Abstract
This study analyzed the effects of thermohydrolysis on the anaerobic conversion efficiency of lignocellulosic biomass, comparing conventional and microwave heating methods. The research aimed to identify the optimal temperature and duration for biomass pre-treatment to maximize biogas output. Four temperatures (100 °C, 130 °C, 150 °C, and 180 °C) and six durations (10, 15, 20, 25, 30, and 40 min) were tested. The results showed that microwave heating increased biogas production compared to conventional heating at the same temperatures and durations. At 150 °C, microwave heating for 20 min produced 1184 ± 18 NmL/gVS of biogas, which was 16% more than the 1024 ± 25 NmL/gVS achieved through conventional heating. Statistically significant differences in biogas output between microwave and conventional heating were observed at 130 °C, 150 °C, and 180 °C, with the greatest difference recorded between 130 °C and 150 °C: 13% for conventional heating and 18% for microwave heating. Notably, increasing the temperature from 150 °C to 180 °C did not result in a statistically significant rise in biogas production. The energy balance analysis revealed that microwave heating, despite its lower efficiency compared to conventional heating, resulted in higher net energy gains. The most favorable energy balance for microwave heating was observed at 150 °C, with a net gain of 170.8 Wh/kg, while conventional heating at the same temperature achieved a gain of 126.2 Wh/kg. Microwave heating became cost-effective starting from 130 °C, yielding an energy surplus of 18.2 Wh/kg. The maximum energy output from microwave conditioning was 426 Wh/kg at 150 °C, which was 158 Wh/kg higher than conventional heating. These findings suggest that microwave thermohydrolysis, particularly at 150 °C for 20 min, enhances both biogas production and energy efficiency compared to conventional methods. The results highlight the potential of microwave pre-treatment as an effective strategy to boost methane fermentation yields, especially at temperatures above 130 °C.
1. Introduction
As a natural source of carbon, lignocellulosic biomass is mainly derived from agricultural, industrial, municipal and forestry residues [1]. It is composed mainly of cellulose, hemicellulose and lignin, and is one of the most accessible resources in the world, with a global abundance estimated at around 80,000 Mt [2]. Lignocellulosic biomass is, therefore, an abundant and sustainable feedstock for biogas production. However, the complex structure and high lignin content of lignocellulosic biomass hinder its biodegradation and limit the efficiency of anaerobic digestion [3]. Operators of agricultural biogas plants have to choose the right raw material and prepare it properly. In order to increase the biogas output, pre-treatment of the substrates is necessary. The application of the right methods of production intensification should result in increasing the economic effectiveness of the operation of a biogas plant.
The degradation of lignocellulosic substrates is a complex process limited by a number of factors that directly affect the efficiency of biogas production. A key barrier is the complex chemical structure of lignocellulose, resulting from the presence of lignin, hemicellulose and cellulose, which form a dense and difficult-to-access matrix that makes degradation difficult for the microorganisms responsible for methane fermentation [4,5,6,7]. The high degree of crystallinity of cellulose further restricts enzyme access to its fibers, slowing their hydrolysis—a key step for the release of simple organic compounds that can be converted into methane and carbon dioxide. Insufficient activity of cellulolytic and hemicellulolytic enzymes can reduce the amount of substrate available to methanogenic microorganisms, which directly reduces the efficiency of biogas production. Environmental conditions such as pH, temperature and oxygen availability are crucial, as unfavorable parameters can inhibit the activity of fermentative and methanogenic bacteria. In addition, the presence of inhibitors, such as phenols released during the partial degradation of lignin, can have a toxic effect on microorganisms, slowing down the entire fermentation process. Water deficiency limits the transport of substrates and enzymes, reducing the rate of reaction, which also negatively affects the amount of biogas produced. The physical properties of the substrate should not be overlooked either; its density and porosity affect the access of microorganisms to the interior of the material and, therefore, the efficiency of its decomposition. Effectively modifying these factors can significantly increase the availability of simple organic compounds to methanogenic microorganisms, thereby optimizing biogas production [8,9,10,11,12,13]. This has increased interest in studies concerning methods of decomposition of lignocellulosic structures and maximization of the effectiveness of bioprocesses. This trend has been observed mainly for methane and alcohol fermentation, which result in the production of biogas and bioethanol. Particular emphasis has been placed on studying the effect of methods of lignocellulosic biomass pre-treatment on the extent of the degradation of polymeric structures and the products of their decomposition, such as five- and six-carbon sugar phenolic and furfural compounds [14,15,16]. The pre-treatment of lignocellulosic biomass must be carried out in such a way as to efficiently separate lignin from cellulose, increase the amount of amorphous cellulose, increase the porosity of the substrate, reduce the loss of sugars and reduce the risk of fermentation inhibitors while minimizing energy costs. A key aspect is to establish process parameters that guarantee efficiency, safety and cost-effectiveness. Incorrectly chosen processing conditions can lead to the synthesis of toxic compounds that impede the metabolism of methanogenic bacteria [14,17,18,19]. One solution that may address these challenges is the use of microwaves as a heating method. Research indicates that microwave pre-treatment can contribute to process efficiency through faster heating rates, lower activation temperatures and reduced reaction times. Scientific papers addressing this issue are available in the literature. For example, Dębowski et al. analyzed the effect of microwave chemical thermohydrolysis on the methane fermentation efficiency of lignocellulosic biomass, indicating the potential benefits of microwave heating over traditional methods [20]. In addition, a review published by Zhang et al. discusses the use of microwaves in biorefineries, suggesting that microwave heating can lead to lower activation temperatures and shorter reaction times compared to conventional heating [21]. In addition, work by Syed et al. examines the use of microwaves in biomass processing, highlighting their advantages, such as faster heating rates and potential improvements in process efficiency over traditional methods [22].
This article presents the impact of thermohydrolysis of lignocellulosic biomass by comparing conventional thermal treatment with microwave heating. Particular attention was given to analyzing the effect of thermohydrolysis on unit biogas production and the dynamics of the fermentation process. The aim of the study was to verify the hypothesis regarding the occurrence of athermal effects resulting from the use of microwaves. It was assessed whether—and if so to what extent—microwave heating affects the degree of biomass disintegration and its susceptibility to fermentation, thus providing important data for further optimization of biotechnological processes.
2. Materials and Methods
The aim of the study was to compare the disintegration effects of plant biomass using microwave heating and conventional heating. The experiment was designed to determine the shortest time and lowest temperature of the disintegration process that provides the highest efficiency of anaerobic biomass conversion.
2.1. Experimental Conditions
Four temperatures were tested: 100, 130, 150, and 180 °C, selected based on commonly used parameters in the literature, balancing effective lignocellulose decomposition with the risk of inhibitor formation. The upper limit of 180 °C was dictated by the technical capacity of the equipment, allowing a maximum internal reactor temperature of 190 °C. Six process durations were examined: 10, 15, 20, 25, 30, and 40 min. The selected times reflected a balance between assessing the short-term impact of microwave and conventional heating on biomass structure (for 10–20 min) and evaluating the effect of longer treatments (25–40 min) on inhibitor formation and process cost-effectiveness.
2.2. Substrate Preparation
Maize silage was used as the raw material. Before processing, the substrate was mechanically crushed to obtain particles of 2–5 mm in size. The characteristics of the raw material are presented in the table (Table 1). Subsequently, the substrate was hydrated to a level of 90% and subjected to a thermohydrolysis process.

Table 1.
Characteristics of native materials.
2.3. Thermohydrolysis
Two methods of disintegration were compared. Microwave heating: This was carried out using the Mars-Solvent Extraction system (CEM, Stallings, NC, USA), which allows adjustable power up to 1600 W and operates at a microwave frequency of 2.45 GHz. Samples were placed in 115 cm3 Teflon Easy Prep dishes. For conventional heating, proprietary thermo-reactors made of acid-resistant steel, identical in size to the Easy Prep vessels, were used. Heating was carried out in a Laboplay O420E oil bath (DanLab, Białystok, Poland) using silicone oil as the heating medium. In both heating methods, the conditions (temperature, pressure) were comparable, and the rate of temperature rise and exposure time were identical. The efficiency of hydrothermal depolymerization was assessed both directly and indirectly: Direct measurement: determination of glucose released into solution during depolymerization by the anthrone method using a glucose standard curve. Soluble sugars were measured after filtering the sample through a membrane filter (pore diameter: 1.2 µm). Indirect measurement: Respirometric analysis of the yield and composition of the biogas produced by methane fermentation.
The mass balance of dissolution was calculated according to the formulae:
where:
ms—soluble sugars in substrate [mg],
mr—soluble sugars in solution [mg],
me—total mass of soluble sugars in solution after time of ∞ = total mass of soluble sugars in the substrate at the initial time (t0).
Change of mass of soluble sugars in substrate:
where:
k—reaction rate constant
Mass of soluble sugars in the substrate as a function of time:
Substitution of ms (3) to (1) yields:
Dividing both sides of (5) by the solution volume yields:
where:
C—concentration of soluble sugars in the solution (mg/L),
Ce—concentration of saturation after the time of ∞ (mg/L).
The coefficients Ce and the constant k were estimated in the Statistica 10.0 software, taking into account the concentration of soluble sugars released to the solution as a function of the duration and temperature of the maize silage pre-treatment process.
2.4. Anaerobic Digestion
The effectiveness of the hydrothermal depolymerization was analyzed indirectly by respirometric measurements of the decomposition of the biomass digested by mesophilic (36 °C) methane fermentation. After thermohydrolysis, the substrate was transferred to OxiTop control-recording units manufactured by WTW (London, UK), which consisted of reaction chambers connected tightly with measuring and recording devices. The reaction chambers were inoculated with an anaerobic sludge from an agricultural biogas plant fed with maize silage. Raw material was added to 100 cm3 of the sludge until the initial load reached 5.0 kg VS/m3; subsequently, the samples were blown through with nitrogen to ensure anaerobic conditions. The hold time was 40 days. The measurement units analyzed changes in partial pressure in the chamber caused by biogas production in anaerobic processes induced by microorganisms. The measurements were made at 36 ± 0.5 °C, and the pressure was measured in the reaction chamber every 90 min.
The biogas composition, particularly the methane concentration, was analyzed by collecting samples from the reactors and examining them with a 7890A gas chromatograph equipped with a thermal conductivity detector (TCD) from AGILENT TECHNOLOGIES (Santa Clara, CA, USA).
2.5. Analysis of Phenolic and Furan Compounds
Once the shortest disintegration time was determined, inhibitor analysis was carried out. Phenolic compounds were determined by cuvette tests according to Hach (LCK 345). Furan compounds were analyzed by the modified phenol–sulfur method according to the Rao and Pattabiraman procedure [23].
2.6. Statistical Analyses
Each experimental option (time, temperature, heating method) was tested in 10 replicates. The Shapiro–Wilk test was used to test the hypothesis of the normality of the distribution of the variables. To test the significance of the differences between the variables (time, temperature, heating method), a one-way analysis of variance (ANOVA) was performed. The significance of differences between the values obtained for the variables studied was determined using Tukey’s RIR test. The tests assumed a significance level of p = 0.05. In addition, a Pearson correlation analysis between the amount of inhibitors formed (furfural, 5-HMF and phenolic compounds) was carried out to determine the relationship between their concentration and biogas production efficiency.
3. Results and Discussion
3.1. Efficiency of Releasing Soluble Sugars and the Mass Balance of Dissolution
The first temperature option put to the test, 100 °C, did not result in releasing soluble sugars to the solution regardless of the duration of pre-treatment, either during conventional or microwave heating (Figure 1). Extending the duration of treatment in consecutive temperature options (130, 150 and 180 °C) resulted in an increase in the amount of soluble sugars released to the solution. The application of microwave heating in each of the three options under study resulted in releasing a larger amount of sugars than hydrothermal treatment under conventional conditions. The differences between the methods of heating applied were significant in all of the tested options at a level of significance of α = 0.05.
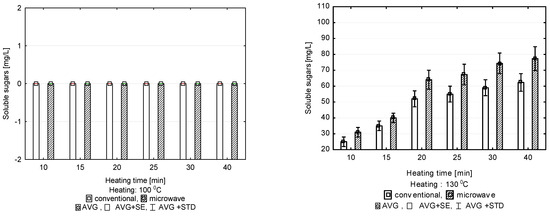
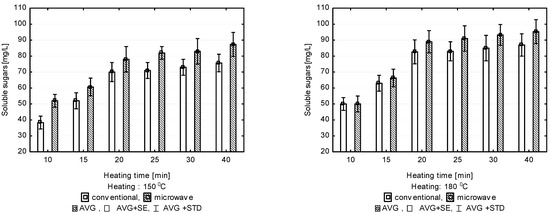
Figure 1.
Concentration of soluble sugars released after pre-treatment at 100, 130, 150 and 180 °C, depending on heating time.
The results indicate an increase in the efficiency of the hydrothermal treatment by raising the process temperature from 100 °C to 150 °C. A further increase in the temperature, up to 180 °C, did not bring about a statistically significant increase in the amount of released soluble sugars. When the temperature of depolymerization was raised from 100 °C to 130 °C, the amount of released soluble sugars was found to increase significantly.
The greatest effect of the method of heating on the effectiveness of hydrolysis at 130 °C was observed when pre-treatment lasted for 20 min. Microwave heating caused 23.1% more soluble sugars to be released to the solution compared to conventional heating. A statistical analysis showed that regardless of the method of heating, extending the time of hydrothermal treatment to 20 min improved the process efficiency significantly. Extending the duration of hydrolysis further did not bring about a significant increase in the amount of soluble sugars released to the solution. When microwave heating lasted for 15 min at 130 °C (Figure 1), the soluble sugar concentration in the solution was 40 ± 2.9 mg/L, whereas 64.3 ± 5.1 mg/L was achieved after 20 min. Extending the duration of heating to 25 min, and further to 30 min, did not result in a statistical increase in the amount of soluble sugars released to the solution.
Raising the hydrolysis temperature to 150 °C resulted in a significant increase in the amount of soluble sugars released to the solution (Figure 1). Applying microwave heating resulted in a larger soluble sugar output in the process of hydrolysis compared to conventional heating. A particularly significant effect of the method of heating was observed when the duration of hydrolysis was shorter (i.e., 10, 15, 20 min). The greatest difference was observed when the heating lasted for 10 min. With microwave heating, 52 mg/L of soluble sugars was released to the solution, and only 39 mg/L when heating was conventional. A statistically significant increase in efficiency with increasing process duration was observed within the range between 10 and 20 min. The increase in the amount of soluble sugars released with longer durations (i.e., 25, 30 and 40 min) was not statistically significant. The amount of soluble sugars released to the solution in the option with microwave heating increased from 78.0 ± 7 mg/L obtained over 20 min to 87.0 ± 7 mg/L recorded after treatment lasting 40 min. Conventional heating resulted in releasing soluble sugars in a mean amount of 70.0 ± 6 mg/L following treatment lasting 20 min; 76.0 ± 7 mg/L of soluble sugars was found in the solution after the time of hydrolysis was extended to 40 min (Figure 1).
Raising the temperature to 180 °C did not bring about a statistically significant increase in the amount of released soluble sugars. The mean concentrations of soluble sugars in the solution at 180 °C (50.0 ± 4 mg/L) were close to those obtained at 150 °C (52 ± 4 mg/L), especially under microwave heating and short process durations (10 min). However, the values were not statistically significant. A similar effect was achieved when conventional heating was applied. Such heating at 180 °C did not result in a significant increase in the amount of soluble sugars in the solution. As at lower temperatures, a positive effect of the hydrothermal digestion duration on the amount of soluble sugars present in the solution was observed only for the first 20 min; continuing the process did not have any statistical effect on the content of soluble sugars in the solution regardless of the heating method (Figure 1).
The conditions of thermohydrolysis for microwave and conventional heating were determined on the basis of regression equations for the rate of cellulose digestion, measured as an increase in soluble sugar concentration in the solution. It was assumed that the process could be interpreted as dissolving soluble sugars released from the hydrolyzed biomass. It was assumed that the soluble sugars in the solution following disintegration came from the cellulose in the plant material.
The highest reaction rate, k, for the microwave heating options was achieved for the temperature of 150 °C. The highest k value in the conventional heating options was recorded at 180 °C, but the value achieved in this process was not statistically different (at the level of significance of 0.05) from the k value obtained for 150 °C (Table 2).

Table 2.
Coefficients r and k, depending on the temperature and method of heating used in the pre-treatment of maize silage.
3.2. Biogas Production
Conventional heating of the substrate for 10 min. at 180 °C resulted in a biogas output of 902 ± 16 NmL/gVS, which is 15.5% more than in the control sample. This was the largest amount of biogas recorded for the batch with conventional heating for 10 min. However, the difference between the highest temperature used in the experiment and a temperature lower by 30 °C was not statistically significant. Biogas in the amount of 882 ± 17 NmL/gVS was obtained from the substrate digested at 150 °C. Microwave heating of the substrate for the same time of 10 min resulted in 6% more biogas output at 150 °C and 7% more at 180 °C compared to conventional heating (Figure 2). The same relationship was observed for 15 min of depolymerization between the process temperature and the biogas output from converted biomass. The greatest statistically significant difference in the biogas output was recorded when the pre-treatment temperature increased from 130 °C to 150 °C; the difference was 13% for conventional heating and 18% for microwave heating. Heating at 180 °C did not increase the biogas output compared to the amount achieved at 150 °C. Extending the time of pre-treatment by another 5 min resulted in larger biogas output in each heating method. When conventional heating was applied, the biogas output increased from 938 ± 28 NmL/gVS at 130 °C to 1024 ± 25 NmL/gVS at 150 °C. With microwave heating of the substrate, the output of biogas grew to a similar extent, from 1022 ± 59 NmL/gVS to 1184 ± 18 NmL/gVS. This means that microwave heating of the substrate for 20 min at 150 °C produced 16% more biogas than conventional heating. Statistically significant differences between the amount of biogas produced from substrates converted in the two heating options were observed at temperatures of 130 °C, 150 °C and 180 °C. An analysis of the 25 min pre-treatment option showed that the biogas output in respirometric tests with conventional heating was lower by ca. 2% than with a 20-min treatment, which was not a statistically significant difference. Likewise, the output of biogas with microwave heating lasting for 25 min was not significantly different from that obtained when the treatment lasted for 20 min. The differences between the biogas output in these time options were ±2%. Extending the pre-treatment duration by another 5 and 15 min also did not increase the biogas output significantly. However, considerable differences were observed depending on the method of heating the substrate.
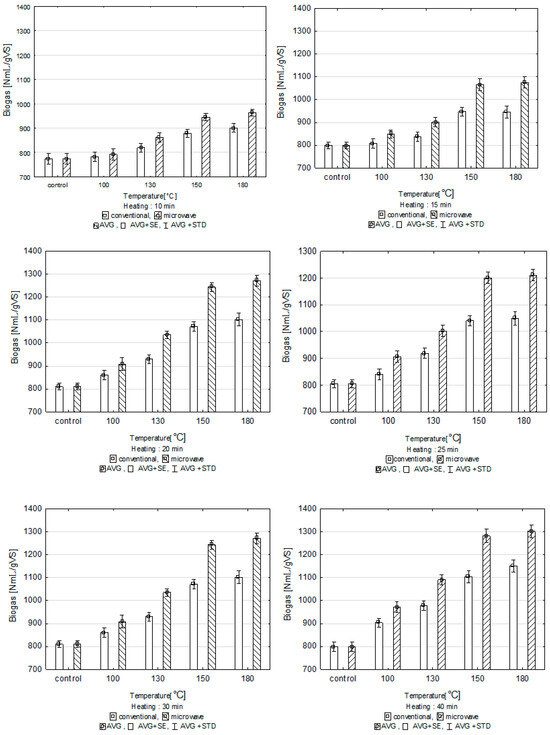
Figure 2.
Biogas production after pre-treatment for 10, 15, 20, 25, 30 and 40 min, depending on process temperature.
The literature provides examples of the application of microwaves as a conditioning factor in methane fermentation. Substrate exposure to microwave radiation increases the solubilization of substrates and breaks the network of exopolysaccharides, speeding up the process of methane fermentation [8,24,25].
Microwave heating of the substrate resulted in greater biogas output than conventional heating in all the thermal pre-treatment options tested. Microwave radiation can change the ultrastructure of cellulose and bring about the degradation of lignin and hemicellulose in the lignocellulosic complex, thereby increasing its susceptibility to biodegradation. Microwave pre-treatment in the presence of water has been found to increase the productivity of hydrolysis of lignocellulosic structures [26]. Jackowiak et al. (2011) focused on microwave optimization of methane fermentation of wheat straw. Four temperature options were tested in the experiment: 100 °C, 120 °C, 150 °C and 180 °C. The biogas output increased considerably following the microwave treatment; the methane output increased by 28% for the most effective temperature option of 150 °C. Similarly, this study found that 150 °C is the optimum thermohydrolysis temperature for microwave heating. A 15% increase in biogas output from the substrate was achieved after microwave conditioning compared to conventional heating and a 50% increase compared to the control sample. It is not justified to apply higher temperatures, as biomass susceptibility to anaerobic decomposition does not increase [27]. Sapci (2013) studied the production of biogas from barley, spring wheat, winter wheat and oat straw using microwave radiation at temperatures of 200 °C and 300 °C. The results of the experiments did not show any positive effect of microwave radiation on the fermentation productivity [28]. Saelor et al. (2024) compared different pre-treatment methods, including microwave, chemical, thermal and mechanical treatment, in the context of methane production from lignocellulosic waste (palm stumps). The authors indicated that chemical treatment with acids (sulfuric acid) was the most effective in increasing methane production, however, microwave processing has also shown significant potential in this area. Chemical treatment was the most effective, reaching 395 mL/g, which was the highest methane production value compared to other methods. Microwave treatment also showed high efficiency, reaching 311 mL/g VS. Thermal treatment led to methane production of 226 mL/g VS. Mechanical treatment had the least impact, reaching 193 mL/g vs. [29].
Low-temperature (<200 °C) microwave treatment can increase the energy value of biomass. The process productivity in microwave treatment depends largely on the type of material converted, its physical parameters, structure, conductivity and dielectric properties. In conventional thermal treatment, heat is supplied by convection and conduction; when microwave radiation is applied, energy is supplied directly to the material being heated. The use of microwaves in processes has significant benefits, not least due to the ability of the waves to penetrate the material, where the energy accumulates and generates heat throughout the sample volume. The use of this method reduces heating time, allows for better process control and increases energy efficiency. As shown by Budarin et al. (2009), microwaves interact strongly with cellulose molecules, which significantly accelerates their decomposition. These interactions, occurring at lower temperatures, lead to products that, with conventional heating, require temperatures above 200 °C [30].
3.3. Production of Phenolic and Furanic Compounds
The amount of furanic and phenolic compounds formed during the thermohydrolysis was determined for the process duration of 20 min, for all of the tested temperature options and for both heating methods, and it was compared to the results for the control sample (Table 3).

Table 3.
The amount of furanic and phenolic compounds produced over 20 min of thermohydrolysis.
A total of 20 mg/L of phenolic compounds were found in the control sample, which was not subjected to thermohydrolysis. The concentration of the compounds after thermohydrolysis increased in each of the temperature options tested. A significantly lower concentration of phenolic compounds was observed in samples heated in a conventional manner. More of such compounds were produced when microwave heating was applied, reaching 28 mg/L at 180 °C. The same dependence was observed for furanic compounds; the concentration of the compounds after thermohydrolysis was higher at 150 °C and 180 °C (Table 3). The experiments proved that the amount of intermediate products did not inhibit the process of methane fermentation. Low-temperature microwave pre-treatment, carried out at 180 °C, resulted in the production of 40% more phenolic compounds than in the control sample without hydrothermal treatment and 25% more than in a conventionally heated sample. Increased production of furan compounds was noted along with the increasing process temperature; the largest quantities were recorded at a temperature of 180 °C with microwave pre-treatment; for furfural, it was 38 mg/L with microwave pre-treatment, and for 5-HMF, 30 mg/L. The inhibiting compounds in the amount produced in the process did not affect the effectiveness of methane fermentation.
In order to investigate the potential effect of furfural, 5-HMF and phenolic compound concentrations on biogas production, a Pearson correlation analysis was conducted between the two variables (Table 4). The analysis resulted in a correlation coefficient close to 1, indicating a very strong positive relationship between the amount of furfural, 5-HMF, phenols and biogas production.

Table 4.
Correlation relationships between the amounts of biogas and inhibitors produced.
Importantly, despite an increase in the concentration of the test substances in the samples, biogas production also increased. This result suggests that the concentration of inhibitors in the samples did not have a negative impact on the biogas production process. This means that in the analyzed range, inhibitor concentration did not inhibit the activity of the microorganisms responsible for methane fermentation. This result may suggest that the inhibitor levels in the samples did not exceed a threshold that could have a toxic effect on the microorganisms. Conclusions from the Pearson correlation analysis indicate that there was no direct inhibitory effect of furfural, 5-HMF and phenols on biogas production, which is an important result in the context of assessing the effectiveness of microwave pre-treatment. These results confirm that increasing the concentration of inhibitors did not negatively affect the efficiency of the methane fermentation process.
Pre-treatment of lignocellulose can lead to the formation of fermentation inhibitors such as furan derivatives and phenolic compounds [31]. Furan derivatives such as furfural and 5-hydroxymethylfurfural (5-HMF) are formed by the dehydration of pentoses and hexoses, which are released from cellulose and hemicellulose. A wide range of phenolic compounds (such as vanillin, coniferyl aldehyde and 4-hydroxy-benzoic acid) are generated during lignin decomposition [19]. The formation of furan and phenol derivatives is observed during thermal and thermochemical treatment of biomass; the amount of furfural formed in the process may vary depending on the type of biomass and the method of conditioning applied, from 0.08 g/L in the treatment of rice husks at 25 °C in the presence of hydrogen peroxide [32] to 1.332 mg/L in the pre-conditioning of macroalgae Gelidium amansii of the red algae family [33]. The largest amount of 5-HMF (4.3 mg/L) was observed by Yun et al. (2013) in microalgae Chlorella vulgaris treated with hydrochloric acid [34]. Rios-Gonzalez et.al. analyzed the effects of different factors on ethanol yields from agave pomace. In the study, a high-pressure reactor was used to carry out an autohydrolysis process under different conditions, and the marc was then subjected to enzymatic hydrolysis and fermentation. The authors investigated, among other things, the amounts of 5-HMF and furfural in samples dissolved at temperatures from 140 °C to 200 °C; no 5-HMF was detected in any of the samples, and trace amounts of furfural were detected at 180 °C and 200 °C [35].
Both phenolic compounds and furfural derivatives can negatively affect the methanogenesis process [36]. Furfural and 5-HMF, which belong to the group of furanic compounds, restrict microbial growth, cause damage to genetic material and inhibit enzymes responsible for glycolysis. Phenolic compounds, on the other hand, destabilize microbial cell membranes, leading to the loss of intracellular components and the inactivation of key enzyme systems. Furanic compounds (i.e., furfural and 5-HMF) are known to have detrimental effects on microorganisms by inhibiting cell growth, inducing DNA damage and inhibiting several enzymes of the glycolysis pathway. Phenolic compounds damage microbial cells by selectively altering the membrane permeability, causing leakage of intracellular components and inactivation of essential enzymatic systems [37,38]. Zupančič et al. studied the effect of polyphenolic compounds on the anaerobic digestion process of pepper processing waste. They proved that the obtention of polyphenols (maximum concentration 364.77 mg/L minimum 48.62 mg/L) did not adversely affect the biogas production efficiency [39]. Other studies have shown that polyphenols reduce the ability of methanogens to produce methane; at concentrations of phenolic compounds in the range of 120 to 594 mg/L, biogas production decreased by up to 50%. However, polyphenol content in the range of 2.1–21.4 g/kg vs. had no effect on the biogas potential of grape pomace methane. This indicates that anaerobic microorganisms show a certain degree of tolerance to phenols and that low concentrations of phenols may even favor anaerobic fermentation [40].
3.4. Energy Balance
Comparisons of energy efficiency between conditioning using microwave heating and conventional heating were carried out for a heating time of 20 min (Table 5). The energy inputs necessary to heat 1 kg of substrate were calculated, taking into account the efficiency of microwave heating of 50% and conventional heating of 90%. Then, the increases in the energy value of biogas obtained from 1 kg of dry organic mass of substrate were determined in relation to the control sample. In the final energy balance, the differences between the increase in the amount of energy (contained in methane) and the theoretical minimum inputs in heating the biomass were taken into account.

Table 5.
Energy balance of the methane fermentation process using microwave and conventional conditioning.
Microwave heating, despite lower efficiency compared to conventional heating, led to a greater increase in the production of energy contained in the biogas. The greatest increase in energy production after microwave conditioning was recorded at 150 °C (426 Wh/kg), which was 158 Wh/kg more than after conventional heating. The difference between usable energy and energy input showed that microwave conditioning of biomass becomes cost-effective from 130 °C onwards, where the energy gain was 18.2 Wh/kg. The most favorable energy balance for microwave heating was recorded at 150 °C (170.8 Wh/kg), indicating that this is the optimum temperature for the process. The most favorable energy balance for microwave heating was recorded at 150 °C (170.8 Wh/kg), indicating that this is the optimum temperature for this process. The results suggest that microwave conditioning may be an effective way to increase the methane fermentation yield, especially at temperatures above 130 °C.
4. Conclusions
The results showed that microwave heating applied to the hydrothermal disintegration of lignocellulosic biomass resulted in a higher release efficiency of soluble sugars compared to conventional heating, reaching a maximum increase of 14% at 180 °C in 30 min. The optimal pre-treatment conditions, determined by the mass balance of soluble sugars and respiration measurements, were 150 °C and 20 min for both heating methods. Microwave heating increased biogas production by 15% relative to the conventionally heated sample and by as much as 50% compared to biomass not subjected to hydrothermal depolymerization. Analysis of the results also showed that microwave heating at 150 °C for 20 min yielded 1184 ± 18 NmL/gVS of biogas, an increase of 16% compared to the 1024 ± 25 NmL/gVS obtained during conventional heating. Statistically significant differences between the two heating methods were observed at 130 °C, 150 °C and 180 °C, with the clearest difference between 130 °C and 150 °C: 13% for conventional heating and 18% for microwave heating, respectively. Importantly, although increasing the temperature to 180 °C did not result in a further statistically significant increase in biogas production, the energy balance analysis showed that microwave heating resulted in higher net energy gains. The most favorable energy balance was recorded at 150 °C, where the net gain was 170.8 Wh/kg, compared to 126.2 Wh/kg for conventional heating. Microwave conditioning of biomass became energetically viable from 130 °C onwards, achieving an energy surplus of 18.2 Wh/kg, with a maximum energy yield of 426 Wh/kg–158 Wh/kg more than conventional heating.
In summary, the results confirm that microwave thermohydrolysis, especially at 150 °C for 20 min, increases both biogas production and energy efficiency compared to traditional methods, making it a promising strategy for pretreating lignocellulosic biomass, especially at temperatures above 130 °C. Furthermore, the results obtained may suggest the existence of athermal effects associated with the use of microwaves.
Author Contributions
Conceptualization, A.N. and M.Z.; methodology, A.N. and M.Z.; validation, A.N., M.M., I.B. and M.Z.; formal analysis, A.N., M.D. (Magda Dudek) and M.D. (Marcin Dębowski); investigation, and M.Z.; writing—original draft preparation, A.N. and M.Z.; writing—review and editing, A.N. and M.Z.; visualization, A.N.; supervision, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The project was financed by the National Center for Sciences from funds granted under decision no. DEC-2012/05/N/ST8/02667 and a Faculty Grant from the Faculty of Environmental Sciences of the University of Warmia and Mazury entitled: “Effect of furan derivatives and phenolic compounds on inhibition of methane fermentation of lignocellulosic biomass”.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jaffur, N.; Jeetah, P.; Kumar, G. A Review on Enzymes and Pathways for Manufacturing Polyhydroxybutyrate from Lignocellulosic Materials. 3 Biotech 2021, 11, 483. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Mamimin, C.; O-Thong, S.; Reungsang, A. Enhancing Biogas Production from Hemp Biomass Residue through Hydrothermal Pretreatment and Co-Digestion with Cow Manure: Insights into Methane Yield, Microbial Communities, and Metabolic Pathways. J. Environ. Manag. 2024, 370, 123039. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Liyanage, S.; Abidi, N.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, R.; Barrio, A.; Tamminen, T. Determination of Hemicellulose, Cellulose, and Lignin Content in Different Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model (TGA-PKM Method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, K.; Zhang, Y.; Wang, M.; Yong, C.; Chen, L.; Qu, P.; Huang, H.; Sun, E.; Pan, M. Lignocellulose Dissociation with Biological Pretreatment towards the Biochemical Platform: A Review. Mater. Today Bio 2022, 16, 100445. [Google Scholar] [CrossRef]
- Martínez-Gutiérrez, E. Biogas Production from Different Lignocellulosic Biomass Sources: Advances and Perspectives. 3 Biotech 2018, 8, 233. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current Understanding of the Correlation of Lignin Structure with Biomass Recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef]
- Martín, C.; Dixit, P.; Momayez, F.; Jönsson, L.J. Hydrothermal Pretreatment of Lignocellulosic Feedstocks to Facilitate Biochemical Conversion. Front. Bioeng. Biotechnol. 2022, 10, 846592. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, Y.; Kwak, S.Y. Comparing the Influence of Acetate and Chloride Anions on the Structure of Ionic Liquid Pretreated Lignocellulosic Biomass. Biomass Bioenergy 2016, 93, 243–253. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic Digestion of Lignocellulosic Biomass: Substrate Characteristics (Challenge) and Innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production from Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 472405. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.R.; Sugitha, S.; Kavitha, S.; Kannah, R.Y.; Merrylin, J.; Kumar, G. Lignocellulosic Biomass Pretreatment for Enhanced Bioenergy Recovery: Effect of Lignocelluloses Recalcitrance and Enhancement Strategies. Front. Energy Res. 2021, 9, 646057. [Google Scholar] [CrossRef]
- Su, C.; Wang, X.; Deng, Y.; Tian, Z.; Huang, C.; Fang, G. Comprehensive Insights of Pretreatment Strategies on the Structures and Bioactivities Variation of Lignin-Carbohydrate Complexes. Front. Bioeng. Biotechnol. 2024, 12, 1465328. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Ballesteros, M. Steam Explosion as Lignocellulosic Biomass Pretreatment. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 349–368. [Google Scholar] [CrossRef]
- Mahmud, N.; Rosentrater, K.A. Low Moisture Anhydrous Ammonia Pretreatment of Four Lignocellulosic Materials—Distillers Dried Grains with Solubles, Corn Gluten Feed, Corn Fiber, and Oil Palm Frond. Front. Energy Res. 2021, 9, 682522. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam Explosion Pretreatment of Lignocellulosic Biomass: A Mini-Review of Theorical and Experimental Approaches. Front. Chem. 2021, 9, 705358. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Nowicka, A.; Kazimierowicz, J. Influence of Microwave-Assisted Chemical Thermohydrolysis of Lignocellulosic Waste Biomass on Anaerobic Digestion Efficiency. Energies 2024, 17, 4207. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Liu, Z.; Prasetyatama, Y.D.; Oh, W.K.; Yu, I.K.M. Microwave-Assisted Biorefineries. Nat. Rev. Clean. Technol. 2025, 1–19. [Google Scholar] [CrossRef]
- Syed, N.R.; Zhang, B.; Mwenya, S.; Aldeen, A.S. A Systematic Review on Biomass Treatment Using Microwave-Assisted Pyrolysis under PRISMA Guidelines. Molecules 2023, 28, 5551. [Google Scholar] [CrossRef]
- Rao, P.; Pattabiraman, T.N. Reevaluation of the Phenol-Sulfuric Acid Reaction for the Estimation of Hexoses and Pentoses. Anal. Biochem. 1989, 181, 18–22. [Google Scholar] [CrossRef]
- Appels, L.; Houtmeyers, S.; Degrève, J.; Van Impe, J.; Dewil, R. Influence of Microwave Pre-Treatment on Sludge Solubilization and Pilot Scale Semi-Continuous Anaerobic Digestion. Bioresour. Technol. 2013, 128, 598–603. [Google Scholar] [CrossRef]
- Mitraka, G.C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Zouboulis, A.I.; Kougias, P.G. A Comprehensive Review on Pretreatment Methods for Enhanced Biogas Production from Sewage Sludge. Energies 2022, 15, 6536. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of Biogas Yield from Lignocellulosic Materials with Different Pretreatment Methods: A Review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef]
- Jackowiak, D.; Bassard, D.; Pauss, A.; Ribeiro, T. Optimisation of a Microwave Pretreatment of Wheat Straw for Methane Production. Bioresour. Technol. 2011, 102, 6750–6756. [Google Scholar] [CrossRef] [PubMed]
- Sapci, Z. The Effect of Microwave Pretreatment on Biogas Production from Agricultural Straws. Bioresour. Technol. 2013, 128, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Saelor, S.; Kongjan, P.; Prasertsan, P.; Mamimin, C.; O-Thong, S. Enhancing Thermophilic Methane Production from Oil Palm Empty Fruit Bunches through Various Pretreatment Methods: A Comparative Study. Heliyon 2024, 10, e39668. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Lanigan, B.A.; Shuttleworth, P.; Breeden, S.W.; Wilson, A.J.; Macquarrie, D.J.; Milkowski, K.; Jones, J.; Bridgeman, T.; et al. The Preparation of High-Grade Bio-Oils through the Controlled, Low Temperature Microwave Activation of Wheat Straw. Bioresour. Technol. 2009, 100, 6064–6068. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.S.; Lee, D.J. Inhibitor Formation and Detoxification during Lignocellulose Biorefinery: A Review. Bioresour. Technol. 2022, 361, 127666. [Google Scholar] [CrossRef]
- Banerjee, S.; Sen, R.; Mudliar, S.; Pandey, R.A.; Chakrabarti, T.; Satpute, D. Alkaline Peroxide Assisted Wet Air Oxidation Pretreatment Approach to Enhance Enzymatic Convertibility of Rice Husk. Biotechnol. Prog. 2011, 27, 691–697. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, J.J.; Park, H.D.; Kim, Y.J.; Lim, D.J.; Kim, S.H. Feasibility of Biohydrogen Production from Gelidium amansii. Int. J. Hydrogen Energy 2011, 36, 13997–14003. [Google Scholar] [CrossRef]
- Yun, Y.M.; Jung, K.W.; Kim, D.H.; Oh, Y.K.; Cho, S.K.; Shin, H.S. Optimization of Dark Fermentative H2 Production from Microalgal Biomass by Combined (Acid+ultrasonic) Pretreatment. Bioresour. Technol. 2013, 141, 220–226. [Google Scholar] [CrossRef]
- Ríos-González, L.J.; Medina-Morales, M.A.; Rodríguez-De la Garza, J.A.; Romero-Galarza, A.; Medina, D.D.; Morales-Martínez, T.K. Comparison of Dilute Acid Pretreatment of Agave Assisted by Microwave versus Ultrasound to Enhance Enzymatic Hydrolysis. Bioresour. Technol. 2021, 319, 124099. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of Lignocellulosic Biomass for Enhanced Biogas Production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Arteaga, J.E.; Rivera-Becerril, E.; Le Borgne, S.; Sigala, J.C. Influence of Furfural on the Physiology of Acinetobacter Baylyi ADP1. FEMS Microbiol. Lett. 2024, 371, 59. [Google Scholar] [CrossRef]
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M. Progress in the Production of Biogas from Maize Silage after Acid-Heat Pretreatment. Energies 2021, 14, 8018. [Google Scholar] [CrossRef]
- Zupančič, G.D.; Lončar, A.; Ranilović, J.; Šubarić, D.; Panjičko, M. The Influence of Polyphenolic Compounds on Anaerobic Digestion of Pepper Processing Waste during Biogas and Biomethane Production. Processes 2024, 12, 913. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielinska, M. Individual Phenolic Acids in Distillery Stillage Inhibit Its Biomethanization. Energies 2022, 15, 5377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).