Abstract
Emerging contaminants such as per- and polyfluoroalkyl substances (PFASs) pose significant challenges for conventional wastewater treatment technologies. Non-thermal plasma (NTP) has gained attention as a promising advanced oxidation process capable of degrading persistent pollutants via hydrated electrons and reactive oxygen/nitrogen species under ambient conditions. This review summarizes recent progress in the application and scale-up of NTP for water treatment, with a focus on reactor configurations, degradation mechanisms, and energy efficiency. Key plasma reactor types—including dielectric barrier discharge, corona discharge, plasma jets, and gliding arc discharge—are evaluated for their suitability in large-scale applications. Pilot-scale studies addressing pharmaceuticals, dyes, and PFASs are reviewed to assess scalability, cost, and operational viability. Although NTP systems consistently achieve >80% contaminant removal, optimizing energy use and maintaining performance across complex water matrices remain critical challenges. Hybrid systems integrating NTP with ozonation, ultrafiltration, or cavitation show potential to improve treatment efficacy and reduce energy demands. Future research priorities include reactor design optimization, contaminant-specific plasma tuning, and technoeconomic analysis to support the translation of NTP technologies from lab-scale innovation to field-scale implementation.
1. Introduction
It is well known that rapid and continuous population growth and industrialization drive the constant demand for clean water [1,2]. However, these industrial activities also contribute to the tremendous amount of wastewater released, severely harming human health and ecology. It is worth noting that the World Health Organization (WHO) estimated that water pollution is responsible for approximately 3.1% of global deaths. Thus, wastewater treatment has become a global concern, pushing the scientific community to develop efficient and cost-effective wastewater treatment technologies [3].
Among different water pollutants, the aqueous film-forming foam (AFFF) has been employed extensively for training purposes in fire and firefighting operations which involve class B fires, i.e., combustible and flammable liquids at the different installation of the US Department of Defense (DOD) such as the local fire station, airports, and other locations for emergency responses across the globe. AFFF has a complex formulation with proprietaries that include (non)fluorinated surfactants, solubilizes, stabilizers, and other chemicals [4]. It is interesting to note that around 3–6% by weight, the total formulation of AFFF comprises poly- and perfluorinated alkyl substances (PFASs) which is itself a complex group of more than 14,000 chemicals composed of carbon chains that have at least fully fluorinated either methylene (-CF2-) or methyl (-CF3-) functional group [5,6,7,8]. As mentioned previously, the elevated concentration levels of PFASs have been detected in the groundwater at almost all sites and airports of the DOD where AFFF has been utilized [9,10,11,12]. It is worth noting that the some of the PFASs have demonstrated their potential of bioaccumulation in the animals and humans leading to associated health risks such as developmental and reproductive impacts, potential immunotoxicity, and carcinogenicity [10,13,14]. This situation led the European Chemical Agency (ECHA) to recommend restrictions on almost all types of PFAS productions and their subsequent use [15]. The US environmental protection agency (EPA) has also taken several actions to protect public health and environmental welfare in April 2024 via the US EPA’s PFAS Strategic Roadmap [16].
To date, several technologies have been developed for the efficient destruction of PFAS compounds in wastewater, including incineration which is limited by the PFAS remains in leachate, aur, and ash [17,18] near the incineration facilities, mechanochemical treatment, electrochemical treatment, sonolysis, photocatalysis, sonochemical degradation, direct photolysis, advanced reduction, thermo-chemical treatment, microwave hydrothermal, subcritical degradation supercritical water oxidation (SCWO), and plasma treatment [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54].
As the scope of global water pollution continues to broaden, the treatment of emerging contaminants has become a central focus in the development of advanced water and wastewater technologies. Traditional treatment systems such as activated sludge, sedimentation, membrane filtration, and granular activated carbon have proven effective at removing bulk pollutants, suspended solids, and a range of conventional organic and inorganic substances [1]. However, as detection technologies improve, a growing number of trace-level contaminants are being identified in effluents from municipal and industrial facilities. These include pharmaceuticals, personal care products, pesticides, and synthetic industrial compounds, many of which are only partially removed by conventional systems [2,3]. This trend has motivated the search for novel treatment approaches that not only capture, but also chemically degrade persistent and toxic contaminants.
One of the most promising technologies in this space is non-thermal plasma (NTP), a form of electrical discharge that creates a partially ionized gas containing electrons, ions, radicals, and UV photons. Unlike thermal plasmas, NTP systems operate under near-ambient conditions, allowing for scalable integration into water treatment processes without significant heat production [55]. When discharged in contact with or above water, plasma produces a variety of reactive oxygen and nitrogen species (RONS) such as hydroxyl radicals (•OH), hydrogen peroxide (H2O2), ozone (O3), nitric oxide (NO), and solvated electrons, which can participate in complex degradation reactions [1,3]. This mixture of oxidative and reductive chemistry allows NTP to target a wide range of chemical structures, including aromatic rings, halogenated compounds, and low-biodegradability organics [56,57].
Over the past two decades, numerous studies have demonstrated NTP’s efficacy for degrading various classes of contaminants. Laboratory-scale reactors have successfully degraded dyes, antibiotics, endocrine-disrupting compounds, and other recalcitrant pollutants under optimized conditions [1,3]. Systems based on dielectric barrier discharge (DBD), corona discharge, and gliding arc discharge have each been studied, offering different advantages in terms of energy input, electrode configuration, and interaction with the liquid phase [55]. Research has also explored combining NTP with other treatment methods such as ozonation, photocatalysis, and microbubble injection to enhance mass transfer and synergistically improve degradation efficiency [58,59].
A particularly urgent application of plasma technologies involves the treatment of per- and polyfluoroalkyl substances (PFASs), a large class of synthetic fluorinated compounds used in consumer and industrial products for their resistance to heat, water, and oils. PFAS compounds, including perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), and newer alternatives such as GenX, are highly persistent in the environment and resistant to conventional biological and chemical degradation processes [60,61]. While physical removal methods such as granular activated carbon and reverse osmosis can isolate PFASs from water, they do not destroy the compounds, resulting in concentrated waste streams that require additional treatment. Unlike these technologies, NTP has demonstrated the ability to break the carbon–fluorine bonds in PFAS molecules through mechanisms involving hydrated electrons and radical species [60,62,63].
Recent studies have shown that NTP systems can achieve significant levels of PFAS degradation in various water matrices, including groundwater, secondary effluent, and synthetic wastewater. However, challenges remain, particularly in achieving complete mineralization, managing byproduct formation, and maintaining energy efficiency across different PFAS structures and concentrations [61,62,64]. Additionally, matrix effects and energy utilization remain critical concerns as researchers attempt to scale up bench-scale demonstrations to pilot- and full-scale systems [62,64]. Research on plasma reactor design continues to emphasize the need for improved gas–liquid contact, optimization of residence time, and integration with complementary technologies for long-term viability [60,63].
This review synthesizes current research on non-thermal plasma systems for the treatment of both PFASs and non-PFAS contaminants in water and wastewater, with an emphasis on degradation mechanisms, reactor configurations, and scale-up considerations. The strengths and limitations of major NTP reactor designs, survey key pilot- and lab-scale studies are evaluated, and hybrid technologies aimed at improving performance are examined. Finally, engineering challenges and future research opportunities that must be addressed are identified for NTP to become a practical, field-deployable solution for water and wastewater remediation.
2. Application Examples of NTP for Water/Wastewater Treatment
Non-thermal plasma and its applicability to wastewater treatment has been the subject of lab-scale experimental studies for decades. NTP discharge in both gaseous and liquid media can generate reactive oxygen species (ROS) such as H, OH, O3, and H2O2 [59] and reactive nitrogen species (RNS) including NO, NO2−, and NO3− [65], among others. These reactive species can participate in chemical reactions with components of cell membranes, intracellular components, genetic material, and others to degrade or defunctionalize them, leading to the deaths of affected microorganisms. They can also be used as an “advanced oxidative process” to mineralize and remove chemical pollutants such as dyes [66].
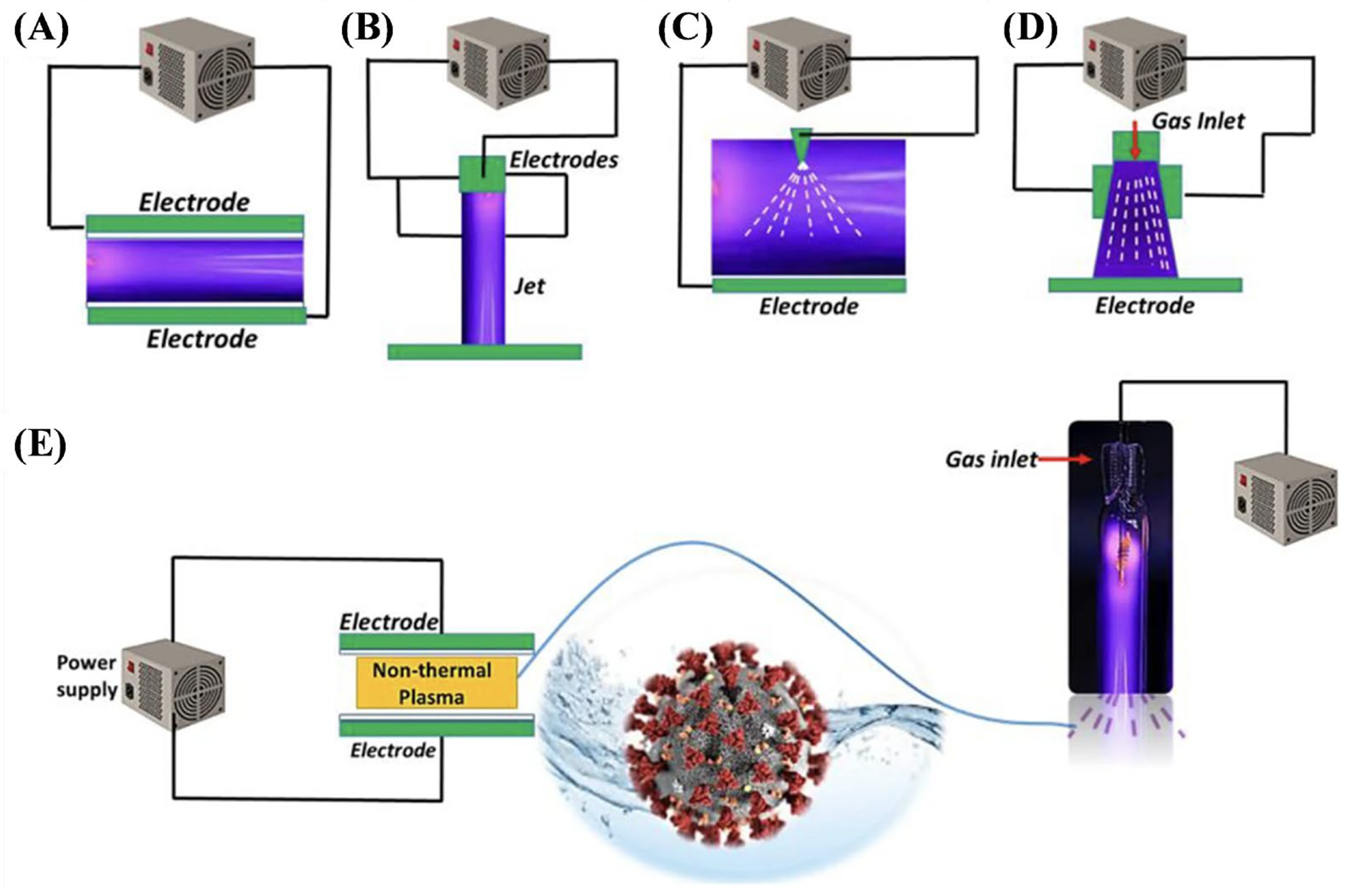
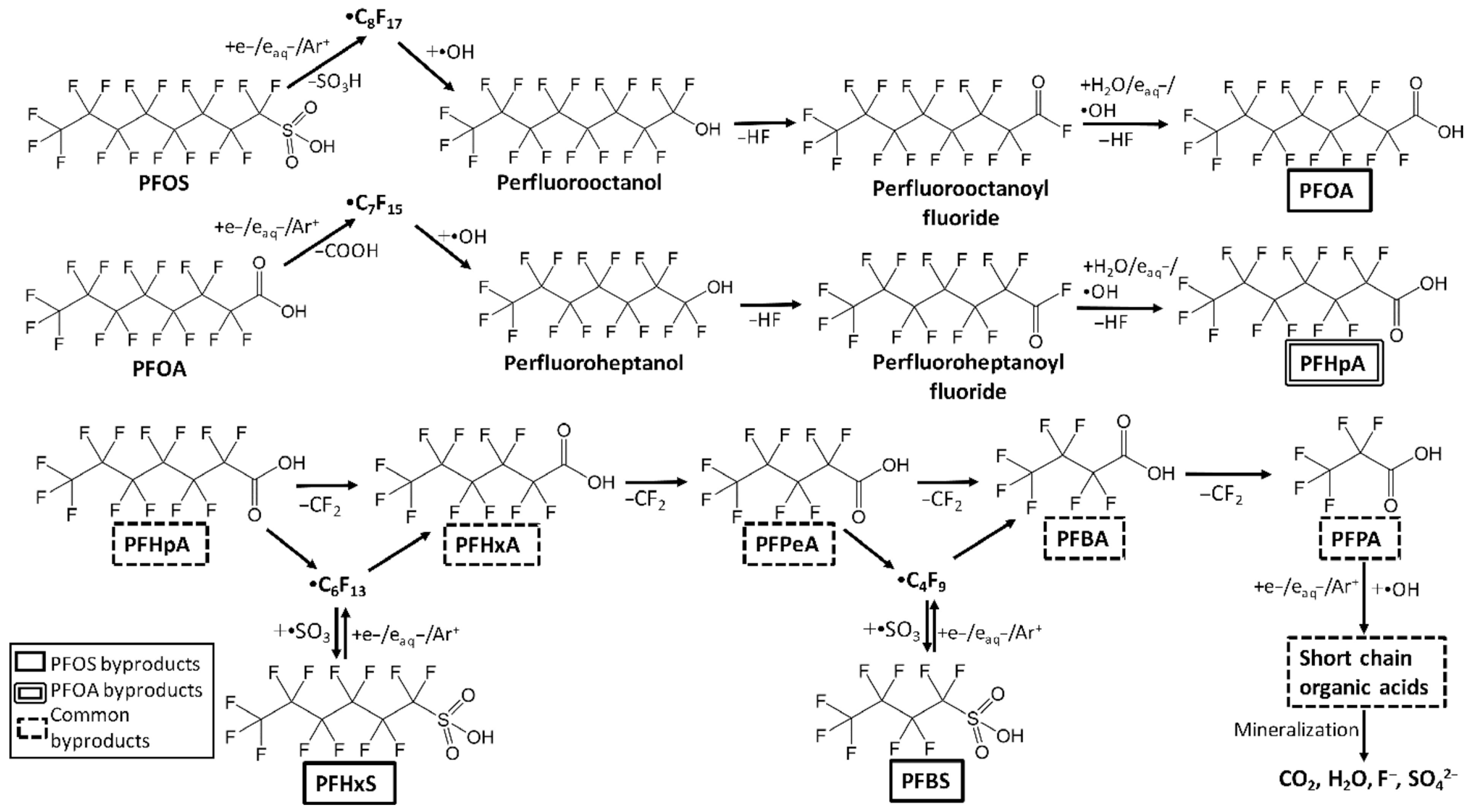
How these reactive species are administered to polluted wastewater varies, with many different reactor configurations being possible and no approach being standardized for wastewater treatment. Figure 1 shows some general examples of such reactor configurations, with additional considerations including the medium of discharge (gas vs. liquid), the composition of said gas/liquid, the volume/area coverage of water to be treated, applied voltage, electrode location and composition, batch vs. continuous treatment, and so on [67]. Each configuration will be discussed separately in detail below.
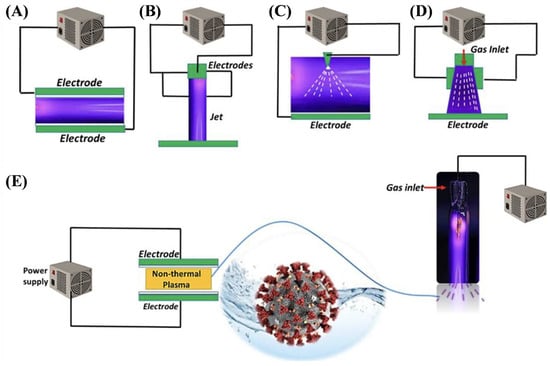
Figure 1.
Representative non-thermal plasma reactor designs relevant to wastewater treatment: (A) dielectric barrier discharge (DBD), (B) plasma jet, (C) corona discharge, (D) gliding arc discharge, and (E) inactivation of COVID virus in waster by non-thermal plasma. Each configuration influences plasma–liquid interaction dynamics and energy efficiency differently [67].
2.1. DBD
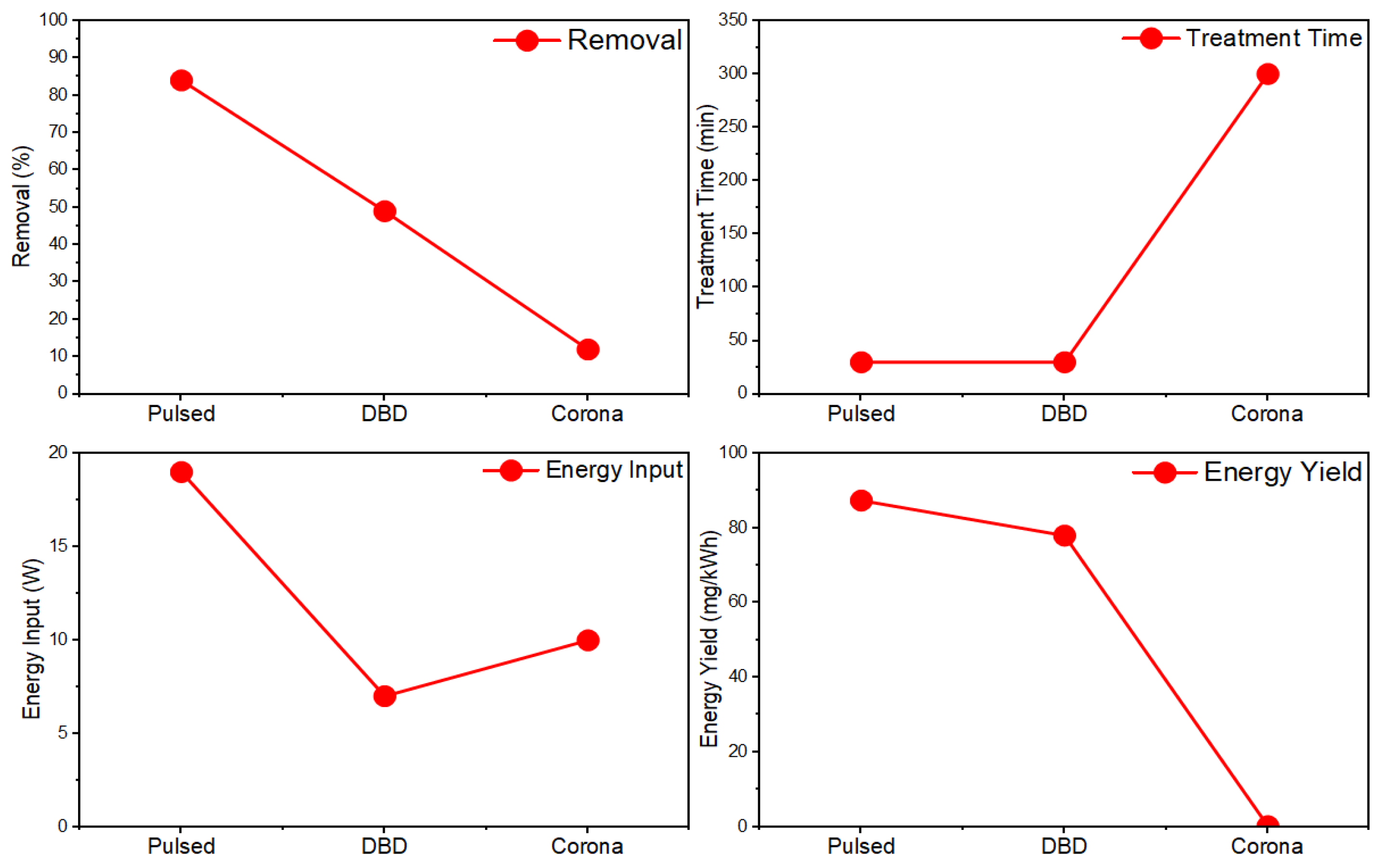
A popular NTP configuration is dielectric barrier discharge (DBD), in which a barrier made of a dielectric material is placed between electrodes to mitigate the discharge current and grants greater stability and design customizability than other configurations. One study investigated the effectiveness of a DBD reactor employing a semi-continuous design with a cylindrical quartz glass barrier in removing various textile dyes from wastewater [68]. A collection of 13 dyes including azo dyes, indole dyes, and more were used to produce model wastewater which was then treated in a semi-continuous DBD reactor to evaluate degradation. Such pollutants can be relatively stable and difficult to remove via conventional means, yet the results showed between 90% and 95% removal for all dyes after 600 s of plasma treatment, exemplifying the technology’s promise as a powerful wastewater treatment option for difficult pollutants. A comparison of the performance of some non-thermal plasma reactor configurations for PFOA degradation in water under the same conditions is provided in Figure 2 [69].
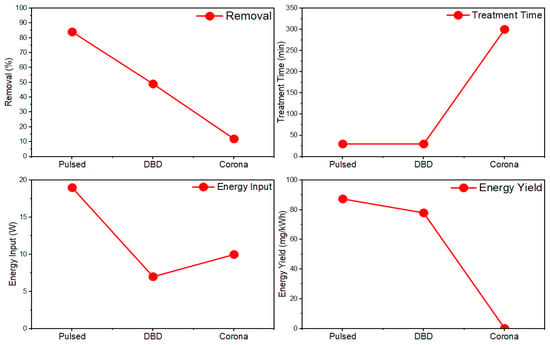
Figure 2.
Comparison of the non-thermal plasma reactor configurations for the degradation of PFOA in water [69].
2.2. Jet
Although not quite as commonly employed in wastewater treatment, plasma jets are another type of discharge which can be used and has been explored in a recent publication [70]. With the goal of remediating the textile dye methylene blue (MB) in an aqueous solution, a plasma jet system was developed using pin-to-plate style discharge between a stainless steel HV rod and a steel mesh ground electrode to discharge into gas just above the liquid sample. H2O2 was identified as the most critical ROS for pollutant degradation, and across possible input gases of air, nitrogen, and argon, argon was found to result in the best performance. With that setup, it was found that over 99% MB degradation at 30 ppm concentration could be achieved in 40 min of treatment, dropping only a couple percentage points for concentrations up to 50 ppm.
2.3. Corona
One of the most widely studied NTP configurations in the context of wastewater treatment is corona discharge, which typically involves a sort of pin-to-pin or pin-to-plate electrode pairing and is often favored for its relative simplicity, lower energy consumption, and effectiveness of plasma generation compared to other methods. One study [71] provided an exploration of the use of pulsed corona discharge plasma (PCDP) for the inactivation of waterborne viruses. Water samples contaminated with spring viremia of carp virus (SVCV) were treated in a PCDP reactor under multiple exposure lengths and voltage intensities before being injected into zebrafish to evaluate for survivability and virus activity. It was shown that a 4 log10 reduction in viral infectivity could be achieved after 120 s of treatment and that water samples treated for 240 s showed no significant difference from non-viral control samples in terms of fish survivability, revealing the effectiveness of this NTP configuration for water treatment.
2.4. Gliding Arc
Dye removal can similarly be accomplished through gliding arc discharge (GAD) plasma, as shown in the work of Merouani et al. [72]. In their study, a gas-phase reactor utilized a discharge between diverging electrodes, which were used to generate and amplify a gliding arc discharge over a target solution consisting of the dyes Alizarin red (ARS) and Orange G (OG) both separately and in mixtures. It was found that after 120 min of treatment, over 61% degradation and 81% discoloration of the dyes could be achieved, establishing the useability of GAD as an option for wastewater treatment if it could be further optimized.
2.5. Ozonation
Ozonation is an established sanitation process in industry, and while it can be employed without the use of NTP, the ability of NTP to generate ROS such as ozone makes it a viable mechanism for driving ozonation. One study [73] took advantage of this feature to evaluate the effectiveness and efficiency of plasma-induced ozonation in the treatment of both chemical and microbial contaminants in various water samples. Oxygen gas was supplied to a chamber in which discharge between a pair of hollow copper electrodes generated reactive species, which then passed through an outlet into the wastewater chamber. It was found that ozone generation could vary greatly across different reactor settings, with a discharge voltage of 16 kV for a treatment time of 15 s that produced the highest gaseous concentration of 490 ppm, while 16 kV for 40 min achieved a maximum liquid concentration of 51.74 ppm. This generated ozone was also shown to be effective against wastewater pollutants, reducing total dissolved solids in sewage and tap water samples from 462 mg/L and 355 mg/L to 282 mg/L and 212 mg/L, respectively.
Overall, the DBD reactors offer stable and controllable plasmas, achieving high PFAS removal rates of up to 97%. However, their energy efficiency is moderate, and discharge uniformity is constrained. Plasma jets, originating from dielectric barrier discharge (DBD) systems, facilitate the localized and continuous treatment of intricate matrices; however, their limited active zones pose challenges for scaling up. Corona discharges are cost-effective and straightforward; however, they exhibit the lowest PFAS degradation, approximately 12%, attributed to inadequate plasma–liquid interactions and irregular streamer formation. Gliding-arc plasmas, in contrast, integrate non-thermal electrons with moderate heating, resulting in superior PFAS removal rates exceeding 99% and effective energy transfer. However, this approach incurs higher gas temperatures and potential electrode degradation. In summary, DBD and gliding-arc systems are identified as the most effective options for PFAS abatement. However, all NTP technologies continue encountering issues related to high energy consumption, incomplete mineralization of short-chain PFAS, and a limited understanding of the underlying mechanisms [69].
3. Scale-Up of NTP for Wastewater Treatment
Historically, scaling up NTP in wastewater treatment applications has largely been a challenge of achieving adequate treatment effectiveness while maintaining commercially attractive energy and cost efficiency [74]. Most research on NTP systems takes place at the bench scale treating smaller volumes than would be expected in real-world wastewater treatment applications, and prior reviews on this topic typically conclude that more work is needed to prove the technology’s feasibility in an industrial setting. This section aims to examine recent examples of scale-up work and analysis in the literature and provide commentary on its status and potential future directions.
3.1. Scale-Up Microbial and Pharmaceutical Wastewater Treatment
3.1.1. Plasma Jet + Cavitation
Microbial and pharmaceutical contaminants in wastewater are a significant health hazard, which is one that can be effectively tackled by NTP. Plasma-activated water (PAW) is an approach in which a plasma discharge is used to produce reactive species in water, which is then in turn used as a disinfectant. It is a common and powerful strategy for achieving microbial deactivation and can be applied to wastewater effluent either through gas–liquid diffusion or directly into the water itself.
One study [58] utilized PAW in conjunction with hydrodynamic cavitation (HC) for the degradation of antibiotics sulfathiazole and norfloxacin, as well as azo dye methylene blue (MB). The experimental setup employed a continuous water flow loop, in which microbubbles produced in a Venturi tube were meant to boost gas-to-liquid mass transfer of reactive species. It was found that every pollutant could be degraded by over 80% under a volume of 500 mL and a flow rate of 5 L/min. Scale-up potential was also demonstrated by applying the treatment to 2 L and 10 L volumes of MB, for which up to 96% of MB was found to be degraded after 30 min of treatment in the PAW system, though effectiveness was heavily dependent on dye concentration. It is claimed that this was the first published use of NTP for dye waste treatment at such a scale, and that the novel, continuous PAW flow system design used was robust for scaling up to larger volumes.
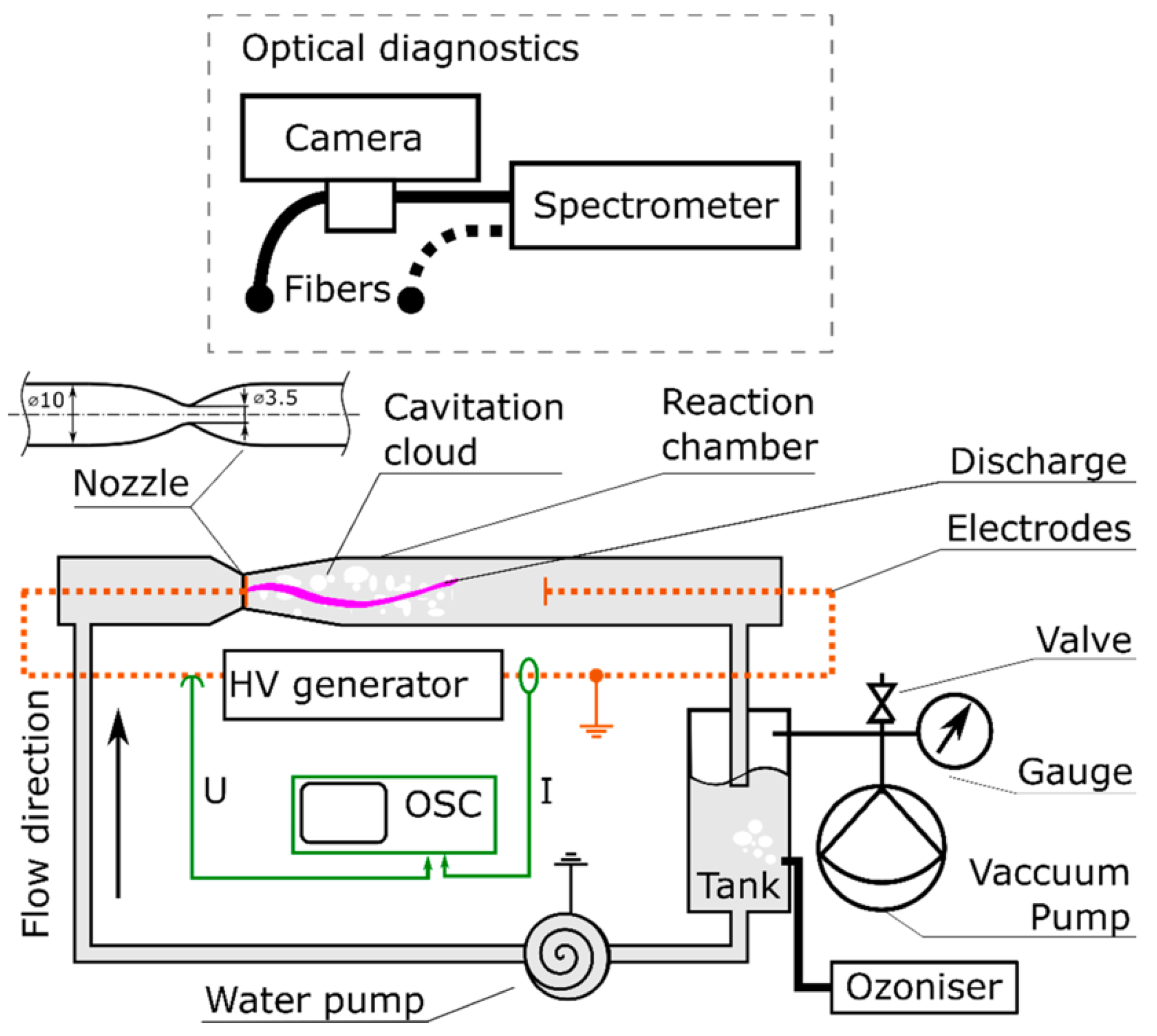
While many studies related to PAW microbial deactivation focus on medical settings and pathogens, it is applicable to microbial deactivation in any setting. One study [75] provided insight into its use against environmental microbes such as cyanobacteria and algae. Here, a hydrodynamic cavitation plasma jet (HCPJ) configuration, showcased in Figure 3, was employed to combine NTP discharge with hydrodynamic cavitation as an effective and relatively efficient means of producing mass quantities of reactive species. Results showing a 0.55 m3/h maximum production rate for PAW placed the performance of the experimental beyond all other PAW production methods found in the prior literature by at least 15 folds. A single 13 s cycle in a 1:1 batch treatment was sufficient to neuter all cyanobacterial metabolic activity and achieve complete sterilization after 24 h. More experimental replication and larger, pilot-scale studies may be needed to validate the scalability of this approach, but the findings nonetheless represent a significant step forward in making PAW-based treatments more accessible and cost-effective.
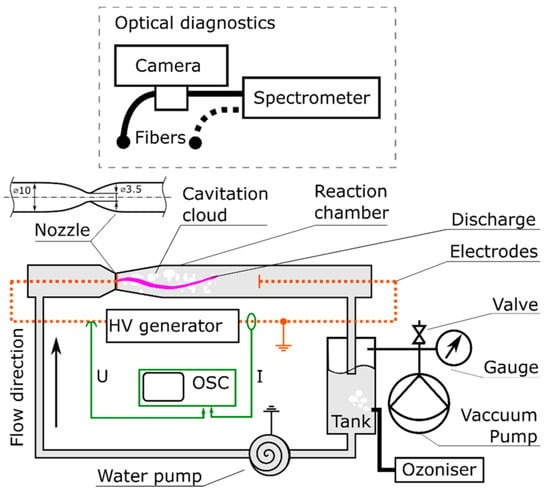
Figure 3.
Experimental setup consisting of the HCPJ unit, vacuum unit, ozonizer, and electrical/optical diagnostics [75].
Boosted PAW generation appears to be one of the more promising approaches for scale-up, due to the diversity of pollutants it can destroy, as well as not requiring overly complex or specific equipment to operate. It has proven highly effective at the lab scale for microbial inactivation and in some chemical pollutant removal applications. However, as with most NTP-based solutions, more work is needed to prove a combination of scalability and cost-effectiveness compared to existing market solutions. High energy consumption and generation time for PAW remain the biggest hurdles, so efforts should be focused on streamlining existing processes to generate more PAW at reduced energy and time.
3.1.2. Corona Discharge—Advancements and Comparisons
As one of the most common NTP configurations seen in the literature, corona discharge is valued for its relative energy efficiency and high generation of reactive species. Among recent works, one study used a pilot-scale pulsed corona discharge (PCD) in combination with membrane ultrafiltration (UF) to degrade pharmaceuticals in hospital sewage and in the water of a healthcare center after biological treatment [76]. The configuration is shown in Figure 4, in which the reactor was part of a water circulation system that included a tank with a capacity of 50 L, with water circulating through at a rate of 15 L/min for experiments. A total of twenty-nine pharmaceuticals were identified in wastewater of the Etelä-Karjala Central Hospital, and all but a few—caffeine, hydrochlorothiazide, and metronidazole—were found to be readily oxidized by the NTP treatment. In the Rinnekoti Foundation’s biologically treated effluent, sufficient oxidation of all seventeen observed pharmaceuticals was achieved to render all of them below quantification. Since ultrafiltration was noted as having a minimal impact on pollutant reduction, NTP contributed to the removal of most pollutants. The compact circulation design enabled effective contaminant contact and demonstrated >87% pharmaceutical removal, highlighting the viability of PCD for scaled operations. A lack of market equivalents prevented an accurate capital cost assessment, but the NTP process was noted as being a more thorough treatment approach than alternatives such as absorption, which may require additional treatment steps.
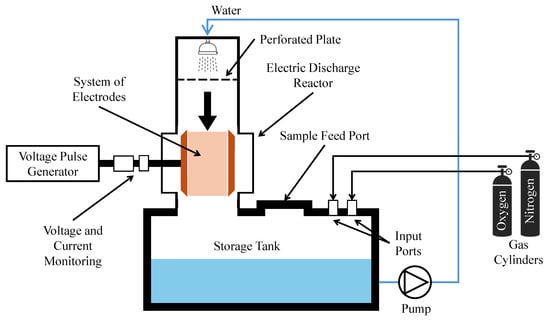
Figure 4.
Experimental PCD configuration for treatment of 50 L batches (not to scale) [76,77].
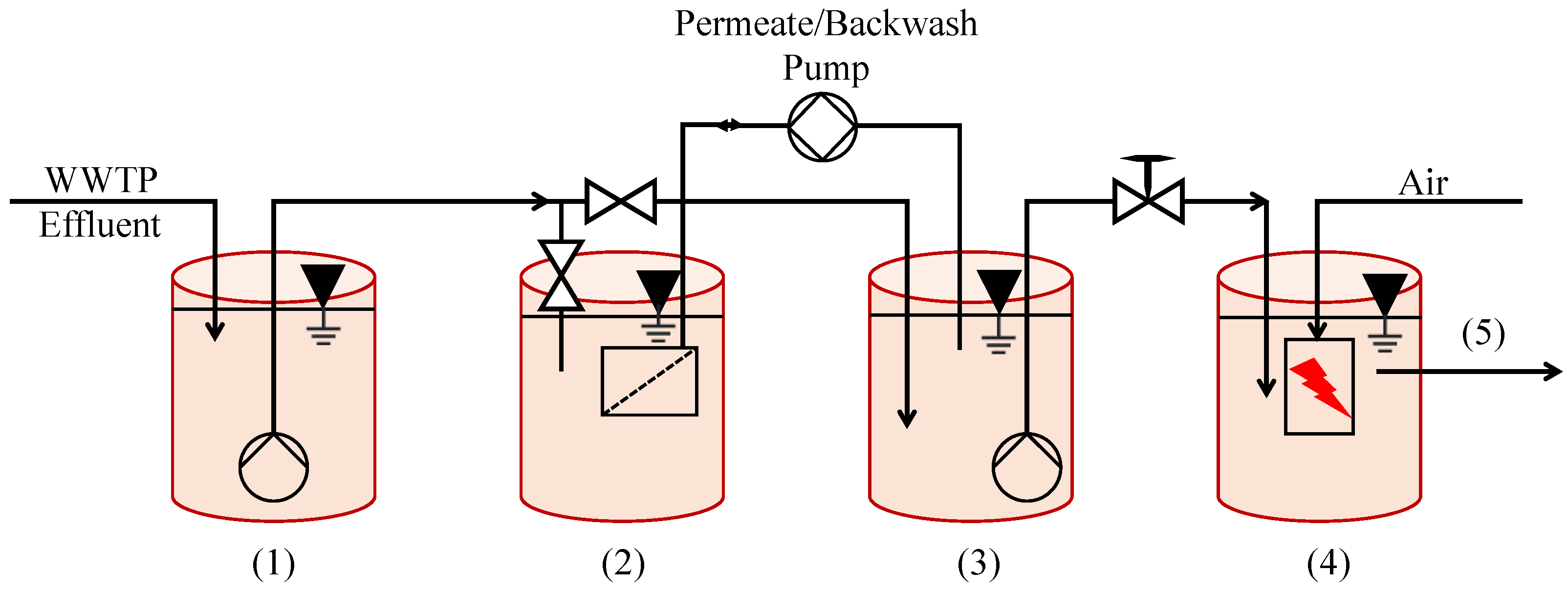
Despite the above conclusions, however, ultrafiltration may still be a viable means of enhancing the commercial viability of NTP. Another pilot-scale approach combining NTP with UF was conducted in Bad Reichenhall, Germany to treat activated sludge effluent (Figure 5) [78]. Selected treatment products included diclofenac (DCF), carbamazepine (CBM), and sulfamethoxazole (SMX), all known pharmaceuticals. Design and process optimization culminated in a setup capable of achieving a >90% reduction in the pollutants, with retention times of 20–40 min. Ultrafiltration could not outperform NTP on its own, but when they were used together, its energy- and cost-efficiency could allow greater volumes of wastewater to be effectively treated.
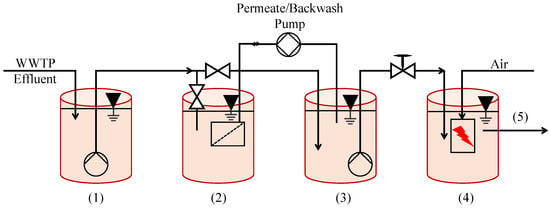
Figure 5.
Scheme of the pilot plant used in Bad Reichenhall, Germany, (1) WWTP effluent, (2) fil-tration tank, (3) permeate tank (backwash), and (4) downstream NTP tank and (5) final treated effluent [78].
3.1.3. Corona Reactor Design Advancements
Another study [79] also evaluated corona discharge reactors for the remediation of select pharmaceutically active compounds (PhAcs), but with greater emphasis on variations in reactor design and discharge setup as well as a more thorough cost analysis. Sequential flow plasma reactor (SFR), continuous-flow top-discharge plasma reactor (TDPR), and continuous-flow side-discharge plasma reactor (SDPR) configurations were compared in terms of diclofenac (DCF) and verapamil hydrochloride (VPL) degradation. The SPDR was found to have the best overall performance, achieving 99% removal of DCF and VPL at hydraulic retention times (HRTs) of 9 and 12 min, respectively. The continuous processes showed mass transfer coefficients higher than what was seen in many other studies, as well as superior cost-effectiveness with the SDPR that had an operating cost of 0.25 $/m3. These findings bring corona NTP a step closer towards more widespread use in tertiary wastewater treatment.
Put together, there are many directions in which corona-based wastewater treatment could be developed. Several pilot studies have already proved effective in real-world applications, with optimized reactor designs, synergetic combinations with other processes, and larger treatment volumes, which all bring the technology closer to adoption at an industrial scale. Examined more broadly, corona discharge can be desirable due to typically higher reactive species generation than other discharge types but can be held back by the limited controllability and limits on power applications allowable for the corona discharge itself. These can prove especially challenging to tackle when attempting to scale up the technology, where versatility and cost-effectiveness are of primary concern.
3.1.4. DBD—Advancements and Life-Cycle Analyses (LCAs)
Dielectric barrier discharge (DBD) stands as one of the most used reactor configurations in NTP research. DBD reactors employ a dielectric barrier between electrodes to help control current and maintain uniformity within a plasma discharge, making treatments more controllable while retaining most of the advantages of NTP processes. At the same time, however, adding a dielectric element may reduce the allowable volume in the reactor chamber and add additional considerations in terms of materials and cost, as well as affect the reactor’s energy efficiency. All of these must be justified by the reactor’s performance compared to non-DBD configurations.
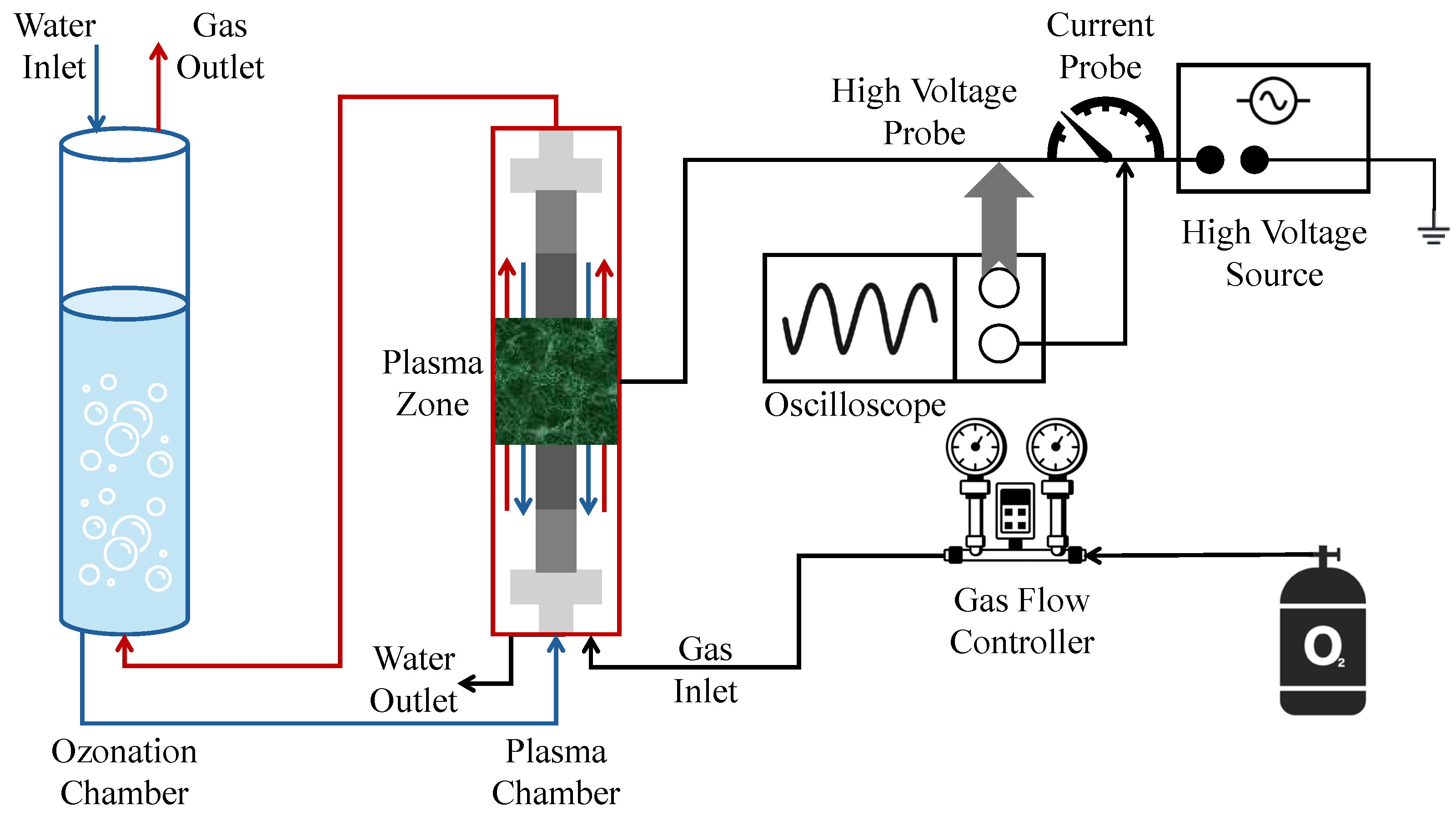
A treatment system consisting of a DBD reactor followed by an ozonation chamber was used in the degradation of emerging micropollutants (µPs) with an eye towards industry-scale applicability (Figure 6) [80]. µPs include pesticides, herbicides, pharmaceuticals, and other such pollutants that may be troublesome for traditional activated sludge treatments to handle. µP degradation efficiency falls in the range of 84–98% across seven chosen µPs, and energy consumption ranges from 2.4 to 5.3 kW/m3. The use of a varied wastewater sample and a continuous process are targeted at addressing industry needs to avoid the limitations of simplistic synthetic wastewater and a batch process.
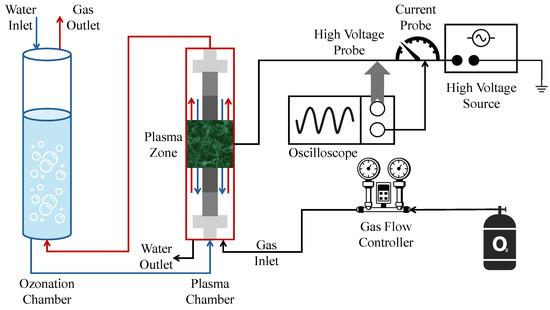
Figure 6.
Schematics of the DBD + ozonation setup with plasma chamber (right) and ozonation chamber (left) [80].
Despite often being criticized for high energy consumption compared to alternatives, NTP treatment processes can offer unique advantages that are not always quantified or appreciated. In one work [81], a life-cycle analysis (LCA) was performed on NTP in a DBD configuration compared against quaternary wastewater treatment methods including sand filtration (SF), nanofiltration (NF), ultrafiltration (UF), ozonation (OZ), reverse osmosis (RO), and UV disinfection for the removal of pharmaceutical compounds (PhCs) in water. Pilot-scale units for all processes were operated at Portuguese wastewater treatment plant (WWTP) in accordance with the regulations of ISO 14040 [82] and ISO 14044 [83], the results were upscaled for a comparative cradle-to-gate LCA. Among the various treatments and combinations attempted, NTP joined the majority by achieving >80% pharmaceutical removal, as well as the lowest operating costs despite having the highest energy consumption at 0.24 € m−3 compared to UF + RO at 0.31 € m−3, UF + NF at 0.33 € m−3, and FA + OZ at 0.41 € m−3. This is attributed to the lack of reagents and relatively low equipment maintenance costs of NTP systems compared to others, which also allow it to score as one of the most environmentally friendly options in the LCA. Thus, while high energy consumption remains a challenge in NTP scale-up and commercialization, the other benefits in which it can save costs and promote environmental responsibility compared to alternatives may still make it a competitive market solution.
3.2. Scale-Up NTP for Industrial Wastewater Treatment
3.2.1. Corona Discharge Advancements and Comparisons
In addition to pharmaceuticals, much experimental work on NTP-based wastewater treatment makes use of dyes as a representative for industrial wastewater effluent. For example, dye byproducts can frequently be found in the textile industry and are known to have adverse health effects for both humans and the environment [84]. At the same time, existing wastewater treatment methods such as photolysis and biodegradation are proving insufficient and/or inefficient for dealing with such pollutants [85]. Compared to medical and/or pharmaceutical wastewater effluents, pollutants of industrial processes tend to be non-living chemical waste products that must be oxidized and/or separated from the waste discharge in some manner.
Another study [86] explored the use of a corona dielectric barrier discharge (cDBD) reactor for the remediation of methylene blue (MB) with a special focus on making the process energy-efficient and environmentally friendly. After optimizations, a 98% removal rate for MB was achieved within 10 min, while calculated energy and capital costs were several orders of magnitude lower. Although the microreactor’s 10 mL volume was small in scale, the practical analysis revealed that the plasma treatment was both highly efficient and highly cost-effective compared to other existing AOPs such as ozonation, UV light, photocatalysis, and ultrasonification, among others. Having these advantages, while also being chemical-free and without long-lasting by-products, makes NTP a front runner in next-generation wastewater treatment.
3.2.2. DBD and DBD-like Discharge—Energy Efficiency and Cost Analysis
From the standpoint of industrial pollutants, much experimental work has focused on the treatment of textile dyes, which contribute heavily to chemical contamination in water bodies of all types. Industrial dye decolorization has been examined recently using DBD reactor setups [87], aiming to provide optimizations that will make the reactor more energy-efficient while retaining its effectiveness for remediating aquatic environments. Methylene blue is again the dye target, whereas for the reactor, having a larger dielectric and outer electrode represents a significant departure from conventional DBD designs to allow for greater gas flow that provides enhanced cooling of the electrodes. The reactor designed was shown to cut the chromaticity of industrial dye wastewater by 80% after 30 min, with an energy cost nearly half that of standard DBD and even more so beyond photocatalysis and UV light. Although demonstrably high energy efficiency should be verified in a larger industrial-scale model, the level of efficiency achieved compared to other processes was impressive.
3.2.3. Optimization of Reactive Species Generation
A seemingly less common but still valuable research approach to enhancing NTP scale-up viability is through analysis and control of the reactive species themselves to obtain optimal conditions to produce certain types of species for a given application. This is often overlooked in studies aiming to evaluate novel reactor designs and/or system configurations, and it remains an area of potential optimizations in terms of NTP treatment effectiveness and costs associated with reactive species generation. The quantity of species needed can be mitigated if certain species can be established to be more effective in given settings.
This is the perspective taken by a recent publication on the subject [88], which utilized different electrode configurations and reactor conditions to favor certain reactive species for the degradation of MB dye. The reactor itself was an innovative fountain DBD (FDBD) design, and four distinct configurations were evaluated against one another for reactive species production and economic/energy efficiency, differing in composition of the central tube (copper, Cu or alumina ceramic, Ce) and whether a water-cooling system was used. It was found that Ce setups produced a greater quantity of reactive oxygen species (ROS), especially H2O2. Cu setups produced less H2O2, and water cooling in both cases resulted in more NO2− production while lacking H2O2. Consequently, the Ce setup proved to be most effective and efficient in terms of MB degradation, whereas the water-cooled setups were both less efficient and more prone to generating toxic byproducts, culminating in a nearly 30% difference in cost between the most- and least-efficient configurations. A cost analysis also revealed that a hypothetical scaled-up version of the FDBD reactor system would be cheaper than most AOP alternatives in both setup and operating costs, which is highly promising. These findings exemplify the continued advancement of newer and better NTP reactor designs for wastewater treatment, while also highlighting the importance of tuning based on desired reactive species.
3.3. Scale-Up and Pilot Studies of NTP for PFAS Treatment
While laboratory-scale investigations have established the potential of non-thermal plasma to degrade PFAS, translating these findings into field-ready technologies requires addressing several engineering and operational challenges. A growing body of research has begun to explore the behavior of NTP systems under pilot-scale and near-field conditions to offer insight into the limitations and opportunities for broader adoption.
One of the most prominent field demonstrations was conducted by Nau-Hix et al. [89], who deployed a trailer-mounted DBD plasma reactor system at Wright-Patterson Air Force Base. The system operated at flow rates up to 8.4 L/min and achieved over 90% removal of long-chain PFAS, including PFOA and PFOS, in a single pass. The authors noted that the removal of short-chain PFAS remained limited; however, the introduction of a cationic surfactant could yield modest improvements. Importantly, this study demonstrated that plasma-based systems could be mobilized and operated under field conditions while maintaining performance consistency—a key step forward in demonstrating scalability.
In a complementary effort, Singh et al. [90] examined a mobile, skid-mounted plasma reactor using pulsed corona discharge technology for the treatment of investigation-derived waste (IDW) collected from 13 Department of Defense sites. Their 4 L batch reactor achieved rapid degradation of long-chain PFAAs and demonstrated robustness under variable influent compositions. Energy efficiencies ranged from 9.2 to 31 kWh/m3, with an average of 16 ± 5.8 kWh/m3 and estimated treatment costs were approximately $7.30 per 1000 gallons. These findings directly support the feasibility of plasma systems as cost-competitive, field-deployable technologies and contribute empirical energy data for scale-up benchmarking.
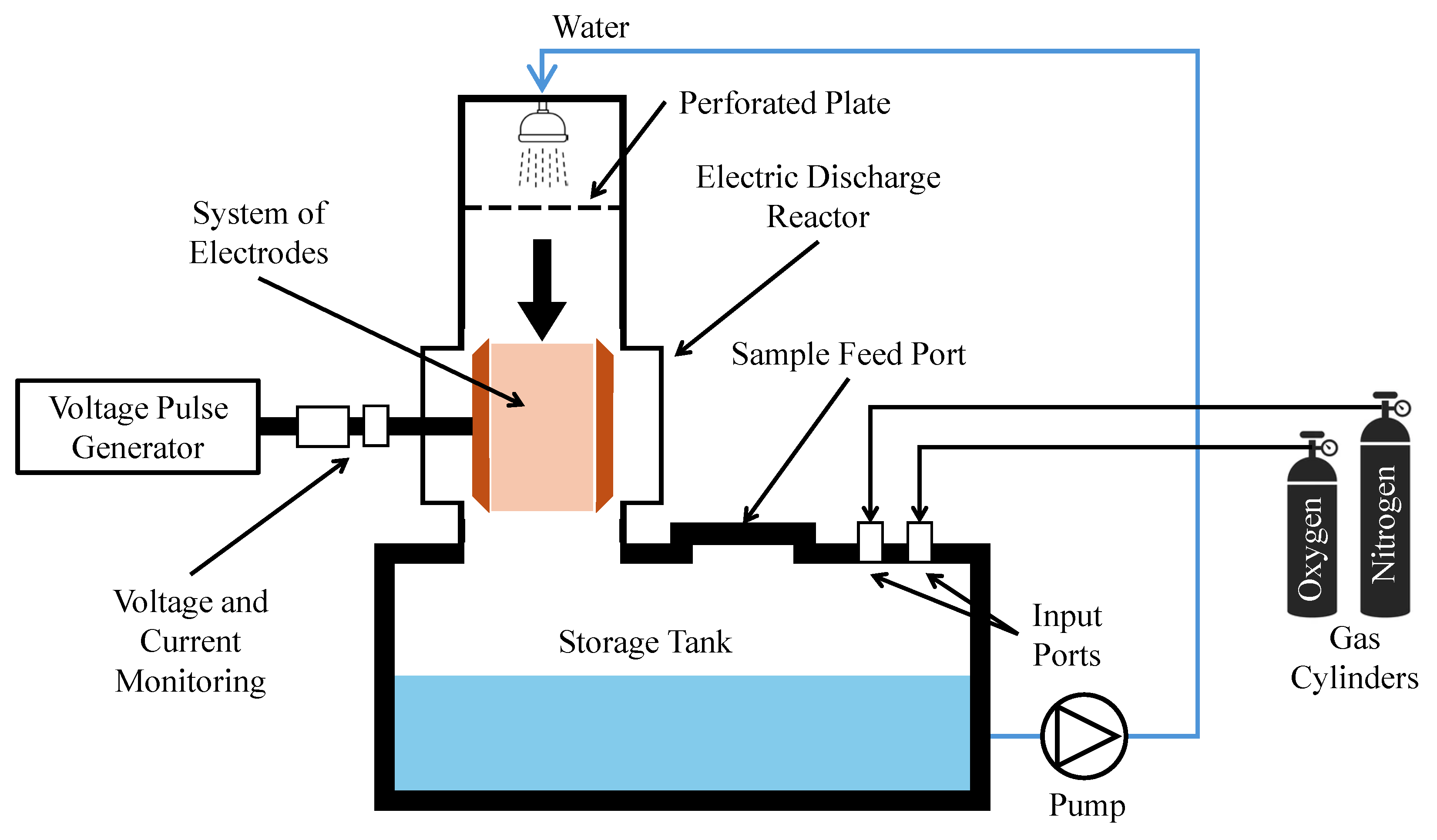
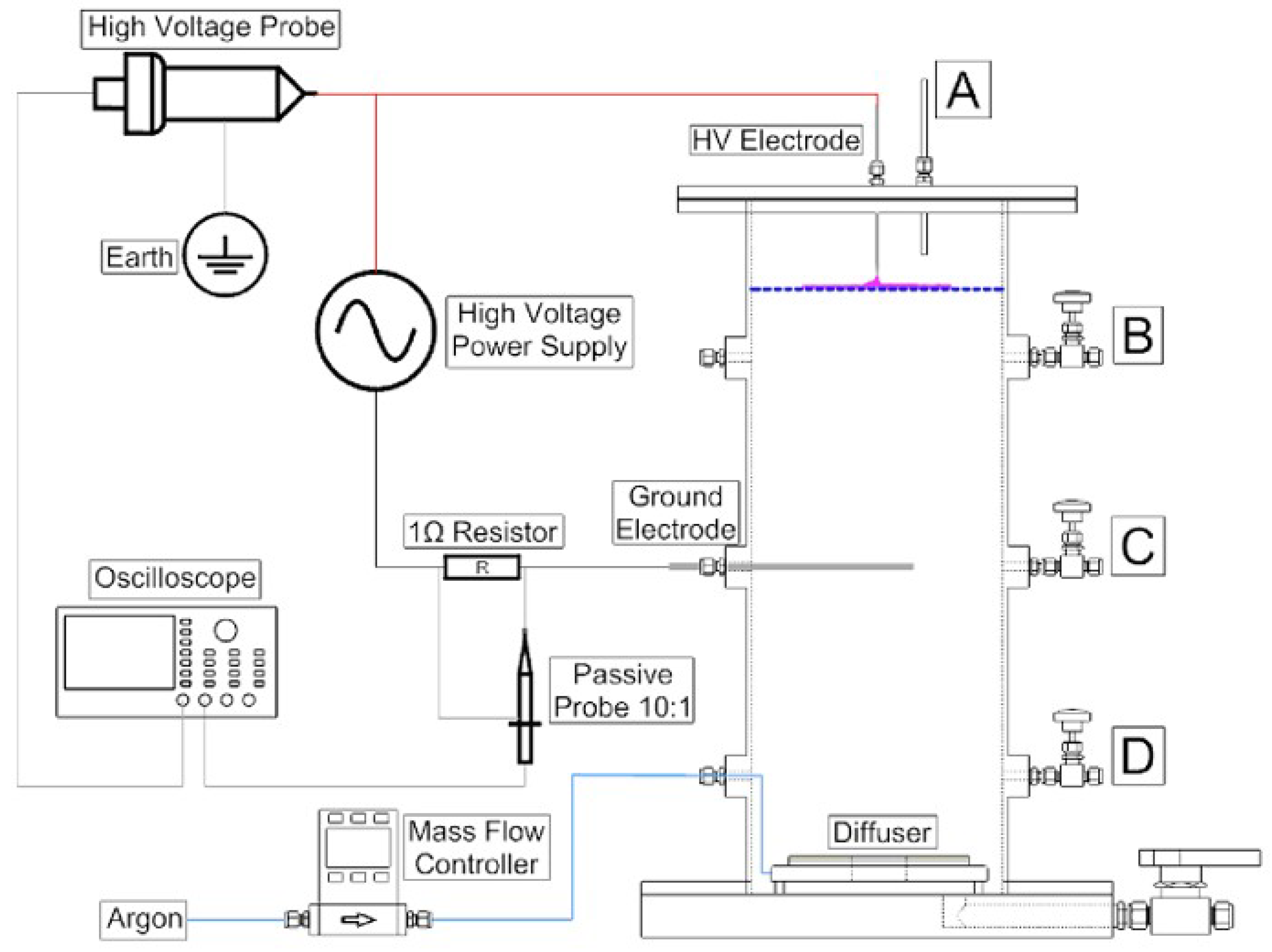
Recent pilot studies have also focused on optimizing plasma reactor designs for PFAS degradation under realistic flow conditions. Figure 7 and Figure 8 illustrate two distinct approaches: a bubble-driven plasma reactor and a falling-film plasma reactor, respectively. Alam et al. [91] investigated PFOS degradation in a 25 L pilot-scale bubble-driven NTP reactor. Incorporating fine bubble diffusers significantly increased the interfacial contact area between plasma species and aqueous PFOS (Figure 7). The fine bubble system produced a degradation rate constant of 0.16 ± 0.01 min−1, approximately 290% higher than that of medium bubble diffusers. A plug flow model was also developed and validated to predict degradation kinetics. This work provides critical insight into how reactor geometry and hydrodynamic parameters influence degradation performance and offers tools for guiding the scale-up of diffusion-limited plasma systems.
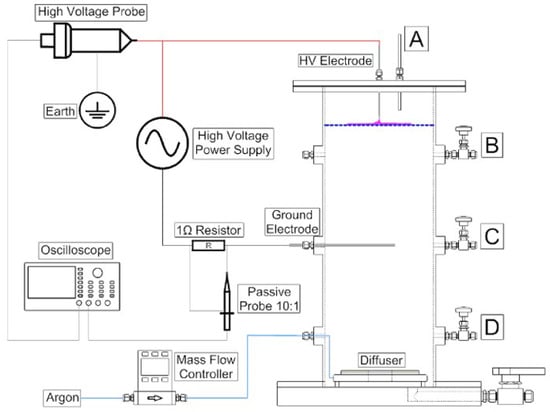
Figure 7.
Schematic diagram of the 25 L plasma treatment reactor with four manual (A) from surface and (B–D) from column wall liquid sample drawn points [91].
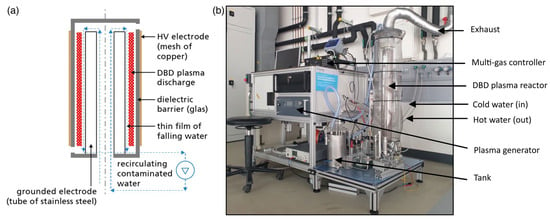
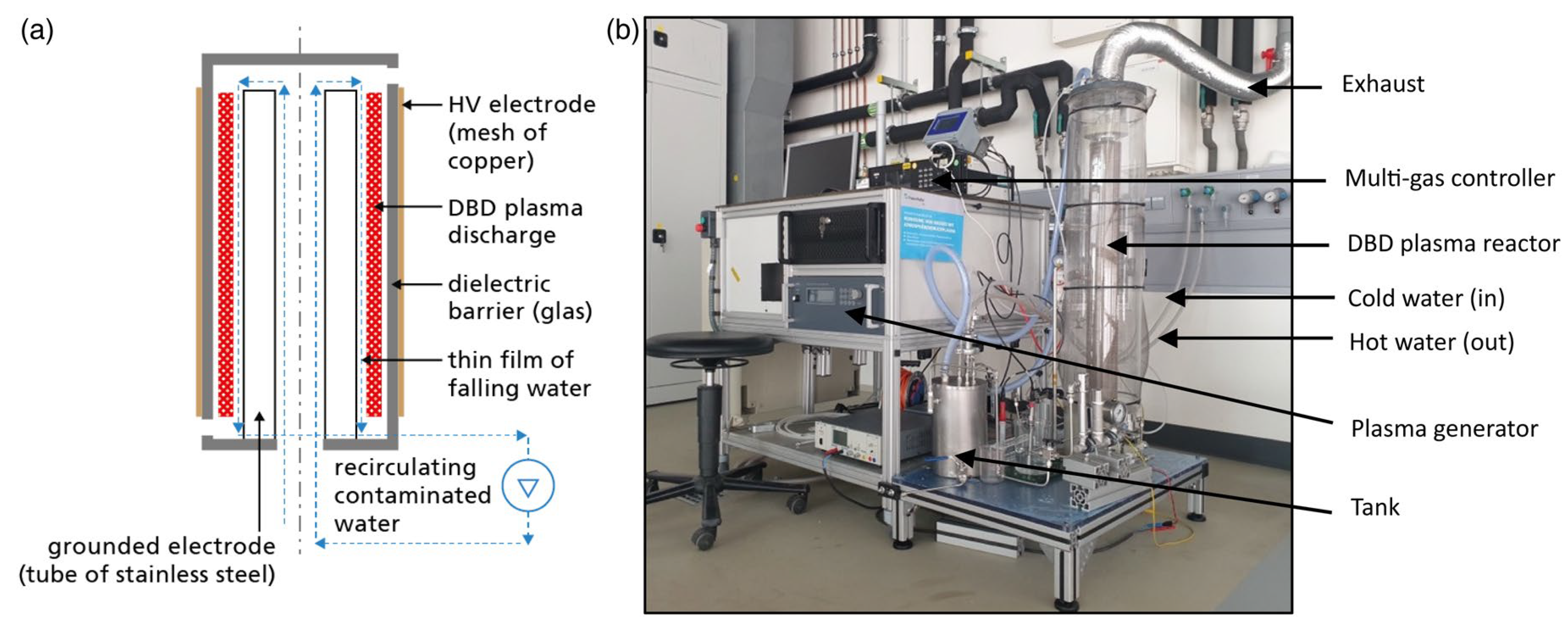
Figure 8.
(a) Schematic diagram of the experimental setup and (b) a DBD plasma reactor at a pilot plant scale with 5 L water tank [92].
A separate effort by Tamang et al. [92] utilized a 5 L falling film DBD reactor to treat both synthetic and real PFAS-contaminated groundwater sourced from fire-training areas (Figure 8). Their work focused on assessing the effects of carrier gas composition and applied voltage on degradation performance. They reported energy efficiency values between 0.31 and 15.31 mg/kWh and observed that plasma generated using air could achieve up to 63.75% PFOA removal. These results highlight the substantial influence of operating conditions on both treatment efficacy and energy consumption, reinforcing the importance of plasma tuning and optimization in scale-up designs. Thus, while the bubble-driven system achieved superior interfacial mass transfer rates, the falling-film configuration offered improved energy efficiency depending on operating voltage and carrier gas.
Collectively, these studies demonstrate growing momentum toward scalable, field-capable plasma technologies for PFAS treatment. Each example underscores different aspects of the scale-up challenge from energy efficiency and cost modeling to reactor design and matrix sensitivity and highlights the importance of integrated design approaches moving forward. Continued efforts in model development, material selection, and pilot-scale testing will be essential for establishing non-thermal plasma as a viable and sustainable solution for PFAS remediation at scale.
Among emerging configurations, bubble-enhanced systems stand out for scalability because gas–liquid interfacial area and mass transfer can be engineered at pilot scale; microbubbles improve energy yield and degradation kinetics, with bubble size shown to control PFAS removal performance and thus offer a rational scale-up handle [58,89,90,91]. DBD-based plasma-activated-water (PAW) generators—including fountain-style variants of DBD contactors—offer tunability of oxidant speciation (RONS) for matrix-adaptable treatment and can be produced in large volumes for distributed use. Still, they typically require post-contact handling/storage and careful control of species stability [75,88]. Pulsed-corona and underwater discharge reactors demonstrate compelling pilot-scale throughput for complex effluents (hospital wastewater, and dyes) with manageable energy intensities and pathway clarity via radical/molecular oxidants. Yet, they face challenges around absolute energy demand and optimization across variable water chemistries [76,79,80,87]. Hybrid configurations that pair membranes with plasma, lower specific energy by pre-concentrating targets, and mitigating scavengers, are promising for plant-level integration. However, fouling/pretreatment logistics add complexity [78]. Cross-cutting assessments indicate that while non-thermal plasma can deliver rapid removal even for recalcitrant-like PFAS at pilot/field scales, comparative life-cycle burdens hinge on electricity mix and reactor efficiency—underscoring the need to report standard metrics (mg·kWh−1, kWh·m−3) and to benchmark designs under identical matrices and flow regimes to substantiate claims of scalability and sustainability [58,76,79,81,89,90].
In plasma treatment, the liquid-phase byproducts primarily comprised shorter-chain perfluoroalkyl acids (PFCAs) and sulfonates, which were produced through stepwise defluorination and decarboxylation of parent compounds like PFOA and PFOS. The substances identified were PFHpA, PFHxA, PFPeA, and PFBA, in addition to PFHxS and PFBS, resulting from the transformation of PFOS. Oxygenated intermediates and fluorinated organo-sulfonates were identified as transient products. The liquid phase included short-chain organic acids, specifically trifluoroacetic, acetic, and formic acid, alongside fluoride ions and CO2, suggesting near-complete mineralization in the later stages (Figure 9). The detected gaseous byproducts were mainly cyclic perfluoro-alkanes, which were formed via intramolecular radical cyclisation reactions initiated by plasma-generated electrons and hydroxyl radicals. Their total concentration was two orders of magnitude lower than that of the aqueous byproducts, and they underwent further degradation with continued plasma exposure. Plasma treatment effectively reduces PFAS levels; however, monitoring short-chain PFAS and gaseous fluorocarbon byproducts is essential to mitigate secondary pollution risks [26].
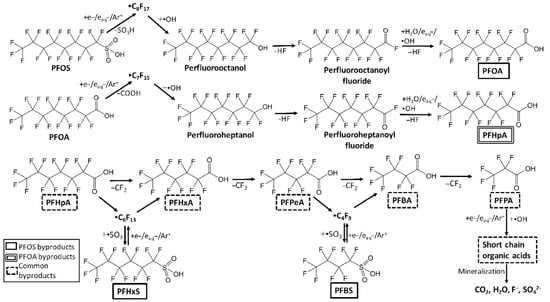
Figure 9.
Proposed degradation pathways for the PFOA and PFOS degradation via plasma technology [26].
4. Perspectives on Scaling Up NTP for Wastewater Treatment
The results summarized in Table 1 demonstrate that recent work has made meaningful progress toward establishing non-thermal plasma (NTP) as a feasible candidate for scale-up in wastewater treatment. Broadly speaking, studies can be categorized by their efforts to optimize pollutant degradation performance, reduce energy and capital costs, and extend treatment to more complex water matrices. Several pilot-scale studies have reached volumes ranging from tens to hundreds of liters, with several of them achieving >80% removal of pharmaceutical and industrial pollutants in real wastewater conditions [63,81,87]. In one particularly large study, a glow discharge–ultrafiltration system achieved >90% removal in treating 410 L of activated sludge effluent [78]. However, these scaled-up treatments still fall short of the removal efficiencies often reported in small-volume, lab-scale studies using synthetic wastewater, where >99% degradation can be achieved [79].

Table 1.
Summary of results and perspectives on scale-up of wastewater treatment with NTP.
The new PFAS-focused pilot studies further enrich this discussion by addressing a class of contaminants that are uniquely persistent and structurally resistant to conventional treatment. The work of Nau-Hix et al. [89] is especially noteworthy as a true field demonstration of a trailer-mounted DBD system, which achieved >90% removal of long-chain PFAS at flow rates up to 8.4 L/min. The exploration of surfactant-enhanced treatment also illustrated how interfacial chemistry might be leveraged to address short-chain PFAS, a key challenge in real-world deployments. Singh et al. [90] contributed essential operational metrics from a pulsed corona discharge system, including cost benchmarks and energy efficiencies ranging from 9.2 to 31 kWh/m3, providing a much-needed frame of reference for technoeconomic evaluations.
A recurring theme across both legacy and PFAS-specific studies is the effectiveness of combined treatment approaches. NTP coupled with ultrafiltration, ozonation, or cavitation often yields higher removal rates than NTP alone. These combinations tend to mitigate NTP’s limitations such as short residence times, limited diffusion depth, and reactive species decay by enhancing contact time, facilitating mass transfer, or preconditioning the wastewater. For example, the 410 L glow discharge study [78] showed significantly improved energy efficiency when paired with ultrafiltration. Similarly, earlier studies [58,76,80] demonstrated that hybrid processes could achieve faster pollutant removal across multiple pollutant classes.
The studies by Alam et al. [91] and Tamang et al. [92] deepened the conversation around reactor design and gas–liquid interface dynamics, both of which are critical for scale-up success. The use of a 25 L reactor by the former to compare fine and medium bubble diffusion showed that enhanced mass transfer increased PFOS degradation rates by 290%, supported by plug flow modeling. The falling film reactor studied by the latter, on the other hand, tied plasma input gas composition and voltage directly to energy efficiency, with PFOA removal reaching 63.75% in real groundwater. These insights reinforce the idea that reactor architecture and hydrodynamic control are not merely supportive variables but are central to optimizing performance in scaled systems.
Despite these promising developments, several persistent challenges remain. Chief among them is the inconsistency in how energy consumption and degradation performance are reported. Metrics like energy per order (EE/O) or mg/kWh are increasingly used, but not universally, making comparisons across studies difficult. Furthermore, while some studies incorporate cost analysis, these are often internal estimates or limited to electricity use. Broader lifecycle cost assessments, like those found in [81], remain rare but are necessary to fully evaluate NTP viability in real-world utilities.
Another area ripe for development is contaminant-specific optimization. Some studies, like those by Kooshki et al. [88], explored the tuning of reactor inputs to favor the generation of specific reactive species, which could significantly improve performance against target compounds. In the context of PFAS, this could mean selectively producing hydrated electrons or perfluoroalkyl radical intermediates under controlled plasma environments.
Taken together, the existing body of work suggests that NTP scale-up is no longer just a matter of treating larger volumes—it is about integrating chemical, physical, and operational insights to tailor systems for specific contaminants and applications. The recent emergence of PFAS-focused pilot studies offers some of the strongest arguments yet for NTP’s role in next-generation water treatment, provided that future work continues to address the open questions of long-term stability, byproduct toxicity, and cost-competitiveness at scale.
For advanced oxidation processes, the standard measure is electrical energy per order (EE/O, kWh·m−3·order−1). Reported NTP EE/O ranges from approximately 1 to 6 kWh.m−3 for certain antibiotics and PFAS treatments in water by using optimized bubble/DBD reactors [91,93], whereas other NTP studies indicate values in the tens to hundreds for challenging targets or non-optimized matrices (e.g., PFOS/PFOA: approximately 23 to 213 kWh.m−3) [94]. Energy yields are also documented as g.kWh−1, varying from around 0.14 to over 6 g.kWh−1, depending on the contaminant and reactor [95], with recent bubble–plasma research reporting 4.68 g.kWh−1 at approximately 6.75 kJ/L input [96]. In contrast, optimally designed ozonation/UV-AOP systems often attain EE/O values of around 1–5 kWh.m−3 for various micropollutants, with ozonation energy demands corresponding to about 22–33 kWh/g-O3 produced (air-fed) [97,98]; UV-ARP metrics are similarly aligned for the targeted chemicals [99]. Recent evaluations indicate that NTP may achieve EE/O < 1 kWh.m−3 under certain setups and aims, although it is significantly influenced by reactor and matrix variables, highlighting the need for energy optimization at scale (gas–liquid mass transfer, residence–time distribution, and pulsed power) [63,93,100]. Ultimately, comparative life-cycle assessment indicates that NTP may be advantageous compared to many traditional quaternary alternatives when accounting for grid composition and integration; nevertheless, site-specific techno-economic analysis is crucial [81].
In conclusion, the reviewed studies indicate a transition from laboratory proof-of-concept systems to modular, field-deployable plasma configurations incorporating hydrodynamic, cavitation, and hybrid contact designs for enhanced scalability. The threshold of field viability for non-thermal plasma (NTP) reactors is identified within an energy efficiency range of 5–20 kWh.m−3 for advanced oxidation targets, including PFAS and pharmaceuticals. This conclusion is supported by recent pilot and field trials utilizing trailer- and skid-mounted DBD or PCD reactors. Research on microbubble-assisted and falling-film reactors indicates that interfacial control and flow geometry are critical factors for achieving energy-efficient scale-up, facilitating removal rates exceeding 90% in volumes greater than 25 L. The synthesis reveals that scaling NTP involves more than mere size; it requires a balance between plasma–liquid interfacial design, modular reactor integration, and measurable energy benchmarks delineating the transition from laboratory feasibility to industrial readiness.
Author Contributions
Conceptualization, B.M. and S.W.; methodology, B.M. and S.W.; software, A.M.; validation, S.S., A.M. and J.S.; formal analysis, B.M. and S.S.; investigation, B.M. and S.S.; resources, A.M., J.S. and S.W.; data curation, S.S., A.M. and S.W.; writing—original draft preparation, B.M.; writing—review and editing, S.S., A.M. and S.W.; visualization, B.M. and A.M.; supervision, S.W.; project administration, S.W.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Defense Strategic Environmental Research and Development Program (DoD SERDP) Project ER21-3564, USDA National Institute of Food and Agriculture (NIFA) Foundational and Applied Science Program Grant # 2021-67021-34204, and USDA NIFA Hatch project IDA01723, United States.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kyere-Yeboah, K.; Bique, I.K.; Qiao, X.-C. Advances of non-thermal plasma discharge technology in degrading recalcitrant wastewater pollutants. A comprehensive review. Chemosphere 2023, 320, 138061. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Murugesan, P.; Moses, J.; Anandharamakrishnan, C. Water decontamination using non-thermal plasma: Concepts, applications, and prospects. J. Environ. Chem. Eng. 2020, 8, 104377. [Google Scholar] [CrossRef]
- Leeson, A.; Thompson, T.; Stroo, H.F.; Anderson, R.H.; Speicher, J.; Mills, M.A.; Willey, J.; Coyle, C.; Ghosh, R.; Lebrón, C.; et al. Identifying and managing aqueous film-forming foam-derived per-and polyfluoroalkyl substances in the environment. Environ. Toxicol. Chem. 2021, 40, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per-and Polyfluoroalkyl Substances (PFASs)? ACS Publications: Washington, DC, USA, 2017. [Google Scholar]
- U.S. EPA (Ed.) CompTox Chemicals Dashboard; U.S. EPA: Washington, DC, USA, 2023.
- Wang, Z.; Buser, A.M.; Cousins, I.T.; Demattio, S.; Drost, W.; Johansson, O.; Ohno, K.; Patlewicz, G.; Richard, A.M.; Walker, G.W.; et al. A new OECD definition for per-and polyfluoroalkyl substances. Environ. Sci. Technol. 2021, 55, 15575–15578. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef]
- Barzen-Hanson, K.A.; Roberts, S.C.; Choyke, S.; Oetjen, K.; McAlees, A.; Riddell, N.; McCrindle, R.; Ferguson, P.L.; Higgins, C.P.; Field, J.A. Discovery of 40 classes of per-and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. [Google Scholar] [CrossRef]
- Dickman, R.A.; Aga, D.S. A review of recent studies on toxicity, sequestration, and degradation of per-and polyfluoroalkyl substances (PFAS). J. Hazard. Mater. 2022, 436, 129120. [Google Scholar] [CrossRef]
- Houtz, E.F.; Higgins, C.P.; Field, J.A.; Sedlak, D.L. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 2013, 47, 8187–8195. [Google Scholar] [CrossRef]
- Backe, W.J.; Day, T.C.; Field, J.A. Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from US military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ. Sci. Technol. 2013, 47, 5226–5234. [Google Scholar] [CrossRef]
- Huang, Q.; Luo, L.; Han, X.; Li, F.; Zhang, X.; Tian, M. Low-dose perfluorooctanoic acid stimulates steroid hormone synthesis in Leydig cells: Integrated proteomics and metabolomics evidence. J. Hazard. Mater. 2022, 424, 127656. [Google Scholar] [CrossRef]
- McDonough, C.A.; Choyke, S.; Barton, K.E.; Mass, S.; Starling, A.P.; Adgate, J.L.; Higgins, C.P. Unsaturated PFOS and other PFASs in human serum and drinking water from an AFFF-impacted community. Environ. Sci. Technol. 2021, 55, 8139–8148. [Google Scholar] [CrossRef] [PubMed]
- ECHA. ECHA Publishes PFAS Restriction Proposal; European Chemicals Agency: Helsinki, Finland, 2023. [Google Scholar]
- U.S. EPA. PFAS Strategic Roadmap: EPA’s Commitments to action 2021–2024; Environmental Protection Agency: Washington, DC, USA, 2021; Volume 20460.
- Winchell, L.J.; Ross, J.J.; Wells, M.J.; Fonoll, X.; Norton, W.N., Jr.; Bell, K.Y. Per-and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: A state of the science review. Water Environ. Res. 2021, 93, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, B.G.; Sajwan, K.S.; Sinclair, E.; Kumar, K.S.; Kannan, K. Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia. Water Res. 2007, 41, 4611–4620. [Google Scholar] [CrossRef] [PubMed]
- Rosansky, S.; Al-Dirani, S.M.; Scheitlin, C.G.; Dasu, K.; Dzurnak, M.; Xia, X.; Orth, C.; McCauley, M.; Mullins, L. Field Demonstration of PFAS Destruction in Various Alcohol-Resistant AFFFs Using Supercritical Water Oxidation (SCWO). ACS EST Water 2024, 4, 4486–4496. [Google Scholar] [CrossRef]
- Liu, X.; Huang, X.; Wei, X.; Zhi, Y.; Qian, S.; Li, W.; Yue, D.; Wang, X. Occurrence and removal of per-and polyfluoroalkyl substances (PFAS) in leachates from incineration plants: A full-scale study. Chemosphere 2023, 313, 137456. [Google Scholar] [CrossRef]
- Espana, V.A.A.; Mallavarapu, M.; Naidu, R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA): A critical review with an emphasis on field testing. Environ. Technol. Innov. 2015, 4, 168–181. [Google Scholar] [CrossRef]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in landfill groundwater: Environmental matrix effects. Environ. Sci. Technol. 2008, 42, 8057–8063. [Google Scholar] [CrossRef]
- Carter, K.E.; Farrell, J. Oxidative destruction of perfluorooctane sulfonate using boron-doped diamond film electrodes. Environ. Sci. Technol. 2008, 42, 6111–6115. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Eng. China 2009, 3, 129–151. [Google Scholar] [CrossRef]
- Stratton, G.R.; Dai, F.; Bellona, C.L.; Holsen, T.M.; Dickenson, E.R.; Mededovic Thagard, S. Plasma-based water treatment: Efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ. Sci. Technol. 2017, 51, 1643–1648. [Google Scholar] [CrossRef]
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process. Environ. Sci. Technol. 2019, 53, 2731–2738. [Google Scholar] [CrossRef]
- Wu, B.; Hao, S.; Choi, Y.; Higgins, C.P.; Deeb, R.; Strathmann, T.J. Rapid destruction and defluorination of perfluorooctanesulfonate by alkaline hydrothermal reaction. Environ. Sci. Technol. Lett. 2019, 6, 630–636. [Google Scholar] [CrossRef]
- Berg, C.; Crone, B.; Gullett, B.; Higuchi, M.; Krause, M.J.; Lemieux, P.M.; Martin, T.; Shields, E.P.; Struble, E.; Thoma, E.; et al. Developing innovative treatment technologies for PFAS-containing wastes. J. Air Waste Manag. Assoc. 2022, 72, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; de Souza, B.B.; Casarini, M.M.; Kewalramani, J.A. A review of PFAS destruction technologies. Int. J. Environ. Res. Public Health 2022, 19, 16397. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, R.; Liu, J.; Xiao, X.; Maizel, A.C.; Higgins, C.P.; Schaefer, C.E.; Strathmann, T.J. Destruction of per-and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam (AFFF) with UV-sulfite photoreductive treatment. Environ. Sci. Technol. 2020, 54, 6957–6967. [Google Scholar] [CrossRef]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in groundwater: Kinetic effects of matrix inorganics. Environ. Sci. Technol. 2010, 44, 445–450. [Google Scholar] [CrossRef]
- Kalra, S.S.; Cranmer, B.; Dooley, G.; Hanson, A.J.; Maraviov, S.; Mohanty, S.K.; Blotevogel, J.; Mahendra, S. Sonolytic destruction of Per-and polyfluoroalkyl substances in groundwater, aqueous Film-Forming Foams, and investigation derived waste. Chem. Eng. J. 2021, 425, 131778. [Google Scholar] [CrossRef]
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ. Sci. Technol. 2005, 39, 3388–3392. [Google Scholar] [CrossRef]
- Giri, R.; Ozaki, H.; Morigaki, T.; Taniguchi, S.; Takanami, R. UV photolysis of perfluorooctanoic acid (PFOA) in dilute aqueous solution. Water Sci. Technol. 2011, 63, 276–282. [Google Scholar] [CrossRef]
- Giri, R.R.; Ozaki, H.; Okada, T.; Taniguchi, S.; Takanami, R. Factors influencing UV photodecomposition of perfluorooctanoic acid in water. Chem. Eng. J. 2012, 180, 197–203. [Google Scholar] [CrossRef]
- Cao, M.; Wang, B.; Yu, H.; Wang, L.; Yuan, S.; Chen, J. Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation. J. Hazard. Mater. 2010, 179, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, P. Photodegradation of perfluorooctanoic acid in water under irradiation of 254 nm and 185 nm light by use of persulfate. Water Sci. Technol. 2006, 54, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Zhang, P.-Y.; Jian, L. Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light. J. Environ. Sci. 2007, 19, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Noma, Y.; Sakai, S.-I.; Shibata, Y. Photodegradation of perfluorooctane sulfonate by UV irradiation in water and alkaline 2-propanol. Environ. Sci. Technol. 2007, 41, 5660–5665. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, P. Photochemical decomposition of perfluorooctane sulfonate (PFOS) in an anoxic alkaline solution by 185 nm vacuum ultraviolet. Chem. Eng. J. 2015, 280, 241–247. [Google Scholar] [CrossRef]
- Hori, H.; Yamamoto, A.; Koike, K.; Kutsuna, S.; Osaka, I.; Arakawa, R. Photochemical decomposition of environmentally persistent short-chain perfluorocarboxylic acids in water mediated by iron (II)/(III) redox reactions. Chemosphere 2007, 68, 572–578. [Google Scholar] [CrossRef]
- Hori, H.; Yamamoto, A.; Koike, K.; Kutsuna, S.; Osaka, I.; Arakawa, R. Persulfate-induced photochemical decomposition of a fluorotelomer unsaturated carboxylic acid in water. Water Res. 2007, 41, 2962–2968. [Google Scholar] [CrossRef]
- Song, Z.; Tang, H.; Wang, N.; Zhu, L. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. J. Hazard. Mater. 2013, 262, 332–338. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, C.; Li, F.; Chen, J.; Zhou, Q. Photo-reductive defluorination of perfluorooctanoic acid in water. Water Res. 2010, 44, 2939–2947. [Google Scholar] [CrossRef]
- Park, H.; Vecitis, C.D.; Cheng, J.; Choi, W.; Mader, B.T.; Hoffmann, M.R. Reductive defluorination of aqueous perfluorinated alkyl surfactants: Effects of ionic headgroup and chain length. J. Phys. Chem. A 2009, 113, 690–696. [Google Scholar] [CrossRef]
- Liu, C.; Higgins, C.; Wang, F.; Shih, K. Effect of temperature on oxidative transformation of perfluorooctanoic acid (PFOA) by persulfate activation in water. Sep. Purif. Technol. 2012, 91, 46–51. [Google Scholar] [CrossRef]
- Liu, C.; Shih, K.; Wang, F. Oxidative decomposition of perfluorooctanesulfonate in water by permanganate. Sep. Purif. Technol. 2012, 87, 95–100. [Google Scholar] [CrossRef]
- Hori, H.; Nagaoka, Y.; Murayama, M.; Kutsuna, S. Efficient decomposition of perfluorocarboxylic acids and alternative fluorochemical surfactants in hot water. Environ. Sci. Technol. 2008, 42, 7438–7443. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shih, K. Adsorption of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on alumina: Influence of solution pH and cations. Water Res. 2011, 45, 2925–2930. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Lo, S.-L.; Chiueh, P.-T.; Chang, D.-G. Efficient decomposition of perfluorocarboxylic acids in aqueous solution using microwave-induced persulfate. Water Res. 2009, 43, 2811–2816. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Lo, S.-L.; Chiueh, P.-T.; Liou, Y.-H.; Chen, M.-L. Microwave-hydrothermal decomposition of perfluorooctanoic acid in water by iron-activated persulfate oxidation. Water Res. 2010, 44, 886–892. [Google Scholar] [CrossRef]
- Lee, Y.; Lo, S.; Kuo, J.; Hsieh, C. Decomposition of perfluorooctanoic acid by microwaveactivated persulfate: Effects of temperature, pH, and chloride ions. Front. Environ. Sci. Eng. 2012, 6, 17–25. [Google Scholar] [CrossRef]
- Hori, H.; Nagaoka, Y.; Yamamoto, A.; Sano, T.; Yamashita, N.; Taniyasu, S.; Kutsuna, S.; Osaka, I.; Arakawa, R. Efficient decomposition of environmentally persistent perfluorooctanesulfonate and related fluorochemicals using zerovalent iron in subcritical water. Environ. Sci. Technol. 2006, 40, 1049–1054. [Google Scholar] [CrossRef]
- Hori, H.; Nagaoka, Y.; Sano, T.; Kutsuna, S. Iron-induced decomposition of perfluorohexanesulfonate in sub-and supercritical water. Chemosphere 2008, 70, 800–806. [Google Scholar] [CrossRef]
- Suresh, R.; Rajoo, B.; Chenniappan, M.; Palanichamy, M. Treatment possibilities of electrical discharge non-thermal plasma for industrial wastewater treatment-review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1055, 012018. [Google Scholar] [CrossRef]
- Yusuf, A.; Amusa, H.K.; Eniola, J.O.; Giwa, A.; Pikuda, O.; Dindi, A.; Bilad, M.R. Hazardous and emerging contaminants removal from water by plasma-based treatment: A review of recent advances. Chem. Eng. J. Adv. 2023, 14, 100443. [Google Scholar] [CrossRef]
- Barjasteh, A.; Dehghani, Z.; Lamichhane, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Recent progress in applications of non-thermal plasma for water purification, bio-sterilization, and decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Shahsavari, N.; Zhang, X. Microbubble-enhanced cold plasma activation for water decontamination: Degradation dynamics and energy yield in relation to pollutant concentration, total volume and flow rate of water. J. Water Process Eng. 2023, 55, 104169. [Google Scholar] [CrossRef]
- Cui, Y.; Cheng, J.; Chen, Q.; Yin, Z. The types of plasma reactors in wastewater treatment. IOP Conf. Ser. Earth Environ. Sci. 2018, 208, 012002. [Google Scholar] [CrossRef]
- Thagard, S.M. Reactor design in plasma-liquid systems for wastewater treatment. Curr. Opin. Green Sustain. Chem. 2025, 53, 101023. [Google Scholar] [CrossRef]
- Chen, C.; Ma, C.; Yang, X.; Gromov, M.; Tian, Y.; Demeestere, K.; Nikiforov, A.; Van Hulle, S.W. Degradation of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in water by use of a nonthermal plasma-ozonation cascade reactor: Role of different processes and reactive species. Chem. Eng. J. 2024, 486, 150218. [Google Scholar] [CrossRef]
- Topolovec, B.; Jovanovic, O.; Puac, N.; Skoro, N.; Lumbaque, E.C.; Petrovic, M. Plasma water treatment for PFAS: Study of degradation of perfluorinated substances and their byproducts by using cold atmospheric pressure plasma jet. J. Environ. Chem. Eng. 2024, 12, 112979. [Google Scholar] [CrossRef]
- Palma, D.; Richard, C.; Minella, M. State of the art and perspectives about non-thermal plasma applications for the removal of PFAS in water. Chem. Eng. J. Adv. 2022, 10, 100253. [Google Scholar] [CrossRef]
- Blotevogel, J.; Thagard, S.M.; Mahendra, S. Scaling up water treatment technologies for PFAS destruction: Current status and potential for fit-for-purpose application. Curr. Opin. Chem. Eng. 2023, 41, 100944. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Kwak, H.S.; Park, J.H.; Uhm, H.S.; Bogaerts, A.; Choi, E.H.; Attri, P. Bacterial inactivation by plasma treated water enhanced by reactive nitrogen species. Sci. Rep. 2018, 8, 11268. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Raju, B.R.; Karuppiah, J.; Reddy, E.L.; Subrahmanyam, C. Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Guesmi, A.; Cherif, M.M.; Baaloudj, O.; Kenfoud, H.; Badawi, A.K.; Elfalleh, W.; Ben Hamadi, N.; Khezami, L.; Assadi, A.A. Disinfection of corona and myriad viruses in water by non-thermal plasma: A review. Environ. Sci. Pollut. Res. 2022, 29, 55321–55335. [Google Scholar] [CrossRef]
- Tichonovas, M.; Krugly, E.; Racys, V.; Hippler, R.; Kauneliene, V.; Stasiulaitiene, I.; Martuzevicius, D. Degradation of various textile dyes as wastewater pollutants under dielectric barrier discharge plasma treatment. Chem. Eng. J. 2013, 229, 9–19. [Google Scholar] [CrossRef]
- Saleem, M.; Biondo, O.; Sretenović, G.; Tomei, G.; Magarotto, M.; Pavarin, D.; Marotta, E.; Paradisi, C. Comparative performance assessment of plasma reactors for the treatment of PFOA; reactor design, kinetics, mineralization and energy yield. Chem. Eng. J. 2020, 382, 123031. [Google Scholar] [CrossRef]
- Chandana, L.; Reddy, P.M.K.; Subrahmanyam, C. Atmospheric pressure non-thermal plasma jet for the degradation of methylene blue in aqueous medium. Chem. Eng. J. 2015, 282, 116–122. [Google Scholar] [CrossRef]
- Song, K.; Wang, H.; Jiao, Z.; Qu, G.; Chen, W.; Wang, G.; Wang, T.; Zhang, Z.; Ling, F. Inactivation efficacy and mechanism of pulsed corona discharge plasma on virus in water. J. Hazard. Mater. 2022, 422, 126906. [Google Scholar] [CrossRef] [PubMed]
- Merouani, D.; Abdelmalek, F.; Taleb, F.; Martel, M.; Semmoud, A.; Addou, A. Plasma treatment by gliding arc discharge of dyes/dye mixtures in the presence of inorganic salts. Arab. J. Chem. 2015, 8, 155–163. [Google Scholar] [CrossRef]
- Qasim, M.; Rafique, M.S.; Naz, R. Water purification by ozone generator employing non-thermal plasma. Mater. Chem. Phys. 2022, 291, 126442. [Google Scholar] [CrossRef]
- Zeghioud, H.; Nguyen-Tri, P.; Khezami, L.; Amrane, A.; Assadi, A.A. Review on discharge Plasma for water treatment: Mechanism, reactor geometries, active species and combined processes. J. Water Process Eng. 2020, 38, 101664. [Google Scholar] [CrossRef]
- Čech, J.; St’ahel, P.; Rahel, J.; Prokes, L.; Rudolf, P.; Marsalkova, E.; Marsalek, B. Mass Production of Plasma Activated Water: Case Studies of Its Biocidal Effect on Algae and Cyanobacteria. Water 2020, 12, 3167. [Google Scholar] [CrossRef]
- Ajo, P.; Preis, S.; Vornamo, T.; Mänttäri, M.; Kallioinen, M.; Louhi-Kultanen, M. Hospital wastewater treatment with pilot-scale pulsed corona discharge for removal of pharmaceutical residues. J. Environ. Chem. Eng. 2018, 6, 1569–1577. [Google Scholar] [CrossRef]
- Preis, S.; Panorel, I.; Kornev, I.; Hatakka, H.; Kallas, J. Pulsed corona discharge: The role of ozone and hydroxyl radical in aqueous pollutants oxidation. Water Sci. Technol. 2013, 68, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Back, J.O.; Obholzer, T.; Winkler, K.; Jabornig, S.; Rupprich, M. Combining ultrafiltration and non-thermal plasma for low energy degradation of pharmaceuticals from conventionally treated wastewater. J. Environ. Chem. Eng. 2018, 6, 7377–7385. [Google Scholar] [CrossRef]
- Nippatlapalli, N.; Ramakrishnan, K.; Philip, L. Enhanced degradation of complex organic compounds in wastewater using different novel continuous flow non-Thermal pulsed corona plasma discharge reactors. Environ. Res. 2021, 203, 111807. [Google Scholar] [CrossRef]
- Chen, C.; Ma, C.; Yang, Y.; Yang, X.; Demeestere, K.; Nikiforov, A.; Van Hulle, S. Degradation of micropollutants in secondary wastewater effluent using nonthermal plasma-based AOPs: The roles of free radicals and molecular oxidants. Water Res. 2023, 235, 119881. [Google Scholar] [CrossRef]
- Surra, E.; Paíga, P.; Baptista, I.; Jorge, R.; Marinheiro, L.; Löblich, S.; Delerue-Matos, C. Comparative life cycle assessment of non-thermal plasma for the removal of pharmaceuticals from wastewater. J. Environ. Manag. 2024, 370, 122728. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Genewa, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Genewa, Switzerland, 2006.
- Yaseen, D.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Dhote, J.; Ingole, S.; Chavhan, A. Review on wastewater treatment technologies. Int. J. Eng. Res. Technol. 2012, 1, 1–10. [Google Scholar]
- Xiao, Y.; Tian, Y.; Zhan, Y.; Zhu, J. Optimization of a Low-Cost Corona Dielectric-Barrier Discharge Plasma Wastewater Treatment System through Central Composite Design/Response Surface Methodology with Mechanistic and Efficiency Analysis. Sustainability 2024, 16, 605. [Google Scholar] [CrossRef]
- Kim, M.W.; Kim, H.K.; Lee, H.; Kim, K.; Hong, Y.C. Decolorization of industrial dye wastewater using an underwater non-thermal plasma system. J. Water Process Eng. 2024, 67, 106149. [Google Scholar] [CrossRef]
- Kooshki, S.; Pareek, P.; Janda, M.; Machala, Z. Selective reactive oxygen and nitrogen species production in plasma-activated water via dielectric barrier discharge reactor: An innovative method for tuning and its impact on dye degradation. J. Water Process Eng. 2024, 63, 105477. [Google Scholar] [CrossRef]
- Nau-Hix, C.; Multari, N.; Singh, R.K.; Richardson, S.; Kulkarni, P.; Anderson, R.H.; Holsen, T.M.; Thagard, S.M. Field demonstration of a pilot-scale plasma reactor for the rapid removal of poly-and perfluoroalkyl substances in groundwater. ACS EST Water 2021, 1, 680–687. [Google Scholar] [CrossRef]
- Singh, R.K.; Multari, N.; Nau-Hix, C.; Anderson, R.H.; Richardson, S.D.; Holsen, T.M.; Thagard, S.M. Rapid removal of poly-and perfluorinated compounds from investigation-derived waste (IDW) in a pilot-scale plasma reactor. Environ. Sci. Technol. 2019, 53, 11375–11382. [Google Scholar] [CrossRef]
- Alam, D.; Lee, S.; Hong, J.; Fletcher, D.F.; Liu, X.; McClure, D.; Cook, D.; Carfort, J.l.N.d.; Krühne, U.; Cullen, P.; et al. Influence of bubble size on perfluorooctanesulfonic acid degradation in a pilot scale non-thermal plasma treatment reactor. Chem. Eng. J. 2024, 489, 151349. [Google Scholar] [CrossRef]
- Tamang, S.G.; Umlauf, G.; Barz, J.; Ghomi, M.R. Degradation of PFOA solutions and PFAS-contaminated groundwater using atmospheric non-thermal plasma treatment. Water Pract. Technol. 2024, 19, 2645–2654. [Google Scholar] [CrossRef]
- Tang, X.; Júnior, A.D.F.; Karu, K.; Campos, L.C.; Kim, M. Atmospheric pressure dielectric barrier discharge plasma for in-situ water treatment using a bubbling reactor. J. Environ. Manag. 2024, 370, 122574. [Google Scholar] [CrossRef]
- Lewis, A.J.; Joyce, T.; Hadaya, M.; Ebrahimi, F.; Dragiev, I.; Giardetti, N.; Yang, J.; Fridman, G.; Rabinovich, A.; Fridman, A.A.; et al. Rapid degradation of PFAS in aqueous solutions by reverse vortex flow gliding arc plasma. Environ. Sci. Water Res. Technol. 2020, 6, 1044–1057. [Google Scholar] [CrossRef]
- Giardina, A.; Lofrano, G.; Libralato, G.; Siciliano, A.; Marotta, E.; Paradisi, C. Air non-thermal plasma, a green approach for the treatment of contaminated water: The case of sulfamethoxazole. Front. Environ. Chem. 2024, 5, 1416702. [Google Scholar] [CrossRef]
- Li, S.; Huang, B.; Gao, Y.; Dong, Y.; Wang, Q.; Zhang, L.; Zhang, C.; Yu, J.; Yang, M. Spatially-confined bubble plasma oxidation induced by microchannel discharge to boost energy efficiency in water treatment. Water Res. 2025, 287, 124452. [Google Scholar] [CrossRef]
- Zoschke, K.; Dietrich, N.; Börnick, H.; Worch, E. UV-based advanced oxidation processes for the treatment of odour compounds: Efficiency and by-product formation. Water Res. 2012, 46, 5365–5373. [Google Scholar] [CrossRef]
- Shahrestani, M.M.; Rahimi, A. Evaluation of electrical energy consumption in UV/H2O2 advanced oxidation process for simultaneous removal of NO and SO2. Environ. Eng. Res. 2019, 24, 389–396. [Google Scholar] [CrossRef]
- Fennell, B.D.; Mezyk, S.P.; McKay, G. Critical review of UV-advanced reduction processes for the treatment of chemical contaminants in water. ACS Environ. Au 2022, 2, 178–205. [Google Scholar] [CrossRef]
- Naicker, K.-I.; Kaweesa, P.; Daramola, M.O.; Iwarere, S.A. Non-thermal plasma review: Assessment and improvement of feasibility as a retrofitted technology in tertiary wastewater purification. Appl. Sci. 2023, 13, 6243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).