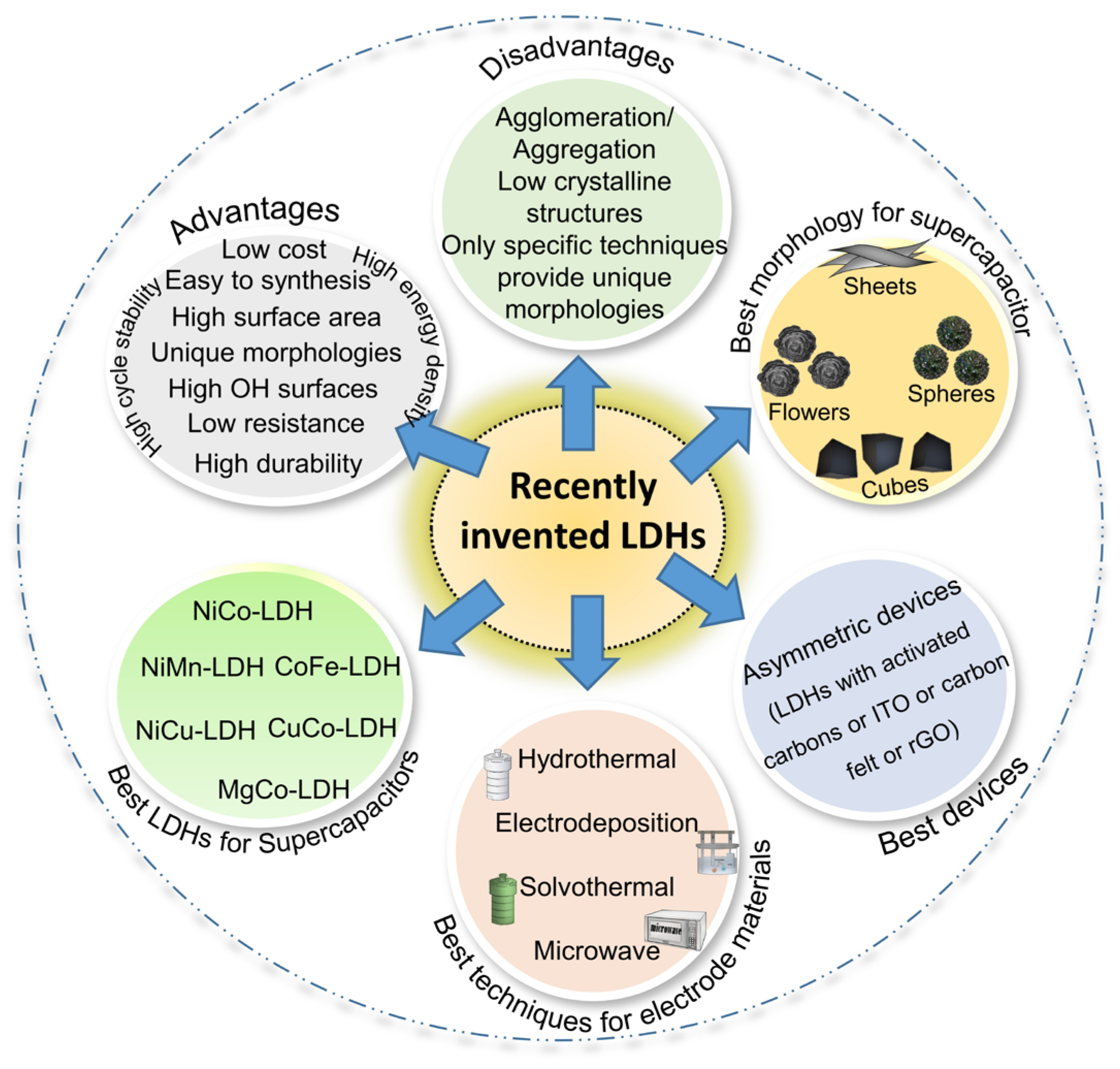
Recent Progress in the Synthesis of Layered Double Hydroxides and Their Surface Modification for Supercapacitor Application
Abstract
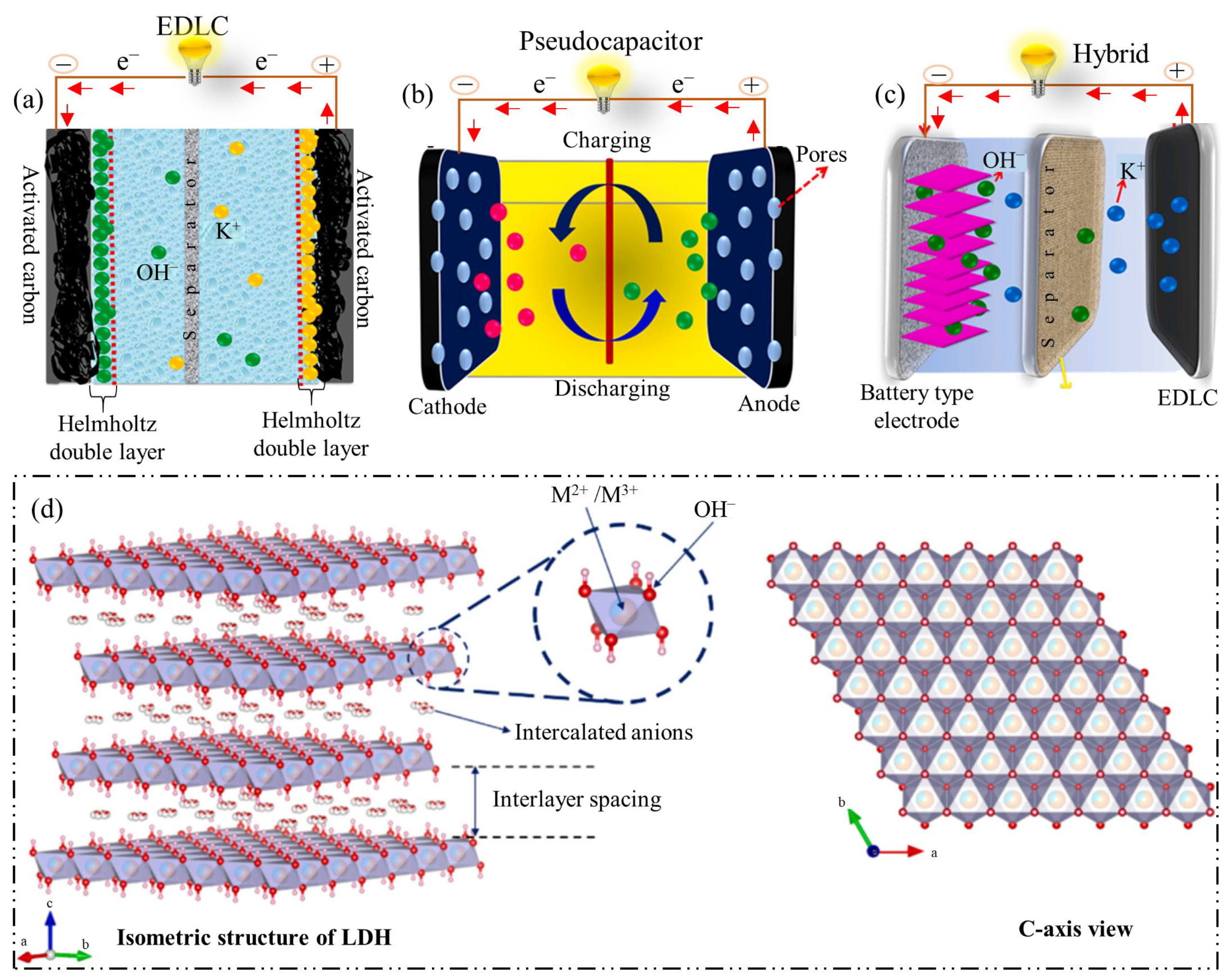
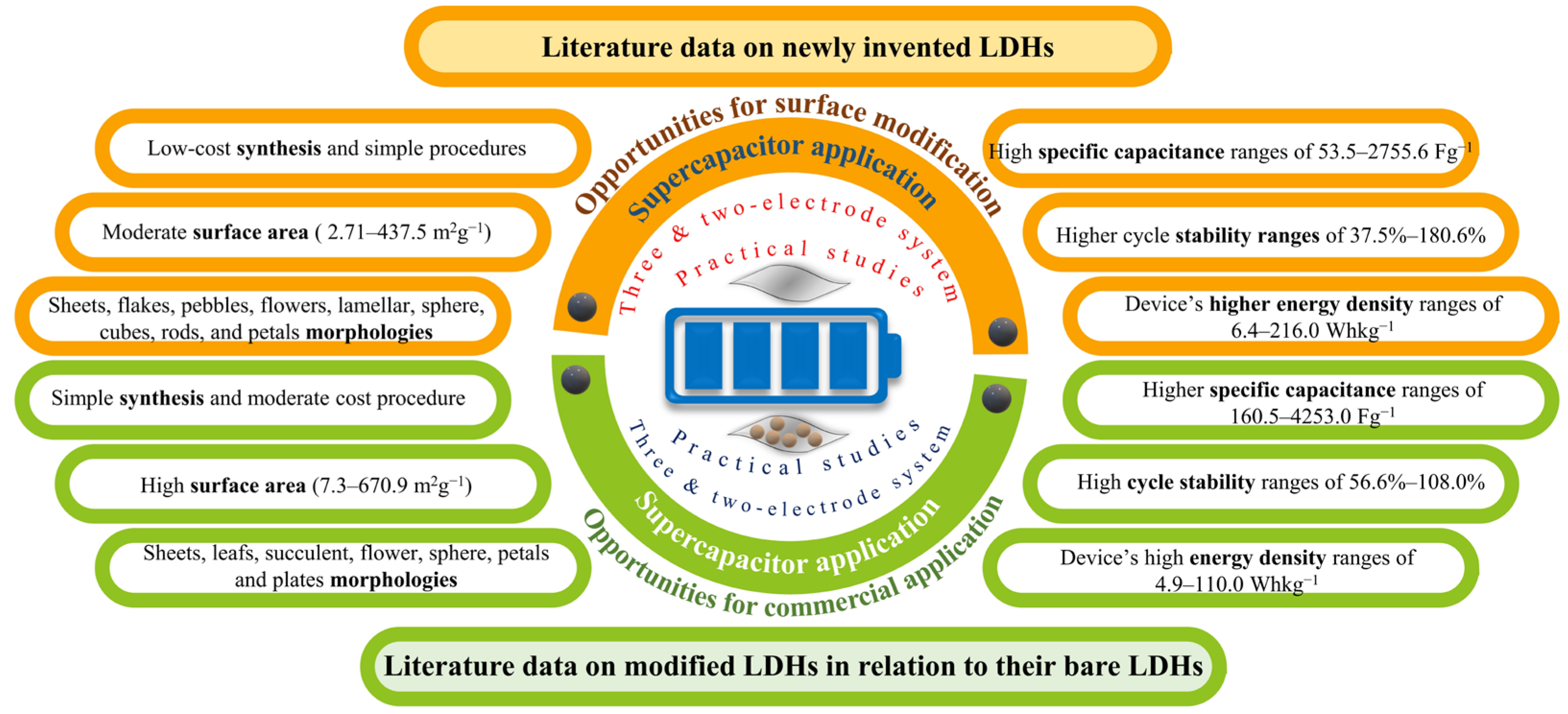
1. Introduction
2. Recent Advances in the Synthesis of Various LDHs for SCs
2.1. CoCe-LDH
2.2. CoV-LDH
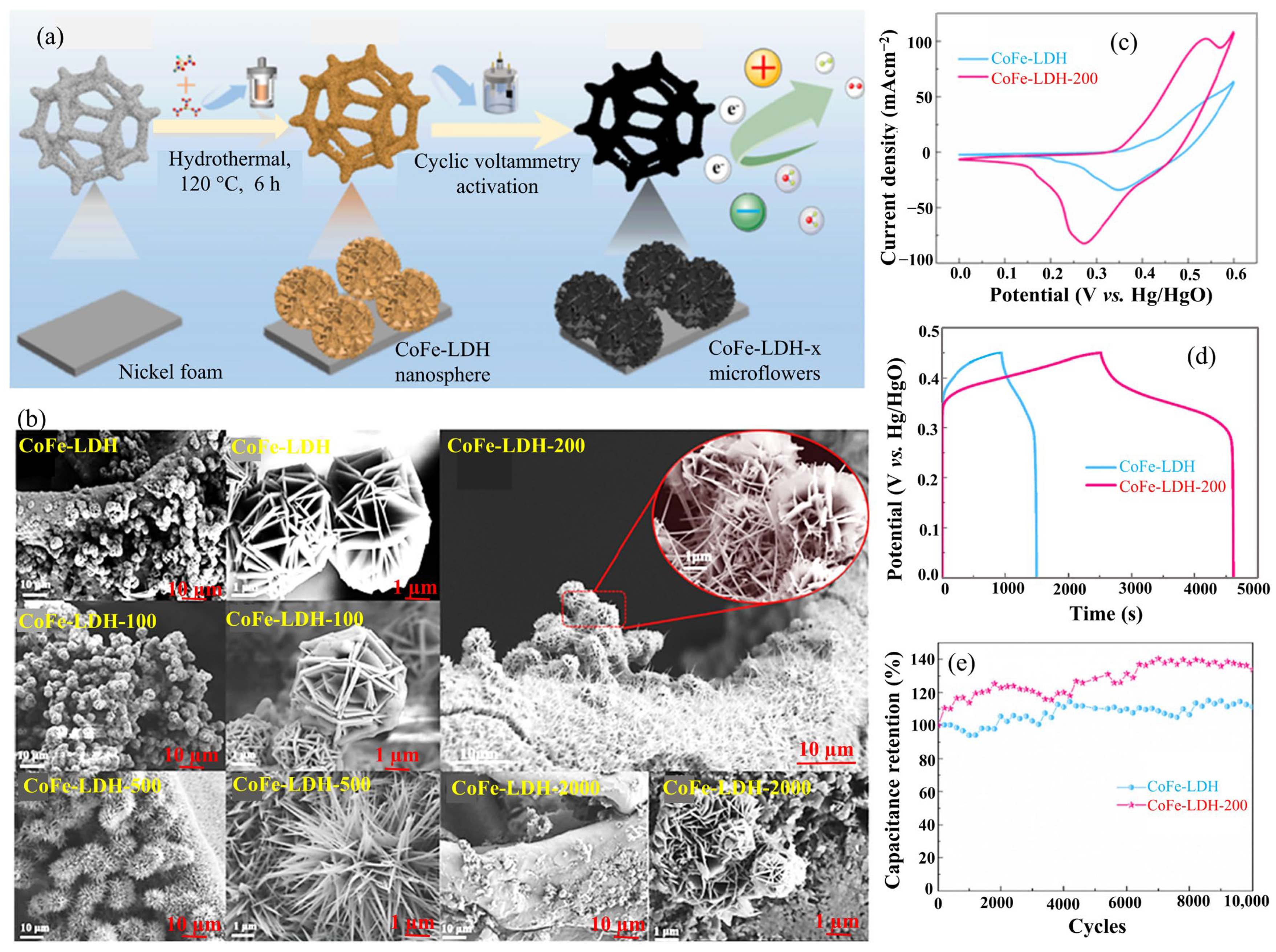
2.3. CoFe-LDH
2.4. FeCo-LDH
2.5. ZnCo-LDH
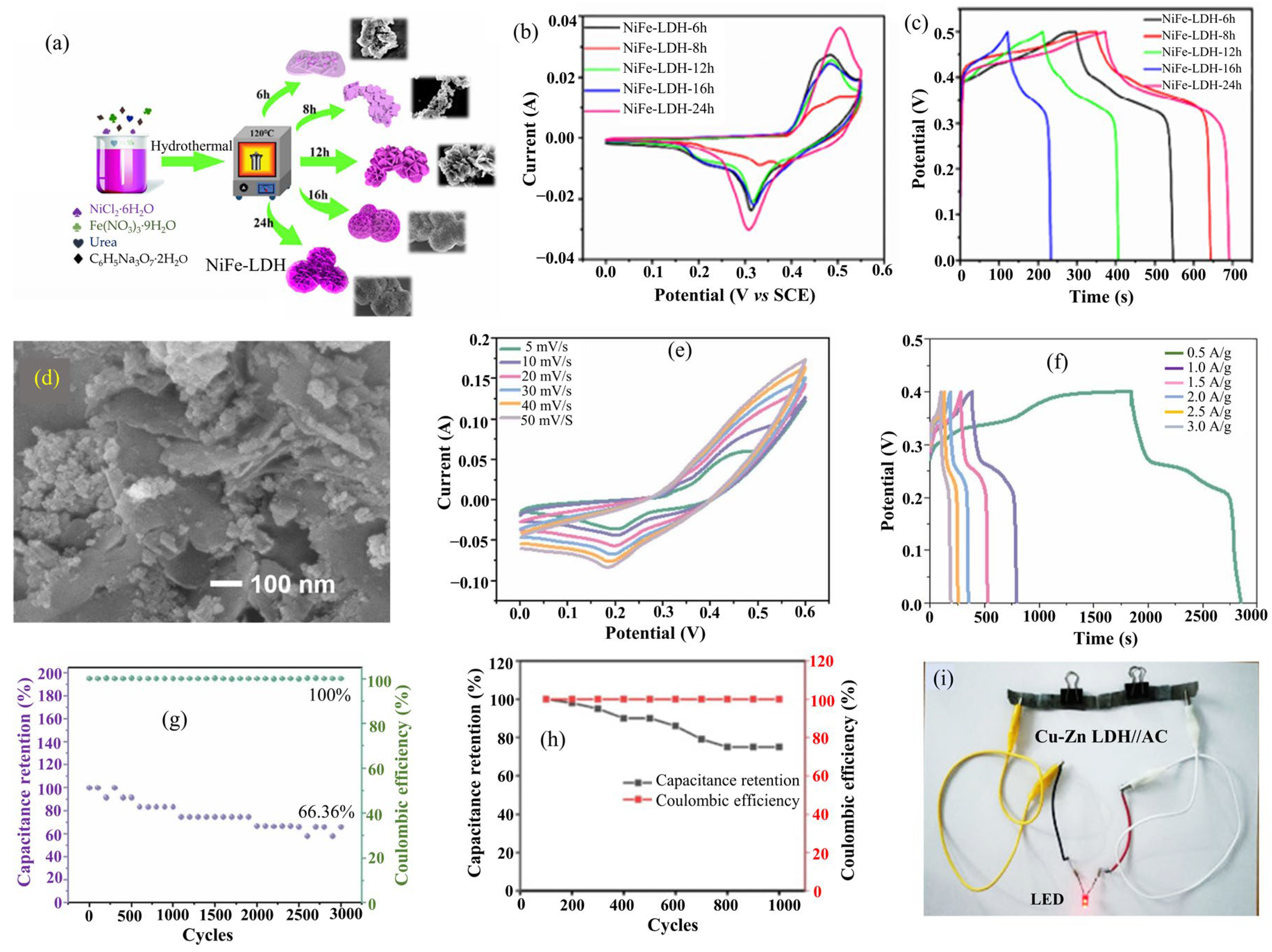
2.6. NiFe-LDH
2.7. NiCu-LDH
2.8. CuZn-LDH
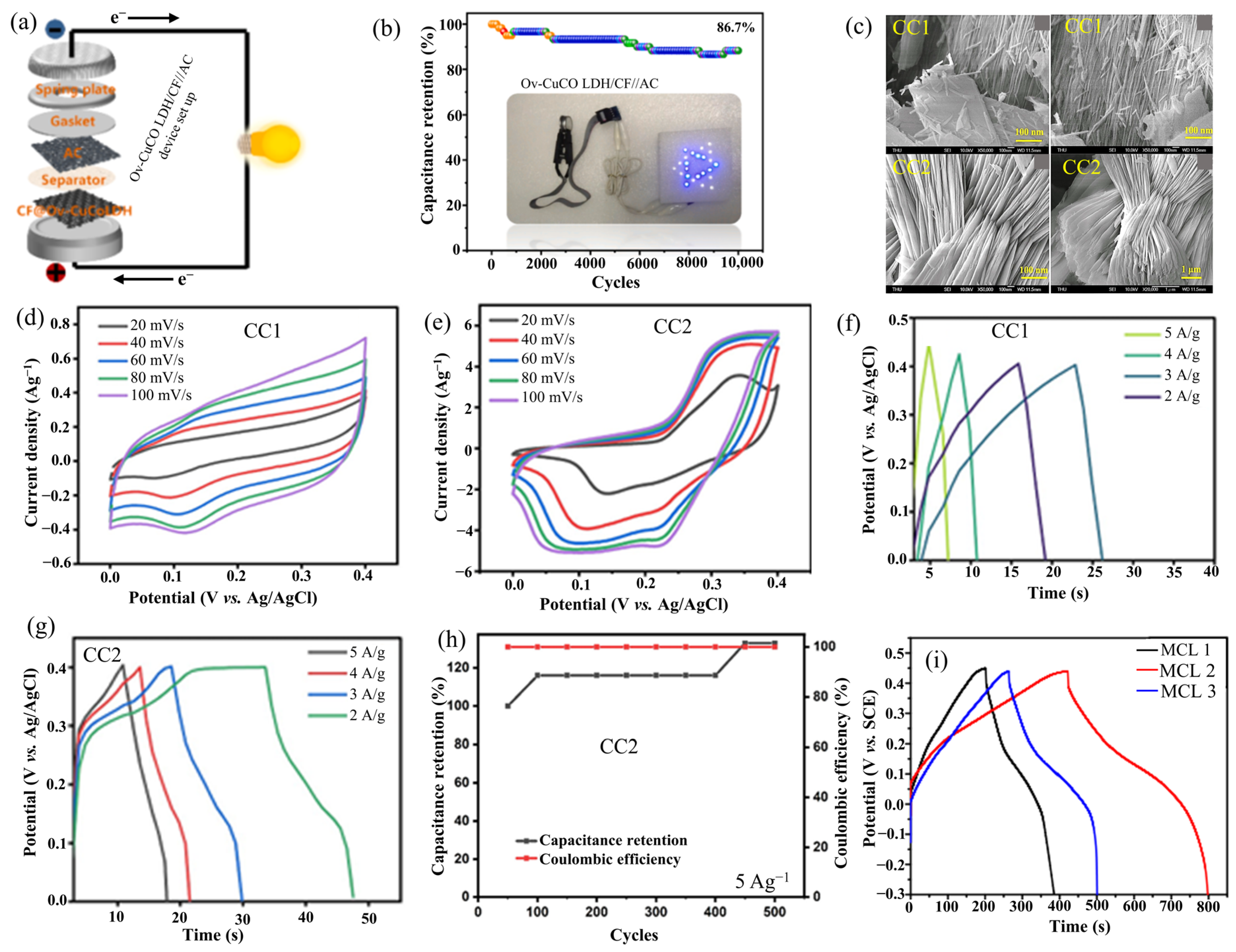
2.9. CuCo-LDH
2.10. MgCo-LDH
2.11. NiAl-LDH
2.12. ZnAl-LDHs
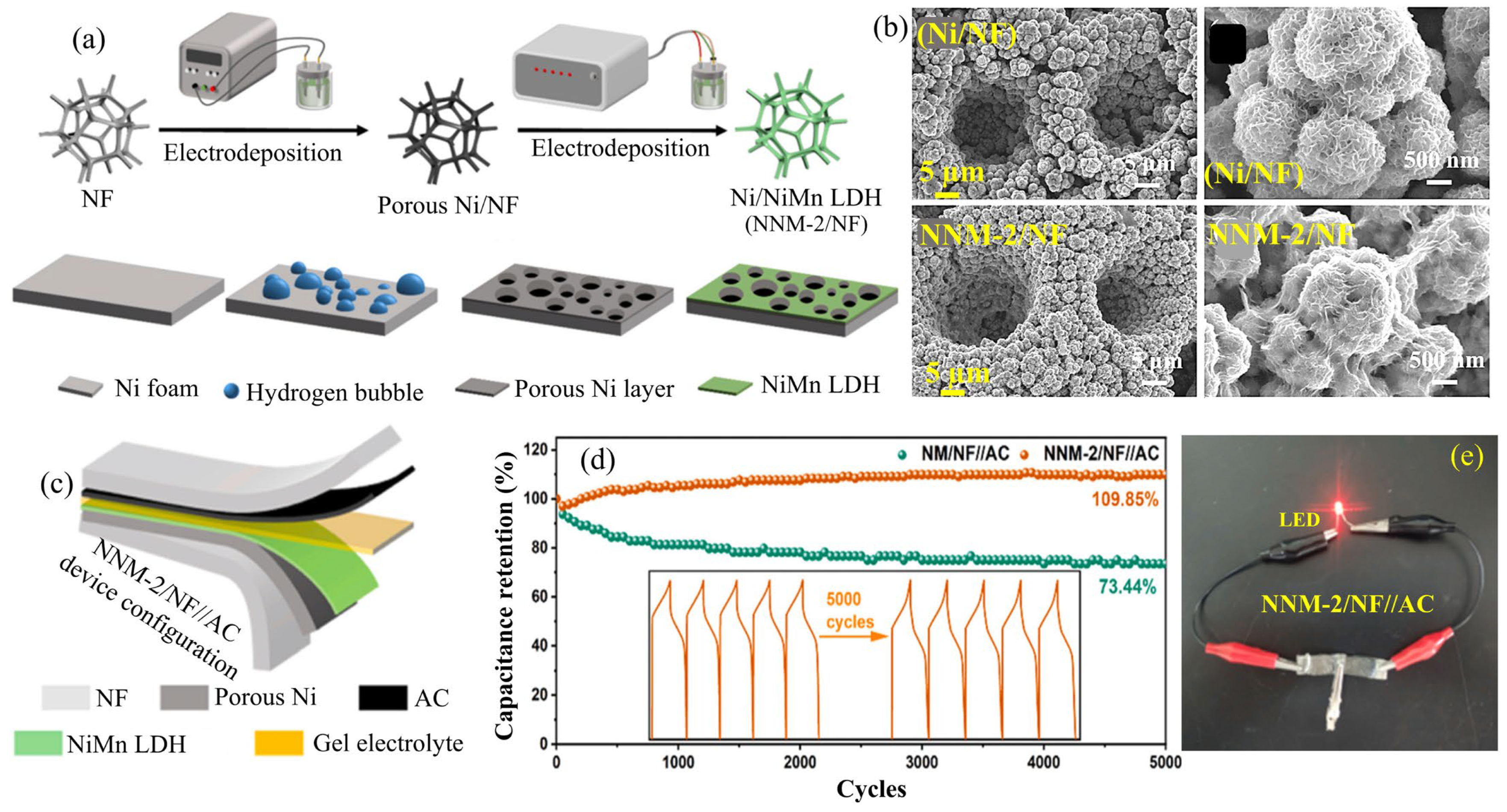
2.13. NiMn-LDHs
2.14. CoMn-LDH
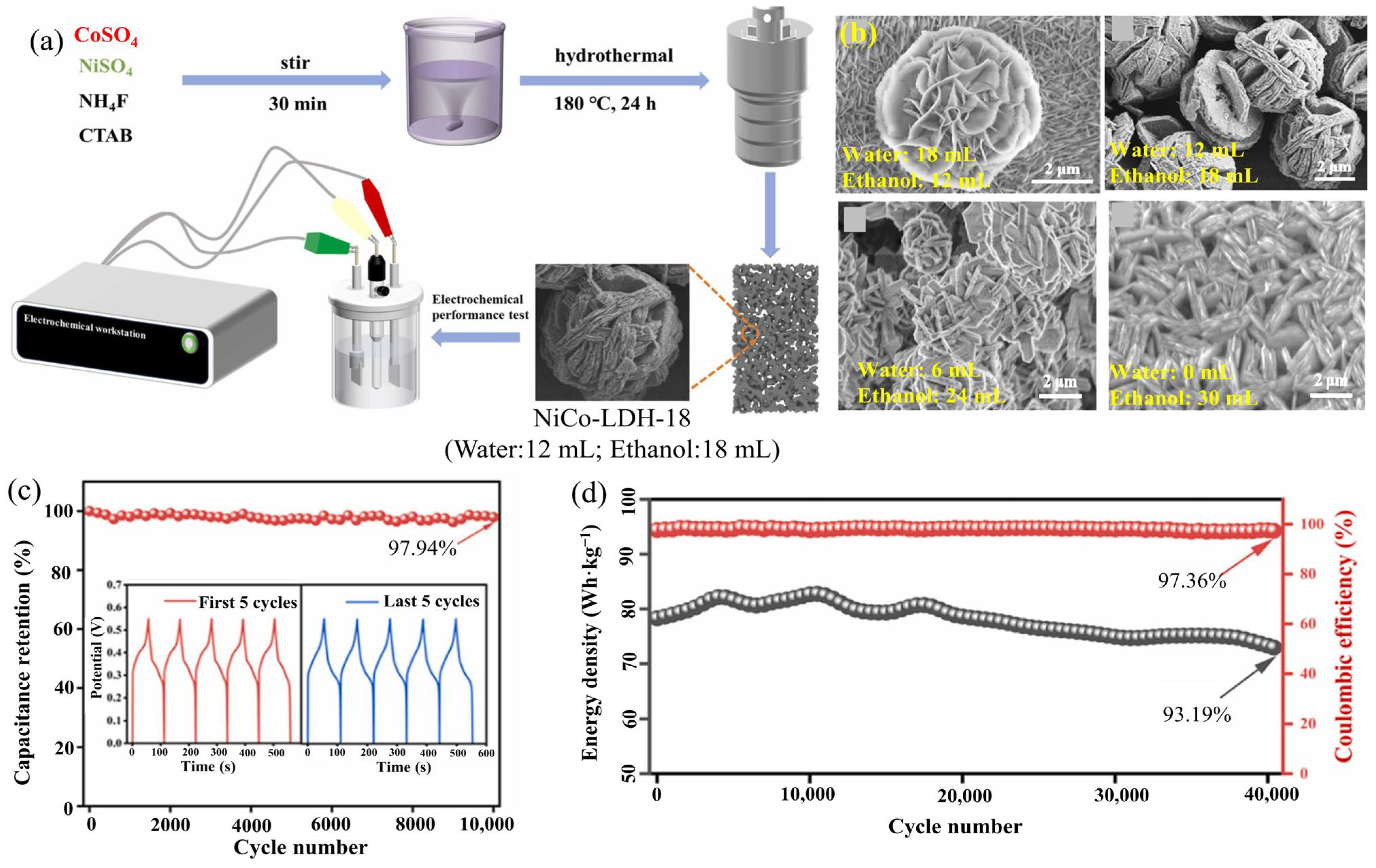
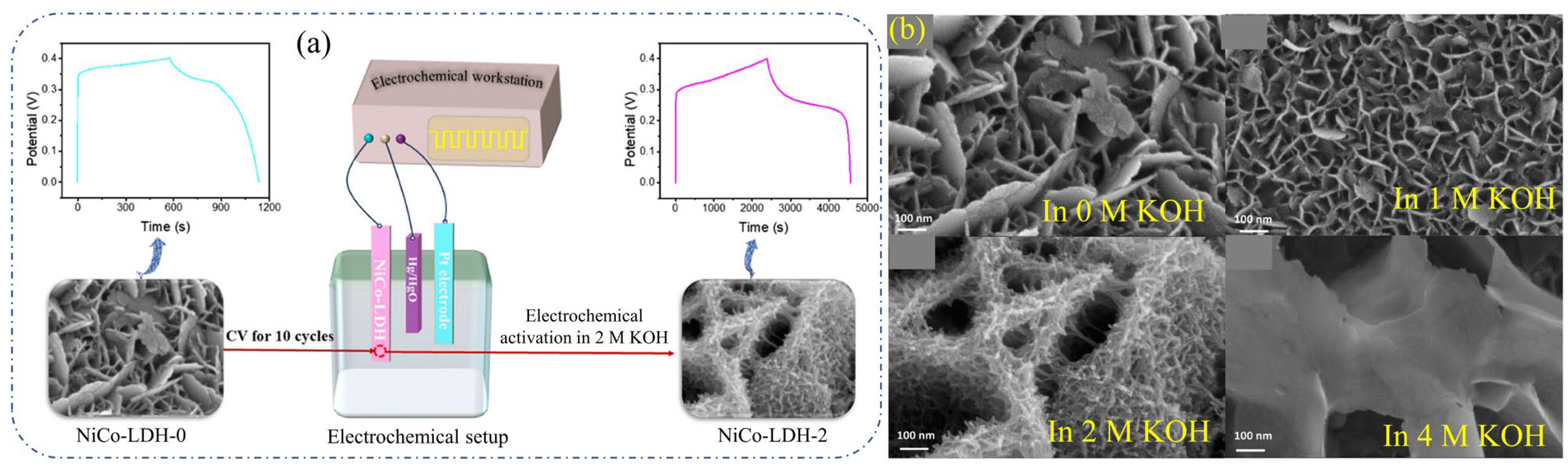
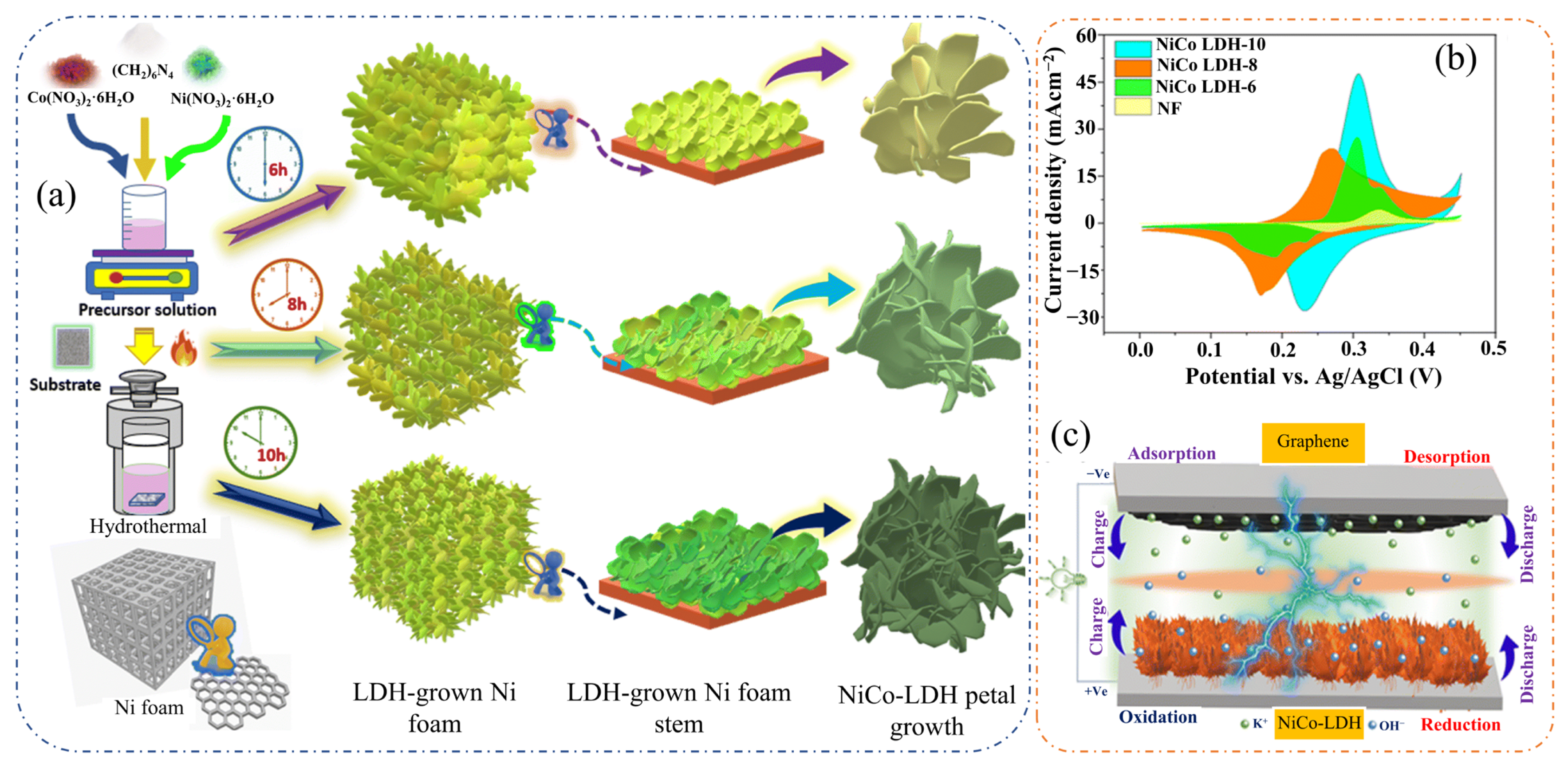
2.15. NiCo-LDHs
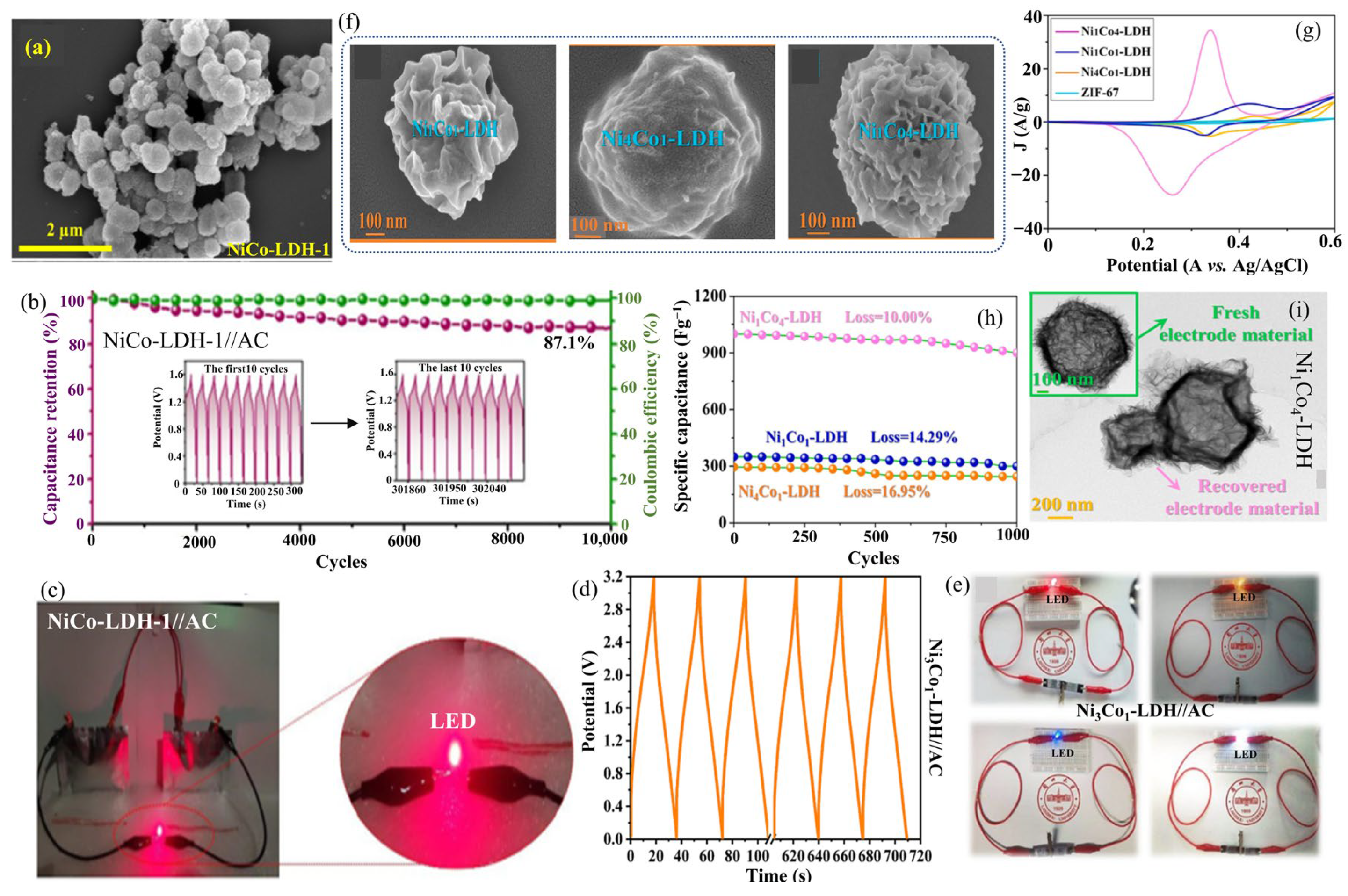

2.16. CoNi-LDH
| Electrode Materials | Synthesis | Morphology | Three Electrodes | Two Electrodes | EL | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| Cs at Cd | Cycle Stability | Devices | Ed | Pd | |||||
| CoCe-LDH | |||||||||
| CoCe-LDH | Urea hydrolysis | Sheet/spherical (95.4 m2·g−1) | - | 96.93%, after 20,000 cycles | CoCe-LDH// CoCe-LDH | 2.98 μWhcm−2 | 375.1 μWcm−2 | 3 M KOH | [45] |
| CoV-LDH | |||||||||
| CoV-LDH (CVL1-120) | Hydrothermal | Nanosheets (15.7 m2·g−1) | 314.4 at 1.0 | 97%, after 5000 cycles | CVL-1-120//AC | 36.5 | 1208.2 | 1 M KOH | [46] |
| CoFe-LDH | |||||||||
| CoFe-LDH-200 | Hydrothermal | Microflowers | 2222.0 at 1.0 | 100%, after 10,000 cycles | - | - | - | 3 M KOH | [47] |
| FeCo-LDH | |||||||||
| FeCo-LDH /NF (FC3) | Hydrothermal | Dense nanoflakes (84.7 m2·g−1) | 1448.0 at 20 mAcm−2 | 87%, after 5000 cycles | FC3//AC-NF | 157.0 | 4210.0 | 1 M KOH | [48] |
| ZnCo-LDH | |||||||||
| Ov-ZnCo-LDH/CC (Ov-LDH(3)) | Hydrothermal | Nanosheets (113 m2·g−1) | - | 84.2%, after 5000 cycles | Ov-LDH(3)//AC | 57.9 | 849.4 | 3 M KOH | [49] |
| NiFe-LDH | |||||||||
| NiFe-LDH (NFS) | Hydrothermal | Hexagonal plates (50 m2·g−1) | - | - | NFS//AC | 11.0 | 5500.0 | 6 M KOH | [50] |
| NiFe-LDH- 24 h | Hydrothermal | Porous nanoflowers | 635.8 at 1.0 | 58%, after 500 cycles | - | - | - | 2 M KOH | [51] |
| Ni5Fe1-LDH | Hydrothermal | Combined sheets and particles (136.9 m2·g−1) | - | 66.3%, after 3000 cycles | Ni5Fe1-LDH//AC | 35.8 | 800.0 | 6 M KOH | [52] |
| NiCu-LDH | |||||||||
| L-Ni4Cu-LDH | Co-precipitation | Layered sheets | 1656.9 at 1.0 | - | L-Ni4Cu-LDH//AC | 45.4 | 800.0 | 2 M KOH | [53] |
| CuZn-LDH | |||||||||
| CuZn-LDH | Co-precipitation | Flower petals | 265.0 at 1.0 mAcm−2 | - | CuZn-LDH//AC | 7.1 | 121.9 | 3 M KOH | [54] |
| CuCo LDH | |||||||||
| Ov-CuCo LDH/CF | Solution method/NaBH4 reduction | Nanosheet arrays | 1392.4 at 1.0 | - | Ov-CuCo LDH/CF//AC | 58.2 | 850.0 | - | [55] |
| CuCo-LDH (CC2) | Sonochemical | Bundled rice straw | 50.0 at 20 mVs−1 | 103%, after 500 cycles | - | - | - | 6 M KOH | [56] |
| MgCo-LDH | |||||||||
| MgCo-LDH/Ni (MCL-2) | Vertical autoclave hydrothermal | Sheet | 1431.2 at 6.0 mAcm−2 | 75%, after 5000 cycles | - | - | - | 1 M KOH | [57] |
| NiAl-LDH | |||||||||
| NiAl-LDH-60 | Hydrothermal | Nanoflakes (160.6 m2·g−1) | - | 85.9%, after 5000 cycles | NiAl-LDH-60//NiAl-LDH-60 | 71.3 | 2400.0 | 3 M KOH | [58] |
| NiAl-LDH/ITO film | Hydrothermal | Micropebbles (117.4 m2·g−1) | 589.3 at 1.0 | 95%, after 5000 cycles | NiAl-LDH/ITO// AC/ITO | 216.0 | 5200.0 | 1 M KOH | [59] |
| NiAl-LDH/CC | Electrodeposition | Microsheets (437.5 m2·g−1) | - | 92%, after 2000 cycles | NiAl-LDH/CC//CC | 0.465 Whcm−3 | 8.23 Wcm−3 | 1 M KOH | [60] |
| ZnAl-LDH | |||||||||
| ZnAl-LDH/Al flexible device | Chemical bath deposition | Nanosheets | 596.5 at 10 mVs−1 (Flat mode) | - | - | 6.4 | 46,000.0 | 2 M H2SO4 | [61] |
| NiMn-LDH | |||||||||
| NiMn-LDH | Co-precipitation | Thick sheets | 905.0 at 1.0 | 94.8%, after 5000 cycles | - | - | - | 1 M KOH | [62] |
| Ni3Mn1-T100 | Hydrothermal | 3D Flowers (36.2 m2·g−1) | 1708.0 at 1.0 | 72.7%, after 10,000 cycles | Ni3Mn1-T100//rGO | 60.0 | 965.0 | 3 M KOH | [63] |
| Ni75Mn25-LDH/NF | Hydrothermal | 3D Microflower (23.4 m2·g−1) | 1008.0 at 1.0 mAcm−2 | 72.7%, after 3000 cycles | - | - | - | 1 M KOH | [64] |
| NNM-2/NF | Electrodeposition | 3D Flowers | 2755.6 at 1.0 | 71.2%, after 5000 cycles | NNM-2/NF//AC | 41.7 | 2700.0 | 6 M KOH | [65] |
| NiMn-LDH/Ni foil | Reflux condensation method | Pebbles (127.9 m2·g−1) | 416.0 at 4.0 mAcm−2 | 92.8%, after 3000 cycles | NiMn-LDH/Ni foil//AC/Ni foil | 10.0 | 900.0 | 2 M KOH | [66] |
| CoMn-LDH | |||||||||
| CoMn-LDH/NF | Electrodeposition | Lamellar | - | 83.6%, over 7000 cycles | CoMn-LDH/NF//AC | 0.409 mWh cm−2 | 0.798 mW cm−2 | 2 M KOH | [67] |
| CoMn-LDH-6 | Hydrothermal | Porous solid nanosphere | 53.5 at 0.67 | 56.2%, after 1000 cycles | - | - | - | 6 M KOH | [68] |
| NiCo-LDH | |||||||||
| NiCo-LDH (NCLDH-21) | Solvothermal | Thin nanosheets (47 m2·g−1) | 2054.0 at 1.0 | 93.6%, after 5000 cycles | NCLDH-21//AC | 67.6 | 839.9 | 6 M KOH | [69] |
| NiCo-LDH-18/NF | Hydrothermal | Microflower (372.9 m2·g−1) | 2266.6 at 1.0 | 97.4% after 10,000 cycles | NiCo-LDH-18//AC | 104.8 | 3118.0 | 6 M KOH | [70] |
| NiCo-LDH (LDH-A0.3) | Hydrothermal | Nanosheets | 2237.5 at 1.0 | - | - | - | - | 6 M KOH | [71] |
| NiCo-LDH/NF-100 | Hydrothermal | Microporous nanosheets | 1156.0 at 1.0 | 95.3%, after 5000 cycles | NiCo-LDH/NF-100//AC | - | - | 2 M KOH | [72] |
| NiCo-LDH/NF-140 | Hydrothermal | Nanofibrous | - | - | NiCo-LDH/NF-140//AC | 51.5 μWh cm−2 | 1.125 mW cm−2 | 3 M KOH | [73] |
| NiCo-LDH-2 | Hydrothermal | Nanoflowers | - | 72.7%, after 3000 cycles | NiCo-LDH-2// carbon | 36.0 | 732.0 | 1 M KOH | [74] |
| NiCo-LDH (AL-LDH) | Electrodeposition | Interconnected nanoflakes | - | Poor stability over 500 cycles | Mg-Hybrid SC (Al-LDH/VS2) | 48.4 | 937.4 | 1 M KOH or MgSO4 | [75] |
| Binder-free NiCo-LDH/SS film | Electrodeposition | Wrinkled nanosheets | 1406.7 at 3.0 | 83%, over 5000 cycles | - | - | - | 1 M KOH | [76] |
| NiCo-LDH-1 | Reduction/ etching | Nano cubes (90.4 m2·g−1) | 1671.0 at 1.0 | 67.9%, after 10,000 cycles | NiCo-LDH-1//AC | 59.0 | 935.7 | 1 M KOH | [78] |
| NiCo-CH-180 | Solvothermal | Nanorods (52.6 m2·g−1) | 762.0 at 1.0 | - | NiCo-CH-180//AC | 52.0 | 1500.0 | 2 M KOH | [79] |
| NiCo-LDH-10 | Hydrothermal | Nanopetals (56.1 m2·g−1) | - | - | NiCo-LDH-10//graphene | 22.6 | 169.9 | 3 M KOH | [77] |
| Ni0.7Co0.3-LDH | Solvothermal | 3D hierarchical Flowers (132 m2·g−1) | 2052.0 at 1.0 | 72%, after 6000 cycles | Ni0.7Co0.3-LDH//AC | 54.0 | 750.0 | 6 M KOH | [80] |
| Ni3Co1-LDH | Solvothermal | 3D microflowers (143.8 m2·g−1) | 1978.2 at 1.0 | 70.6%, after 10,000 cycles | Ni3Co1-LDH//AC | 54.8 | 779.4 | 3 M KOH | [81] |
| Ni1Co4-LDH | Solvothermal | 3D nanosphere | 1300.0 at 1.0 | 90.0%, after 1000 cycles | - | - | - | 3 M KOH | [82] |
| NiCo-LDH-5:5 | Microwave radiation | 3D nanospheres (38.3 m2·g−1) | 2156.0 at 1.0 | - | NiCo-LDH-5:5//AC | 41.6 | 8000.0 | 2 M KOH | [83] |
| NiCo-LDH0.6 | Ammonia diffusion method | 3D nanoflower | 1463.2 at 1.0 | 87.9%, after 2000 cycles | NiCo-LDH0.6//AC | 23.4 | 900.0 | 2 M KOH | [84] |
| NiCo-LDH (NCDH-1) | Hydrothermal | 3D hierarchical Flowers (47.3 m2·g−1) | 2199.0 at 1.0 | - | NCDH-1//AC | 72.5 | 837.0 | 6 M KOH | [85] |
| NiCo-LDH film | Hydrothermal | Network porous flakes (23.3 m2·g−1) | 700.4 at 1.0 | 56.9%, after 2000 cycles | - | - | - | 1 M KOH | [86] |
| NiCo-LDH-6/NF | Electrodeposition | Thick films (5.7 m2·g−1) | 953.0 at 3 mAcm−2 | 37.5%, after 5000 cycles | - | - | - | 1 M KOH | [87] |
| NiCo-LDH-Vo/NF | Electrodeposition | Interconnected nano-wall arrays (4.0 m2·g−1) | - | 91.8%, after 5000 cycles | NiCo-LDH-Vo//AC | 56.46 | 800.0 | 1 M KOH | [88] |
| NiCo-LDH/Ni/CC | Electrodeposition | Thick, porous films (2.71 m2·g−1) | 1545.3 at 3 mAcm−2 | 180.6%, over 5000 cycles | NiCo-LDH/Ni/ CC//AC/Ni/CC | 49.3 | 750 | 3 M KOH | [89] |
| CF/CRBI-NiCo-LDH | Electrodeposition | Nanosheets (14.5 m2·g−1) | 1661.6 at 1.0 | 96.7%, after 3000 cycles | CF/CRBI-NiCo-LDH// AC/CF | 57.2 | 820.0 | 6 M KOH | [90] |
| CoNi-LDH | |||||||||
| CoNi-LDH nanoflower | Solvothermal | 3D Nanoflowers (101.3 m2·g−1) | - | - | CoNi-LDH NF//AC | 37.1 | 748.0 | 2 M KOH | [91] |
| CoNi-LDH7.5 | Sonochemical | Nanopetals | - | - | CoNi-LDH7.5// AC | 35.6 | 781.1 | 2 M KOH | [92] |
| CA-CoNi-LDH/CC | Hydrothermal/ electrochemical activation | Broccoli structures (45.5 m2·g−1) | 695.2 at 5 mAcm−2 | 103.1%, after 5000 cycles | CA-CoNi-LDH/CC// AC cloth | 37.2 | 206.8 | 6 M KOH | [93] |
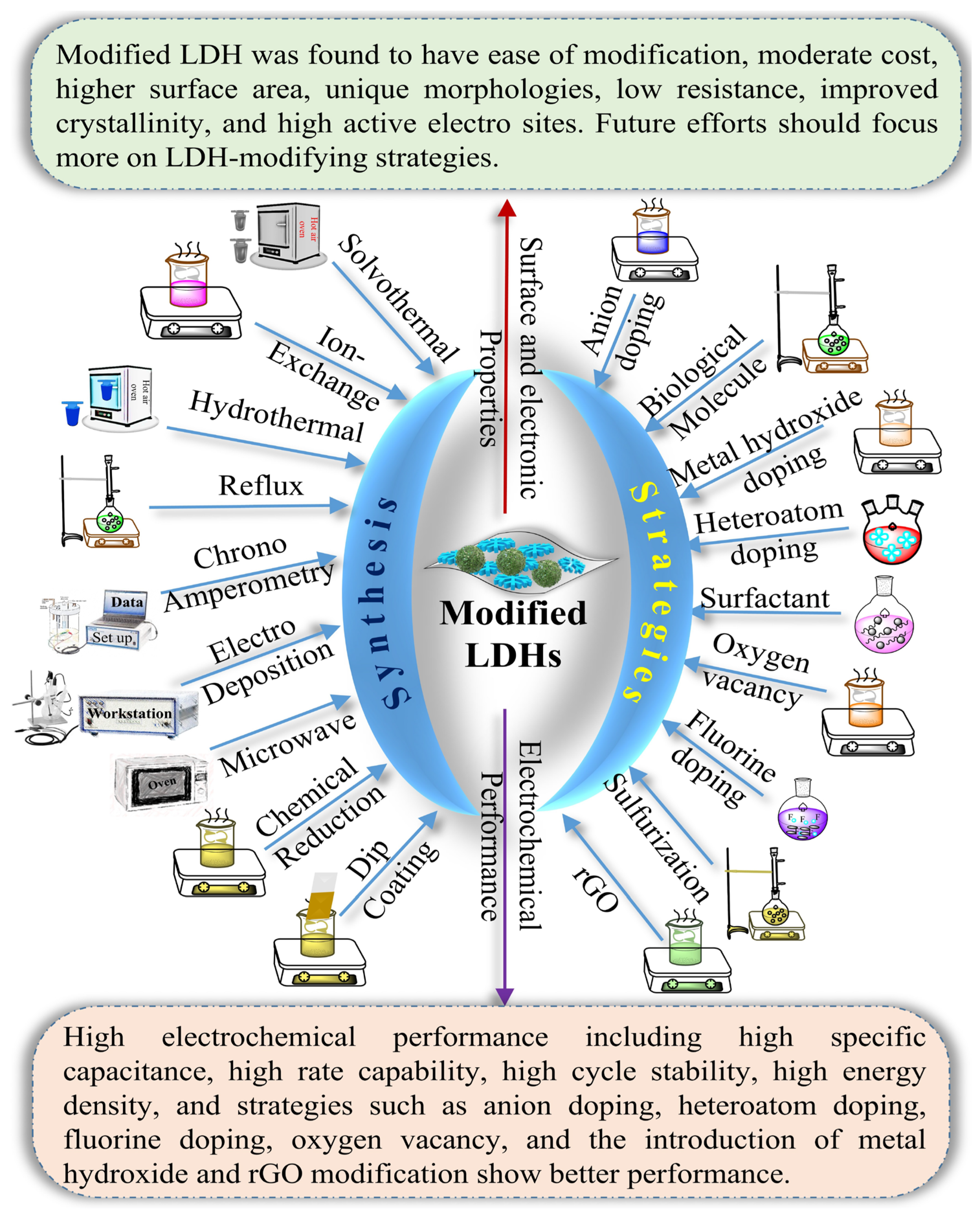
3. Strategies for Modifying LDHs and Their Electrochemical Performance
3.1. Sulfurized LDHs
3.2. Heteroatom-Doped LDHs
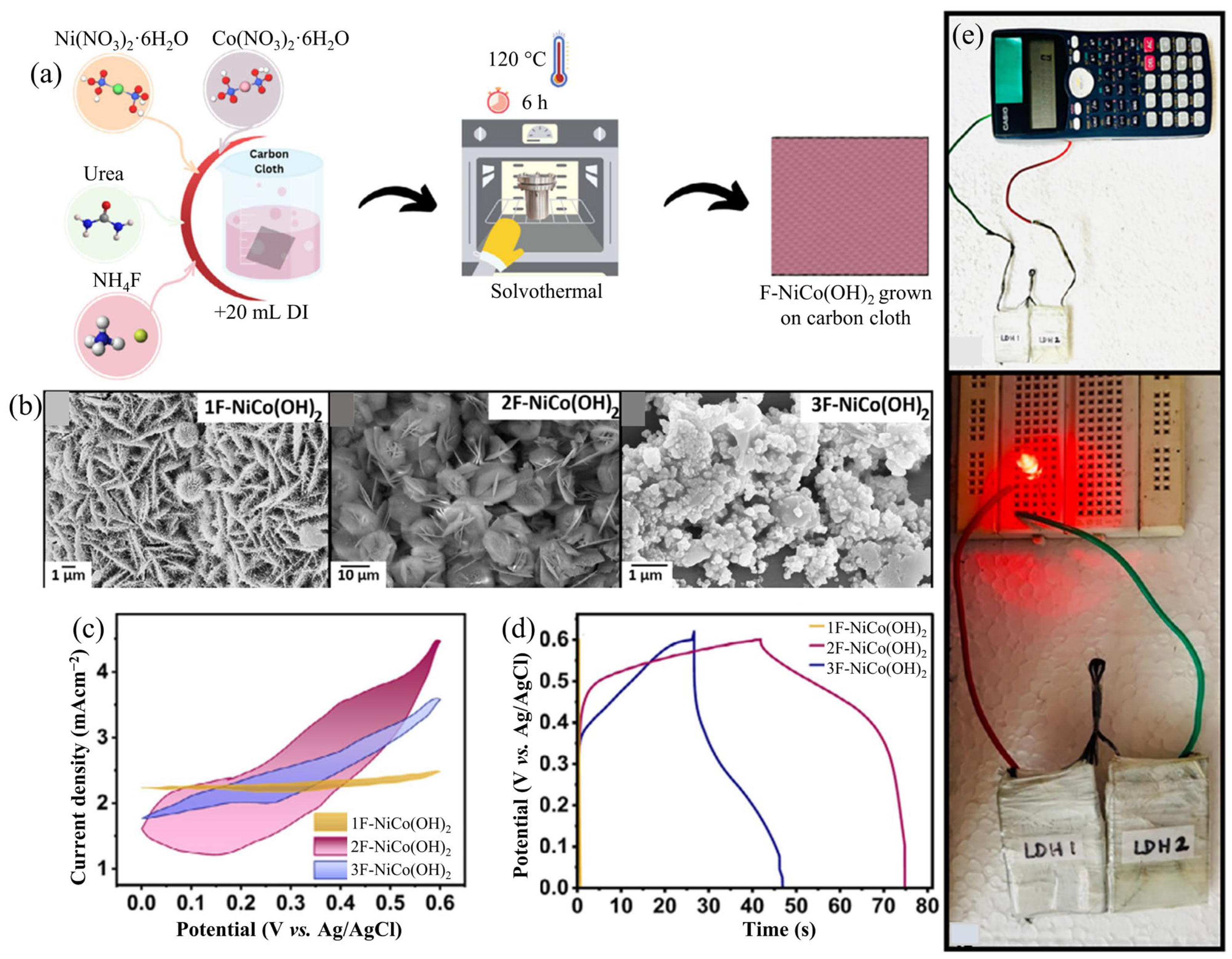
3.3. Fluorine-Doped LDH
3.4. Biological Molecule-Modified LDH
3.5. Oxygen Vacancy-Rich Modified LDH
3.6. Anion-Incorporated LDH
3.7. Metal Cation-Doped LDHs
3.8. Surfactant-Modified LDH

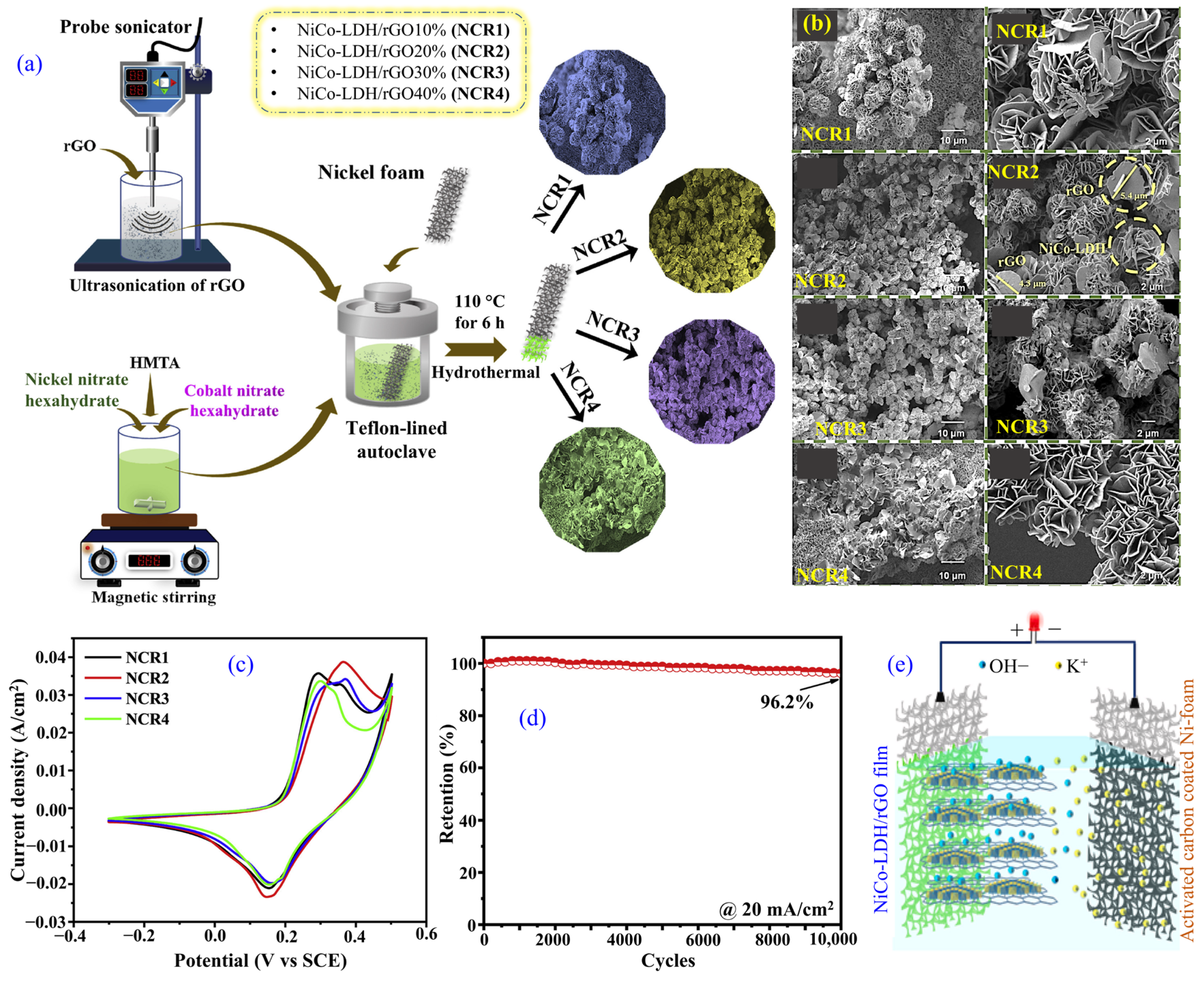
3.9. Reduced Graphene Oxide-Modified LDH
| Electrode Materials | Synthesis | Morphology | Three Electrodes | Two Electrodes | EL | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| Cs at Cd | Cycle Stability | Devices | Ed | Pd | |||||
| Sulfurized LDHs | |||||||||
| CoNiS-10 | Ion exchange | Agglomerated sheets (30.0 m2·g−1) | 954.0 at 1.0 | - | CoNiS-50//AC | 37.8 | 750.0 | 6 M KOH | [94] |
| CoMn-LDH/CoMn-S/NF | Chronoamperometric | Cross-linked nanosheets | - | - | CoMn-LDH/CoMn-S/NF//AC | 82.6 | 985 | 3 M KOH | [95] |
| Heteroatom-doped LDHs | |||||||||
| S-Ni0.5Co0.5-LDH | Electrodeposition | Thick cracked nanosheet | - | 56.6%, after 10,000 cycles | S-Ni0.5Co0.5-LDH //AC | 4.9 | 83.2 | 1 M KOH | [96] |
| CoMoS/NF | Hydrothermal | Leaf structure (7.3 m2·g−1) | 1655.1 at 10.0 | - | CoMoS/NF//rGO | 37.14 | 700.0 | 3 M KOH | [97] |
| Fluorine-doped LDH | |||||||||
| 2F-NiCo(OH)2 | Solvothermal | Succulent | 683.0 at 1.0 mAcm−2 | - | 2F-NiCo(OH)2//AC | 67.0 | 10,666.0 | 1 M KOH | [98] |
| F-NiFe-LDH-24 h | Hydrothermal | Flower structure | 1942.0 at 1.0 | 91.55%, after 1000 cycles | - | - | - | 2 M KOH | [51] |
| Biological molecule-modified LDH | |||||||||
| NiCoLDH-0.05 | Hydrothermal | Flower structure (18.8 m2·g−1) | 959.0 at 1.0 | - | NiCoLDH-0.05//AC | 32.7 | 400 | 6 M KOH | [27] |
| Oxygen-vacancy-rich modified LDH | |||||||||
| Ov-NCM | Hydrothermal | Spherical protrusions/ nanopetals | 4253.0 at 1.0 | 89%, after 5000 cycles | Ov-NCM //AC | 71.0 | 801 | 2 M KOH | [99] |
| Anion-incorporated LDH | |||||||||
| NiCo-LDH-P | Electrodeposition | Nanosheets | 2070.0 at 1.0 | - | NiCo-LDH-P//AC | 49.2 | 375.0 | 1 M KOH | [100] |
| NiCoLDH-ClO3− | Solvothermal | Microflower (25.6 m2·g−1) | - | 60%, after 1000 cycles | NiCoLDH-ClO3−//AC | 15.06 | 1910.0 | 1 M KOH | [101] |
| Metal cation-doped LDHs | |||||||||
| NiCoAl0.1 | Hydrothermal/ solvothermal | Interconnected nanosheets (69.06 m2·g−1) | - | 108%, after 40,000 cycles | NiCoAl0.1//ACC | 0.84 mWh cm−2 | 10.0 mWcm−2 | 2 M KOH | [102] |
| NiCo-LDH-Mo0.5 | Hydrothermal | Cauliflower structured microsphere (91.7 m2·g−1) | 160.5 at 1.0 | 82.7%, after 1500 cycles | NiCo-LDH-Mo0.5//AC | 41.3 | 850.0 | 6 M KOH | [103] |
| MoNiCo-LDH-0.05/CC | Microwave | Cross-liked nanosheets (178.8 m2·g−1) | 3002.0 at 1.0 | 90.7%, after 3000 cycles | MoNiCo-LDH-0.05/ CC//AC | 103.3 | 750.0 | 2 M KOH | [104] |
| ZnNiCo−G−H-0.5 | Two-step hydrothermal | Flower structures (670.9 m2·g−1) | 1145.0 at 1.0 | 75.68%, after 5000 cycles | ZnNiCo−G−H-0.5//AC | 40.0 | 825.0 | 6 M KOH | [105] |
| NCS-LDH-Br1 | Electrodeposition | Cross-linked nanoplates (211.4 m2·g−1) | 1994.0 at 1.0 | 70.4%, after 15,000 cycles | NCS-LDH-Br1//AC | 31.4 | 360.0 | 3 M KOH | [106] |
| Surfactant-modified LDH | |||||||||
| NiCo-LDH-PVP | Electrodeposition | Nanoflower | - | 57.0%, after 2000 cycles | NiCo-LDH-PVP//AC | 741.0 μWhcm−2 | 800.0 μWcm−2 | 3 M KOH | [107] |
| rGO-modified LDH | |||||||||
| rGO/NiCo-LDH | Hydrothermal | Sheets (124.8 m2·g−1) | 1975.0 at 1.0 | 98.5%, after 10,000 cycles | rGO/NiCo-LDH//AC | 54.1 | 789.0 | 3 M KOH | [108] |
| rGO/NiCo-LDH/Ni foam | Hydrothermal | Honeycomb structure (42.5 m2·g−1) | 2702.0 at 0.25 | 108%, after 10,000 cycles | NiCo-LDH/rGO/Ni foam//AC/Ni foam | 48.5 | 200.0 | 2 M KOH | [109] |
| rGO/NiCo-LDH/Ni foam (NCR2) | Hydrothermal | Microflower (70.2 m2·g−1) | 2823.0 at 4.0 mAcm−2 | 96.2%, after 10,000 cycles | rGO/NiCo-LDH/Ni foam//AC/Ni foam | 63.9 | 7080.0 | 1 M KOH | [110] |
| BC/rGO0.75-NiCo-LDH | Reflux | Spherical particle embedded sheet | - | - | - | - | - | 1 M H2SO4 | [111] |
| NiCoLDH/ rGO-SCC30 | Microwave- assisted hydrothermal | Nanoflower modified sheet | 2818.0 at 1.0 | - | NiCoLOH/rGO-SCC//AC | 35.9 | 375.0 | 6 M KOH | [112] |
| rGO/ZnMgAl-LDH (1:1) | Hydrothermal | Sheets/spherical (78.5 m2·g−1) | 656.6 at 1.0 | 81.0%, after 10,000 cycles | rGO/ZnMgAl-LDH//AC | 31.7 | 784.0 | 1 M KOH | [113] |
| rGO-15/F-NiFe-LDH | Hydrothermal | Nanoflower/sheets | 2860.0 at 1.0 | 102%, after 1000 cycles | F-NiFe-LDH/rGO//AC | 110.0 | 1250.0 | 2 M KOH | [114] |
| rGO/NiMnB-16 | Chemical reduction | Core–shell porous structured particles | 916.0 at 1.0 | 96.1%, after 4000 cycles | NiMnB/rGO-16//AC | 50.2 | 800.4 | 6 M KOH | [115] |
| rGO/NiMn-LDH/Ni foam | Dip coating | Porous nanoflake/sheet (99.5 m2·g−1) | 1090.0 at 1.0 | 92.3%, after 10,000 cycles | NiMn-LDH/Ni foam/rGO//AC | 45.8 | 623.0 | 3 M KOH | [116] |
4. Advantages and Disadvantages of Three- and Two-Electrode Systems
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACs | Activated carbons |
| AC | Activated carbon |
| Ag−1 | Amperes per gram |
| Al foil | Aluminum foil |
| BC/rGO0.75-NiCo-LDH | Bacterial cellulose/reduced graphene oxide-NiCo-LDH |
| CC | Carbon cloth |
| CC1 | Cu1Co1 |
| CC2 | Cu2Co1 |
| Cd | Current density |
| Cg−1 | Coulombs per gram |
| CPs | Conducting polymers |
| CF/CRBI-NiCo-LDH | Carbon felt/coral reef-bioinspired ultrathin NiCo-LDH |
| CTAB | Cetyltrimethylammonium bromide |
| Cs | Specific capacitance |
| CNTs | Carbon nanotubes |
| Co(OH)2 | Cobalt(II) hydroxide |
| CuCo2O4 | Copper cobaltite |
| CV | Cyclic voltammetry |
| DMF | N,N-Dimethylformamide |
| Ed | Energy density |
| EDLCs | Electrochemical double-layer capacitors |
| EL | Electrolyte |
| F | Fluorine |
| FC3//AC-NF | 12 h hydrothermally prepared FeCo-LDH//activated carbon-nickel foam |
| 1F-NiCo(OH)2 | 6 mM NH4F is used in the synthesis of 1F-NiCo(OH)2 |
| 2F-NiCo(OH)2 | 12 mM NH4F is used in the synthesis of 2F-NiCo(OH)2 |
| 3F-NiCo(OH)2 | 24 mM NH4F is used in the synthesis of 3F-NiCo(OH)2 |
| Fg−1 | Farads per gram |
| GCD | Galvanostatic charge/discharge |
| GO | Graphene oxide |
| HSs | Hybrid supercapacitors |
| H2O2 | Hydrogen peroxide |
| ITO | Indium tin oxide |
| LED | Light-emitting diode |
| L-Ni4CuLDH | Low-temperature chemical co-precipitation synthesized nickel-copper layered double hydroxide |
| µWhcm−2 | Micro-watt-hours per square centimeter |
| mAcm−2 | Milliamperes per square centimeter |
| mAhg−1 | Milliampere-hours per gram |
| mFcm−2 | Milli-Femto Farads per square centimeter |
| m2·g−1 | Square meters per gram |
| MgSO4 | Magnesium sulfate |
| mVs−1 | Millivolts per second |
| mWcm−2 | Milliwatts per square centimeter |
| mWhcm−2 | Milliwatt-hours per square centimeter |
| 2-MI | 2-Methylimidazole |
| Mn2O3 | Manganese(III) oxide |
| Mo | Molybdenum |
| N | Nitrogen |
| NCDH-1 | 1:1 ratio of Ni and Co acetate precursors is used in the synthesis of NiCo-LDH |
| NF | Nickel foam |
| NFC | NiFe-LDHs with carbonate anions |
| NFS | NiFe-LDHs with sulfate anions |
| NH4F | Ammonium fluoride |
| NiAl-LDH-60 | Nickel-foam-supported NiAl-LDH soaks in a solution of ice water containing NaBH4 for 60 min |
| Ni/NF | Nickel/nickel foam |
| NiCo-CH-x | NiCo-layered double hydroxide carbonate hybrid |
| NiCo-CH-180 | NiCo-layered double hydroxide carbonate hybrid processed at 180 °C |
| NiCo-LDH-Mo0.5 | 0.50 molar Na2MoO4·2H2O was used in the synthesis of NiCo-LDH-Mo |
| Ni3Co1-LDH | 3:1 ratio of Ni and Co nitrate precursors is used in the synthesis of LDH |
| Ni1Co4–LDH | 1:4 ratio of Ni and Co nitrate precursors is used in the synthesis of LDH |
| NiCoSc-LDH-Br1 | 1 mmol Sc(NO3)3·H2O is used in the synthesis of NiCoSc-LDH-Br |
| NM/NF//AC | NiMn-LDH/Nickel foam/Activated carbon |
| Ov | Oxygen vacancy |
| PO43 | Phosphate ions |
| Pd | Power density |
| PCs | Pseudocapacitors |
| PC | Pseudocapacitor |
| rGO | Reduced graphene oxide |
| Rct | Charge transfer resistance |
| S | Sulfur |
| SS | Stainless steel |
| SCs | Supercapacitors |
| rGO-SCC30 | 30 mg of reduced graphene oxide derived from aluminum electrolytic spent cathode carbon |
| SDS | Sodium dodecyl sulfate |
| SC | Supercapacitor |
| V(OH)2 | Vanadium(II) hydroxide |
| VS2 | Vanadium disulfide |
| Wkg−1 | Watts per kilogram |
| Wh·kg−1 | Watt-hours per kilogram |
| ZMAG2 | rGO/ZnMgAl (1:1) |
| ZnNiCo-G-H-0.5 | 0.5 mmol ZnCl2 was used in the synthesis of Zn-doped hydrolyzed NiCo-glycerate nanosphere |
References
- Chen, M.; Zhang, Y.; Xing, G.; Chou, S.-L.; Tang, Y. Electrochemical energy storage devices working in extreme conditions. Energy Environ. Sci. 2021, 14, 3323–3351. [Google Scholar] [CrossRef]
- Shan, X.-Y.; Li, F.; Wang, D.-W.; Cheng, H.-M. The smart era of electrochemical energy storage devices. Energy Storage Mater. 2016, 3, 66–68. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, H.; Kumar, G.; Sharma, S.; Aneja, R.; Sharma, A.K.; Kumar, R.; Kumar, P. Progress and challenges in electrochemical energy storage devices: Fabrication, electrode material, and economic aspects. Chem. Eng. J. 2023, 468, 143706. [Google Scholar] [CrossRef]
- Yu, L.; Chen, G.Z. Supercapatteries as high-performance electrochemical energy storage devices. Electrochem. Energy Rev. 2020, 3, 271–285. [Google Scholar] [CrossRef]
- Rudra, S.; Seo, H.W.; Sarker, S.; Kim, D.M. Supercapatteries as hybrid electrochemical energy storage devices: Current status and future prospects. Molecules 2024, 29, 243. [Google Scholar] [CrossRef]
- Shi, C.; Wang, Y.; Kulaots, I.; Zhu, H.; Sheldon, B.W. Water-in-salt battery electrolyte for high-voltage supercapacitors: A Fundamental study on biomass and carbon fiber electrodes. J. Electrochem. Soc. 2024, 171, 110526. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Liu, Y.; Gong, X. Electrochemical energy storage devices-batteries, supercapacitors, and battery-supercapacitor hybrid devices. ACS Appl. Electron. Mater. 2025, 7, 2233–2270. [Google Scholar] [CrossRef]
- Singh, N.; Singh, V.; Bisht, N.; Negi, P.; Dhyani, A.; Sharma, R.K.; Tewari, B.S. A comprehensive review on supercapacitors: Basics to recent advancements. J. Energy Storage 2025, 121, 116498. [Google Scholar] [CrossRef]
- Sriram, G.; Kurkuri, M.; Oh, T.H. Recent trends in highly porous structured carbon electrodes for supercapacitor Applications: A review. Energies 2023, 16, 4641. [Google Scholar] [CrossRef]
- Girirajan, M.; Bojarajan, A.K.; Pulidindi, I.N.; Hui, K.N.; Sangaraju, S. An insight into the nanoarchitecture of electrode materials on the performance of supercapacitors. Coord. Chem. Rev. 2024, 518, 216080. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, R.; Joanni, E.; Singh, R.K.; Shim, J.-J. Advances in pseudocapacitive and battery-like electrode materials for high performance supercapacitors. J. Mater. Chem. A 2022, 10, 13190–13240. [Google Scholar] [CrossRef]
- Aman, M.; Konduparty, P.; Sharma, S.; Srivastava, R.P.; Bhattacharyya, S.; Sharma, V.; Balani, K.; Jha, S.K.; Omar, S. Layered double hydroxide based composites for energy storage applications: Insights into supercapacitors and batteries. J. Energy Storage 2025, 116, 116093. [Google Scholar] [CrossRef]
- Xiong, C.; Su, Y. Recent progress of transition metal-based oxide composite electrode materials in supercapacitor. Adv. Sustain. Syst. 2025, 9, 2400578. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Liu, Q.; Li, T.; Lan, D.; Ma, Y. Recent progress on covalent organic frameworks and their composites as electrode materials for supercapacitors. Adv. Compos. Hybrid Mater. 2024, 8, 86. [Google Scholar] [CrossRef]
- Muralee Gopi, C.V.V.; Alzahmi, S.; Narayanaswamy, V.; Vinodh, R.; Issa, B.; Obaidat, I.M. Supercapacitors: A promising solution for sustainable energy storage and diverse applications. J. Energy Storage 2025, 114, 115729. [Google Scholar] [CrossRef]
- Sriram, G.; Hegde, G.; Dhanabalan, K.; Kalegowda, Y.; Mouraliraman, D.; Vishwanath, R.S.; Kurkuri, M.; Oh, T.H. Recent trends in hierarchical electrode materials in supercapacitor: Synthesis, electrochemical measurements, performance and their charge-storage mechanism. J. Energy Storage 2024, 94, 112454. [Google Scholar] [CrossRef]
- Sahoo, G.; Rout, C.S. MXene-transition metal chalcogenide hybrid materials for supercapacitor applications. Chem. Commun. 2025, 61, 6439–6461. [Google Scholar] [CrossRef]
- Karwasra, R.; Siwatch, P.; Gupta, Y.; Sharma, K.; Tripathi, S.K. Recent advancements in layered double hydroxides as an electrode material for high-performance asymmetric supercapacitors. J. Solid State Electrochem. 2025, 1–31. [Google Scholar] [CrossRef]
- Oladele, I.O.; Adelani, S.O.; Taiwo, A.S.; Akinbamiyorin, I.M.; Olanrewaju, O.F.; Orisawayi, A.O. Polymer-based nanocomposites for supercapacitor applications: A review on principles, production and products. RSC Adv. 2025, 15, 7509–7534. [Google Scholar] [CrossRef]
- Manfo, T.A.; Laaksonen, H. A review of carbon-based hybrid materials for supercapacitors. New Carbon Mater. 2025, 40, 81–110. [Google Scholar] [CrossRef]
- Sriram, G.; Arunpandian, M.; Dhanabalan, K.; Sarojamma, V.R.; David, S.; Kurkuri, M.D.; Oh, T.H. Recent progress using graphene oxide and its composites for supercapacitor applications: A review. Inorganics 2024, 12, 145. [Google Scholar] [CrossRef]
- Kumar, J.; Solangi, N.H.; Neiber, R.R.; Bai, F.; Sharma, B.P.; Charles, V.; Zhai, P.; Wang, Z.; Yang, X. Chemical perspectives on synthesis, functionalization, artificial intelligence, and energy storage applications of layered double hydroxides-based nanomaterials: A comprehensive review. Coord. Chem. Rev. 2025, 537, 216705. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, K.; Hua, Y.; Peng, L.; Meng, X.; Zhang, C.; Su, R.; Tao, X.; Hu, H.; Guo, Y.; et al. LDHs and their derivatives for electrochemical energy storage and conversion systems: Design, insights and applications. ChemElectroChem 2024, 11, e202400156. [Google Scholar] [CrossRef]
- Sadavar, S.P.; Mulik, S.V.; Koyale, P.A.; Sadavar, S.V.; Delekar, S.D. Advances in anion-intercalated layered double hydroxides for supercapacitors: Study of chemical modifications and classifications. Mater. Horiz. 2025, 12, 5031–5052. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.; Shakir, I. Recent advances in layered double hydroxides as electrode materials for high-performance electrochemical energy storage devices. J. Energy Storage 2017, 13, 103–122. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Q.; Li, B.; Xue, H.; Pang, H.; Chen, C. Recent progress in layered double hydroxide based materials for electrochemical capacitors: Design, synthesis and performance. Nanoscale 2017, 9, 15206–15225. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, L.; Li, L.; Wu, H.; Zheng, J.; Sun, J.; Li, G. One-step hydrothermal synthesis of glucose-induced low crystallinity nico-based layered double hydroxides for high-performance asymmetric supercapacitors. Chem.-Eur. J. 2025, 31, e202403439. [Google Scholar] [CrossRef]
- Ahmad, S.; Tariq, M.; Ni, H.; Albaqawi, H.S.; Alabbad, E.A.; Althagafi, T.M.; Tahir, K.; Almarhoon, Z.M.; Zaki, M.E.A.; Khan, A.U. Constructing Fluorine modified NiCo-LDH nanocomposite for enhanced supercapacitor performance through active site and structural optimization. Electrochim. Acta 2025, 532, 146452. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Qiao, Q.Q.; Li, G.R.; Gao, X.P. PO43− polyanion-doping for stabilizing Li-rich layered oxides as cathode materials for advanced lithium-ion batteries. J. Mater. Chem. A 2014, 2, 7454–7460. [Google Scholar] [CrossRef]
- Jia, X.; Zheng, T.; Gao, R.; Chen, Y.; Liu, L.; Wei, Q.; Liu, J.; Chen, D.; Xu, D.; Chi, J.; et al. Controllable construction of porous yolk shell phosphate-functionalized Co/Ni-layered double hydroxides for high-performance supercapacitor. Electrochim. Acta 2025, 537, 146853. [Google Scholar] [CrossRef]
- Malavekar, D.; Pujari, S.; Jang, S.; Bachankar, S.; Kim, J.H. Recent development on transition metal oxides-based core–shell structures for boosted energy density supercapacitors. Small 2024, 20, 2312179. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, C.; Chen, H. A review on the synthesis of CuCo2O4-based electrode materials and their applications in supercapacitors. J. Mater. 2021, 7, 98–126. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Luo, B. Designed nickel cobalt-layered double hydroxide/CuCo2O4 nanorod heterostructure for high-performance supercapacitors. ACS Appl. Energy Mater. 2025, 8, 12058–12070. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Ulfah, F.F.; Nahda, D.P.N.; Luhur, S.P.; Sanjaya, A.R.; Krisnandi, Y.K.; Sumboja, A.; Zulfia, A.; Ryu, K.-S.; Akbar, Z.A. Hierarchical rod-like structure MnO2/NiCo-layered double hydroxide on nickel foam for a high-performance supercapacitor electrode. Mater. Res. Bull. 2025, 184, 113289. [Google Scholar] [CrossRef]
- Tai, P.-Y.; Sakthivel, M.; Peng, Y.-J.; Kubendhiran, S.; Lin, L.-Y.; Ho, K.-C. In Situ Growing Te-doped Ni–Mn layered double hydroxide on a cetyltrimethylammonium bromide-modified MXene conductive layer for binder-free supercapacitors. ACS Appl. Energy Mater. 2025, 8, 4122–4133. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Jamal, R.; Abdiryim, T.; Abdurexit, A.; Yang, H.; Song, K.; Li, J.; Chen, J.; Zhang, W. N-doped hollow mesoporous carbon spheres modified with cobalt–nickel hydroxide for supercapacitor applications. Chem. Eng. J. 2025, 513, 162944. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y.; Chen, Y.; Zhou, N.; Dai, J.; Li, D.; Guo, H.; Qin, Q.; Zeng, B.; Yuan, C.; et al. Polyaniline-modified NiCo layered double hydroxide hollow nanocages for aqueous ammonium-ion supercapacitors. J. Colloid Interface Sci. 2025, 686, 691–700. [Google Scholar] [CrossRef]
- Jaramillo-Hernández, C.; Seijas-Da Silva, A.; Abellán, G. Crystallographic Phase-dependent electrochemical properties of layered hydroxides for energy applications. Eur. J. Inorg. Chem. 2025, 28, e202400754. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Li, C.-E.; Yan, X.-H.; Zhang, Y.-Y.; Yin, R.-B.; Wei, Y.-F.; Gao, K.-Z.; Gao, H.-L. Advanced strategies for enhancing electrochemical performance of NiAl LDH electrodes in supercapacitors. Coord. Chem. Rev. 2025, 531, 216497. [Google Scholar] [CrossRef]
- Jafari, M.; Ganjali, F.; Eivazzadeh-Keihan, R.; Maleki, A.; Geranmayeh, S. Recent advances in applications of graphene-layered double hydroxide nanocomposites in supercapacitors and batteries. FlatChem 2024, 45, 100658. [Google Scholar] [CrossRef]
- Luo, F.; San, X.; Wang, Y.; Meng, D.; Tao, K. Layered double hydroxide-based electrode materials derived from metal–organic frameworks: Synthesis and applications in supercapacitors. Dalton Trans. 2024, 53, 10403–10415. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, X.; Zhang, H.; Xu, B.B.; Wasnik, P.; Li, H.; Singh, M.V.; Ma, Y.; Li, T.; Guo, Z. Research progress of MXenes and layered double hydroxides for supercapacitors. Inorg. Chem. Front. 2023, 10, 4358–4392. [Google Scholar] [CrossRef]
- Ray, P.K.; Mohanty, R.; Parida, K. Recent advancements of NiCo LDH and graphene based nanohybrids for supercapacitor application. J. Energy Storage 2023, 72, 108335. [Google Scholar] [CrossRef]
- Li, X.; Ren, J.; Sridhar, D.; Xu, B.B.; Algadi, H.; El-Bahy, Z.M.; Ma, Y.; Li, T.; Guo, Z. Progress of layered double hydroxide-based materials for supercapacitors. Mater. Chem. Front. 2023, 7, 1520–1561. [Google Scholar] [CrossRef]
- Samuel, J.M.; Navaneeth, P.; Satheesh Babu, T.G.; Suneesh, P.V. Fabrication of flexible printed supercapacitor with high cycling stability using Cobalt-Cerium layered double hydroxide. J. Ind. Eng. Chem. 2025, 150, 620–631. [Google Scholar] [CrossRef]
- Moorthi, K.; Padaki, M.; Mohan, S. Insights into the Morphological effects of 1D, 2D, and 3D CoV-layered double hydroxides on their electrochemical performance in supercapacitors. Langmuir 2025, 41, 3922–3937. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Chai, H.; Zhang, Y.; Wang, R.; Xie, M.; Xu, Y.; Chen, J.; Jiao, Y. Electrochemical activation strategy assisted morphology engineering Co-Fe layered double hydroxides for oxygen hydrogen evolution and supercapacitor. J. Colloid Interface Sci. 2023, 632, 186–195. [Google Scholar] [CrossRef]
- Chougale, N.B.; Gaikwad, M.A.; Shembade, U.V.; Bhosale, T.T.; Magadum, M.G.; Wategaonkar, S.B.; Ghatage, S.R.; Pillai, P.K.; Khot, J.A.; Hatshan, M.R.; et al. Advanced self-supported FeCo-LDHs electrocatalyst for combined energy storage and water oxidation. Colloids Surf. A 2025, 711, 136299. [Google Scholar] [CrossRef]
- Luo, C.-W.; Zhang, K.; Zeng, H.-Y.; Wang, H.-B.; Xu, K.-W.; Feng, B.; Wu, G.-Z. Engineering oxygen vacancies into ZnCo hydrotalcite for boosting performances for flexible supercapacitor. J. Power Sources 2025, 633, 236403. [Google Scholar] [CrossRef]
- Nishad, H.S.; Kotha, V.; Sarawade, P.; Chaskar, A.C.; Mane, S.; Lee, J.; Walke, P.S. Exchanging interlayer anions in NiFe-LDHs nanosphere enables superior battery-type storage for high-rate aqueous hybrid supercapacitors. J. Mater. Chem. A 2024, 12, 9494–9507. [Google Scholar] [CrossRef]
- Li, Y.; He, G.; HuangFu, H.; Mi, Y.; Zhang, H.; Zheng, D.; Wu, M.; Yuan, H. Morphology evolution of NiFe layered double-hydroxide nanoflower clusters from nanosheets: Controllable structure–performance relation for green energy storage. Energy Technol. 2024, 12, 2300749. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Li, H.; Yao, H.; Wang, X. Influence of different Ni:Fe molar ratios on the properties of NiFe-LDH as electrode materials for supercapacitor applications. Int. J. Electrochem. Sci. 2024, 19, 100590. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Xiao, Q.; Cheng, C.; Miao, Y.; Zhu, J. Synthesis of nickel-copper layered double hydroxides for asymmetric supercapacitors by low-temperature chemical co-precipitation method. J. Mater. Sci. Mater. Electron. 2024, 35, 733. [Google Scholar] [CrossRef]
- Kanimozhi, S.N.; Vasudevan, B.; Souwaileh, A.A.; Subbiah, J.; Anandan, S. Cu-Zn layered double hydroxides as high-performance electrode for supercapacitor applications. Electrochim. Acta 2024, 507, 145106. [Google Scholar] [CrossRef]
- Xu, X.; Lu, H.; Xu, D.; Zhou, P.; Ying, Y.; Li, L.; Liu, Y. Oxygen-rich vacancies CuCoLDH with 1D/2D nanoarray structure for high performance asymmetric supercapacitor. Appl. Surf. Sci. 2023, 614, 156174. [Google Scholar] [CrossRef]
- Aljafari, B.; Kanimozhi, S.N.; Wu, J.J.; Anandan, S. Synthesis of nano-architectured copper-cobalt layered double hydroxide for energy storage applications. Inorg. Chem. Commun. 2024, 159, 111748. [Google Scholar] [CrossRef]
- Yadav, A.A.; Redekar, R.S.; Patil, K.V.; Kshirsagar, V.P.; Tarwal, N.L. Development of the nickel foam supported hydrothermally grown binder-free and highly porous magnesium-cobalt hydroxide films for supercapacitor application. J. Energy Storage 2024, 81, 110292. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Li, R.; Li, R.; Jia, R.; Nie, K.; Xie, H.; Xu, X.; Lin, L. Fabrication of oxygen-vacancy abundant NiAl-layered double hydroxides for ultrahigh capacity supercapacitors. Dalton Trans. 2025, 54, 821–831. [Google Scholar] [CrossRef]
- Duddi, R.; Shivani; Dhiman, S.; Singh, A.K.; Kamboj, N.; Kumar, S. Hydrothermally synthesised porous NiAl-LDH as an efficient pseudocapacitive material in asymmetric supercapacitors. Hybrid Adv. 2025, 8, 100372. [Google Scholar] [CrossRef]
- Duddi, R.; Shivani; Dhiman, S.; Kumar Singh, A.; Kamboj, N.; Kumar, S. Superior cycle stability and enhanced pseudocapacitive performance of NiAl-LDH decorated carbon cloth for supercapacitor application. Appl. Surf. Sci. 2025, 679, 161298. [Google Scholar] [CrossRef]
- Hammood, S.A.; Al-Asadi, A.S.; Al-Mudhaffer, M.F. Synthesis and properties of flexible supercapacitor based on zinc-aluminum layered doubled hydroxide. J. Alloys Compd. 2024, 1009, 176925. [Google Scholar] [CrossRef]
- Marimuthu, M.; Muniappan, E.; Jayaraj, M.; Bojarajan, A.K.; El-marghany, A.; Duraisamy, P.; Sangaraju, S. Synthesis and unveiling properties of nanocomposite NiO/Mn2O3 and Ni–Mn-Based layered double hydroxide as efficient supercapacitor electrodes. Energy Technol. 2025, 2402451. [Google Scholar] [CrossRef]
- Kidie, A.E.; Dhakal, G.; Sahoo, S.; Tuma, D.; Shim, J.-J. Interlayer spacing-controlled carnation flower-like microstructure of nickel-manganese layered double hydroxide for enhancing hybrid supercapacitor performance. J. Ind. Eng. Chem. 2024, 133, 550–560. [Google Scholar] [CrossRef]
- Jituri, S.D.; Nikam, S.M.; Bane, T.S.; Inamdar, A.I.; Mujawar, S.H. Porous cauliflower-like nanoarchitectures of NiMn-layered double hydroxide as a promising electrode for oxygen evolution reaction and supercapacitor applications. Electrochim. Acta 2025, 513, 145544. [Google Scholar] [CrossRef]
- Shi, Y.; Xue, J.; Yu, Y.; Liu, N.; Tang, C. Construction of binder-free 3-D porous NiMn LDH electrode materials for high performance asymmetric supercapacitors using dynamic hydrogen bubbles as template. J. Alloys Compd. 2024, 970, 172513. [Google Scholar] [CrossRef]
- Sankannavar, R.; Bhosale, R.; Jadhav, R.; Kolekar, S.; Kim, J.H.; Manjanna, J. High-efficiency pseudocapacitive layered double hydroxide on Ni-foil as an electrode for superior solid-state over liquid-state asymmetric supercapacitor. Inorg. Chem. Commun. 2025, 172, 113741. [Google Scholar] [CrossRef]
- Emin, A.; Ding, A.; Mateen, M.; Tul Muntaha, S.; Parkash, A.; Ali, S.; Li, Q. An efficient electrodeposition approach for preparing CoMn-hydroxide on nickel foam as high-performance electrodes in aqueous hybrid supercapacitors. Fuel 2025, 381, 133335. [Google Scholar] [CrossRef]
- Sreenidhi, L.; Verma, V.; Kour, S.; Kour, P.; Sharma, A.L. Investigation of the electrochemical performance of manganese based layered double hydroxides for energy storage applications. Mater. Chem. Phys. 2025, 332, 130229. [Google Scholar]
- Sivakumar, P.; Raj, C.J.; Savariraj, A.D.; Manikandan, R.; Karuppasamy, K.; Alfantazi, A.; Jung, H. The interwoven structured two-dimensional nico layered double hydroxide tortuous nanosheet as performance-enhanced electrode material toward battery-type supercapacitor. Int. J. Energy Res. 2024, 2024, 3149906. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, H.; Sa, R.; Lin, Q.; Li, Y. Synergistic coupling of NiCo-LDH in high-performance supercapacitors via hydrothermal method: Optimal utilization of the potential window. J. Alloys Compd. 2025, 1010, 177091. [Google Scholar] [CrossRef]
- Han, Z.; Guo, K.; Ding, J.; Liu, J.; Yang, X.; Hu, P.; Fan, H.; Teng, F. Influence of alkaline sources for the electrochemical performance of NiCo layered double hydroxide nanostructures. J. Energy Storage 2024, 78, 110165. [Google Scholar] [CrossRef]
- Bai, S.; Jia, B.; Zhang, Y.; Yang, H.; Qiang, X.; Wu, X. Mitigating self-discharge in supercapacitors through strategic mesoporous structural modification of NiCo layered double hydroxides. J. Alloys Compd. 2024, 1002, 175441. [Google Scholar] [CrossRef]
- Li, R.-Y.; Shen, X.-Y.; Li, J.; Zhao, D.-P.; Zhao, R.-D.; Wu, F.-F. Enhanced Electrochemical performance of NiCo-layered double hydroxides: Optimal synthesis conditions and supercapacitor Applications. Adv. Sustain. Syst. 2025, 9, 2400753. [Google Scholar] [CrossRef]
- Lei, W.; Liu, S.; Liu, Q.; Zou, X.; Xia, H. Morphology reconstruction of nickel cobalt layered double hydroxides induced by electrolyte concentrations triggers high performance of supercapattery storage. Micro Nano Lett. 2024, 19, e12201. [Google Scholar] [CrossRef]
- Xing, H.; Deng, X.; Wang, X. Alkaline capacity decay induced vacancy-rich LDH for high-performance magnesium ions hybrid supercapacitor. J. Colloid Interface Sci. 2025, 679, 43–53. [Google Scholar] [CrossRef]
- Kuku, M.; Arishi, M. Flexible and binder-free nickel-cobalt layered double hydroxides for high specific capacity supercapacitor applications. Mater. Chem. Phys. 2025, 332, 130230. [Google Scholar] [CrossRef]
- Das, J.P.; Nardekar, S.S.; Kesavan, D.; Bhunia, K.; Ravichandran, V.; Kim, S.-J. Unveiling the effect of growth time on bifunctional layered hydroxide electrodes for high-performance energy storage and green energy conversion. J. Mater. Chem. A 2024, 12, 20179–20190. [Google Scholar] [CrossRef]
- Jiang, H.; Ke, Q.; Qiu, X.; Chen, J.; Chen, P.; Wang, S.; Luo, X.; Rao, B. NiCo layered double hydroxide nanocages for high-performance asymmetric supercapacitors. Inorg. Chem. Front. 2023, 10, 2154–2164. [Google Scholar] [CrossRef]
- Kumar, S.; Satpathy, B.K.; Pradhan, D. Morphology-controlled synthesis of a NiCo-carbonate layered double hydroxide as an electrode material for solid-state asymmetric supercapacitors. Mater. Adv. 2024, 5, 2271–2284. [Google Scholar] [CrossRef]
- Wang, G.; Meng, Y.; Chi, C.; Liu, Z. Acetate ion-intercalated NiCo-LDH with quasi-theoretical capacitance for high energy/power density aqueous supercapacitors. Inorg. Chem. Front. 2024, 11, 863–873. [Google Scholar] [CrossRef]
- Yang, S.; Jia, Y.; Cao, H.; Li, Q.; Guo, Y.; Mi, Y.; He, J.; Du, P. Fabrication of three-dimensional hierarchical NiCo-LDH micro-flowers for enhanced charge storage in battery-type supercapacitors. J. Energy Storage 2024, 97, 112822. [Google Scholar] [CrossRef]
- Yu, L.; Ma, X.; Liang, Q. Facile synthesis of layered double hydroxides nanospheres and their electrochemical supercapacitor property dependent morphology. Mater. Lett. 2024, 355, 135519. [Google Scholar] [CrossRef]
- Yang, X.; He, Q.; Hu, L.; Wang, W.; Chen, W.; Fang, X.; Liu, J. Ultrafast microwave-assisted synthesis of porous nico layered double hydroxide nanospheres for high-performance supercapacitors. Molecules 2024, 29, 2546. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Wu, W.; Sun, P.; Zhou, Y.; Li, X.; Meng, C.; Wang, X. Preparation and electrochemical properties of NiCo-layered double hydroxide supercapacitor. Mater. Lett. 2024, 355, 135522. [Google Scholar] [CrossRef]
- Sivakumar, P.; Raj, C.J.; Savariraj, A.D.; Manikandan, R.; Jung, H. Developing a high-performance hybrid supercapacitor with the controlled assembly of a hierarchical 3D flower-like NiCo double hydroxide nano/microarchitecture electrode material. Surf. Interfaces 2024, 51, 104641. [Google Scholar] [CrossRef]
- Dighe, P.S.; Bhoite, A.A.; Patil, V.L.; Tarwal, N.L.; Sarawade, P.B. Development of ultrathin nanoflakes of Ni–Co LDH films by hydrothermal route for energy storage application. J. Phys. Chem. Solids 2024, 185, 111772. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Song, X.; Gao, J.; Liu, Y.; Yang, S. Preparation and capacitance performance of NiCo layered double hydroxide by multi-step constant current electrodeposition method. Electrochim. Acta 2024, 497, 144514. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, K.; Su, Y.; Li, S.; Ma, M.; Wu, T.; Huan, Y.; Shen, X.; Wei, T. In situ grown oxygen-vacancy rich NiCo-LDHs for high-performance supercapacitors. ChemistrySelect 2025, 10, e202403969. [Google Scholar] [CrossRef]
- Zhao, N.; Fu, P.; Cui, X.; Luo, M.; Huang, J. Electrodeposition of nickel cobalt layered double hydroxides on conductive nickel-coated carbon cloth: A strategy for boosting flexible supercapacitor electrode performance. J. Energy Storage 2025, 110, 115355. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Mozaffari, S.A.; Rasche, B.; Ebrahimi, F. Bio-inspired coral reef-like NiCo-LDH nanostructure fabricated on carbon felt for high performance flexible supercapacitor. Sci. Rep. 2025, 15, 10542. [Google Scholar] [CrossRef]
- Bao, E.; Ren, X.; Wang, Y.; Zhang, Z.; Luo, C.; Liu, X.; Xu, C.; Chen, H. Advanced hybrid supercapacitors assembled with CoNi LDH nanoflowers and nanosheets as high-performance cathode materials. J. Energy Storage 2024, 82, 110535. [Google Scholar] [CrossRef]
- Chen, H.; Bao, E.; Sun, H.; Ren, X.; Han, X.; Wang, Y.; Zhang, Z.; Luo, C.; Xu, C. Sonochemical synthesis of CoNi layered double hydroxide as a cathode material for assembling high performance hybrid supercapacitor. J. Colloid Interface Sci. 2024, 664, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Quan, H.; Tu, Z.; Zhang, Z.; Chen, D. Crystalline-amorphous hybrid CoNi layered double hydroxides for high areal energy density supercapacitor. J. Colloid Interface Sci. 2025, 683, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Y.; Ye, H.; Chen, J.; Wan, L.; Du, C.; Zhang, Y.; Xie, M. Gradually sulfurized Co-Ni LDH as electrode for highly-stable asymmetric supercapacitor. J. Energy Storage 2024, 91, 112117. [Google Scholar] [CrossRef]
- Moradi, M.; Afkhami, A.; Madrakian, T.; Moazami, H.R.; Tirandaz, A. Partial hydrothermal sulfidation of electrosynthesized Co-Mn layered-double-hydroxide as an active material for supercapacitor applications. J. Power Sources 2025, 629, 235993. [Google Scholar] [CrossRef]
- Li, A.; Liang, Y.; Zhang, Z.; Fang, B. Synthesis of sulfur-doped nickel cobalt hydroxide nanostructures on stainless steel substrates by electrodeposition and their characteristics as supercapacitor electrodes. Ionics 2025, 31, 2069–2084. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Kubendhiran, S.; Chung, R.-J.; Kongvarhodom, C.; Husain, S.; Yougbaré, S.; Chen, H.-M.; Wu, Y.-F.; Lin, L.-Y. Investigating energy storage ability of cobalt molybdenum hydroxide, sulfide and boride as active materials of battery supercapacitor hybrids. J. Energy Storage 2025, 112, 115530. [Google Scholar] [CrossRef]
- Caroline, S.C.; Ravindran, A.; Ghosh, K.; Batabyal, S.K. Growth of succulent shaped fluorine incorporated Ni-Co LDH (F-NiCo(OH)2): Elevating supercapacitor efficiency. Small 2025, 21, 2411641. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, T.; Ha, Y.; Chen, X.; Zhu, X.; Tao, J.; Liu, Y. Mn-doped NiCo LDH nanosheets with rich oxygen vacancies for high-performance supercapacitors and efficient oxygen evolution. J. Energy Storage 2025, 106, 114848. [Google Scholar] [CrossRef]
- Cui, S.; Ren, X.; Yin, H.; Fan, H.; Wang, C.; Zhang, M.; Tang, Y.; Yuan, H.; Xin, Y. One-step electrodeposited nickel-cobalt-layered double hydroxide nanosheets with anion intercalation for supercapacitor. J. Energy Storage 2024, 85, 111092. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Wang, J.; Zhang, S.; Xie, H.; Gu, Z. Preparation of nickel cobalt hydroxide with oxygen vacancies by intercalation of oxidizing anions as a high-performance electrode for supercapacitors. Chem. Eng. J. 2025, 511, 162080. [Google Scholar] [CrossRef]
- Huang, X.; Chu, B.; Han, B.; Wu, Q.; Yang, T.; Xu, X.; Wang, F.; Li, B. 2D-on-2D Al-doped NiCo LDH nanosheet arrays for fabricating high-energy-density, wide voltage window, and ultralong-lifespan supercapacitors. Small 2024, 20, 2401315. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, Z.; Hu, J.; Lu, S.; Guo, T.; Yang, W.; Xie, Q.; Ruan, Y. Hierarchical Mo-doped NiCo hydroxides cauliflower-like microspheres via a rapid microwave hydrothermal synthesis for supercapacitors. J. Energy Storage 2024, 81, 110393. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Z.; Wang, G.; Sang, H.; Xu, Z.; Li, W. Regulating the oxygen vacancy and electronic structure of NiCo Layered double hydroxides by molybdenum doping for high-power hybrid supercapacitors. Small 2024, 20, 2306382. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Wei, D.; Qiu, S.; Xu, F.; Cao, W.; Wang, Q.; Sun, L.; Chu, H. Zinc ions doping in layered bimetallic nickel-cobalt glycerol nanosheets for enhanced electrochemical performance of supercapacitors. ChemCatChem 2024, 16, e202400604. [Google Scholar] [CrossRef]
- Hu, B.; Hu, W.; Qian, X.; Zuo, M.; Wu, Z.; Song, Y.; Zheng, Q.; Shan, G.; Du, M. Scandium doping enables superior cycling performance of NiCo-LDHs-based supercapacitors via NH4Br-assisted electrodeposition. J. Energy Storage 2024, 92, 112069. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Zhao, Z.; Yang, W.; Zou, H.; Chen, S. Nanoflower-like nickel–cobalt bimetallic hydroxide electrode materials for asymmetric supercapacitor. ACS Appl. Energy Mater. 2024, 7, 6322–6333. [Google Scholar] [CrossRef]
- Vanasundari, K.; Sureka, P.; Mahalakshmi, G. Fabrication of porous Ni–Co LDH@rGO nanocomposites as efficient electrode materials for asymmetric supercapacitor. J. Mater. Sci. Mater. Electron. 2024, 35, 1083. [Google Scholar] [CrossRef]
- Lokhande, P.E.; Jagtap, C.; Kadam, V.; Udayabhaskar, R.; Shaikh, S.F.; Ali, R.; Pathan, H.M. Synergistic electrochemical behaviour of hydrothermally deposited Ni·Co LDH/rGO nanocomposite on nickel foam. J. Energy Storage 2024, 97, 112910. [Google Scholar] [CrossRef]
- Dighe, P.S.; Redekar, R.S.; Tarwal, N.L.; Sarawade, P.B. Unveiling the performance of nickel cobalt-layered double hydroxide/reduced graphene oxide composite for high performance aqueous supercapacitor. J. Power Sources 2025, 634, 236474. [Google Scholar] [CrossRef]
- Muhammad Afdhal Saputra, A.; Marpongahtun; Andriayani; Alemin Barus, D.; Goei, R.; Tok, A.; Ibadurrahman, M.; Ramadhan, H.T.S.R.; Irvan Hasibuan, M.; Peijs, T.; et al. Facile synthesis and electrochemical performance of bacterial cellulose/reduced graphene oxide/NiCo-layered double hydroxide composite film for self-standing supercapacitor electrode. Mater. Sci. Energy Technol. 2025, 8, 66–81. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, L.; Lu, J.; Sun, Y.; Shu, J.; Xiong, Y.; Zhang, W. Preparation of NiCoLDH/rGO-SCC composites from aluminum electrolytic spent cathode carbon for hybrid supercapacitor. Chem. Eng. J. 2025, 514, 163478. [Google Scholar] [CrossRef]
- Siwach, P.; Gaba, L.; Aggarwal, K.; Dahiya, S.; Punia, R.; Maan, A.S.; Singh, K.; Ohlan, A. Novel three-dimensional architectured ZnMgAl ternary layered double hydroxide@reduced graphene oxide nanocomposites as electrode material for high-performance supercapacitor. J. Energy Storage 2024, 98, 113055. [Google Scholar] [CrossRef]
- Li, Y.; He, G.; Huang, Z.; Huangfu, H.; Li, Z.; Qiao, Y.; Shi, Z. High-performance NF-loaded F-NiFe-LDH/rGO nanoflower/nanosheet composite electrode material achieving enhanced specific capacitance and energy density for supercapacitor. J. Energy Storage 2024, 101, 113954. [Google Scholar] [CrossRef]
- Li, Z.; Chen, G.; Yao, M.; Gou, S.; Deng, X.; Hu, Z.; Lu, X. Preparation of core-shell NiMnB/rGO composite electrodes and their application in hybrid supercapacitors. J. Alloys Compd. 2024, 992, 174605. [Google Scholar] [CrossRef]
- Priyadharshini, P.; Mahalakshmi, G. Facile synthesis of reduced graphene oxide nanosheet modified NiMn-LDH nanoflake arrays as a novel electrode for asymmetric supercapacitor. Diamond Relat. Mater. 2025, 151, 111833. [Google Scholar] [CrossRef]
- Lin, Y.; Mou, D.; Pu, X.; Li, B.; Sun, S.; Zhu, X. One-step hydrothermal synthesis of reduced graphene oxide/NiMn layered double hydroxide composites for high-performance hybrid supercapacitors. J. Power Sources 2025, 654, 237834. [Google Scholar] [CrossRef]
- Wu, C.; Tang, Y.; Zou, A.; Li, J.; Meng, H.; Gao, F.; Wu, J.; Wang, X. Recommended electrochemical measurement protocol for oxygen evolution reaction. DeCarbon 2025, 8, 100108. [Google Scholar] [CrossRef]
- Abdullin, K.; Gabdullin, M.; Kalkozova, Z.; Kudryashov, V.; Mirzaeian, M.; Yelemessov, K.; Baskanbayeva, D.; Serikkanov, A. Enhancing the Electrochemical performance of ZnO-Co3O4 and Zn-Co-O supercapacitor electrodes due to the in situ electrochemical etching process and the formation of Co3O4 Nanoparticles. Energies 2024, 17, 1888. [Google Scholar] [CrossRef]
- Rajivgandhi, P.; Thirumal, V.; Sekar, A.; Kim, J. Sustainable Sugarcane bagasse-derived activated carbon for high-performance symmetric supercapacitor devices applications. Nanomaterials 2025, 15, 1028. [Google Scholar] [CrossRef]
- Raccichini, R.; Amores, M.; Hinds, G. Critical Review of the use of reference electrodes in li-ion batteries: A diagnostic perspective. Batteries 2019, 5, 12. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, B.; Ni, M.; Xu, H. A mini-review on three-electrode configuration for Solid Oxide Cells. Energy Rev. 2025, 4, 100128. [Google Scholar] [CrossRef]
- Harris, A.R.; Grayden, D.B.; John, S.E. Electrochemistry in a Two- or three-electrode configuration to understand monopolar or bipolar configurations of platinum bionic implants. Micromachines 2023, 14, 722. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Zanello, P.; Nervi, C.; Fabrizi de Biani, F. Fundamentals of Electrode Reactions. In Inorganic Electrochemistry Theory, Practice and Application; The Royal Society of Chemistry: London, UK, 2011. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriram, G.; Dhanabalan, K.; Oh, T.H. Recent Progress in the Synthesis of Layered Double Hydroxides and Their Surface Modification for Supercapacitor Application. Energies 2025, 18, 4846. https://doi.org/10.3390/en18184846
Sriram G, Dhanabalan K, Oh TH. Recent Progress in the Synthesis of Layered Double Hydroxides and Their Surface Modification for Supercapacitor Application. Energies. 2025; 18(18):4846. https://doi.org/10.3390/en18184846
Chicago/Turabian StyleSriram, Ganesan, Karmegam Dhanabalan, and Tae Hwan Oh. 2025. "Recent Progress in the Synthesis of Layered Double Hydroxides and Their Surface Modification for Supercapacitor Application" Energies 18, no. 18: 4846. https://doi.org/10.3390/en18184846
APA StyleSriram, G., Dhanabalan, K., & Oh, T. H. (2025). Recent Progress in the Synthesis of Layered Double Hydroxides and Their Surface Modification for Supercapacitor Application. Energies, 18(18), 4846. https://doi.org/10.3390/en18184846







