Abstract
Rapeseed oil transesterification reaction with ethanol under supercritical fluid conditions was performed either in the presence of catalysts or without them. The catalysts were Al2O3 and AlOOH, obtained after Al2O3 hydrothermal processing, and CaO/Al2O3 and CaO/AlOOH, obtained after permeation. The obtained product was measured for dynamic viscosity and density. Based on these data, kinematic viscosity was calculated. Biodiesel fuel was separated via centrifugation to extract more viscous ethyl esters of saturated fatty acids and unreacted triglycerides in order to comply with the standards for biodiesel fuel. Analyses have found that the maximum content of obtained ethyl esters of fatty acids in a reaction product before separation is reached, in the case of using the CaO/AlOOH catalyst, is in the amount of 93.34% by mass; and none of the samples’ kinematic viscosity values comply with the standards for biodiesel fuel. Performing centrifugation allowed us to reduce viscosity and increase biodiesel fuel concentration to reach the EN14214 standard requirements. Also, a significant deterioration of the initial catalysts’ strength after the singular experiment has been observed: Al2O3 by 22.4%, AlOOH by 13.89%, CaO/Al2O3 by 25.13%, and CaO/AlOOH by 17.27%.
1. Introduction
The fast-paced depletion of global oil resource deposits, as well as the increased threat of environmental crisis caused by atmosphere pollution, motivate the global community to look for ways to replace hydrocarbon fuel with alternative energy sources. For example, an option in liquid fuel for vehicles can be the environmentally friendly biodiesel fuel, which is composed of fatty acid esters obtained through transesterification of vegetable oils or animal-based fats with low alcohols (methanol, ethanol) [1]. Exhaust fumes from vehicles powered by biodiesel fuel are significantly safer than fumes from engines powered by fossil oil-based diesel fuel [2]. In addition, biodiesel is fireproof; it decomposes within a month to form harmless products, and it has a high lubricating ability [1].
Technology for obtaining biodiesel fuel includes commercial developments using homogeneous alkaline or acidic catalysts [3,4]. However, this technology has disadvantages related to quality in multiple stages, which makes it necessary to separate reaction mix components: biodiesel, excessive alcohol, saponification products, glycerin, etc. Inefficient separation and washing of biodiesel cause serious problems for diesel engines, including filter clogging, nozzle gumming-up, more carbon residue, increased engine wear, flingers sticking, engine knock, lubrication oil thickening, and jellification [5]. The final product may also have esters with high viscosity. These esters include the ones obtained from transesterification of saturated fatty acids (stearinic, palmitimic, etc.), found in large amounts in palm oil, shea butter, etc. Their viscosity is slightly higher than the viscosity of unsaturated fatty acid esters obtained through transesterification of, for example, rapeseed or sunflower oil with high unsaturated fatty acid content. This leads to the understanding that the obtained fuel can have higher viscosity than that set by global biofuel standards EN 14214 [6] and ASTM 6751 [7]. The kinematic viscosity at 40 °C and atmospheric pressure, following the EN 14214 standard, is within the range of 3.5–5.0 mm2/s, following the ASTM 6751 standard, and is within the range of 1.9–6.0 mm2/s. Obtaining biodiesel with acceptable viscosity values is an important task for improving biodiesel quality.
There are methods of separating biodiesel from reaction byproducts; glycerin using ionic liquids [8], membrane separation [9], ultrafiltration [10], and gravity sedimentation [11]. All these methods are limited in terms of obtaining the final product, washing efficiency, the time needed for separation, and complex equipment. A method that does not have these drawbacks is biodiesel separation from unwanted elements through centrifugation. Centrifuges are used in medicine, agriculture, the food industry, etc. [12,13,14]. A large number of studies are devoted to the extraction of fatty acids present in plant-based and animal-based fats, oils, and waxes [15,16,17]. There are also studies on oil extraction from microalgae, which are new generation materials for obtaining biodiesel fuel [18,19,20]. Nevertheless, the use of centrifuges to separate unwanted products from biodiesel is limited by the way glycerin is extracted [11] and does not impact other components of transesterification products.
Transesterification under supercritical fluid (SCF) conditions can be considered the most advanced technology for obtaining biodiesel fuel, which allows for the acquisition of biodiesel without free glycerin [21,22,23]. In addition to a lack of glycerin, an advantage of obtaining biodiesel fuel through SCF transesterification is the high yield at minimal process time without the use of catalysts. Moreover, there are no saponification products, and there is no need for some post-reaction steps, including neutralization, washing, and drying. Thus, the use of water and power related to its disposal is minimized.
Table 1 shows data on oil transesterification under SCF conditions as well as process parameters, which allow it to achieve biodiesel yield over 90% by mass.

Table 1.
Conditions on non-catalytic transesterification reaction under SCF conditions.
Considering Table 1 results, it can be noted that achievement of high biodiesel yield requires either high molar ratios of initial products or high temperatures. Both of these factors cause high costs and hinder SCF technology implementation on an industrial scale. One of the ways to optimize oil transesterification parameters can be through the use of heterogeneous catalysts.
The transesterification reaction often involves zeolite-based heterogeneous catalysts and their modified forms, with the application of alkaline-earth metals’ alkali and oxides onto their surface [34,35,36], which is caused by their lower solubility and corrosion resistance [37,38]. Also, there are studies on transesterification using catalysts based on magnesium oxides and magnesium-aluminum hydrocarbonates [39,40,41,42], cerium [43], molybdenum [44], strontium [45], zirconium [46].
Table 2 summarizes the data on catalytic SCF oil transesterification.

Table 2.
Conditions on catalytic transesterification reaction under SCF conditions.
According to the data in Table 1 and Table 2, the use of catalysts provides a temperature fall by 42 °C on average, a lower pressure by 13 MPa, and a shorter reaction time by 9 min, compared to the non-catalytic reaction under SCF conditions. Generally, most studies on catalytic transesterification under SCF conditions deal with the use of various metal oxides on the Al2O3 carrier (Table 2), but studies on the use of metal oxides on the boehmite (AlOOH) carrier are limited by the activities carried out in traditional transesterification [56,57]. The use of AlOOH as a catalyst carrier instead of Al2O3 is explained by high hydrothermal stability at higher temperatures and pressures typical for SCF conditions in reactions carried out in organic homogeneous solutions, including water-containing ones. The catalysts’ work stability under the SCF conditions of transesterification is also important for the further scale-up of the studied technology.
Despite significant progress in SCF transesterification, high reaction speed over a short time is not often able to provide reaction completion, and apart from biodiesel, the reaction product may include unreacted fatty acids. Efficient methods for such fatty acid and saturated fatty acid ester separation, particularly using centrifugation, remain unstudied.
The object of the study is the centrifugal separation of catalytic and non-catalytic transesterification reaction products obtained under SCF conditions from high-viscosity esters of saturated fatty acids and unreacted fatty acids. The presented results with CaO/AlOOH as a catalyst are also novel. Additionally, there is the elemental composition and compressive strength of the catalysts used before and after experiments, which are needed to estimate the prospects of industrial use under working conditions with high state parameters, typical for SCF conditions.
2. Experimental Part
2.1. Initial Raw Materials
Raw material for transesterification was refined food-quality rapeseed oil from Oily, Belarus, with 99.8% purity and 96% ethanol ( = 1.36242, = 797.1 kg/m3). The use of ethanol in experiments is explained by its lower toxicity compared to methanol when working on a laboratory bench. Choice of rapeseed as an oil base is caused by its high crop yield in moderate climate of Eurasian countries. Oil’s content of fatty acids from manufacturer is given in Table 3.

Table 3.
Fatty acid content of rapeseed oil.
Catalyst carrier was granulated Al2O3 (mixture of gamma- and chi-oxides) based on reference review, low price and availability [58], and AlOOH. Boehmite was obtained through Al2O3 hydrothermal processing in distilled water at water to catalyst mass ratio 2:1 for 9 h at temperature t = 190 °C and corresponding water vapor pressure. For preparation of synthesized catalysts CaO/Al2O3 2% by mass and CaO/AlOOH 2% by mass, single permeation with permeation mixture excess to carrier method was used. Permeation solution was prepared the following way: calculated amount of Ca(NO3)2·4H2O was mixed on a magnetic mixer until true solution formation and then diluted with distilled water in a measuring flask to 100 cm3. Carrier aliquot ~30 g was placed into permeation solution and exposed for ~24 h at room temperature. Then, obtained solution was evaporated for 1 h. Next, vacuum impulse drying was carried out at 373 K and atmospheric pressure until stable sample mass was reached. Then, the catalyst was calcinated in a muffle furnace in air atmosphere at 673 K for 4 h and cooled down.
The use of 2% by mass solution was reasoned in previous studies by the article authors in research with similar catalysts [47,55].
2.2. Experiment Equipment and Techniques
2.2.1. Transesterification Under SCF Conditions
Continuous process was used to obtain biodiesel fuel under supercritical fluid condition of reaction mixture. Equipment unit overview is shown in Figure 1. In order to provide intense mixing of hard-miscible reagents under atmospheric conditions and increase phase contact area, an ultrasonic emulsifier UIP1000HD by Hielscher (Teltow, Germany) was included in the process layout (item 6) for process optimization. Emulsifier working frequency was 20 kHz at emulsifying power of 1000 W. Given parameters recommended by manufacturer, mixture treatment with ultrasound for maximum efficiency was performed at 81 µm oscillating amplitude and under 3.5 bar excessive pressure. Previously, our colleagues [59] proved stability of rapeseed oil and ethanol emulsion obtained through ultrasonic dispersion and revealed effectiveness of emulsification for biodiesel fuel obtaining under SCF conditions using palm oil [60].
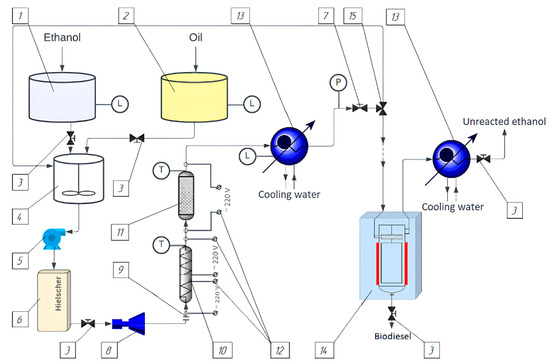
Figure 1.
Overall layout of continuous operation unit with flow-through reactor for biodiesel fuel production under supercritical fluid condition of reaction mixture: 1—ethanol tank; 2—oil tank; 3, 7, 15—shut-off and control valves; 4—mechanical agitator; 5—gear pump; 6—ultrasonic emulsifier by Hielscher; 8—measuring pump; 9—insulator; 10—continuous operation reactor; 11—catalytic reactor with stationary catalyst layer; 12—power unit; 13—coolers; 14—film evaporator.
In addition, the unit also received catalytic reactor with stationary catalyst layer (item 11). The catalytic reactor volume is calculated to be 5% by mass of the total reaction mixture feed. Catalyst layer takes volume of 160 cm3, 0.8 m high. Amount of catalyst used in experiments was 2.5% of initial raw material mass. For complete reactor filling, contact area increase and additional process intensification catalyst is charged with mixed hollow ceramic cylinders. Pumped mixture rate was 300 mL/min, pressure overfall was taken from readings difference of manometers’ installed before and after catalytic reactor and did not exceed 8 KPa. Required temperature control and maintenance are implemented using a cable with magnesial insulation and a nickel–chromium core that receives power through a thermoregulator coaxially rolled onto a catalytic reactor. Temperature taking is performed by chromel–alumel thermocouples at the input and output of the catalytic reactor. Thermal insulation is provided to minimize heat loss.
2.2.2. Chromatography
Obtained transesterification reaction product was evaluated using chromatography performed at quadrupolar mass-spectrometer Agilent 6890N/5973 by Agilent, Santa Clara, CA, USA. Analysis conditions were the following: electronic ionization at ionizing electrons energy 70 eV, ion source temperature 230 °C. Capillary column TG-5MS was used in the process. This column is 30 m long and 0.25 mm wide. Phase layer thickness was 0.25 µm. Helium was used as a carrier gas. Mass-spectral data processing was performed using MSD ChemStation software, Version D.02.00.275 (Agilent Technologies, Santa Clara, CA, USA). Sample was preliminarily dissolved in isopropyl alcohol at 1:10 ratio.
Chromatogram receiving conditions were set as follows: injector temperature—280 °C, split ratio—1:15; column heating was performed in program mode by areas: initial temperature—100 °C (10 min); heating rate 10 °C/min to 280 °C (2 min); heating rate 10 °C/min to 300 °C (10 min); carrier gas flow rate through the column—1 mL/min; temperature of communication unit with mass-spectrometer—320 °C; sample volume—0.5 µL.
2.2.3. Measuring Thermophysical Properties
Density and dynamic viscosity were measured using Stabinger viscometer SVM3000 (AntonPaar, Graz, Austria). This unit allows us to measure dynamic viscosity within 0.2–104 MPa·s, density within 650–2000 kg/m3. Measuring relative error limits as follows: for viscosity—±0.35%; for density—±0.5%; for temperature—±0.02 °C. The working temperature range is the following: 0–100 °C. Kinematic viscosity was calculated as dynamic viscosity to density ratio.
2.2.4. Centrifugation
Reaction product separation was performed in a cooled centrifuge Hanil Scientific Smart 17 Plus, South Korea, equipped with an imperforate basket. Centrifuges of this type are most often used for separation of concentrated suspensions and emulsions. Centrifugation on this unit is based on different particle behavior in centrifugal field. Suspension is put into a glass tube and set into rotor installed on centrifuge drive shaft.
This unit allows to perform separation at top rotation speed, which is 17,000 rpm, within working temperature range of −10–+40 °C. The unit work cycle time can reach 100 min.
Before the experiment, the sample was put into a thermostat set at 0 °C for 12 h for thermal stabilization. This temperature choice is caused by different crystallization temperatures of fatty acids’ ethyl esters and impurities present in biodiesel.
Then, sample was put into Eppendorfs (24 pcs) of 2 mL volume.
The process was performed at the following parameters:
- -
- Temperature—4 °C;
- -
- Rotation speed—6000 rpm;
- -
- Centrifugation time—10 min.
The choice of parameters is explained by preliminary experiments when, at higher temperatures, lower rotations, and longer times, there was no separation and sedimentation even after preliminary cooling.
2.2.5. Catalysts Analysis
Catalysts’ elemental analysis was performed using X-ray fluorescent spectrometer RMS-02 RehnPV/HLF by NTC ExpertCenter (Moscow, Russia).
Since transesterification under SCF conditions runs at high temperatures and pressures, mechanical strength of initial and used catalysts was also analyzed. Catalyst strength before and after experiments was found using LinteL PK-21 by JSC BSKB Neftekhimavtomatika (Ufa, Republic of Bashkortostan, Russia), unit designed for testing catalysts for mechanical strength under static conditions through compression method. Testing of the catalysts in question was performed automatically. The following method was used to find granules’ static strength: 12 granules of a catalyst were taken, and an average granule cross-section was found; then, the granules were placed into special cassettes, and the force was applied to the catalysts using a piston rod. The strength measuring unit takes the value of the strength limit for each sample. Then, the average value (Pop, N/cm2) is calculated according to the following formula:
where N—number of tests; S—average granule cross-section area set before series of tests, cm2; Xi—strength limit value during the test N; static relative error of strength limit measurement is 1%, piston rod lowering speed during catalyst compression is 4.4 N/s.
2.3. Control Measurements
In order to estimate the influence of ultrasonic impact on the reactive environment, authors took control measurements for studied systems without catalysts. Cases of obtaining biodiesel without stirring the initial mixture, with preliminary mechanical stirring, and with preliminary ultrasound impact have been considered. Experiment (t = 334 °C, P = 30 MPa, alcohol to oil molar ratio—25:1, τ = 36 min) results based on biodiesel yield and kinematic viscosity of obtained product are given in Table 4.

Table 4.
Test measurement results in estimation of ultrasound impact on biodiesel yield.
3. Results and Discussion
Non-catalytic and catalytic transesterification were performed experimentally under SCF conditions in a continuous mode. Reaction conditions were chosen based on the average values in Table 1 and Table 2: for non-catalytic reaction—t = 334 °C, P = 30 MPa, alcohol to oil molar ratio—25:1, time—36 min; for catalytic reaction in the presence of Al2O3, CaO/Al2O3 (2% by mass), AlOOH and CaO/AlOOH (2% by mass)—t = 274 °C, P = 18 MPa, alcohol to oil molar ratio—25:1, with catalyst amount 2.5% by mass, time—25 min. After product separation from excess alcohol, dynamic viscosity and density of obtained samples were measured at atmospheric pressure within the temperature range of t = 20–40 °C. The experiment results are shown in Table 5 and Figure 2.

Table 5.
Biodiesel fuel samples’ dynamic viscosity.
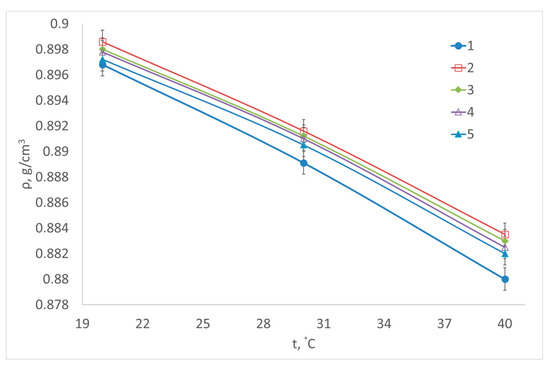
Figure 2.
Change in density depending on temperature for transesterification reaction samples obtained: 1—without catalysts; 2—using Al2O3; 3—using CaO/Al2O3; 4—using AlOOH; 5—using CaO/AlOOH.
Uncertainty value for dynamic viscosity reaches ±1%, for density—±0.1%. Table 6 shows estimated values of kinematic viscosity for biodiesel at atmospheric pressure and various temperatures obtained through dynamic viscosity to density values ratio.

Table 6.
Biodiesel fuel samples’ kinematic viscosity.
Considering Table 5 and Table 6, viscosity decreases with measurement temperature rise. Viscosity measurement results for non-catalytic biodiesel production are slightly lower than the values of samples obtained through a catalytic reaction. This is explained by higher temperatures and longer reaction times for non-catalytic procedures. However, none of the samples complies with international standard EN14214 in terms of kinematic viscosity. Rashid et al. [61], at similar technological parameters but using methanol at T = 313 K, received a kinematic viscosity of 4.85 mm2/s and a density of 0.882 g/cm3 at T = 298 K. Demirbas [62,63] at supercritical transesterification using methanol and vegetable oils received kinematic viscosity within the range of 3.59–4.63 mm2/s. Nascimento et al. [64] measured the properties of biofuel obtained from soybean oil at ethanol to oil molar ratio 39:1 and temperatures between 533 and 573 K and pressure over 10 MPa. Density values at T = 293 K varied within the range of 0.90484–0.91699 g/cm3.
3.1. Transesterificationunder SCF Conditions
Results of biodiesel composition chromatographic analysis for main esters for analyzed samples are shown in Figure 3 and Figure 4.
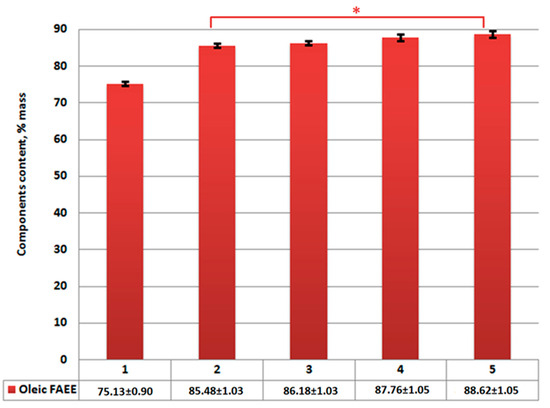
Figure 3.
Ethyl oleate content in reaction product: 1—without catalysts; 2—using Al2O3; 3—using CaO/Al2O3; 4—using AlOOH; 5—using CaO/AlOOH. Statistical significance of Oleic FAEE between catalyst-involving data groups, given that single-tail t-test with p value < 0.04 is marked with one star (*); error bars, mean ± SEM.
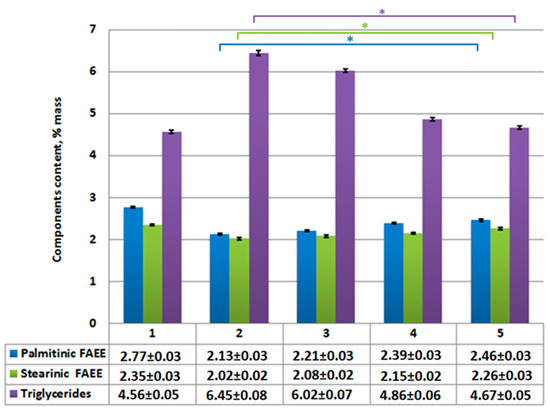
Figure 4.
Content of ethyl palmitate, ethyl stearate, and unreacted triglycerides in reaction product: 1—without catalysts; 2—using Al2O3; 3—using CaO/Al2O3; 4—using AlOOH; 5—using CaO/AlOOH. Statistical significances of palmitinic FAEE, stearinic FAEE, and triglycerides between catalyst-involving data groups, given that single-tail t-test with p value < 0.05 is marked with one star (*); error bars, mean ± SEM.
Considering the chosen reaction conditions, the performed supercritical transesterification experiment showed that a non-catalytic reaction does not allow us to obtain predictable FAEE amount in the reaction product at 90% (Figure 3 and Figure 4). One of the reasons is that the obtained unsaturated oleic fatty acid ethyl esters are less when compared to the content in samples obtained using catalysts (Figure 3). This is the result of this ethyl ester decomposition under supercritical transesterification reaction conditions at temperatures over 300–325 °C, which is also proven by our previous study results and other authors’ studies [54,65,66]. It should also be noted that thermal decomposition is not seen for saturated fatty acids’ (palmitinic, stearinic, etc.) ethyl esters due to even higher decomposition temperatures for these esters.
During catalytic reaction analysis, the declared 90% was achieved using all four catalysts. Insignificant final product changes are seen in the case of using boehmite and synthesized boehmite-based catalyst compared to industrial Al2O3 and CaO/Al2O3 synthesized on its basis.
The maximum content of the obtained FAEE in the reaction product is reached by using CaO/AlOOH in the amount of 93.34% by mass. Meanwhile, none of the samples has reached level of 96.5% by mass in fatty acids esters content in reaction product, which is required by EN 14214 standard.
Also, traces of glycerin, a reaction byproduct, were not found during the reaction either visually or using chromatography. During the reaction process at these temperatures, glycerin decomposes. During the process, it reacts with alcohol:
C3H5(OH)3 + 3C2H5OH = C3H5(OC2H5)3 + 3H2O
Water formed in the reaction interacts with fatty acid triglycerides with formation of fatty acids diglycerides and free fatty acids:
C3H5(OCOR)3 + H2O = C3H5(OCOR)2OH + RCOOH.
Then, free fatty acids turn into fatty acids’ compound esters:
RCOOH + C2H5OH = RCOOC2H5 + H2O.
Such esters are ethyl esters of caprylic, pelargonic, and hendecoic acids formed in minor amounts in reaction products.
3.2. Biodiesel Samples Centrifugation
The obtained biofuel samples do not comply with the EN14214 standard on total FAEE content on reaction products or on kinematic viscosity values. Due to this, biodiesel separation was performed in order to extract unreacted triglycerides and saturated ethylpalmitate and ethylstearate, which have a higher viscosity than ethyloleate.
For this, transesterification reaction product centrifugation was performed. Particles of the products having different densities, shapes, and sizes and charged in 24 Eppendorfs are sedimented at different speeds in a centrifugal field. In the case of the same density, larger particles are settled much faster than smaller ones. The higher the environment viscosity, the slower particles settle down. During the centrifugation process, the centrifugal force pushed heavier biodiesel components toward the Eppendorf walls, thus improving sedimentation. After the experiment, white crystals, which did not separate during centrifugation, stayed on the Eppendorf walls. The repeated mixture run in a centrifuge did not show any separation effect.
One sample separation result is shown in Figure 5. Initial biodiesel (Figure 5A) was separated into two fractions (Figure 5B,C). In the Eppendorfs (Figure 5C), thickened residue traces as white crystals can be seen on the walls. The remaining more liquid fraction (Figure 5B) visually looks more clear compared to the pre-separation mixture (Figure 5A).
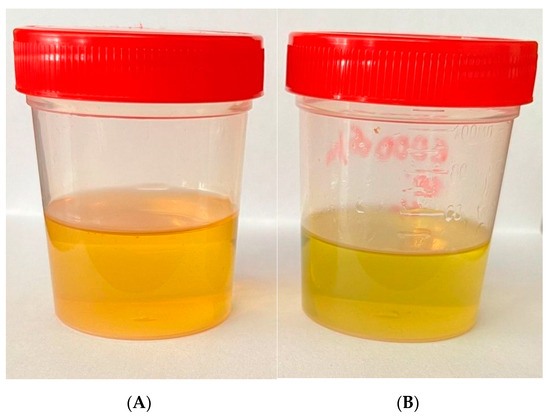
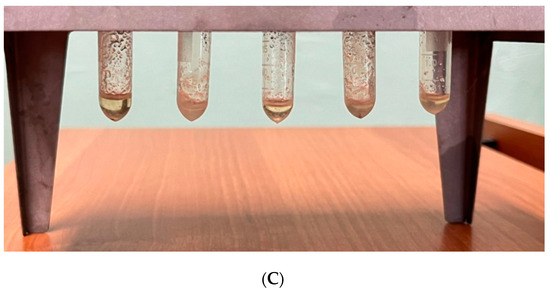
Figure 5.
Biodiesel samples: (A) before separation; (B) after separation; (C) remaining sediment.
Chromatographic analysis results for biodiesel samples after separation (Figure 6 and Figure 7) and the remaining sediment (Figure 8 and Figure 9) were obtained. Figure 8 and Figure 9 results are found based on average values of analyzed samples taken from five Eppendorfs.
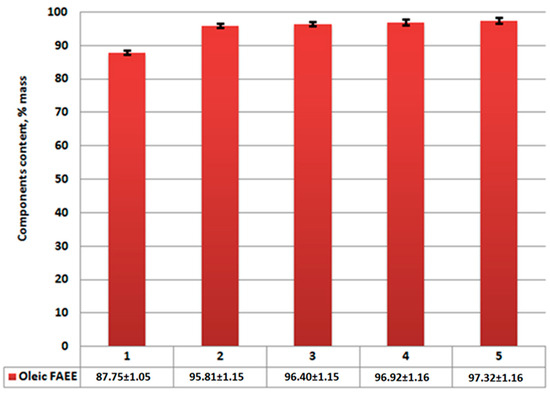
Figure 6.
Ethyl oleate content in reaction product after centrifugation: 1—without catalysts; 2—using Al2O3; 3—using CaO/Al2O3; 4—using AlOOH; 5—using CaO/AlOOH; error bars, mean ± SEM.
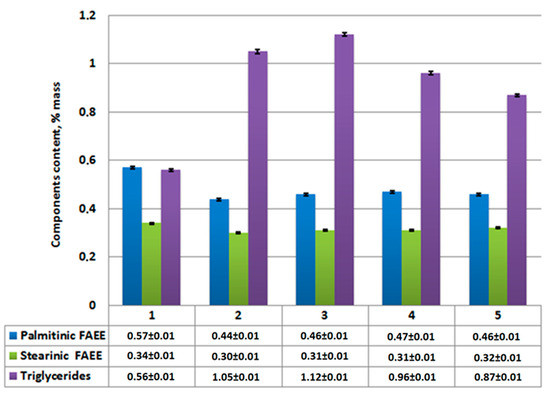
Figure 7.
Content of ethyl palmitate, ethyl stearate, and unreacted triglycerides in reaction product after centrifugation: 1—without catalysts; 2—using Al2O3; 3—using CaO/Al2O3; 4—using AlOOH; 5—using CaO/AlOOH; error bars, mean ± SEM.
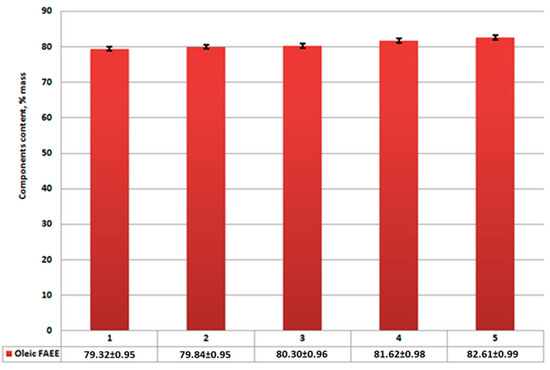
Figure 8.
Ethyl oleate content in sediment product: (1)—without catalysts; (2)—using Al2O3; (3)—using CaO/Al2O3; (4)—using AlOOH; (5)—using CaO/AlOOH; error bars, mean ± SEM.
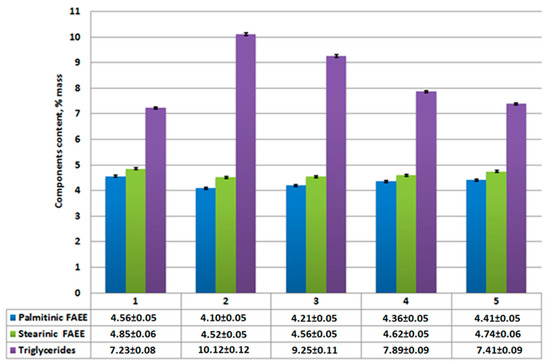
Figure 9.
Content of ethyl palmitate, ethyl stearate, and unreacted triglycerides in sediment product: 1—without catalysts; 2—using Al2O3; 3—using CaO/Al2O3; 4—using AlOOH; 5—using CaO/AlOOH; error bars, mean ± SEM.
According to analysis results (Figure 7), the reduction of unreacted triglycerides and saturated fatty acids (palmitinic, stearinic, etc.) and ethyl esters are compared to Figure 4. Meanwhile, ethyl oleate concentration in the overall mixture (Figure 6) rises compared to Figure 3. Its amount combined with other FAEEs for samples 2–5 (Figure 6 and Figure 7) exceeds 96.5% by mass, which, according to EN14214 standard, makes the fuel obtained after centrifugation suitable for use in terms of the required amount of esters in the reaction product. Unseparated residue results (Figure 9) show a high percentage of saturated fatty acids (palmitinic, stearinic, etc.), ethyl esters, and unreacted triglycerides compared to the mixture’s mass composition.
Samples’ dynamic viscosity and density were measured at t = 40 °C and P = 0.1 MPa, then kinematic viscosity ratios were found. Results are shown in Table 7.

Table 7.
Density, dynamic, and kinematic viscosity of biodiesel fuel samples after separation at t = 40 °C and P = 0.1 MPa.
Table 7 suggests that biodiesel samples after separation have kinematic viscosity equal to or even under 5.0 mm2/s within experiment error.
Also, transesterification product sediment sample density was measured after separation at t = 40 °C and atmospheric pressure (Table 8). Density was measured by the gravimetric method using a glass pycnometer GP 2–10 KSh 7/16 by EximLAB (Khimki, Moscow Region, Russia) (GOST (State standard of the Russian Federation) 22524-77 [67]). Weighing was carried out on analytical balances model BLA-200 (Balances laboratory analytical) and electronic balances “Mettler” model PM600 from “Mettler-Toledo GmbH” company, Greifensee, Switzerland. The use of the Stabinger viscometer is limited by sample size (minimum 10 mL). The sediment sample volume was just over 2 mL. Sediment density is slightly higher than reaction product density after separation (Table 6) due to high fatty acid triglyceride content.

Table 8.
Transesterification product sediment samples density after separation at t = 40 °C and P = 0.1 MPa.
3.3. Catalysts Characteristics
The elemental composition of some used catalysts before and after experiments is shown in Table 9.

Table 9.
Elemental composition of some used catalysts before and after experiments.
The supercritical transesterification process causes aluminum and calcium to leach from the catalyst’s surface. The presence of nickel, chrome, as well as zinc and copper in catalyst samples after the reaction is explained by the presence of these metals in parts of reactors made of stainless steel, brass valves, and zinc-coated connection pipes.
The arithmetic mean strength values for catalysts were calculated according to formula (1) for 12 tested samples of each catalyst and are given in Table 10.

Table 10.
Arithmetic mean values of catalyst strength, N/cm2.
Measurement results (Table 10) show significant deterioration of catalysts’ strength after the singular experiment: Al2O3 by 22.4%; AlOOH by 13.89%; CaO/Al2O3 by 25.13%; CaO/AlOOH by 17.27%. The catalyst AlOOH obtained through hydrothermal processing showed better strength characteristics both before and after the experiment compared to Al2O3. The same is true about the catalyst based on it. The catalysts’ strength decline after the experiment is caused by the high permeability of the supercritical fluid, which is able to penetrate catalysts deeply.
4. Conclusions
Transesterification reaction was performed under supercritical fluid conditions in a flow-through mode for the non-catalytic method (t = 334 °C, P = 30 MPa, alcohol to oil molar ratio—25:1, time—36 min), and in the presence of the catalysts Al2O3, CaO/Al2O3 (2% by mass), AlOOH, and CaO/AlOOH (2% by mass), it was performed at t = 274 °C, P = 18 MPa, an alcohol to oil molar ratio of 25:1, a catalyst amount of 2.5% by mass, and a time of 25 min. For these reaction conditions, the obtained values of kinematic viscosity and biodiesel fuel concentration in the reaction product do not comply with the standard requirements. Sufficiently high parameters for non-catalytic reaction conditions caused ethyloleate decomposition. The performed centrifugation allowed us to separate biodiesel from unreacted triglycerides and highly viscous saturated fatty acid esters. As a result, the reaction product’s kinematic viscosity decreased to a value equal to or under 5.0 mm2/s. The overall content of the main fatty acid ethyl esters increased to values over 96.5% by mass, which allowed us to obtain biodiesel fuel that complied with the EN14214 standard requirements. The initial catalysts’ strength declines after the singular experiment: Al2O3 by 22.4%, AlOOH by 13.89%, CaO/Al2O3 by 25.13%, and CaO/AlOOH by 17.27%. This is caused by the high permeability of supercritical fluid. Based on the present data for the lowest kinematic viscosity, the highest FAEE content in the reaction product, and high enough structural characteristics after the reaction, the CaO/AlOOH catalyst (2% by mass) can be recommended for transesterification reaction under SCF conditions.
Author Contributions
Conceptualization, Y.A.S. and A.U.A.; Methodology, R.R.T.; Software, D.H.K. and R.R.T.; Validation, D.H.K.; Formal analysis, S.V.M. and D.H.K.; Investigation, A.U.A.; Resources, A.U.A.; Data curation, S.V.M. and R.R.T.; Writing—original draft, Y.A.S. and F.M.G.; Writing—review & editing, A.U.A.; Visualization, A.U.A.; Supervision, A.U.A.; Project administration, Y.A.S. and F.M.G.; Funding acquisition, Y.A.S. and S.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out with the support of the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (IRN BR18574219).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was carried out with the support of the Russian Science Foundation (project No. 23-79-10304, https://rscf.ru/project/23-79-10304/ (accessed on 14 August 2023)).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ishola, F.; Adelekan, D.; Mamudu, A.; Abodunrin, T.; Aworinde, A.; Olatunji, O.; Akinlabi, S. Biodiesel production from palm olein: A sustainable bioresource for Nigeria. Heliyon 2020, 6, e03725. [Google Scholar] [CrossRef]
- Umeuzuegbu, J.C.; Okiy, S.; Nwobi-Okoye, C.C.; Onukwuli, O.D. Computational modeling and multi-objective optimization of engine performance of biodiesel made with castor oil. Heliyon 2021, 7, e06516. [Google Scholar] [CrossRef] [PubMed]
- Iweka, S.C.; Falowo, O.A.; Amosun, A.A.; Betiku, E. Optimization of microwave-assisted biodiesel production from watermelon seeds oil using thermally modified kwale anthill mud as base catalyst. Heliyon 2023, 9, e17762. [Google Scholar] [CrossRef]
- Adepoju, T.F.; Rasheed, B.; Olatunji, O.M.; Ibeh, M.A.; Ademiluyi, F.T.; Olatunbosun, B.E. Modeling and optimization of lucky nut biodiesel production from lucky nut seed by pearl spar catalysed transesterification. Heliyon 2018, 4, 1–32. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- EN 14214:2013 V2+A2:2019; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2019.
- ASTM D6751-23a; Standard Specification for Biodiesel Fuel Blendstock (B100) for Middle Distillate Fuels. ASTM: West Conshohocken, PA, USA, 2023.
- Hayyan, M.; Mjalli, F.S.; Hashim, M.A.; Al Nashef, I.M. A novel technique for separating glycerine from palm oil-based biodiesel using ionic liquids. Fuel Process. Technol. 2010, 91, 116–120. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Biodiesel separation and purification: A review. Renew Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Luo, B. Biodiesel production by transesterification of waste cooking oil in the presence of graphitic carbon nitride supported molybdenum catalyst. Fuel 2023, 332, 126309. [Google Scholar] [CrossRef]
- Limmun, W.; Chungcharoen, T.; Rattanamechaiskul, C.; Phetpan, K.; Limmun, W. Enhancing biodiesel yield and purification with a recently developed centrifuge machine: A response surface methodology approach. Heliyon 2024, 10, e29018. [Google Scholar] [CrossRef]
- Mehta, P.; Rasekh, M.; Patel, M.; Onaiwu, E.; Nazari, K.; Kucuk, I.; Wilson, P.B.; Arshad, M.S.; Ahmad, Z.; Chang, M.-W. Recent applications of electrical, centrifugal, and pressurized emerging technologies for fibrous structure engineering in drug delivery, regenerative medicine and theranostics. Adv. Drug Deliv. Rev. 2021, 175, 113823. [Google Scholar] [CrossRef]
- Grause, G.; Kuniyasu, Y.; Chien, M.; Inoue, C. Separation of microplastic from soil by centrifugation and its application to agricultural soil. Chemosphere 2022, 288, 132654. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.; Nielsen, B.S.; Nielsen, F.C.; Ray, A.C.; Lund, N.M. Impact of UHT treatment and storage on liquid infant formula: Complex structural changes uncovered by centrifugal field-flow fractionation with multi-angle light scattering. Food Chem. 2021, 348, 129145. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, T.; Li, S.; Nugroho, Y.K.; Li, B.; Cao, J.; Show, P.-L.; Hiltunen, E. Effects of operating parameters on algae Chlorella vulgaris biomass harvesting and lipid extraction using metal sulfates as flocculants. Biomass Bioenergy 2020, 132, 105433. [Google Scholar] [CrossRef]
- Guldhe, A.; Misra, R.; Singh, P.; Rawat, I.; Bux, F. An innovative electrochemical process to alleviate the challenges for harvesting of small size microalgae by using non-sacrificial carbon electrodes. Algal Res. 2016, 19, 292–298. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Samhan, F.A.; Salama, A.A.; Hamdy, R.M.; Ali, G.H. Cationic starch: Safe and economic harvesting flocculant for microalgal biomass and inhibiting E. coli growth. Int. J. Biol. Macromol. 2018, 116, 1296–1303. [Google Scholar] [CrossRef]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of microalgae by centrifugation for biodiesel production: A review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Sdrula, N. A study using classical or membrane separation in the biodiesel process. Desalination 2010, 250, 1070–1072. [Google Scholar] [CrossRef]
- Abu-Shamleh, A.; Najjar, Y.S.H. Optimization of mechanical harvesting of microalgae by centrifugation for biofuels production. Biomass Bioenergy 2020, 143, 105877. [Google Scholar] [CrossRef]
- Vol’eva, V.B.; Belostotskaya, I.S.; Komissarova, N.L.; Koverzanova, E.V.; Kurkovskaya, L.N.; Usmanov, R.A.; Gumerov, F.M. Synthesis of biodiesel without formation of free glycerol. Russ. J. Org. Chem. 2015, 51, 915–917. [Google Scholar] [CrossRef]
- Aimaretti, N.; Manuale, D.L.; Mazzieri, V.M.; Vera, C.R.; Yori, J.C. Batch study of glycerol decomposition in one-stage supercritical production of biodiesel. Energy Fuels 2009, 23, 1076–1080. [Google Scholar] [CrossRef]
- Marulanda, V.F.; Anitescu, G.; Tavlarides, L.L. Investigations on supercritical transesterification of chicken fat for biodiesel production from low-cost lipid feedstocks. J. Supercrit. Fluids 2010, 54, 53–60. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel Fuel from Rapeseed Oil as Prepared in Supercritical Methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Román-Figueroa, C.; Olivares-Carrillo, M.; Palacios-Nereo, P.F.J.; Quesada-Medina, J. High-yield production of biodiesel by non-catalytic supercritical methanol transesterification of crude castor oil (Ricinus communis). Energy 2016, 107, 165–171. [Google Scholar] [CrossRef]
- Tiguntceva, N.P.; Fomina, E.S.; Evstaf’ev, S.N. Transesterification of sunflower oil in a sub- and supercritical dimethyl carbonate medium. Appl. Chem. Biotechnol. 2019, 9, 773–778. [Google Scholar] [CrossRef]
- García-Martínez, N.; Andreo-Martínez, P.; Quesada-Medina, J.; de los Ríos, A.P.; Chica, A.; Beneito-Ruiz, R.; Caratala-Abril, J. Optimization of non-catalytic transesterification of tobacco (Nicotiana tabacum) seed oil using supercritical methanol to biodiesel production. Energy Convers. Manag. 2017, 131, 99–108. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Dadashev, M.N.; Barkov, A.V. Method for Producing Biodiesel Fuel. Patent RU 2,412,236, 15 December 2008. Available online: https://patents.google.com/patent/RU2412236C2/ru (accessed on 20 February 2011).
- Outili, N.; Kerras, H.; Nekkaba, C.; Merouani, R.; Meniai, A.H. Biodiesel production optimization from waste cooking oil using green chemistry metrics. Renew. Energy 2020, 145, 2575–2586. [Google Scholar] [CrossRef]
- Mazanov, S.V.; Kouagou, Z.-M.; Hounkpatin, D.D.; Fonkou, M.J.; Usmanov, R.A.; Zaripov, Z.I.; Gumerov, F.M.; Shapovalov, Y.A. Transesterification of Shea (Karite) and Palm Oils in Supercritical Ethanol. Russ. J. Phys. Chem. B 2022, 16, 1347–1353. [Google Scholar] [CrossRef]
- Usmanov, R.A.; Mazanov, S.V.; Gabitova, A.R.; Miftakhova, L.K.; Gumerov, F.M.; Musin, R.Z.; Abdulagatov, I.M. The effect of fatty acid ethyl esters concentration on the kinematic viscosity of biodiesel fuel. J. Chem. Eng. Data 2015, 60, 3404–3413. [Google Scholar] [CrossRef]
- Gabitova, A.R.; Mazanov, S.V.; Usmanov, R.A.; Zaripov, Z.I.; Gumerov, F.M.; Abdulagatov, I.M. Viscometry as a method for determining concentration of fatty acid ethyl esters in biodiesel fuel. Chem. Technol. Fuels Oils 2017, 53, 77–86. [Google Scholar] [CrossRef]
- Akkarawatkhoosith, N.; Bangjang, T.; Kaewchada, A.; Jaree, A. Biodiesel production from rice bran oil fatty acid distillate via supercritical hydrolysis–esterification–transesterification in a microreactor. Energy Rep. 2022, 9, 5299–5305. [Google Scholar] [CrossRef]
- Kusuma, R.; Hadinoto, J.; Ayucitra, A.; Soetaredjo, F.; Ismadji, S. Natural zeolite from Pacitan Indonesia, as catalyst support for transesterification of palm oil. Appl. Clay Sci. 2013, 74, 121–126. [Google Scholar] [CrossRef]
- Leclercq, E.; Finiels, A.; Moreau, C. Transesterification of Rapeseed Oil in the Presence of Basic Zeolites and Related Solid Catalysts. J. Am. Oil Chem. Soc. 2001, 78, 1161–1165. [Google Scholar] [CrossRef]
- Suppes, G.J.; Dasari, M.A.; Doskocil, E.J.; Mankidy, P.J.; Goff, M.J. Transesterification of soybean oil with zeolite and metal catalysts. Appl. Catal. A General. 2004, 257, 213–223. [Google Scholar] [CrossRef]
- Mootabadi, H.; Salamatinia, B.; Bhatia, S.; Abdullah, A.Z. Ultrasonic-Assisted Biodiesel Production Process from Palm Oil Using Alkaline Earth Metal Oxides as the Heterogeneous Catalysts. Fuel 2010, 89, 1818–1825. [Google Scholar] [CrossRef]
- Serio, M.D.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous Catalysts for Biodiesel Production. Energy Fuels 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Ashok, A.; Kennedy, L.J. Magnetically Separable Zinc Ferrite Nanocatalyst for an Effective Biodiesel Production from Waste Cooking Oil. Catal. Lett. 2019, 149, 3525–3542. [Google Scholar] [CrossRef]
- Rasouli, H.; Esmaeili, H. Characterization of MgO nanocatalyst to produce biodiesel from goat fat using transesterification process. 3 Biotech 2019, 9, 429. [Google Scholar] [CrossRef]
- Zeng, H.-Y.; Feng, Z.; Deng, X.; Li, Y.-Q. Activation of Mg–Al hydrotalcite catalysts for transesterification of rape oil. Fuel 2008, 87, 3071–3076. [Google Scholar] [CrossRef]
- Olutoye, M.A.; Hameed, B.H. Production of biodiesel fuel by transesterification of different vegetable oils with methanol using Al2O3 modified MgZnO catalyst. Bioresour. Technol. 2013, 132, 103–108. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Haapaniemi, E.; Sillanpaa, M. Nano-magnetic potassium impregnated ceria as a catalyst for the biodiesel production. Renew. Energy 2019, 139, 14281436. [Google Scholar] [CrossRef]
- Gunasekaran, V.; Harichandran, G.; Ganesan, R.; Yuvakkumar, R. Sustainable synthesis of biodiesel and jet-fuel range hydrocarbons from poisonous Abrus Precatorius seed oil over MoO3-HPW/Ga-KIT-6. Renew. Energy 2024, 224, 120–130. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Zhao, X.; Feng, P. Sulfonated ordered mesoporous carbon for catalytic preparation of biodiesel. Carbon 2008, 46, 1664–1669. [Google Scholar] [CrossRef]
- Gupta, A.R.; Chiplunkar, P.P.; Pratap, A.P. Esterification of palm fatty acid distillate for FAME synthesis catalyzed by superacid catalyst HClSO3–ZrO2. Biomass Waste Valor 2021, 12, 281–292. [Google Scholar]
- Mazanov, S.V.; Gabitova, A.R.; Usmanov, R.A.; Gumerov, F.M.; Labidi, S.; Ben Amar, M.; Passarello, J.-P.; Kanaev, A.; Volle, F.; Le Neindre, B. Continuous production of biodiesel from rapeseed oil by ultrasonic assist transesterification in supercritical ethanol. J. Supercrit. Fluids 2016, 118, 107–118. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from sunflower oil in supercritical methanol with calcium oxide. Energy Convers. Manag. 2007, 48, 937–941. [Google Scholar] [CrossRef]
- Elst, K.; Adriansens, W.; Willems, L.; Van Ginneken, L. Method for Producing Biodiesel Using an Immobilized Catalyst. U.S. Patent 8067624B2, 27 September 2011. Available online: https://patents.google.com/patent/EP1884559A1/it (accessed on 29 November 2011).
- Yoo, S.J.; Lee, H.-S.; Veriansyah, B.; Kim, J.; Kim, J.-D.; Lee, Y.-W. Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalysts. Bioresour. Technol. 2010, 101, 8686–8689. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Gupta, R.; Modak, J.M.; Madras, G. ZnO catalyzed transesterification of Madhuca indica oil in supercritical methanol. Fuel 2019, 242, 323–333. [Google Scholar] [CrossRef]
- Ceran, Z.D.; Demir, V.; Akgün, M. Optimization and kinetic study of biodiesel production from Jatropha curcas oil in supercritical methanol environment using ZnO/γAl2O3 catalyst. Biomass Convers. Biorefinery 2024, 15, 3903–3914. [Google Scholar] [CrossRef]
- Hoang, D.; Bensaid, S.; Saracco, G.; Pirone, R.; Fino, D. Investigation on the conversion of rapeseed oil via supercritical ethanol condition in the presence of a heterogeneous catalyst. Green Process Synth. 2017, 6, 91–101. [Google Scholar] [CrossRef]
- Mazanov, S.V.; Gabitova, A.R.; Miftahova, L.K.; Usmanov, R.A.; Gumerov, F.M.; Zaripov, Z.I.; Vasil’ev, V.A.; Karalyn, E.A. Preparing biodiesel fuel in supercritical fluid conditions with heterogeneous catalysts. Russ. J. Phys. Chem. B 2016, 10, 1099–1107. [Google Scholar] [CrossRef]
- Mazanov, S.V.; Usmanov, R.A.; Kuagu, J.M.; Unkpaten, D.D.; Fonkou, M.D.; Gumerov, F.M.; Zaripov, Z.I.; Shapovalov, Y.A.; Nauryzbayev, M.K. Experimental study of non-catalytic and catalytic reaction of transesterification of rapeseed oil under supercritical fluid conditions in a flow-type installation. Ind. Kazakhstan 2019, 1, 90–92. [Google Scholar]
- Mohamed, M.M.; El-Faramawy, H. An innovative nanocatalyst α-Fe2O3/AlOOH processed from gibbsite rubbish ore for efficient biodiesel production via utilizing cottonseed waste oil. Fuel 2021, 297, 120741. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Bayoumy, W.A.; El-Faramawy, H.; El-Dogdog, W.; Mohamed, A.A. A novel α-Fe2O3/AlOOH(γ-Al2O3) nanocatalyst for efficient biodiesel production from waste oil: Kinetic and thermal studies. Renew. Energy 2020, 160, 450–464. [Google Scholar] [CrossRef]
- Lee, J.S.; Saka, S. Biodiesel production by heterogeneous catalysts and supercritical technologies: Review. Bioresour. Technol. 2010, 101, 191–200. [Google Scholar] [CrossRef]
- Gabitov, R.R.; Usmanov, R.A.; Gumerov, F.M.; Gabitov, F.R. Study of stability of rapeseed oil and ethyl alcohol emulsion obtained by ultrasonic dispersion. Bulletin of Kazan. Techn. Univ. 2012, 7, 129–132. [Google Scholar]
- Biktashev, S.A.; Usmanov, R.A.; Gabitov, R.R.; Gazizov, R.A.; Gumerov, F.M.; Gabitov, F.R.; Abdulagatov, I.M.; Yarullin, R.S.; Yakushev, I.A. Transesterification of rapeseed and palm oils in supercritical methanol and ethanol. Biomass Bioenergy 2011, 35, 2999–3011. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F. Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 2008, 87, 265–273. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel from vegetable oils via transesterification in supercritical methanol. Energy Convers. Manag. 2002, 43, 2349–2356. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel production from vegetable oils by supercritical methanol. J. Sci. Ind. Res. 2005, 64, 858–865. Available online: https://hdl.handle.net/20.500.12395/19611 (accessed on 24 March 2025).
- Nascimento, F.P.; Oliveira, A.R.G.; Paredes, M.L.L.; Costa, A.L.H.; Pesso, F.L.P. Biodiesel production from supercritical ethanolysis of soybean oil. Chem. Eng. Trans. 2013, 32, 829–834. [Google Scholar] [CrossRef]
- Olivares-Carrillo, P.; Quesada-Medina, J. Thermal decomposition of fatty acid chains during the supercritical methanol transesterification of soybean oil to biodiesel. J. Supercrit. Fluids 2012, 72, 52–58. [Google Scholar] [CrossRef]
- Vieitez, I. Effect of temperature on the continuous synthesis of soybean esters under supercritical ethanol. Energy Fuels 2009, 23, 558–563. [Google Scholar] [CrossRef]
- GOST 22524-77; Glass Density Bottles. Specifications. USSR State committee on standards: Moscow, USSR, 1977.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).