Co-Pyrolysis Behavior of Energetic Materials and Pine Sawdust
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermogravimetric Analysis
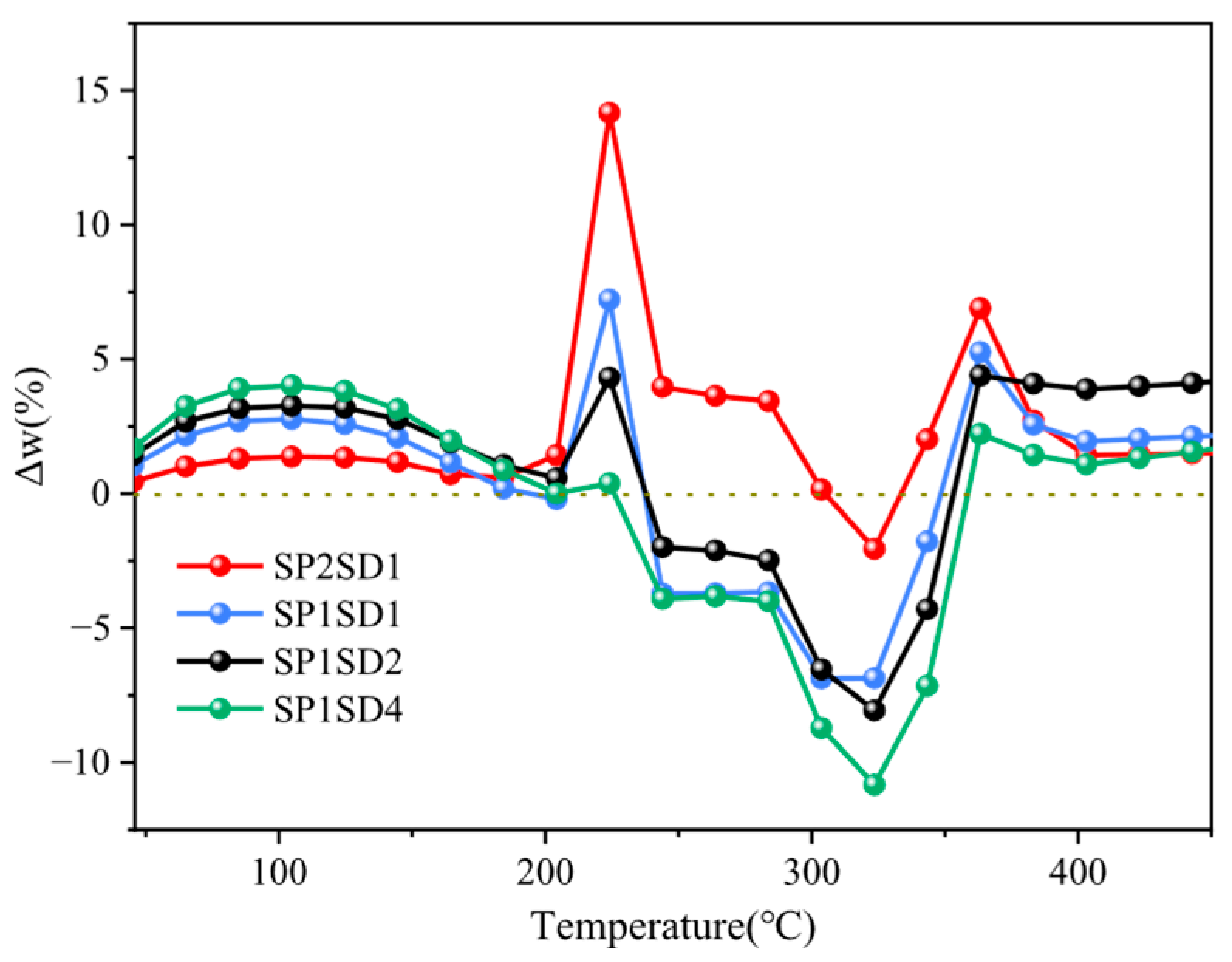
2.3. Co-Pyrolysis Interaction Assessment
2.4. Kinetic Analysis Method for Co-Pyrolysis
2.5. Determination of Enthalpy Change in Co-Pyrolysis
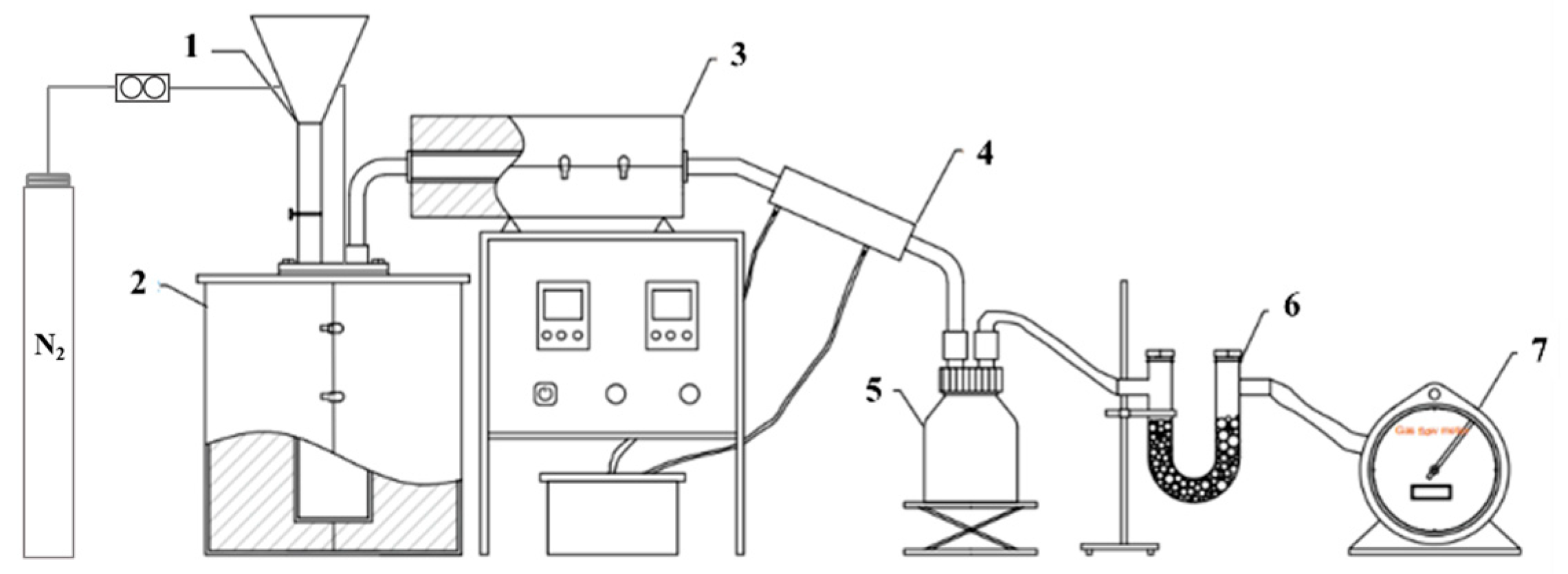
2.6. Fixed-Bed Co-Pyrolysis Experiment
3. Results
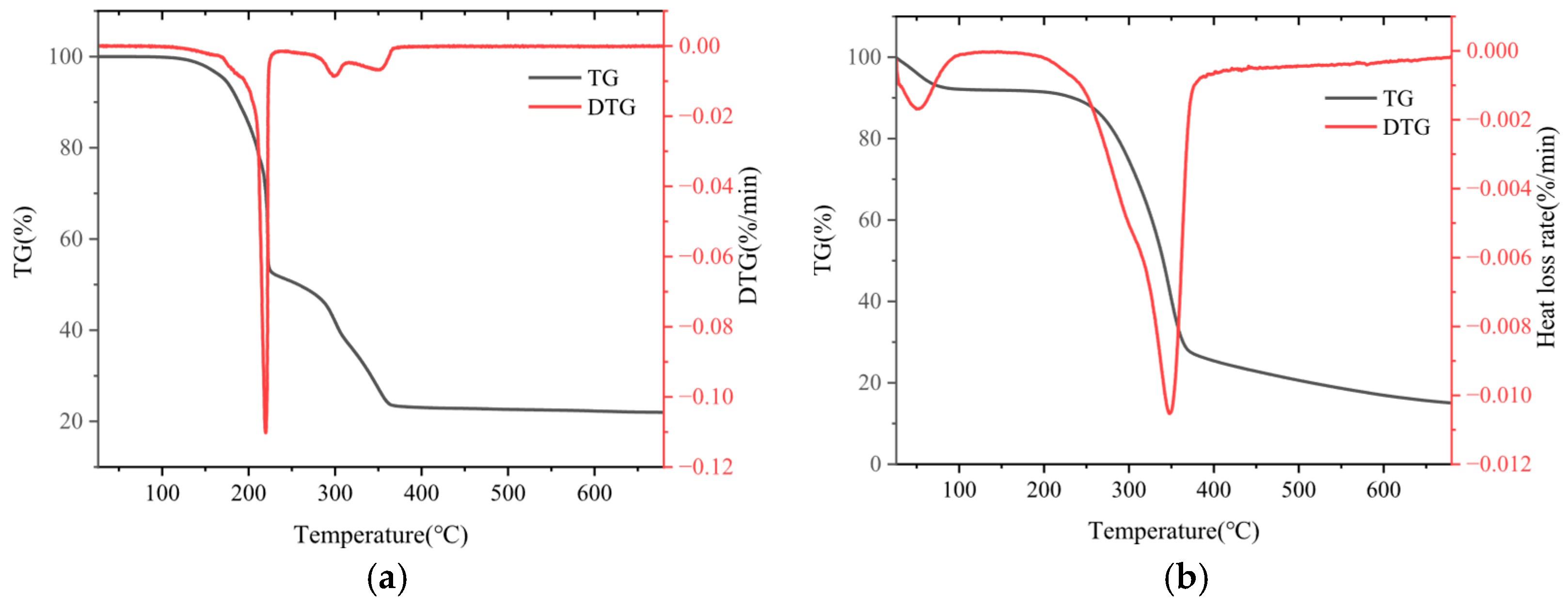
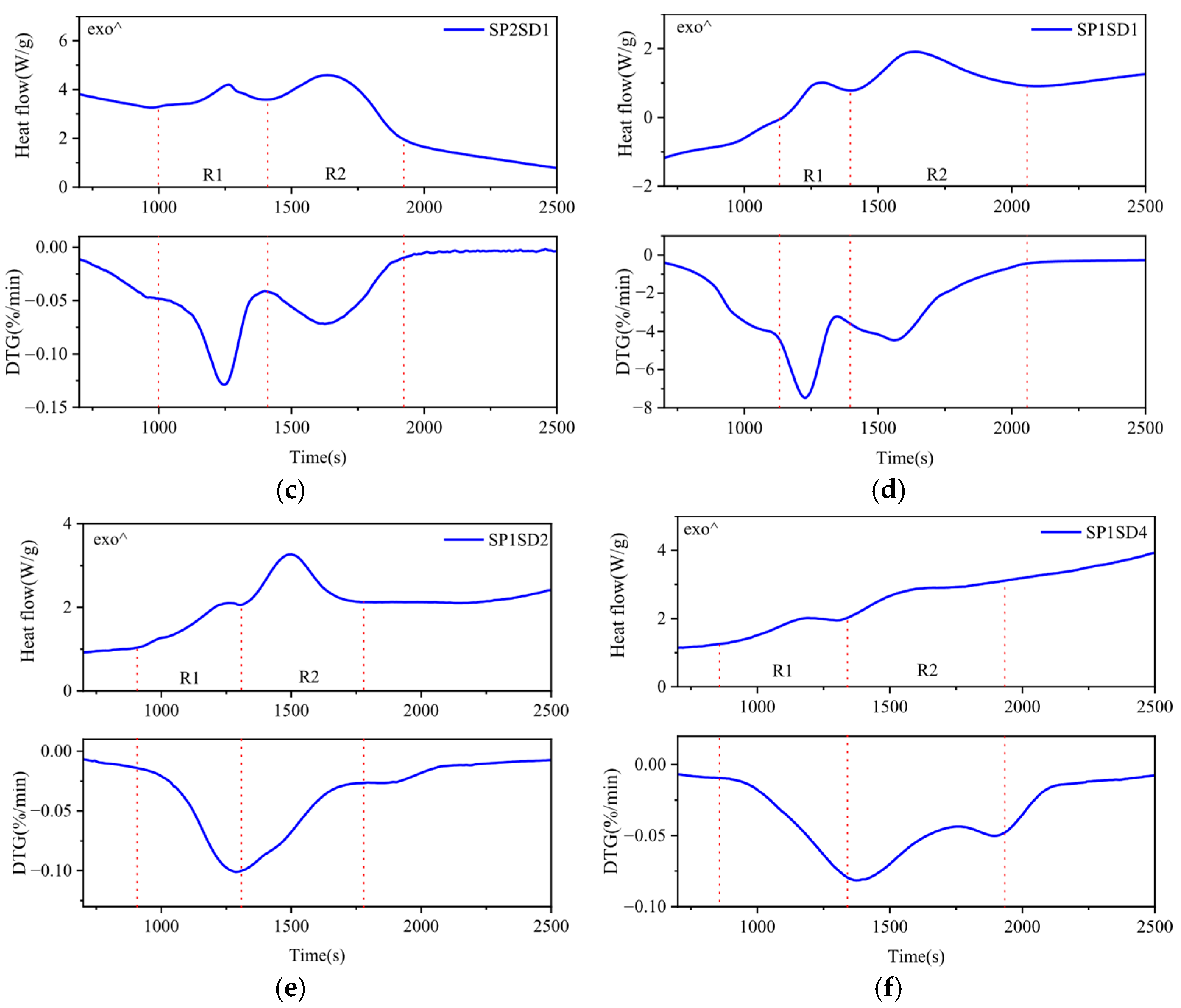
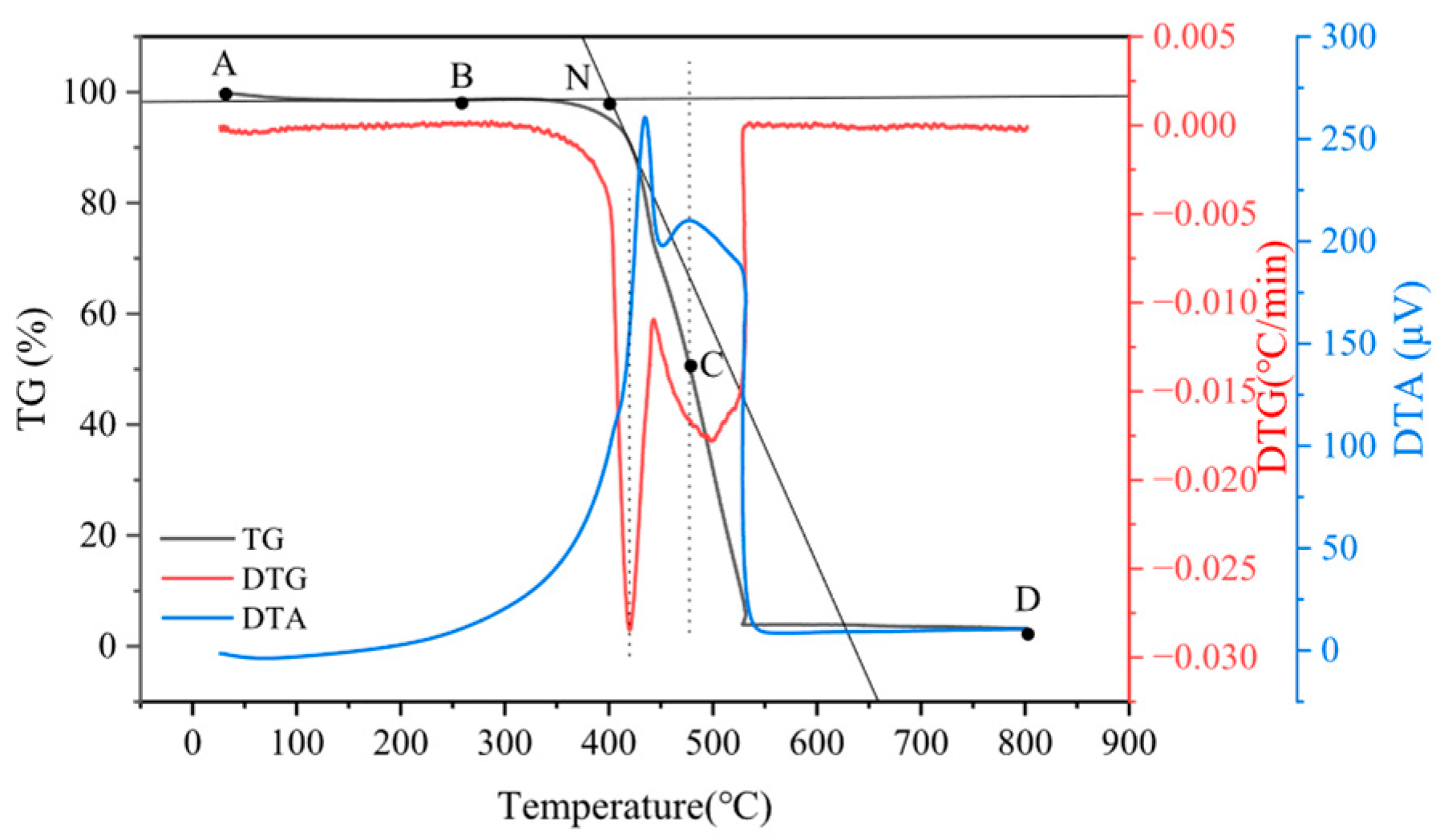
3.1. Thermogravimetric Analysis of SP and SD Pyrolysis
3.2. Kinetic Analysis of the Co-Pyrolysis Process
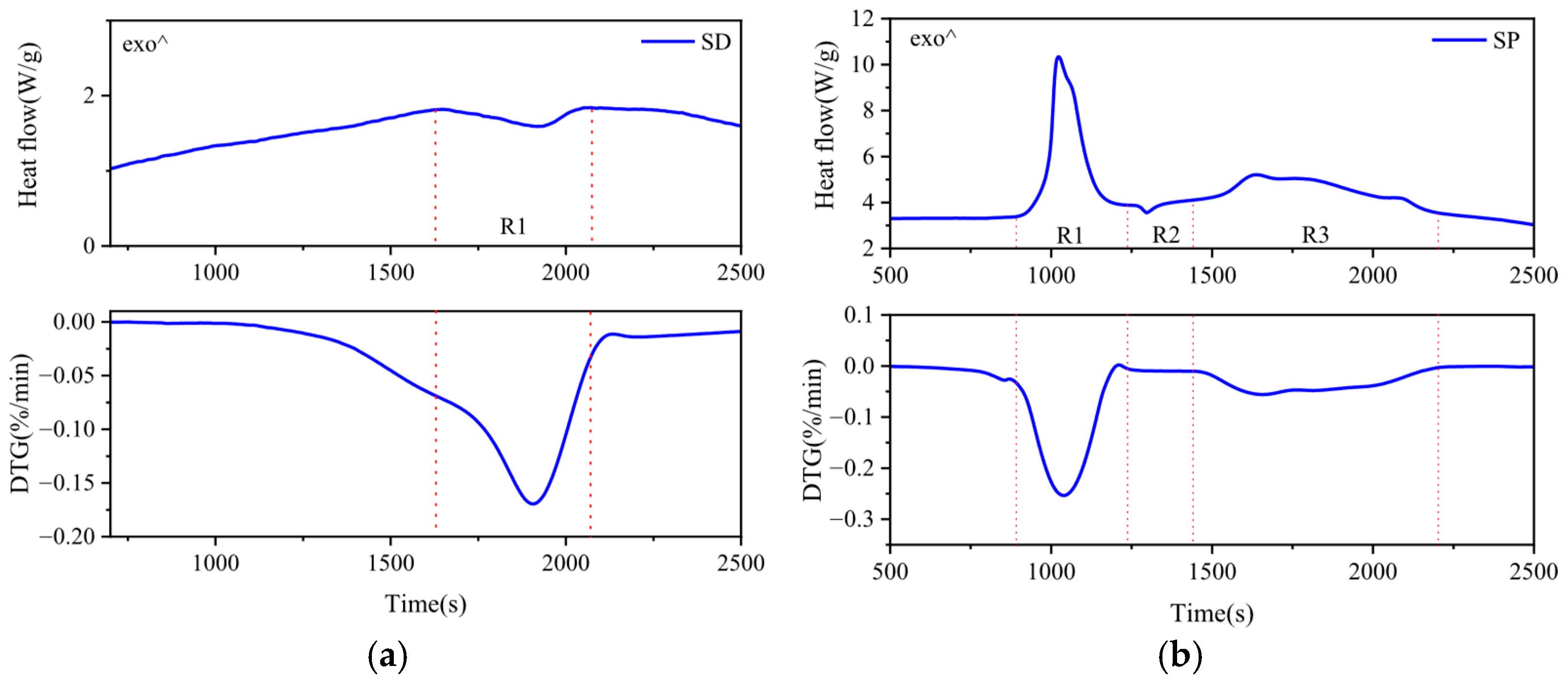
3.3. Enthalpy Change of the Co-Pyrolysis Reaction
3.4. Co-Pyrolysis Product Analysis
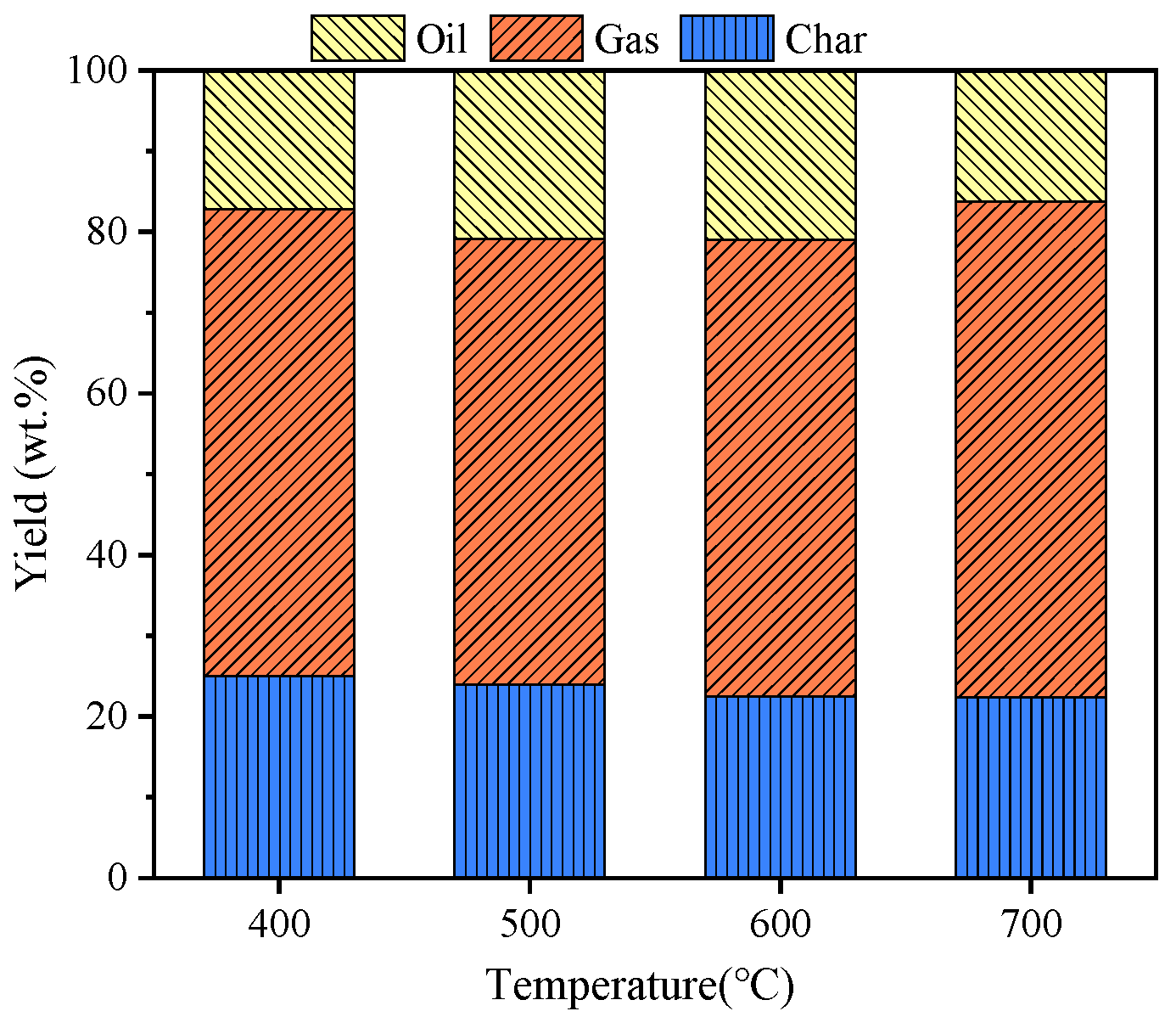
3.4.1. Three-Phase Product Distribution
3.4.2. Co-Pyrolysis Gas Composition Analysis
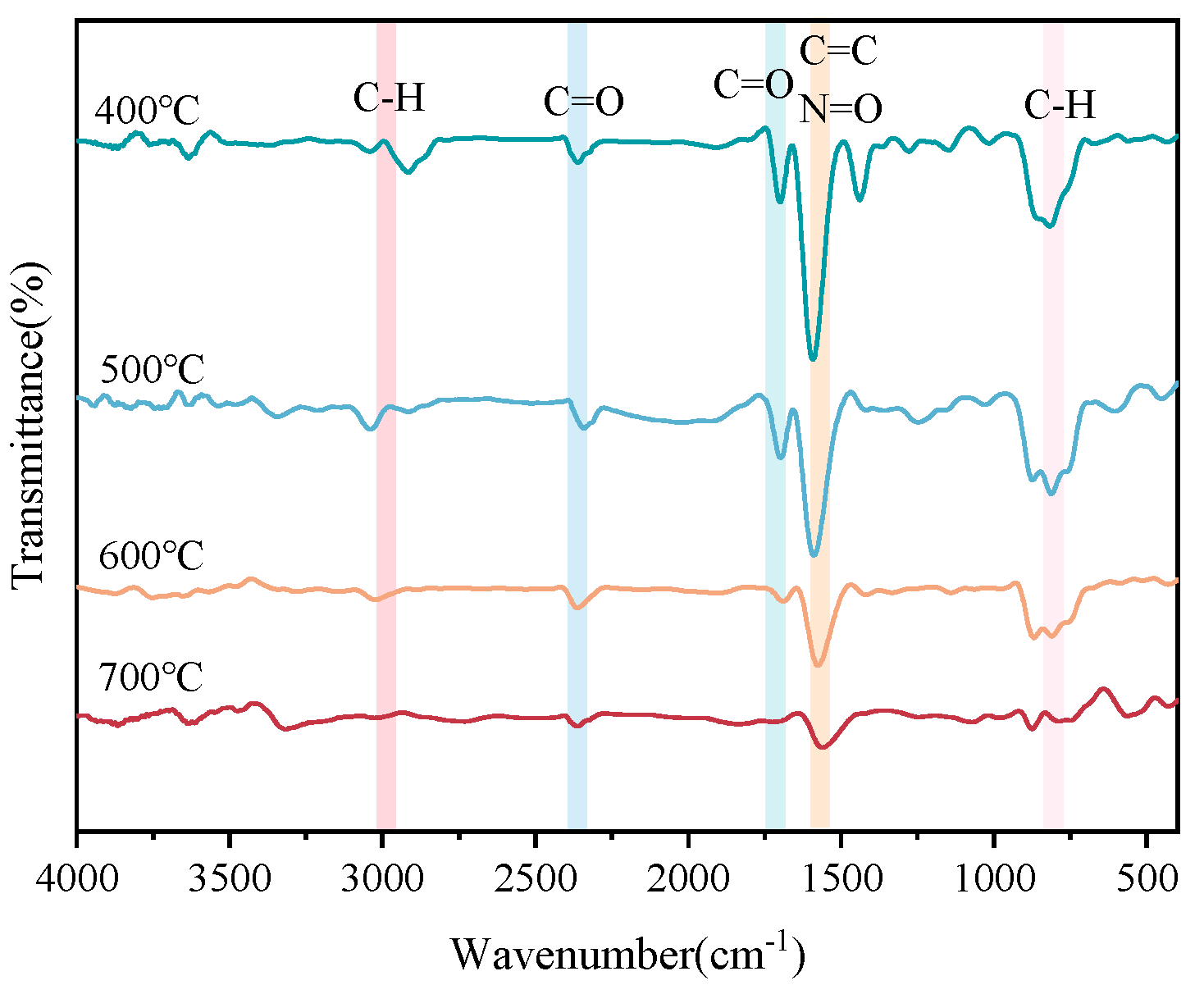
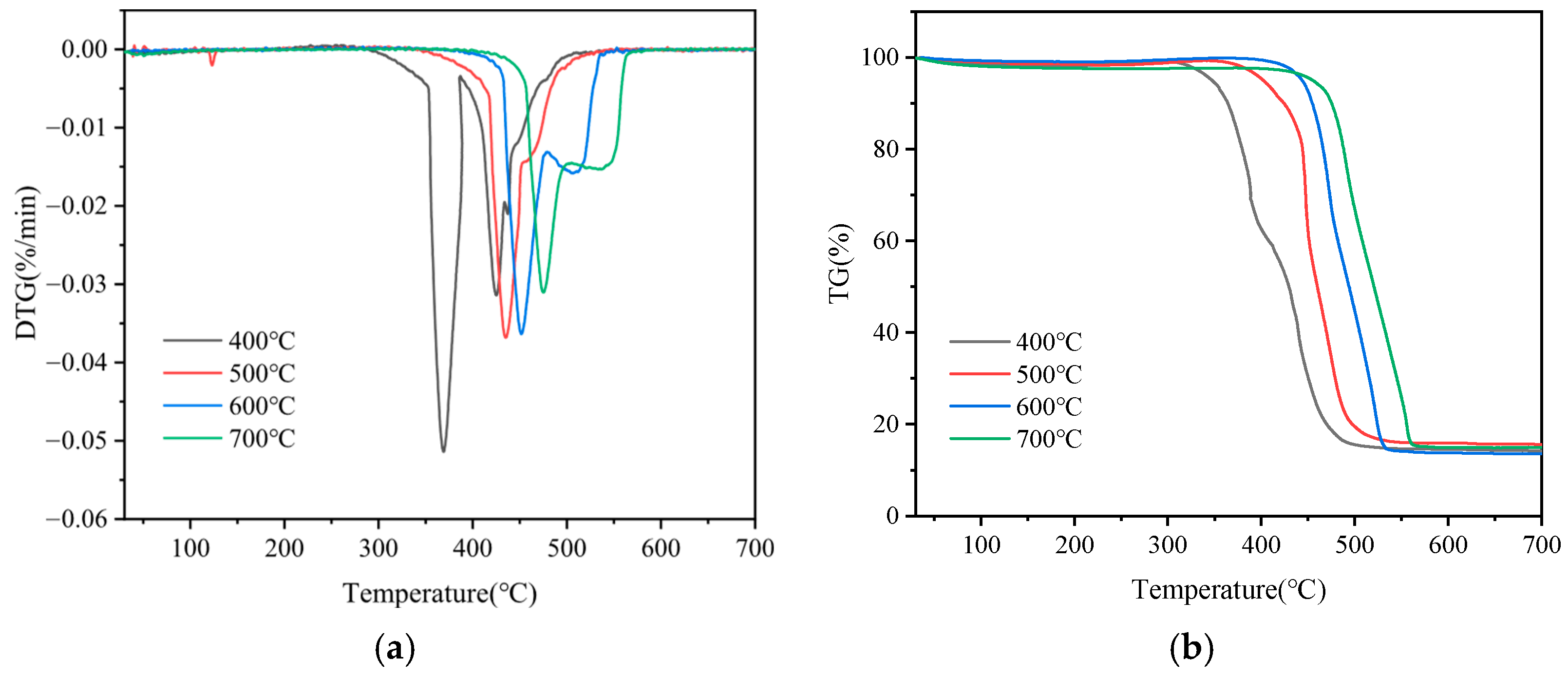
3.4.3. Characterization of Co-Pyrolysis Char
- FTIR analysis of co-pyrolysis char.
- Combustion characteristic analysis of co-pyrolysis char.
3.4.4. Co-Pyrolysis Oil Composition Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naseem, H.; Yerra, J.; Murthy, H.; Ramakrishna, P.A. Ageing studies on AP/HTPB based composites solid propellants. Energetic Mater. Mater. Front. 2021, 2, 111–124. [Google Scholar] [CrossRef]
- Fei, Z.; Sun, M.; Song, Q.; Li, X.; Liu, Y. Freezing-assisted sugaring-out liquid-liquid extraction coupled with LC-MS/MS for quantitative determination of perchlorate in honey. Food Chem. 2024, 435, 137604. [Google Scholar] [CrossRef]
- Liao, J.; Ge, Y.; Liu, C.; Sun, L.; Li, M.; Lu, S. Perchlorate and chlorate in aquatic products from Shenzhen, China, and their implications for human exposure. J. Food Compos. Anal. 2024, 132, 106294. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Trache, D.; Tarchoun, A.F.; Boukeciat, H.; Kadri, D.E.; Hassam, H.; Ouahioune, S.; Sahnoun, N.; Thakur, S.; Klapötke, T.M. Application of co-crystallization method for the production of ammonium perchlorate/ammonium nitrate oxidizer for solid rocket propellants. Chem. Eng. J. 2024, 487, 150654. [Google Scholar]
- Dhabbe, K.I.; Kumari, A.; Manoj, V.; Singh, P.P.; Bhattacharya, B. Development of an eco-friendly method to convert life expired composite propellant into liquid fertilizer. J. Hazard. Mater. 2012, 205–206, 89–93. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhao, A.; Liu, S.; Chen, Y.; Liu, J.; Zhao, W.; Yin, M.; Dong, Q.; Zhang, J.; Zhang, G.; et al. Preparation of nitrogen-containing chemicals from lignocellulosic biomass and nitrogen-rich organic solid waste by pyrolysis: Characteristics, reaction mechanisms, and feedstock interactions. Chem. Eng. J. 2024, 496, 153793. [Google Scholar] [CrossRef]
- Rajput, G.; Liu, B.; Pan, M.; Kumar, A.; Kumari, L.; Farooq, M.Z.; Li, D.; Lin, F.; Ma, W. Valorizing polymeric wastes and biomass through optimized co-pyrolysis for upgraded pyrolysis oil: A study on TG-FTIR and fixed bed reactor. J. Anal. Appl. Pyrolysis 2024, 182, 106686. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, C.; Fan, J.; Ji, T.; Mu, L.; Lu, X.; Zhu, J. Hybrid residual modelling of biomass pyrolysis. Chem. Eng. Sci. 2024, 293, 120096. [Google Scholar] [CrossRef]
- Jin, G.; Wang, M.; Zhang, J.; Chen, L.; Liao, X.; He, W. Efficient reduction of NOx emissions from waste double-base propellant in co-pyrolysis with pine sawdust. Arab. J. Chem. 2023, 16, 104647. [Google Scholar] [CrossRef]
- Islam, A.; Teo, S.H.; Ng, C.H.; Taufiq-Yap, Y.H.; Choong, S.Y.T.; Awual, M.R. Progress in recent sustainable materials for greenhouse gas (NOx and SOx) emission mitigation. Prog. Mater. Sci. 2023, 132, 101033. [Google Scholar] [CrossRef]
- Zhang, Z.; Xi, H.; Zhou, S.; Zhou, W.; Shreka, M. Efficient removal of NOx from simulated marine exhaust by using O3-Na2SO3, Experimental factors and optimization analysis. Fuel 2022, 313, 122659. [Google Scholar] [CrossRef]
- Dong, R.; Tang, Z.; Song, H.; Chen, Y.; Wang, X.; Yang, H.; Chen, H. Co-pyrolysis of vineyards biomass waste and plastic waste: Thermal behavior, pyrolytic characteristic, kinetics, and thermodynamics analysis. J. Anal. Appl. Pyrolysis 2024, 179, 106506. [Google Scholar] [CrossRef]
- Zha, Z.; Li, F.; Ge, Z.; Lu, Q.; Ma, Y.; Zeng, M.; Wu, Y.; Hou, Z.; Zhang, H. Reactivity and kinetics of biomass pyrolysis products for in-situ reduction of NOx in a bubbling fluidized bed. Chem. Eng. J. 2024, 483, 149138. [Google Scholar] [CrossRef]
- Mao, T.; Liu, Z.; Zhang, X.; Feng, H.; Huang, Y.; Xu, Y.; Lin, X.; Zheng, J.; Chen, Z. Interaction evolution and N product distribution during biomass co-pyrolysis for endogenous N-doping bio-carbon. J. Energy Inst. 2025, 118, 101902. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, Y.; Qiang, J.; Deng, Y.; Ren, S.; Elshareef, H.; Li, H.; Dong, R.; Zhou, Y. Experimental study on the regulation of NOx in co-pyrolysis of maize straw and woody biomass. Fuel 2024, 357, 129481. [Google Scholar] [CrossRef]
- Luo, Y.; Mei, W.; Zhou, W.; Gu, J.; Yuan, H.; Yang, G. Co-pyrolysis of hybrid pennisetum and food waste solid digestate: Synergistic effect, products distribution and kinetics. J. Anal. Appl. Pyrolysis 2025, 192, 107243. [Google Scholar] [CrossRef]
- Li, S.; Qu, R.; Hu, E.; Liu, Z.; Xiong, Q.; Yu, J.; Zeng, Y.; Li, M. Co-pyrolysis kinetics and enhanced synergy for furfural residues and polyethylene using artificial neural network and fast heating. Waste Manag. 2025, 195, 177–188. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, Z.; Chen, L.; Liu, P.; Jia, L.; Dong, B.; Li, H.; Xu, S. A study on the decomposition pathways of HTPB and HTPE pyrolysis by mass spectrometric analysis. J. Anal. Appl. Pyrolysis 2023, 170, 105929. [Google Scholar] [CrossRef]
- Arisawa, H.; Brill, T.B. Flash pyrolysis of hydroxyl-terminated polybutadiene (HTPB) I: Analysis and implications of the gaseous products. Combust. Flame 1996, 106, 131–143. [Google Scholar] [CrossRef]
- Li, J.; Yao, X.; Ge, J.; Yu, Y.; Yang, D.; Chen, S.; Xu, K.; Geng, L. Investigation on the pyrolysis process, products characteristics and BP neural network modelling of pine sawdust, cattle dung, kidney bean stalk and bamboo. Process Saf. Environ. Prot. 2022, 162, 752–764. [Google Scholar] [CrossRef]
- Chai, S.; Kang, B.S.; Valizadeh, B.; Valizadeh, S.; Hong, J.; Jae, J.; Lin, K.-Y.A.; Khan, M.A.; Jeon, B.-H.; Park, Y.-K.; et al. Fractional condensation of bio-oil vapors from pyrolysis of various sawdust wastes in a bench-scale bubbling fluidized bed reactor. Chemosphere 2024, 350, 141121. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, S.; Wang, X.; Sun, J.; Liu, J.; Wu, T.; Song, X. Evolution of pyrolysis behaviour of agroforestry biomass waste following bio-board formation. J. Environ. Chem. Eng. 2025, 13, 118763. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Z.; Wang, F.; Wang, X.; Lu, Y.; Zhang, L. Reaction mechanism and kinetic modeling of gas-phase thermal decomposition of prototype nitramine compound HMX. Combust. Flame 2024, 259, 113181. [Google Scholar] [CrossRef]
- Kai, X.; Wang, L.; Yang, T.; Zhang, T.; Li, B.; Liu, Z.; Yan, W.; Li, R. Structure characteristics and gasification reactivity of co-pyrolysis char from lignocellulosic biomass and waste plastics: Effect of polyethylene. Int. J. Biol. Macromol. 2024, 279, 135185. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tian, Y.S.; Zhao, L.X.; Yao, Z.; Meng, H. Industrial analysis and determination of calorific value for biomass based on thermogravimetry. Trans. Chin. Soc. Agric. Eng. 2014, 30, 169–177. [Google Scholar]
- Deng, S.; Xu, K.; Xia, Z.; Yu, S.; Wang, X.; Tan, H.; Ruan, R. Characteristics analysis of char from sewage sludge pyrolysis: Char properties, combustion behavior and ash fusion. J. Environ. Chem. Eng. 2025, 13, 115638. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kameda, R.; Hiratsuka, M.; Kobayashi, J. Initial pyrolysis behavior and char formation characteristics of lignin based on reactive molecular dynamics simulation. Chem. Eng. Sci. 2025, 310, 121531. [Google Scholar] [CrossRef]






| Materials | Proximate Analysis (wt.%, ad) | Ultimate Analysis (wt.%, ad) | HHV (MJ/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | V | M | FC | C | H | O * | N | S | Cl | ||
| SP | 13.63 | 73.90 | 10.84 | 12.47 | 15.42 | 3.36 | 40.02 | 19.39 | 0.17 | 8.01 | 12.52 |
| SD | 0.55 | 82.44 | 6.78 | 17.01 | 50.75 | 6.78 | 40.56 | 1.19 | 0.17 | - | 18.31 |
| α | FWO | KAS | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage 1 (140–260 °C) | Stage 2 (260–420 °C) | Stage 1 (140–260 °C) | Stage 2 (260–420 °C) | |||||
| E (kJ/mol) | R2 | E (kJ/mol) | R2 | E (kJ/mol) | R2 | E (kJ/mol) | R2 | |
| 0.1 | 46.07 | 0.9999 | 95.31 | 0.9999 | 38.88 | 0.9999 | 86.38 | 0.9999 |
| 0.2 | 62.04 | 0.9975 | 102.42 | 0.9976 | 54.53 | 0.9966 | 93.26 | 0.9986 |
| 0.3 | 84.25 | 0.9978 | 109.93 | 0.9958 | 76.58 | 0.9947 | 100.68 | 0.9976 |
| 0.4 | 109.46 | 0.9979 | 112.30 | 0.9921 | 101.48 | 0.9952 | 103.06 | 0.9907 |
| 0.5 | 121.55 | 0.9994 | 118.62 | 0.9840 | 113.65 | 0.9986 | 109.14 | 0.9812 |
| 0.6 | 128.19 | 1 | 117.12 | 0.9933 | 120.21 | 0.9999 | 107.48 | 0.9921 |
| 0.7 | 125.97 | 0.9999 | 110.80 | 0.9999 | 117.83 | 0.9999 | 100.92 | 0.9999 |
| 0.8 | 122.81 | 0.9999 | 111.12 | 0.9995 | 114.67 | 0.9999 | 100.92 | 0.9997 |
| 0.9 | 116.57 | 0.9963 | 116.33 | 0.9980 | 108.35 | 0.9915 | 105.90 | 0.9976 |
| Eavg | 101.87 | 110.44 | 94.02 | 100.86 | ||||
| Samples | Temperature Interval (°C) | Pyrolysis Reaction Heat (kJ/kg) | Sum | |
|---|---|---|---|---|
| SP | R1 | 180–245 | −720.37 | −1080.24 |
| R2 | 245–265 | +15.30 | ||
| R3 | 290–400 | −375.17 | ||
| SD | R1 | 310–380 | +53.98 | +53.98 |
| SP2SD1 | R1 | 220–265 | −70.80 | −565.87 |
| R2 | 265–350 | −495.07 | ||
| SP1SD1 | R1 | 220–265 | −75.48 | −412.88 |
| R2 | 265–320 | −337.40 | ||
| SP1SD2 | R1 | 220–265 | −32.18 | −273.72 |
| R2 | 265–375 | −241.54 | ||
| SP1SD4 | R1 | 210–260 | −58.79 | −165.01 |
| R2 | 260–360 | −106.22 | ||
| Temperatures | Ti (°C) | Tf (°C) | Tm (°C) | dx/dtmax | S (10−9) |
|---|---|---|---|---|---|
| 400 °C | 357.29 | 454.00 | 368.97 | 0.051 | 16.00 |
| 500 °C | 424.93 | 473.00 | 434.77 | 0.037 | 13.00 |
| 600 °C | 445.76 | 522.95 | 451.09 | 0.036 | 7.49 |
| 700 °C | 468.22 | 554.85 | 475.15 | 0.031 | 4.73 |
| Temperatures | Ultimate Analysis (wt.%, ad) | Proximate Analysis (wt.%, ad) | HHV (MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | S | N | O * | M | A | V | FC | ||
| 400 °C | 70.04 | 2.90 | 0.02 | 1.96 | 11.35 | 1.87 | 13.73 | 55.39 | 29.01 | 25.95 |
| 500 °C | 76.96 | 2.61 | 0.01 | 2.45 | 2.36 | 1.62 | 15.61 | 53.32 | 29.45 | 27.51 |
| 600 °C | 80.36 | 2.08 | 0.03 | 2.72 | 1.17 | 0.93 | 13.64 | 53.40 | 32.03 | 29.55 |
| 700 °C | 77.30 | 2.61 | 0.01 | 2.05 | 3.10 | 2.44 | 14.93 | 53.18 | 29.45 | 27.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, C.; Wang, Y.; Gao, N. Co-Pyrolysis Behavior of Energetic Materials and Pine Sawdust. Energies 2025, 18, 4768. https://doi.org/10.3390/en18174768
Quan C, Wang Y, Gao N. Co-Pyrolysis Behavior of Energetic Materials and Pine Sawdust. Energies. 2025; 18(17):4768. https://doi.org/10.3390/en18174768
Chicago/Turabian StyleQuan, Cui, Yufen Wang, and Ningbo Gao. 2025. "Co-Pyrolysis Behavior of Energetic Materials and Pine Sawdust" Energies 18, no. 17: 4768. https://doi.org/10.3390/en18174768
APA StyleQuan, C., Wang, Y., & Gao, N. (2025). Co-Pyrolysis Behavior of Energetic Materials and Pine Sawdust. Energies, 18(17), 4768. https://doi.org/10.3390/en18174768






