Abstract
The concurrent preservation of structural integrity and improvement of electrical conductivity in FeP2 anodes presents a persistent challenge. Herein, FeP2 nanoparticles embedded within a 3D N-doped honeycomb-like carbon framework composite (FeP2@NHC) are synthesized through a phosphorization process with a honeycomb-like Fe3C@NHC as a precursor. The in situ incorporation of FeP2 nanoparticles into the 3D carbon matrix effectively restrains the aggregation, pulverization, and stripping of material during cycling, and significantly enhances reaction kinetics and structural stability, achieving a superior electrochemical performance. Specifically, FeP2@NHC electrodes demonstrate remarkable reversible capacity (1433.9 mA h g−1 at 0.1 A g−1), excellent rate-capability (399.9 mA h g−1 at 10 A g−1), and ultra-long cycle life (631.5 mA h g−1 after 1000 cycles at 2 A g−1). Moreover, XRD analysis reveals that iron-rich Fe3C and Fe3O4 precursors can react with NaH2PO2 to form FeP2 and FeP, respectively. This study offers a rational and practical strategy for designing other phosphorus-rich metal phosphide anode materials.
1. Introduction
Over the past three decades, to address the escalating energy demands of electric vehicles and large-scale energy storage systems, lithium-ion batteries (LIBs) have garnered significant attention [1,2,3]. The anode material plays a crucial role in achieving a fast charging capability for LIBs, meanwhile maintaining high energy density and cycling stability [4]. Recent research has focused on conversion reaction-based transition metal binary compounds (MaXb) resting with their high theoretical capacity and versatile compositional options. However, previous studies predominantly focused on metal oxides [5,6], sulfides [7,8], and selenides [9,10]. Though these materials exhibit impressive reversible capacities, their main capacity-contributing redox reactions typically occur at relatively high oxidation potentials (above 1.5 V vs. Li+/Li), leading to lower voltage outputs and energy densities [11]. In contrast, transition metal phosphides (TMPs), such as CuP2 [12], Cu3P [13], CoP [14], CoP2 [15], Ni2P [16], FeP [17], and FeP2 [18], demonstrate higher specific capacities and lower redox potentials during extended charge–discharge cycles, making them more suitable as anode materials.
Notably, iron is the most abundant transition metal element, and phosphorus reserves are plentiful and readily accessible. Consequently, Fe-P based materials are highly promising candidates for sustainable LIB anodes, with their environmental friendliness, abundant natural resources, and low cost [19]. Iron phosphides encompass various compositions, including Fe4P, Fe3P, Fe2P, FeP, FeP2, and FeP4. The theoretical specific capacity increases with a higher phosphorus content, albeit at the expense of reduced electrical conductivity [20,21]. Although phosphorus-rich FeP2 boasts a notably high theoretical capacity (1365 mA h g−1) and a redox potential of approximately 1 V, its practical implementation is hindered by significant challenges, including irreversible volume changes exceeding 300% and sluggish reaction kinetics, leading to rapid capacity fading [22]. To address these issues, researchers commonly employ a strategy combining nanostructuring with highly conductive carbonaceous materials [23,24,25,26,27]. Nanostructuring enhances complete utilization of active materials in electrochemical reactions and shortens the diffusion distance of Li+ from the surface to the core, thereby improving reaction efficiency [28]. Carbonaceous materials such as carbon nanotubes [29], carbon nanofibers [19], graphene [30], porous carbon [20], and hollow carbon shells [18] can mitigate excessive volume expansion during lithiation and enhance the conductivity of the anode material, and their porous structures facilitate increased Li+ diffusion rates.
The synthesis techniques for TMP anodes primarily encompass high-energy ball milling, temperature-programmed reduction, organometallic synthesis, and solvothermal/hydrothermal methods [31,32,33]. However, TMPs synthesized via ball milling exhibit challenges in morphology control, leading to suboptimal rate performance and long-term stability. Though organometallic synthesis offers precise control over the phase, size, and morphology of TMPs, its multi-step reaction process presents scalability challenges for industrial production. Moreover, nanoparticles produced through solvothermal/hydrothermal routes often suffer from issues of insolubility and aggregation [32]. Notably, the NaH2PO2 reduction method enables the rapid chemical conversion of precursors into TMPs within 300–400 °C while maintaining the structural integrity of the original nanostructures. Although this phosphorization approach has been extensively employed for FeP synthesis, its application in fabricating FeP2 compounds remains unreported in the existing literature [23,34]. Consequently, the rational design of high-performance FeP2 hybrid materials remains a significant challenge.
Herein, we report for the first time in situ embedding of FeP2 nanoparticles within a 3D N-doped honeycomb-like carbon matrix (FeP2@NHC) using sol–gel pyrolysis blowing and the NaH2PO2 phosphorization method. This unique structure offers several advantages. (a) The well-dispersed FeP2 nanoparticles can substantially mitigate the risk of fracture during repeated lithiation/de-lithiation cycles by isotropically distributing stress [35]. (b) The 3D porous carbon matrix provides ample space to accommodate volume expansion and ensures a stable pathway for rapid ion/electron transport. (c) The in situ incorporation of FeP2 nanoparticles within the porous carbon framework prevents the agglomeration and pulverization of active particles, achieving efficient lithium storage. As expected, FeP2@NHC demonstrates outstanding cycling stability and excellent rate capability. Furthermore, the formation mechanisms of FeP and FeP2 are revealed by XRD and XPS analysis.
2. Materials and Methods
2.1. Synthesis of FeP2@NHC Nanocomposite
First, 0.5 g Fe (NO3)3·9H2O (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and 0.5 g polyvinylpyrrolidone (PVP K130, Aladdin, Shanghai, China) were mixed with 50 mL of deionized water to form a homogeneous solution [20]. Then, continuous heating and stirring were carried out until a gel state was achieved. Subsequently, the gel was subjected to heat treatment in Ar at 700 °C for a duration of 3 h to yield the Fe3C@NHC precursor. Finally, the Fe3C@NHC precursor and Na2H2PO2·H2O (Macklin, Shanghai, China) were placed in two porcelain boats with a mass ratio of 1:30 and positioned separately at downstream and upstream locations within a tube furnace. This phosphorization process was carried out at 360 °C for 0.5 h with a heating rate of 4 °C min−1 under Ar atmosphere. The cooled product was named FeP2@NHC.
2.2. Synthesis of FeP-FeP2@C and Pure FeP
The synthesis of FeP-FeP2@C was carried out by replacing PVP K130 with glucose, while all other procedures remained identical to those for the synthesis of FeP2@NHC. The synthesis of pure FeP followed similar procedures to those of FeP-FeP2@C, with the exception that no glucose was added.
2.3. Materials Characterization
The morphology and architecture of the samples were investigated through scanning electron microscopy (SEM, TESCAN) and transmission electron microscopy (TEM, JEM F200, JEOL, Tokyo, Japan). The crystal structures and phases were collected by X-ray powder diffraction (XRD, Smartlab SE, Rigaku, Tokyo, Japan). The chemical states were performed by thermogravimetric analysis (TGA, STA 300, Netzsch, Selb, Germany) and Raman spectroscopy (DXR3, Thermo Fisher Scientific, Waltham, MA, USA). The electronic states of elements in the surface were analyzed by X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Fisher Scientific, Waltham, MA, USA). The N2 adsorption/desorption isotherm was obtained at 77.3 K with a specific surface area analyzer from a Brunauer–Emmett–Teller test (BET, Quadrasorb SI-3MP, Quantachrome, Boynton, FL, USA), while the Barrett–Joyner–Halenda (BJH) method was employed to determine the distributions of pore sizes.
2.4. Electrochemical Measurements
The working electrodes were made by coating a slurry consisting of as-prepared materials, polyvinylidene fluoride (PVDF), acetylene black (mass ratio: 8:1:1), and N-methyl-2-pyrrolidone (NMP) on copper foils and then dried at 80 °C overnight. The composite material loading in each electrode was around 1.0 ± 0.2 mg cm−2. Li-storage measurements were carried out in stainless-steel coin cells (CR2032) assembled in an argon-filled glovebox, with Li foils as counter electrodes, polypropylene membrane (Celgard 2500) as a separator, and 1.0 M LiPF6 dissolved in DMC, EC, and EMC (1:1:1 vol.%), with the addition of 5 vol.% FEC as an electrolyte. The as-prepared materials were characterized using the galvanostatic charge/discharge (GCD) method within 0.01–3 V (vs Li/Li+) on the Land CT2001A. In our work, the total mass of the composite material was utilized in the calculation of specific capacity. The cyclic voltammetry (CV) and electrochemical impedance spectrum (EIS) in the frequency of 100 kHz to 0.01 Hz were measured on an electrochemical workstation (CHI 760E).
3. Results
The synthesis strategy of FeP2@NHC, as illustrated in Figure 1, involved three sequential steps. Firstly, the aqueous solution of Fe (NO3)3·9H2O and PVP K130 was continuously heated and stirred to form a gel. Secondly, the gel underwent a high-temperature heat treatment process to obtain the honeycomb-like 3D porous Fe3C@NHC precursor. PVP played an essential role in the formation of this hierarchical structure. This was due to a substantial amount of gas generated during the decomposition of the colloidal mixture, leading to the formation of bubbles. Once the pressure within the bubble cavities reached a critical threshold, the bubbles burst, and their walls interconnected to form a honeycomb-like structure. Meanwhile, Fe3O4 generated from the decomposition of Fe (NO3)3 was incorporated into the framework. As the temperature increased, the framework underwent gradual carbonization, and Fe3O4 was reduced to form Fe3C. Finally, Na2H2PO2·H2O was used as the phosphorus source, and Fe3C@NHC was rapidly reacted with PH3 under Ar at 360 °C to produce the FeP2@NHC nanocomposite. FeP2@NHC retained the morphology and structure of the Fe3C@NHC precursor, attributing to the resistance of the carbon framework to the phosphorization process, thereby exhibiting a distinctive multi-scale hierarchical structure.
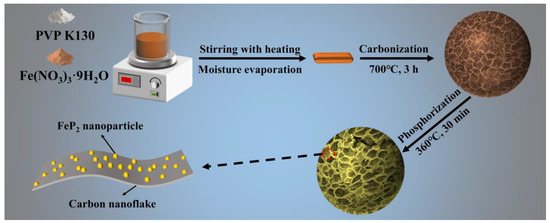
Figure 1.
Schematic illustration of the process for preparing FeP2@NHC nanocomposite.
As shown in Figure 2, the microstructural characteristics and morphological evolution of the synthesized materials were systematically investigated through SEM and TEM analyses. Figure S1a presents the SEM image of the rod-shaped Fe3O4. Pure FeP was observed as micron-sized agglomerates, as shown in Figure S1b. Figure S2a,b depict the aggregates of Fe3C@C and FeP-FeP2@C nano-spherical particles, respectively. As shown in Figures S3 and S4, Fe3C@C and FeP-FeP2@C are encapsulated by a carbon layer, obtained through the high-temperature carbonization of glucose. Notably, FeP2@NHC displayed a unique honeycomb-like 3D porous architecture. In this specific structure, FeP2 was encapsulated within a thin carbon layer and subsequently embedded in two-dimensional N-doped carbon nanosheets. These nanosheets were interconnected, forming a hierarchical three-dimensional framework. The low-magnification TEM image (Figure 2c) confirmed that FeP2 particles, ranging from 20 to 200 nm in size, were embedded within the carbon nanosheets, consistent with the SEM results. This advantageous combination of nanosheets and nanoparticles effectively prevented the aggregation and pulverization of active materials during the cycling process, enhanced the transmission efficiency of electrons and ions, and mitigated volume changes [18,20]. The 2.49 Å lattice fringes observed in the high-resolution TEM (HRTEM) image (Figure 2d) corresponded to the (200) plane of FeP2, indicating excellent crystallinity. Furthermore, the HRTEM image confirmed that FeP2 was uniformly coated with a thin carbon layer. The selected area electron diffraction (SAED) pattern (Figure 2e) showed (200) and (010) crystal planes along the [001] zone axis, corresponding to the FeP2 phase of the orthorhombic crystal system. The EDX analysis (Figure 2f) confirmed the presence of elements such as C, N, Fe, and P within the FeP2@NHC sample. Additionally, the atomic ratio of Fe to P was approximately 1:2, indicating a successful transformation of Fe3C and PH3 into FeP2 following calcination. The TEM mapping images (Figure 2g–k) indicated that Fe and P elements were predominantly localized within the FeP2 nanoparticle regions, whereas C and N elements exhibited a uniform distribution throughout the composite material, suggesting that the FeP2 nanoparticles were effectively encapsulated within the N-doped honeycomb-like carbon matrix.
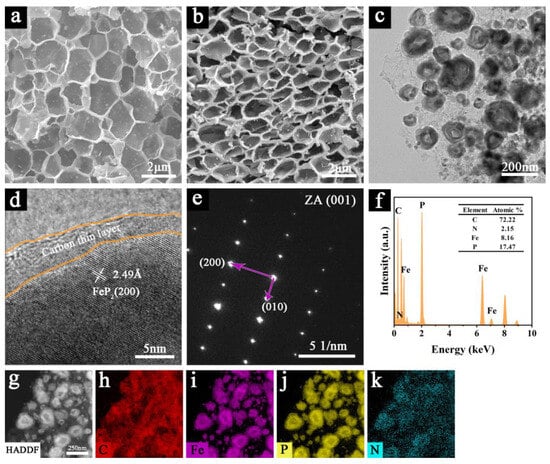
Figure 2.
(a) SEM image of Fe3C@NHC; (b) SEM image of FeP2@NHC; (c) the low-magnification TEM image, (d) HRTEM image, (e) SAED pattern, (f) EDX spectrum of FeP2@NHC; (g–k) STEM image and elemental mapping images of FeP2@NHC.
The crystal structures of the materials and formation mechanisms of FeP and FeP2 were systematically investigated through XRD. As shown in Figure 3a,b, in the presence of a carbon source, Fe (NO3)3 was transformed into Fe3C (ICDD#01-077-0255) at high temperatures. For FeP2@NHC, multiple distinct peaks were observed, indicating that FeP2 (ICDD#01-089-2261) nanoparticles were well-crystallized. The reactions for synthesizing FeP2@NHC can be expressed by Equations (1)–(4). However, when glucose served as the carbon source, Fe3C was encapsulated within the carbon sheets, leading to the formation of aggregates. This structure restricted the diffusion rate of the PH3 gas and reduced the contact area with Fe3C. Consequently, the phosphorization process predominantly proceeded via Equation (5) to form FeP (ICDD#01-078-1443), with only a minor contribution from Equation (4) for the formation of FeP2. Figure 3c illustrates that, in the absence of any assisting polymer, Fe (NO3)3 underwent thermal decomposition to form Fe3O4 (ICDD#01-071-6766), which subsequently reacted with PH3 via Equation (6) to produce pure FeP.
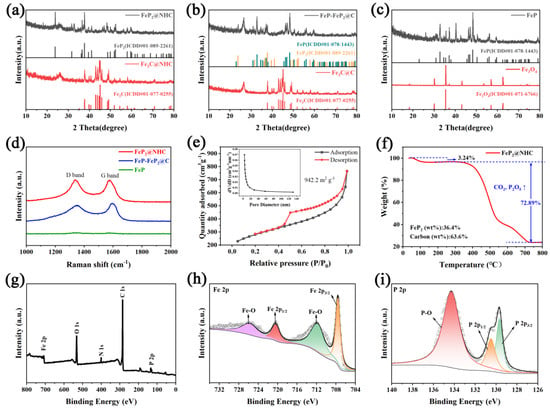
Figure 3.
XRD spectra of (a) FeP2@NHC, (b) FeP−FeP2@C, (c) FeP; (d) Raman spectra, (e) N2 adsorption–desorption isotherms and pore size distribution (inset), (f) TGA analysis, (g) XPS survey spectrum, (h) Fe 2p, and (i) P 2p XPS high solution of FeP2@NHC.
Figure 3d illustrates the Raman spectrum of the materials. FeP2@NHC exhibited two prominent peaks at 1340 cm−1 and 1580 cm−1, corresponding to the D-band and G-band of the carbon sheet, respectively [17,36]. To be specific, the G-band indicated the graphitization level of the carbon atoms, and the D-band reflectred the extent of structural defects in the carbon lattice [37,38]. The intensity ratio of the D-band to the G-band for FeP2@NHC and FeP-FeP2@C was approximately 0.902 and 0.83, respectively, suggesting that the honeycomb-like carbon framework possessed a higher density of defects, enhanced electronic conductivity, and facilitated efficient electron transport. The surface area and porosity characteristics of FeP2@NHC can be characterized using adsorption–desorption isotherms and the pore size distribution curve (Figure 3e). The adsorption–desorption isotherm exhibiteda type IV pattern with a pronounced hysteresis loop, characteristic of mesoporous materials [30,39]. Based on the BET method, the specific surface area of FeP2@NHC was approximately 942.2 m2 g−1. In contrast, the specific surface areas of FeP-FeP2@C and pure FeP were notably lower (Figure S5), measuring 12.23 and 10.54 m2 g−1, respectively. The average pore diameter was estimated to be around 5 nm using the Barrett–Joyner–Halenda (BJH) model. This unique honeycomb-like structure with a large surface area and suitable pore size was anticipated to enhance the contact between the electrolyte and the active materials, provide ample space for accommodating significant volume changes, and facilitate the rapid transport of ions and charges during the electrochemical reaction [35,40]. As illustrated in Figure 3f, TGA was employed to quantify the carbon content in FeP2@NHC. The mass loss below 200 °C can be set with the volatilization of adsorbed water. The substantial mass loss observed from 200 to 800 °C primarily remained with the oxidation of FeP2 into Fe2O3 and P2O5, along with the oxidation of the carbon [41]. The contents of FeP2 and carbon were determined to be 36.4% and 63.6%, respectively. XPS analysis was employed to further characterize the chemical composition and surface state of materials. The XPS survey spectrum in Figure 3g confirmed the presence of C, O, N, Fe, and P elements in FeP2@NHC, with the oxygen signal attributed to partial surface oxidation. The high-resolution Fe 2p spectrum (Figure 3h) indicated that peaks at 711.8 and 725.7 eV were attributed to Fe-O bond, and peaks at 707.6 and 720.4 eV were assigned to Fe-P 2p3/2 and Fe-P 2p1/2, respectively [19,21]. Figure 3i shows the P2p spectrum, in which three peaks observed at 129.7, 130.5, and 134.3 eV corresponded to P 2p3/2, P 2p1/2, and P-O bond, respectively [20,22]. In the high-resolution C 1s spectrum (Figure S6a), the peak at 284.8 eV corresponded to the C-C bond, and peaks at 285.6 and 289 eV were attributed to C-P and C-O bonds, respectively [17,18,29]. Furthermore, the N 1s spectrum (Figure S6b) exhibited three distinct peaks at 400.0, 400.7, and 402 eV, corresponding to pyridinic N, pyrrolic N, and graphitic N, respectively [24]. Abundant nitrogen atoms within the carbon matrix can furnish an abundance of active sites and defects, thus facilitating the diffusion and transport of Li+ ions and enhancing reaction kinetics [42,43]. Additionally, given that the electronegativity of nitrogen exceeds that of carbon, this can boost the conductivity of material. All the above results collectively validated the successful synthesis of FeP2 nanoparticles embedded within a honeycomb-like N-doped carbon matrix. The XPS spectrum of FeP-FeP2@C and pure FeP displayed comparable fitting peaks. The fitted Fe 2p peak position shifted in FeP-FeP2@C to a higher binding energy, attributed to lattice differences in the mixed phosphides. Notably, the oxygen peak in pure FeP was markedly stronger, likely due to the absence of carbon protection, leading to a more pronounced surface oxidation.
The lithium-storage performance of materials was evaluated within a voltage range of 0.01–3.0 V (Figure 4). The first five cyclic voltammogram (CV) curves, recorded at a scan rate of 0.2 mV s−1, are presented in Figure 4a. The peak observed at approximately 1.17 V in the initial cathodic scan suggested the formation of a solid electrolyte interlayer (SEI) film, and this phenomenon disappeared in subsequent cycles. The cathodic peak at 0.73 V and the anodic peak at 1.17 V corresponded to the conversion reaction process of FeP2, respectively, as described by Equations (7) and (8) [20]. In the second and subsequent cycles, the cathodic peak shifted to approximately 0.6 V, while the anodic peak moved to a higher potential, and a broad peak emerged around 1.5 V. These shifts in peak positions can be attributed to the transformation of FeP2 crystals into an amorphous state and the development of strain during the initial lithiation/de-lithiation processes [18,21]. Figure S8a presents the CV curves for the initial five cycles of FeP-FeP2@C. For FeP-FeP2@C, a series of cathodic peaks observed between 0.1 and 1.8 V during the first cycle can be attributed to the formation of an SEI film and the varying voltages associated with the mixed phosphide conversion reactions. Additionally, anodic peaks consistently appearing at 0.16 V across all cycles were indicative of multiple conversion reactions. Figure S8b presents the CV curves of pure FeP, aligning with previous reports [24].
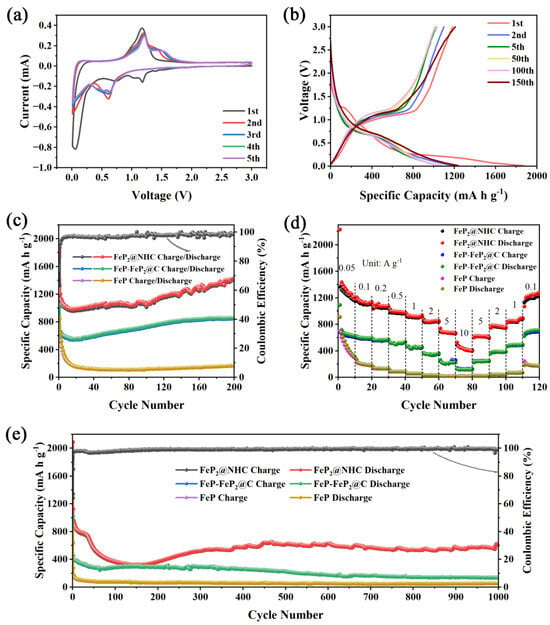
Figure 4.
The electrochemical performance of electrodes in LIBs: (a) CV curves at 0.2 mV s−1, (b) charge–discharge profiles at 0.1 A g−1 of FeP2@NHC; (c) cycling stability at 0.1 A g−1, (d) rate capability, (e) long–cycling performance at 2 A g−1 of FeP2@NHC, FeP–FeP2@C, FeP.
Figure 4b shows the charge–discharge profiles of the FeP2@NHC electrode at 0.1 A g−1. FeP2@NHC exhibited initial lithiation/de-lithiation specific capacities of 1870 mA h g−1 and 1183.3 mA h g−1, respectively, with an initial Coulombic efficiency (CE) of 63.3%. The capacity loss during the first cycle primarily lay with the formation of the SEI film and the irreversible trapping of Li+ within the porous structure [19]. Furthermore, observed charge–discharge voltage plateaus aligned with the findings from the CV curves. As shown in Figure 4c, the capacity of the FeP2@NHC electrode initially decreased and subsequently increased at a current density of 0.1 A g−1, with a reversible capacity of 1433.9 mA h g−1 after 200 cycles. The capacity loss during the initial 20 cycles was attributed to an incomplete redox reaction of FeP2, the continuous formation of the SEI film, and the decomposition of the electrolyte [19]. As the electrode material gradually activated, the capacity began to rise slowly after several dozen cycles. This phenomenon is commonly observed in Fe-based materials [18]. In contrast, the reversible capacities of FeP-FeP2@C and pure FeP after 200 cycles were 859.3 and 168.1 mA h g−1, respectively. These results confirm the superior intrinsic theoretical specific capacity of phosphorus-rich FeP2 and the unique hierarchical structure in the FeP2@NHC composite. The rate performance of FeP-FeP2@C, and FeP was inferior to those of FeP2@NHC under all working conditions. For FeP2@NHC, reversible capacities of 1210.7, 1136.1, 1072.8, 979.9, 934.3, 851.9, 665.2, and 399.9 mA h g−1 were achieved at 0.05, 0.1, 0.2, 0.5, 1, 2, 5, and 10 A g−1, respectively (Figure 4d). Notably, when the current density was reset to 0.1 A g−1, the capacity recovered to a significantly higher value of 1283.8 mA h g−1. To further characterize the stable cycling performance of FeP2@NHC, as illustrated in Figure 4e, all electrodes underwent 1000 cycles at 2 A g−1. FeP2@NHC initially showed a decrease in cycling performance, followed by an increase and eventual stabilization, achieving a reversible capacity of 631.5 mA h g−1. Moreover, compared with previously reported Fe-based metal phosphide anodes, FeP2@NHC exhibited a superior rate capability (Table 1). The SEM and TEM images (Figure S9) of all electrode materials after 200 cycles at 0.1 A g−1 revealed that the distinctive porous honeycomb structure of FeP2@NHC remained intact, indicating the robust structural stability of FeP2@NHC. The superior electrochemical performance can be attributed to the inherent redox reactivity of FeP2 and the innovative structural design that minimized volume changes while facilitating rapid electron–ion transport.

Table 1.
Comparison of rate performance of recently reported Fe-based phosphides in LIBs.
The charge transfer kinetics of electrodes were examined through EIS. As shown in Figure S10, each Nyquist plot comprised a high-frequency semicircle and a low-frequency inclined straight line, corresponding to the charge transfer process at the electrode–electrolyte interface and the Li+ diffusion process, respectively. The charge transfer resistance (Rct) can be estimated by analyzing the diameter of the semicircle observed in the high-frequency region of the impedance plot. The Warburg resistance (W) can be derived from the slope of the straight line [44]. The Rct values of FeP2@NHC before and after cycling were lower than those of FeP-FeP2@C and FeP, indicating that the honeycomb-like N-doped carbon framework enhanced the charge transfer and preserved the structural stability of active materials. The W value of FeP2@NHC indicated a higher rate of Li+ diffusion in this anode material. To further investigate the reaction kinetics of Li+, CV was conducted at various scan rates ranging from 0.2 to 2 mV s−1. As depicted in Figure 5a,d,g, all electrodes exhibited an increase in peak current and a broader potential range as scan rates were increased. The storage mechanism can be examined using the following equations [28,44]:
where i represents the peak current, v denotes the scan rate, and the remaining symbols (a, b) represent related parameters. The b-value can be determined by fitting the slope of the line in logarithmic plots of v versus i, reflecting the type of charge storage mechanism. A b value of 0.5 signifies the dominance of only redox reactions, whereas a value of 1 demonstrates solely capacitive effects. The calculated results of all electrodes, as shown in Figure S11, revealed that b values fell within the range of 0.5 to 1, indicating that the combined action of both mechanisms contributed to the electrochemical performance. The b value of redox peak for FeP2@NHC was lower than that of FeP-FeP2@C and pure FeP, which further corroborated the ultrafast charge transfer rate in FeP2@NHC, along with the enhanced diffusion and reaction rates of lithium ions. Consequently, fewer electrochemical reactions took place at the surface.

Figure 5.
(a,d,g) CV curves of (a) FeP2@NHC, (d) FeP−FeP2@C, (g) FeP electrodes at various scan rates; (b,e,h) diffusion contributions and capacitive contributions to charge storage of (b) FeP2@NHC, (e) FeP−FeP2@C, (h) FeP electrode at 0.8 mV s−1; (c,f,i) normalized contribution proportion of two storage mechanisms at different scan rates.
Moreover, the contribution ratios of diffusion and capacitive control behaviors throughout the process can be quantified by Equations (11) and (12) [45]:
where k1v is associated with the pseudocapacitive contribution, and k2v1/2 is linked to the diffusion contribution. The constants k1 and k2 are determined by the linear plots of iv/v1/2 and ν1/2 at a given potential. The pseudocapacitive contribution at 0.8 mV s−1 is shown in Figure 5b,e,h, respectively, with the green region accounting for approximately 56%, 65%, and 68% of the total area. The contribution percentages presented in Figure 5c,f,i reveal a consistent increase in the capacitive contribution ratio as the scan rate increased. The N-doped honeycomb structure enhanced the charge storage and accelerated the reaction kinetics, which aligned with the fitted b value results.
Additionally, GITT was employed to determine the diffusion coefficients of Li+ in the electrodes, as depicted in Figure 6 and Figure S12. The diffusion coefficients were determined by the subsequent equation [46]:
where τ represents the time of pulse, nB is the number of moles (mol) of the electrode, and Vm is the molar volume of the electrode. Moreover, S denotes the total contacting area between the electrode and the electrolyte. ΔES refers to variations in the steady-state potential during the relevant steps, while ΔEt represents the changes in a single-step experiment by eliminating the IR drop. The calculation results for the Li+ diffusion coefficients are presented in Figure 6b,c. Notably, compared with the FeP-FeP2@C and FeP electrodes, the FeP2@NHC electrode exhibited significantly higher ion diffusion coefficients during both insertion and extraction processes. This finding provides strong evidence explaining the superior rate performance and the excellent cycling stability of FeP2@NHC.
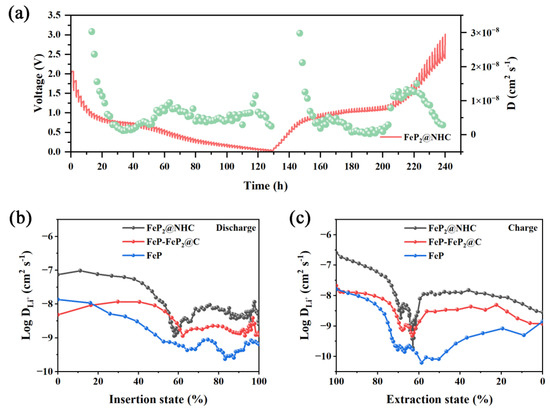
Figure 6.
(a) GITT curve of FeP2@NHC electrode; (b,c) calculation results of Li+ diffusion coefficients of FeP2@NHC, FeP−FeP2@C, FeP.
The exceptional electrochemical performance of the FeP2@NHC electrode lay within the intrinsically high specific capacity of phosphorus-rich FeP2 and its distinctive 3D conductive networks, conferring multiple advantages for a superior performance. As illustrated in Figure 7, in situ generated FeP2 nanoparticles embedded in carbon nanosheets effectively suppressed the grain growth of FeP2. This nanostructure engineering not only mitigated the pulverization of the FeP2 nanoparticles but also shortened the electron diffusion path. Furthermore, FeP2 was encapsulated within a honeycomb-like carbon matrix, which shielded the active material from electrolyte degradation and provided ample space for volume expansion during repeated charge–discharge cycles, thus preserving the structural integrity and electrochemical stability. Furthermore, the 3D interconnected carbon network functioned as a high-speed conduit for both electron and ion transport, while also preventing the aggregation of FeP2 nanoparticles. Benefiting from the optimized structural design, the FeP2@NHC electrode exhibited exceptional rate performance and stable long-term cycling stability.
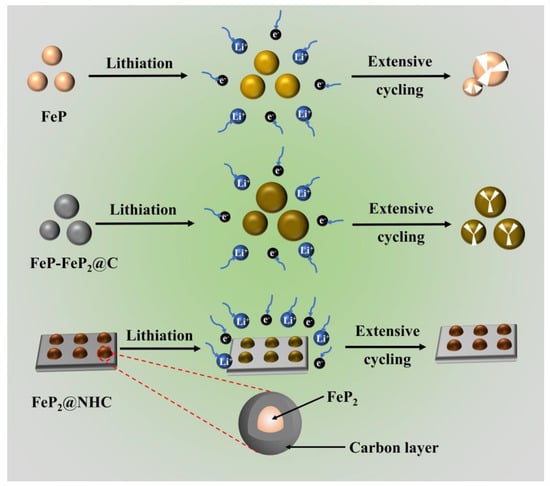
Figure 7.
Structural evolutions during cycling process of anode materials.
4. Conclusions
In conclusion, we successfully synthesized FeP2 nanoparticles embedded in an N-doped honeycomb-like carbon skeleton via a combination of sol–gel pyrolysis blowing and sodium hypophosphite phosphorization methods. FeP2 nanoparticles were embedded into a porous carbon matrix, which effectively prevented the pulverization and aggregation of the nanoparticles, thereby maintaining structural integrity. The 3D honeycomb-like carbon framework provided ample space to accommodate volume expansion during repeated charge–discharge cycles, and offered conductive pathways, along with rapid diffusion channels, for both electrons and ions. Moreover, N doping introduced additional active sites and enhanced electrical conductivity. Benefiting from this innovative structure, the FeP2@NHC electrode demonstrated exceptional reversible capacity (1433.9 mA h g−1 at 0.1 A g−1), outstanding rate capability (399.9 mA h g−1 at 10 A g−1), and excellent cycling stability (631.5 mA h g−1 at 2 A g−1). Furthermore, XRD analysis confirmed that the iron-rich Fe3C and Fe3O4 precursors reacted with NaH2PO2 to form FeP2 and FeP, respectively. This work presents a straightforward approach for the synthesis of phosphorus-rich phosphide composites, which can provide valuable insights for advancements in the energy storage field.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en18061358/s1, Figure S1: SEM images: (a) Fe3O4; (b) FeP; Figure S2: SEM images: (a) Fe3C@C; (b) FeP-FeP2@C; Figure S3: (a) HRTEM image, (b–d) STEM image and elemental mapping images of Fe3C@C. Figure S4: (a) HRTEM image, (b–f) STEM image and elemental mapping images of FeP-FeP2@C. Figure S5: N2 adsorption isotherms and pore size distribution of (a,b) FeP-FeP2@C; (c,d) FeP; Figure S6: (a) C 1s high solution spectrum and (b) N 1s high solution spectrum of FeP2@NHC; Figure S7: (a) XPS survey spectrum, (b) Fe 2p high solution spectrum, (c) P 2p high solution spectrum of FeP-FeP2@C; (d) XPS survey spectrum, (e) Fe 2p high solution spectrum, (f) P 2p high solution spectrum of FeP; Figure S8: CV curves at a scan rate of 0.2 mV s−1 of (a) FeP-FeP2@C, (b) FeP electrode; Figure S9: TEM images of (a) FeP2@NHC, (b) FeP-FeP2@C, (c) FeP electrodes after 200 cycles; Figure S10: Nyquist plots of electrodes in the initial state (a) and after 200 cycles (b); Figure S11: Corresponding log(i) versus log(v) plots at each redox peak (peak current: i, scan rate: v) of (a) FeP2@NHC, (b) FeP-FeP2@C, (c) FeP electrode; Figure S12: GITT curves of (a) FeP-FeP2@C and FeP electrodes.
Author Contributions
Conceptualization, Z.S.; methodology, J.S.; software, J.S.; validation, J.S., and Z.S.; formal analysis, J.S.; investigation, J.S. and X.W.; resources, J.S.; data curation, H.C. and L.H.; writing—original draft preparation, J.S.; writing—review and editing, J.S. and Z.S.; visualization, H.C.; supervision, X.W. and L.H.; project administration, Z.S. and X.W.; funding acquisition, Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC were funded by the National Key R&D Program of China, 2021YFA0715802.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Meng, Z.; Xu, Z.; Li, H.; Xiong, H.; Liu, X.; Qin, C.; Wang, Z. Silicon/biomass carbon composite as a low-cost anode for lithium-ion batteries. Energies 2025, 18, 972. [Google Scholar] [CrossRef]
- Yue, L.; Liang, J.; Wu, Z.; Zhong, B.; Luo, Y.; Liu, Q.; Li, T.; Kong, Q.; Liu, Y.; Asiri, A.M.; et al. Progress and perspective of metal phosphide/carbon heterostructure anodes for rechargeable ion batteries. J. Mater. Chem. A 2021, 9, 11879–11907. [Google Scholar] [CrossRef]
- Yang, H.; Liu, L.; Ma, J.; Zhang, J.; Zhang, Q. Hydrothermal synthesis of hierarchical cage-like Co9S8 microspheres composed of nanosheets as high-capacity anode materials. Energies 2024, 17, 5553. [Google Scholar] [CrossRef]
- Lan, X.; Li, Z.; Zeng, Y.; Han, C.; Peng, J.; Cheng, H.-M. Phosphorus-based anodes for fast-charging alkali metal ion batteries. Ecomat 2024, 6, 12452. [Google Scholar] [CrossRef]
- Diao, G.; Balogun, M.-S.; Tong, S.-Y.; Guo, X.; Huang, X.; Mao, Y.; Tong, Y. Low-valence bicomponent (FeO)x(MnO)1−x nanocrystals embedded in amorphous carbon as high-performance anode materials for lithium storage. J. Mater. Chem. A 2018, 6, 15274–15283. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Wang, H.; Gao, S.; Chen, J.; Chen, Z.; Fu, J. N-doped graphitized carbon-coated Fe2O3 nanoparticles in highly graphitized carbon hollow fibers for advanced lithium-ion batteries anodes. Electrochim. Acta 2023, 467, 143032. [Google Scholar] [CrossRef]
- Wang, S.; Lin, X.; Chai, W.; Yu, W.; Zhang, B.; Li, L.; Wang, H. Iron sulfide quantum dots decorated on porous N-doped carbon for lithium/sodium-ion storage. ACS Appl. Nano Mater. 2024, 7, 26970–26977. [Google Scholar] [CrossRef]
- Sun, C.; Fang, S.; Zhao, K.; Zhang, H.; Qi, L.; Qin, Y.; Bao, H. An innovative double-shell layer nitrogen and sulfur co-doped carbon-encapsulated FeS composite for enhanced lithium-ion battery performance. J. Colloid Interface Sci. 2025, 678, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sun, M.; Wu, S.; Chen, Y.; Li, L.; Zou, X.; Chen, L.; Yang, H.; Pang, H. Interfacial engineering of graphene aerogel encapsulated FeSe2-Fe2O3 heterojunction nanotubes for enhanced lithium storage. J. Alloys Compd. 2023, 934, 167939. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Z.; Yao, S.; Gao, Y.; Jin, X.; Chen, G.; Shen, Z.; Du, F. Polymorph engineering for boosted volumetric Na-ion and Li-ion storage. Adv. Mater. 2021, 33, 2100210. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Q.; Li, H.; Sun, J.; Li, H.; He, Y.; Liu, Z. Iron-chalcogenide-based electrode materials for electrochemical energy storage. J. Mater. Chem. A 2022, 10, 7517–7556. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, S.; Sun, B.; Yang, P.; Zheng, J.; Li, X. Low-temperature synthesis of honeycomb CuP2@C in molten ZnCl2 salt for high-performance lithium ion batteries. Angew. Chem. Int. Ed. 2020, 59, 1975–1979. [Google Scholar] [CrossRef]
- Feng, Q.; Li, T.; Miao, Y.; Sui, Y.; Xiao, B.; Sun, Z.; Qi, J.; Wei, F.; Meng, Q.; Ren, Y.; et al. Polyvinylpyrrolidone assisted transformation of Cu-MOF into N/P-co-doped octahedron carbon encapsulated Cu3P nanoparticles as high performance anode for lithium ion batteries. J. Colloid Interface Sci. 2022, 608, 227–238. [Google Scholar] [CrossRef]
- Zong, H.; Hu, L.; Wang, Z.; Qi, R.; Yu, K.; Zhu, Z. Metal-organic frameworks-derived CoP anchored on MXene toward an efficient bifunctional electrode with enhanced lithium storage. Chem. Eng. J. 2021, 416, 129102. [Google Scholar] [CrossRef]
- Ganesan, V.; Kim, D.-H.; Park, C.-M. Robust CoP2-C hollow nanoboxes: Superior anodes for Li- and Na-ion batteries. J. Energy Storage 2024, 79, 110197. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, M.-C.; Moon, S.-H.; Kim, H.; Park, K.-W. Ni2P/graphitic carbon nanostructure electrode with superior electrochemical performance. Electrochim. Acta 2020, 341, 136045. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Bai, J.; Wang, J.; Zhao, H. Well-dispersed FeP@C nanoparticles anchored on MXene conductive network as outstanding cyclic performance anode for Li/Na-ion batteries. Carbon 2025, 234, 120008. [Google Scholar] [CrossRef]
- Liu, J.; Wu, A.; Tian, R.; Paredes Camacho, R.A.; Zhou, S.; Huang, H.; Yao, M. Progressive lithiation of FeP2 nanoparticles constrained inside the carbon shell. Mater. Today Energy 2020, 18, 100545. [Google Scholar] [CrossRef]
- Zhan, L.; Song, X.; Deng, W.; Wei, T.; Huang, L.; Wei, X.; Wang, C. Facile approach to prepare FeP2/P/C nanofiber heterostructure via electrospinning as highly performance self-supporting anode for Li/Na ion batteries. Electrochim. Acta 2022, 403, 139682. [Google Scholar] [CrossRef]
- Mao, X.; Wu, K.; Li, S.-Q.; Du, F.-H.; Xu, G.; Wu, M.; Liu, H.-K.; Dou, S.-X.; Wu, C. Honeycomb-like 3D carbon skeletons with embedded phosphorus-rich phosphide nanoparticles as advanced anodes for lithium-ion batteries. Nanoscale 2022, 14, 8744–8752. [Google Scholar] [CrossRef]
- Lin, C.; Tang, J.; Wang, S.; Gao, Q.; Liu, Y.; Wu, W.; Wang, X.; Huang, Z.; Yang, L. Fabrication of FeP2/C/CNTs@3D interconnected graphene aerogel composite for lithium-ion battery anodes and the electrochemical performance evaluation using machine learning. J. Alloys Compd. 2024, 996, 174800. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, J.; Wang, Y.; Huang, F.; Peng, J.; Li, J.; Zhai, M. Cactus-like iron diphosphide@carbon nanotubes composites as advanced anode materials for lithium-ion batteries. Electrochim. Acta 2018, 259, 321–328. [Google Scholar] [CrossRef]
- Lin, X.; Ke, Y.; Peng, X.; He, C.; Zhao, X.; Xiao, X.; Lin, X.; Nan, J. Improving the rate capacity and cycle stability of FeP anodes for lithium-ion batteries via in situ carbon encapsulation and copper doping. J. Colloid Interface Sci. 2023, 634, 346–356. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Li, T.; Lv, Z.; Hou, X.; Tang, Y.; Zhang, H.; Zheng, Q.; Li, X. A coral-like FeP@NC anode with increasing cycle capacity for sodium-ion and lithium-ion batteries induced by particle refinement. Angew. Chem. Int. Ed. 2021, 60, 25013–25019. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, W.; Lee, D.C.; Bell, C.; Drexler, M.; Ma, Z.F.; Magasinski, A.; Yushin, G.; Alamgir, F.M. Porous FeP/C composite nanofibers as high-performance anodes for Li-ion/Na-ion batteries. Mater. Today Energy 2020, 16, 100410. [Google Scholar] [CrossRef]
- Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. Rational design of three-dimensional graphene encapsulated with hollow FeP@Carbon nanocomposite as outstanding anode material for lithium ion and sodium ion batteries. ACS Nano 2017, 11, 11602–11616. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, B.; Pan, J.; Li, C.; Liu, C.; Liu, L.; Yang, T.; Li, W.; Li, H.; Wang, Y.; et al. Strongly coupled FeP@reduced graphene oxide nanocomposites with superior performance for lithium-ion batteries. J. Alloys Compd. 2017, 728, 328–336. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Li, J.; Dong, C.; Dai, Y.; Gao, T.; Wang, X.; Yue, K.; Zhou, G. In-situ rooting biconical-nanorods-like Co-doped FeP @carbon architectures toward enhanced lithium storage performance. Chem. Eng. J. 2023, 477, 146996. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, W.; Wang, C.; Zhang, L.; Tang, K.; Zuo, J.; Yang, Q. Electrochemical performance of iron diphosphide/carbon tube nanohybrids in lithium-ion batteries. Electrochim. Acta 2015, 170, 140–145. [Google Scholar] [CrossRef]
- Gao, M.; Liu, X.; Yang, H.; Yu, Y. FeP nanoparticles derived from metal-organic frameworks/GO as high-performance anode material for lithium ion batteries. Sci. China Chem. 2018, 61, 1151–1158. [Google Scholar] [CrossRef]
- Chang, G.; Zhao, Y.; Dong, L.; Wilkinson, D.P.; Zhang, L.; Shao, Q.; Yan, W.; Sun, X.; Zhang, J. A review of phosphorus and phosphides as anode materials for advanced sodium-ion batteries. J. Mater. Chem. A 2020, 8, 4996–5048. [Google Scholar] [CrossRef]
- Liu, W.; Zhi, H.; Yu, X. Recent progress in phosphorus based anode materials for lithium/sodium ion batteries. Energy Storage Mater. 2019, 16, 290–322. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, Q.; Ullah, S.; Yang, X.; Bachmatiuk, A.; Yang, R.; Rummeli, M.H. Phosphorus-based composites as anode materials for advanced alkali metal ion batteries. Adv. Funct. Mater. 2020, 30, 2004648. [Google Scholar] [CrossRef]
- Wang, B.; Wang, G.; Wang, H.; Bai, J. Hierarchically porous carbon nanofibers encapsulating carbon-coated mini hollow FeP nanoparticles for high performance lithium and sodium ion batteries. ChemNanoMat 2018, 4, 924–935. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, C.; Zhang, W.; He, Y.; Li, X.; Yuan, X. Controlled design for integration of FeP into 3D carbon frameworks for superior Na storage. Chem. Eng. J. 2022, 429, 132271. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, Z.; Lin, J.; Zhang, R.; Zhao, C. One-step simultaneously heteroatom doping and phosphating to construct 3D FeP/C nanocomposite for lithium storage. Appl. Surf. Sci. 2020, 500, 144055. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, W.; Bell, C.; Lee, D.-C.; Drexler, M.; Nuli, Y.; Ma, Z.-F.; Magasinski, A.; Yushin, G.; Alamgir, F.M. Iron phosphide confined in carbon nanofibers as a free-standing flexible anode for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 34074–34083. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, Z.; Yue, K.; Li, A.; Liu, H.; Guo, Z.; Qian, L. Ni-FeP @carbon core-shell structure as advanced anode materials for superior lithium storage. Appl. Surf. Sci. 2021, 554, 149666. [Google Scholar] [CrossRef]
- Xu, X.; Feng, J.; Liu, J.; Lv, F.; Hu, R.; Fang, F.; Yang, L.; Ouyang, L.; Zhu, M. Robust spindle-structured FeP@C for high-performance alkali-ion batteries anode. Electrochim. Acta 2019, 312, 224–233. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, R.; Dong, S.; Miao, X.; Zhang, Z.; Wang, C.; Yin, L. Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries. Energy Environ. Sci. 2019, 12, 2422–2432. [Google Scholar] [CrossRef]
- Yang, F.; Gao, H.; Hao, J.; Zhang, S.; Li, P.; Liu, Y.; Chen, J.; Guo, Z. Yolk-shell structured FeP@C nanoboxes as advanced anode materials for rechargeable lithium-/potassium-ion batteries. Adv. Funct. Mater. 2019, 29, 1808291. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liu, P.; Zhu, X.; Ali, R.N.; Naz, H.; Yu, Y.; Xiang, B. Self-supporting hybrid fiber mats of Cu3P-Co2P/N-C endowed with enhanced lithium/sodium ions storage performances. ACS Appl. Mater. Interfaces 2019, 11, 11442–11450. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, Z.; Guo, J.; Guo, S.; Zhou, Z.; Shi, Q.; Zhang, Y.; Chang, Z.; Geng, M. Iron-cobalt phosphide/nitrogen-doped carbon composite derived from prussian blue analogues as anode materials for sodium-ion batteries. J. Energy Storage 2024, 98, 113131. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Zhang, Y.; Jiang, J.; Wu, H.B.; Yu, X.-Y. Copper and carbon-incorporated yolk-shelled FeP spheres with enhanced sodium storage properties. Chem. Eng. J. 2021, 421, 127776. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhao, Z.; Pathak, R.; Hao, S.; Qiu, X.; Qiao, Q. Microstructure controlled synthesis of Ni, N-codoped CoP/carbon fiber hybrids with improving reaction kinetics for superior sodium storage. J. Mater. Sci. Technol. 2022, 99, 184–192. [Google Scholar] [CrossRef]
- Liu, S.; Shi, Q.; Liu, X.; Lin, F.; Geng, M.; Ren, L.; Qin, Z.; Tong, J. Biomass derived 3D N, P co-doped porous carbon incorporated with Ni-Cu bimetallic phosphide: Synergistic effect of Ni-Cu boosting Na+ storage performance. J. Alloys Compd. 2024, 1009, 177013. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).