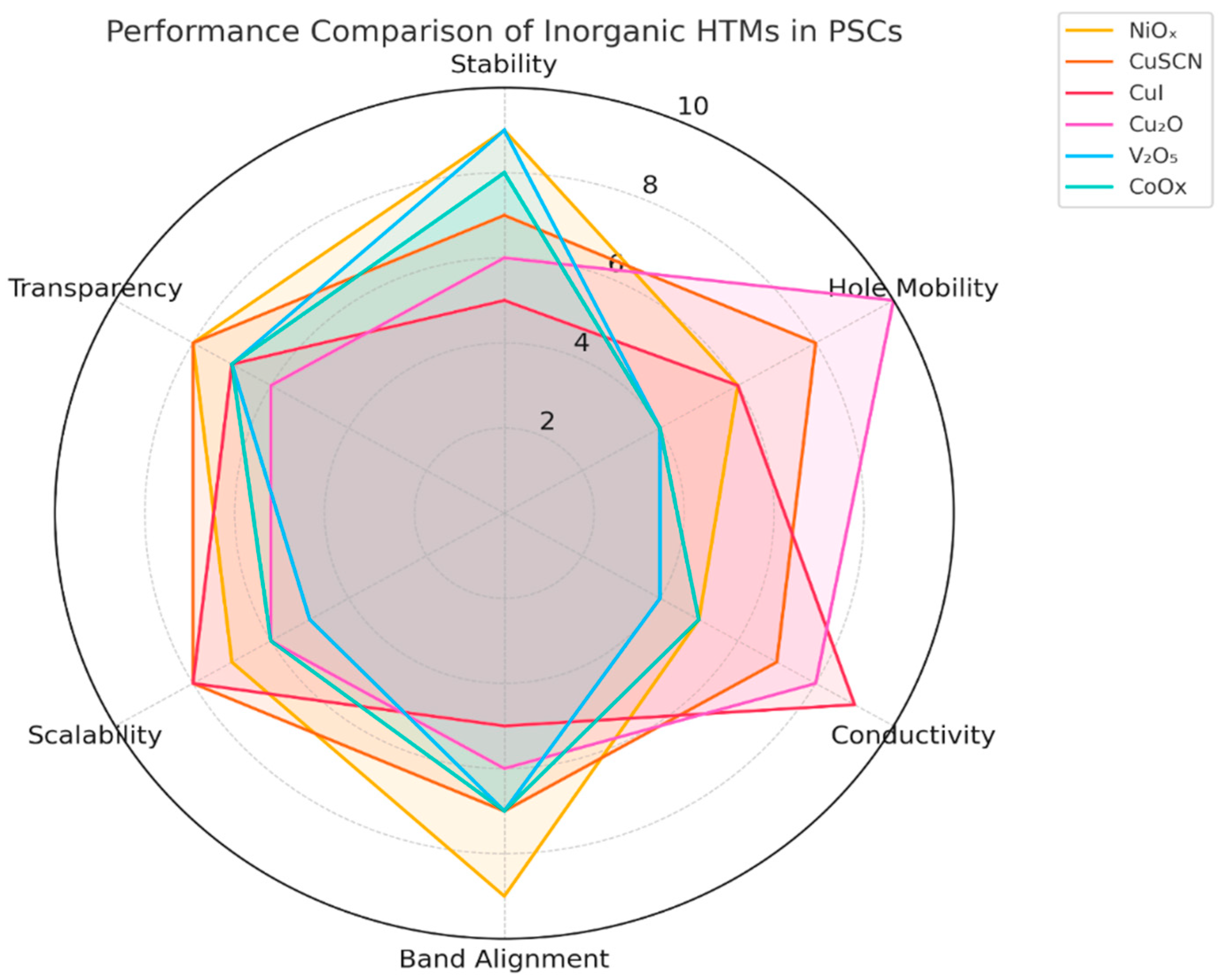
 ), and crystallinity (◇), all of which contribute to the efficiency and stability of PSCs.
), and crystallinity (◇), all of which contribute to the efficiency and stability of PSCs.
 ), and crystallinity (◇), all of which contribute to the efficiency and stability of PSCs.
), and crystallinity (◇), all of which contribute to the efficiency and stability of PSCs.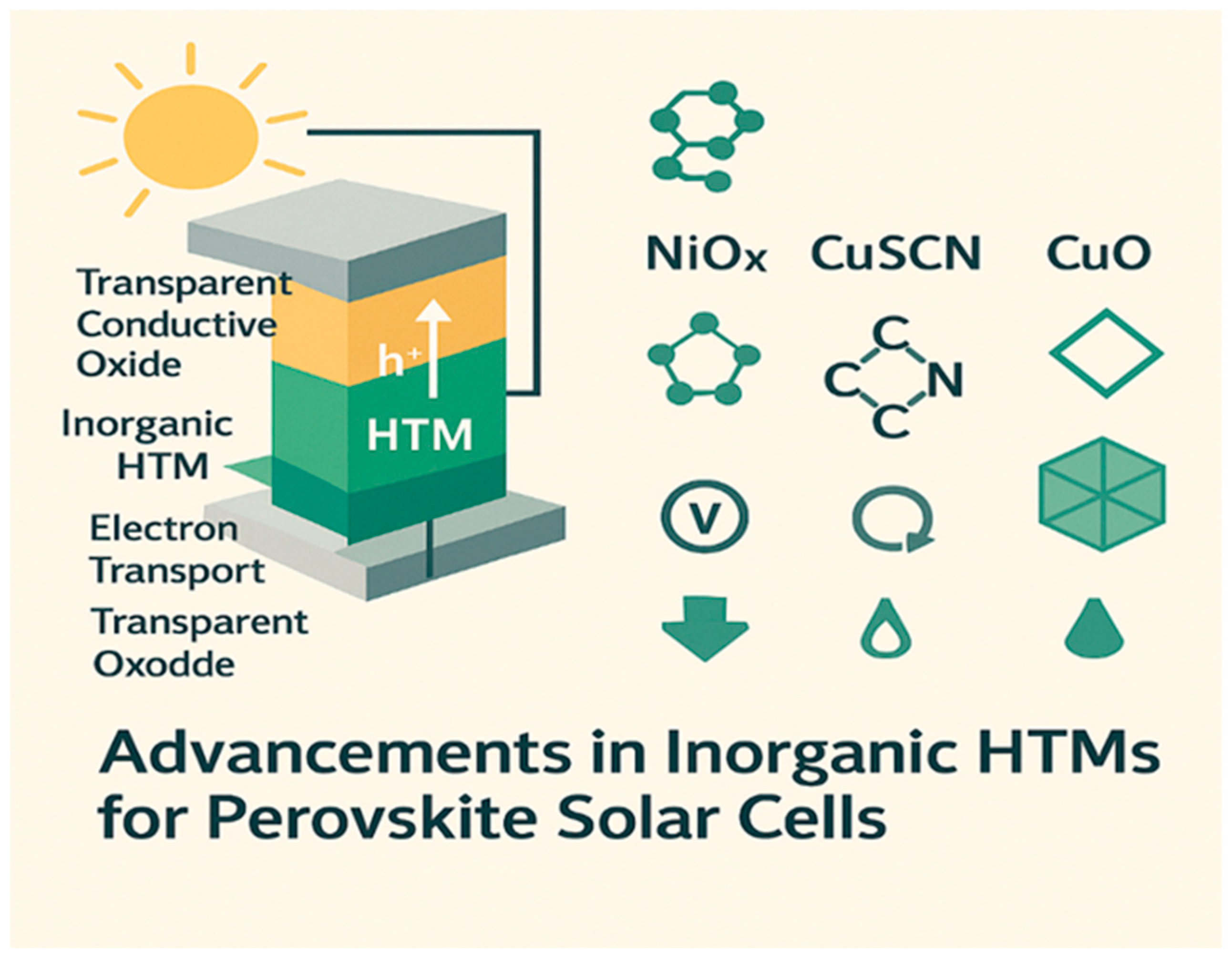
| HTM | Bandgap (eV) | Hole Mobility (cm2/V·s) | Work Function (eV) | Suitable for PSC Type | Stability | Notable Feature |
|---|---|---|---|---|---|---|
| NiOx | +/−3.6 | 10−3–10−1 | ~5.0 | Inverted (p–i–n) | High | Widely used; ALD/sputtered films offer high stability [14,23,36] |
| CuSCN | +/−3.6 | ~10−2 | ~5.3 | Normal (n–i–p) | Moderate | Good mobility and transparency; sensitive to humidity [34,38] |
| CuI | ~3.0–3.1 | ~10−3 | ~5.1 | Normal (n–i–p) | Low–Mod. | Easy processing but suffers from recombination and instability [35] |
| Cu2O | ~2.1 | Up to 256 | ~5.0 | Normal (n–i–p) | Moderate | High hole mobility; lower absorption in the visible range [39] |
| V2O5 | ~2.3–2.8 | ~10−⁴ | ~5.3 | Inverted (p–i–n) | High | Wide bandgap; low conductivity; often used with dopants [40] |
| CoOx | ~2.4–2.8 | ~10−⁴ | ~5.2 | Inverted (p–i–n) | High | Emerging material; promising electrochemical performance [41] |
| Material | Bandgap (eV) | Hole Mobility (cm2/V·s) | Work Function (eV) | Conductivity (S/cm) | Processability | Thermal Stability | References |
|---|---|---|---|---|---|---|---|
| NiOx | 3.6–4.0 | 10−3–10−1 | ~5.0 | 10−⁴–10−2 | ALD; Sputtering; Sol–Gel | Excellent | [13,14,23] |
| CuSCN | ~3.6 | ~10−2 | ~5.3 | 10−2–10−3 | Spin-coating; Electrodeposition | Good | [34,38] |
| CuI | ~3.1 | ~10−3 | ~5.1 | ~1 | Spin-coating; Drop-casting | Moderate | [35] |
| Cu2O | 2.0–2.2 | Up to 256 | ~5.0 | 10−⁴–10−2 | ALD; Oxidation; Sputtering | Moderate | [27,39] |
| V2O5 | 2.3–2.8 | ~10−⁴ | ~5.3 | Low | Thermal evaporation; Sol–Gel | High | [40] |
| CoOx | 2.4–2.8 | ~10−⁴ | ~5.2 | Moderate | Sputtering; Sol–Gel | High | [41] |
| HTM | Compatible Architecture | Typical Deposition Method | Peak PCE Reported (%) | Notes | References |
|---|---|---|---|---|---|
| NiOx | Inverted (p–i–n) | Sputtering; ALD; Sol–gel | 21.50% | Most used inorganic HTM | [14,23,27] |
| CuSCN | Normal (n–i–p) | Solution; Electrodeposition | 16.60% | Transparent and cost-effective | [34,38] |
| CuI | Normal (n–i–p) | Spin-coating; Drop-casting | ~6–12% | Prone to recombination | [35] |
| Cu2O | Normal (n–i–p) | ALD; Oxidation | 13.30% | High mobility and poor long-wavelength absorption | [27,39] |
| V2O5 | Inverted (p–i–n) | Evaporation; Sol–Gel | ~19% | Good chemical barrier | [40] |
| CoOx | Inverted (p–i–n) | Sputtering; Sol–Gel | ~18% | Still under active study | [41] |
| Technique | Type | Cost | Scalability | Suitable HTMs | Key Notes | References |
|---|---|---|---|---|---|---|
| Spin-coating | Solution-based | Low | Moderate | CuI, CuSCN | Lab-scale standard | [34,35] |
| Electrodeposition | Solution-based | Low | High | CuSCN, Cu2O | Low-temperature and scalable | [38,39] |
| Atomic Layer Deposition (ALD) | Vapor-phase | High | High | NiOx, Cu2O | Uniform films; precise control | [14,23] |
| Sputtering | Physical vapor | Medium | High | NiOx, CoOx, Cu2O | High reproducibility | [27,41] |
| Slot-Die Coating | Solution-based | Low | Very High | NiOx (sol–gel) | Roll-to-roll compatible | [23] |
| Blade Coating | Solution-based | Low | Very High | NiOx, CuSCN | Simple; scalable | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbese, J.Z. Advancements in Inorganic Hole-Transport Materials for Perovskite Solar Cells: A Comparative Review. Energies 2025, 18, 2374. https://doi.org/10.3390/en18092374
Mbese JZ. Advancements in Inorganic Hole-Transport Materials for Perovskite Solar Cells: A Comparative Review. Energies. 2025; 18(9):2374. https://doi.org/10.3390/en18092374
Chicago/Turabian StyleMbese, Johannes Zanoxolo. 2025. "Advancements in Inorganic Hole-Transport Materials for Perovskite Solar Cells: A Comparative Review" Energies 18, no. 9: 2374. https://doi.org/10.3390/en18092374
APA StyleMbese, J. Z. (2025). Advancements in Inorganic Hole-Transport Materials for Perovskite Solar Cells: A Comparative Review. Energies, 18(9), 2374. https://doi.org/10.3390/en18092374






